1. Introduction
Bananas are the most widely consumed fresh fruit in the world and are part of the diet of millions of people due to their high production potential and remarkable nutritional value. Cultivation is predominantly concentrated in Asia, Latin America, and Africa. In Brazil, it is the second most important fruit in terms of cultivated area and output, with commercial cultivars inlcuding Pacovan, Prata Anã, Nanica, Nanicão, and Grande Naine, each with specific purposes, whether for direct consumption or industrial applications [
1,
2,
3].
Characterizing the agronomic performance of cultivars in different environments is essential for regional adaptation and the selection of genotypes with short harvest seasons, tolerant to the main pests and diseases, and with reduced size and better vegetative development, with the aim of improving banana production in different regions. Furthermore, the post-harvest properties of fruits are essential parameters in evaluating final quality, directly influencing acceptance by the consumer market [
4,
5,
6,
7,
8,
9].
Bananas stand out as a functional food in the search for healthier foods, recognized by conscious consumers who are looking for foods that promote health and well-being. Bananas have been recognized for their nutritional value, with considerable levels of fibre, minerals, phenolic compounds, and vitamins, among others [
10].
In Brazil, the Prata Anã (AAB) cultivar is the most widely grown banana of the Prata subgroup, recognized for its high yield and adapted to the country’s soil and climate conditions. Its acceptance by producers and consumers is due to the quality of the fruit, with its sweet taste and firm texture. It is cold-tolerant, but susceptible to yellow Sigatoka (
Mycosphaerella musicola, Leach) and black Sigatoka (
Mycosphaerella fijiensis, Morelet), fungal diseases that can reduce productivity and fruit quality [
11,
12]. Cultivar SCS451 Catarina (AAB) is a natural mutant of the Prata subgroup, characterized by greater tolerance to Fusarium wilt, a disease caused by the fungus
Fusarium oxysporum f. sp. cubense, which represents a severe threat to banana cultivation [
12]. This characteristic makes it a promising alternative for regions with a history of the disease, contributing to the sustainability of production. The BRS FHIA Maravilha (AAAB) cultivar is the result of a crossing between the Prata Anã (AAB) cultivar and the SH3142 (AA) diploid. This cultivar stands out for its resistance to black Sigatoka, a fungal disease that causes lesions on the leaves, affecting photosynthesis, as well as the productivity and quality of banana fruit [
2]. In addition, BRS FHIA Maravilha is also resistant to Fusarium wilt, increasing its potential for areas with a history of both diseases [
2]. The BRS Pacoua (AAAB) cultivar is a hybrid between the Pacovan (AAB) and Calcutá (AAA) cultivars, exhibiting advantageous characteristics from both genotypes. It shows resistance to Yellow Sigatoka and Fusarium wilt, and BRS Pacoua exhibits medium resistance to Black Sigatoka, thus complementing its resistance against the main banana diseases [
2].
Banana cultivation faces global challenges such as diseases and excessive use of inputs, which affect the sustainability of production and the quality of the fruit. In view of this, there is a need to look for alternatives that combine productivity, environmental sustainability, and a positive social impact. In this context, one of the promising strategies is intercropping, which can favour ecological balance and reduce input costs, as well as provide diversification of the farmers’ sources of income [
13].
Intercropping consists of planting different crops in the same area, providing multiple benefits between the species [
13]. Banana growing can be integrated into various types of intercropping, both as the main crop, using annual food species (beans, corn, rice, cassava) or cover crops, and as a secondary crop, employing perennial plants such as coffee, oil palm, coconut, cupuaçu and cocoa trees. In addition, banana can be a component of agroforestry systems [
14,
15]. The practice of intercropping, particularly using aromatic species such as lemongrass (
Cymbopogon citratus), can be an option for improving sustainable crop management and promoting additional profits through the sale of products or by-products [
8,
13,
16].
The importance of diversifying banana cultivars and the potential of the intercropping system to improve the sustainability of banana fields requires studies to characterize the impact of these methods on fruit quality. Therefore, this study evaluated the effects of banana cultivars, cropping systems (monocropping and intercropping with lemongrass), and the interaction between these factors on the physicochemical characteristics of ripe and unripe fruit.
2. Materials and Methods
2.1. Experimental Area
The cultivation of banana plants was carried out at the São Manuel Experimental Farm, College of Agriculture, São Paulo State University (UNESP), in the city of São Manuel, state of São Paulo, Brazil, located at 22°44′48″ S, 48°34′37″ W, at an altitude of 740 m a.s.l. According to the Koppen climate classification system [
17], the region is classified as a Cwa climate, or as hot and humid temperate (mesothermal). The soil is classified as a sandy-textured Latossolo Vermelho Distroférrico, according to the nomenclature of the Brazilian Soil Classification System [
18], or as Dystrophic Typic Hapludox [
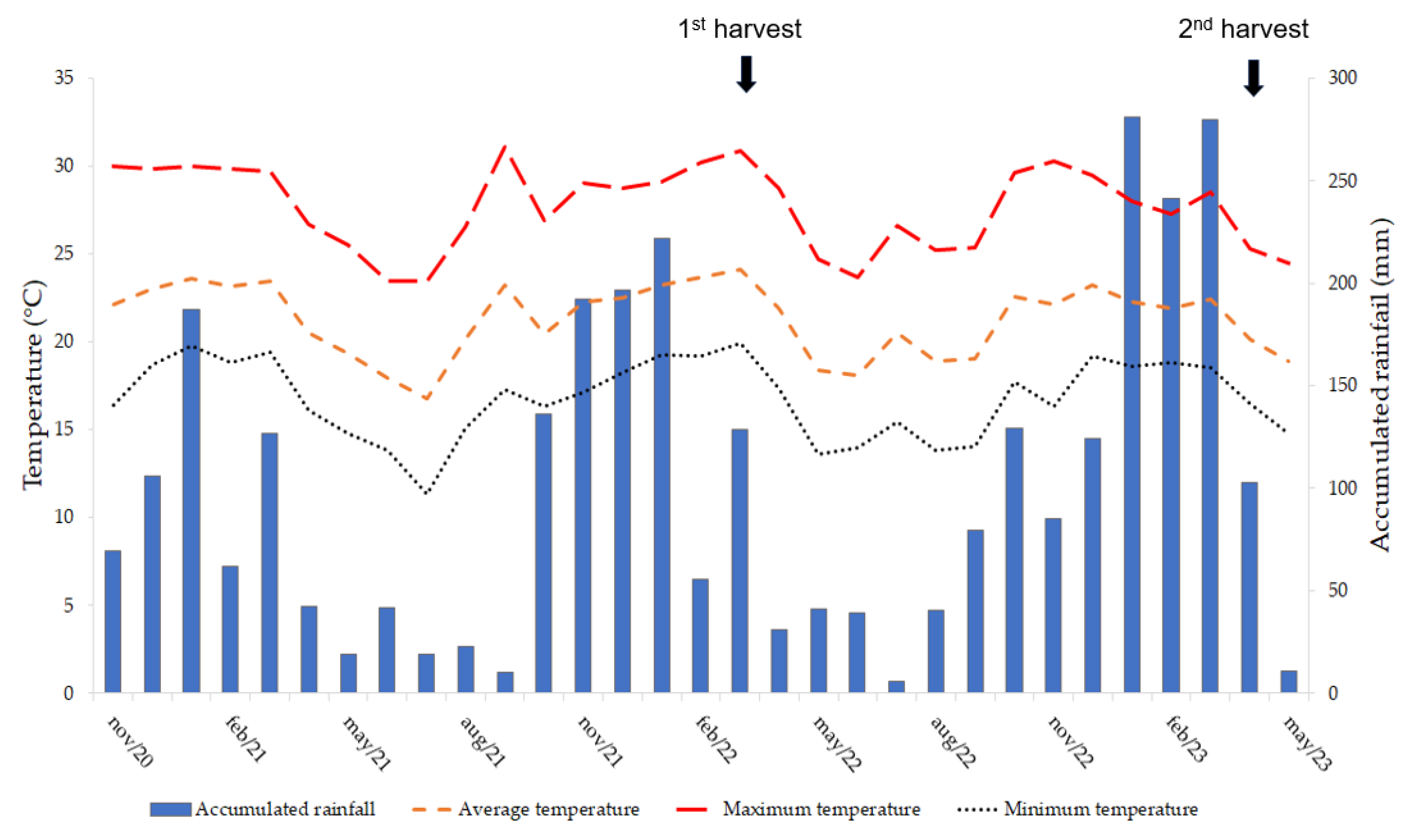
19]. Daily precipitation (mm) levels were obtained from a weather station located 300 m from the experimental area, along with the maximum, minimum, and average temperatures (°C) throughout the experimental period (
Figure 1).
Prior to the implementation of the experiment and during the two harvest seasons, soil samples were collected for chemical analysis from each experimental area (monocropping and intercropping) at a depth of 0–20 cm. The samples comprised 20 sub-samples taken from the plots in the rows and between the rows of the two cropping systems. A clean prober and a bucket were used to collect the samples. After homogenization, each sample consisted of around 300 g of soil, which was sieved (2 mm sieve) and dried in a forced air oven (65 °C), then identified and sent to the laboratory for analysis.
The soil was analysed for pH, organic matter, phosphorus (extracted using anion exchange resin) (P
resin), sulphur (S), potential acidity (H+Al), potassium (K), calcium (Ca), magnesium (Mg), sum of bases, cation exchange capacity, base saturation, iron (Fe), copper (Cu), boron (B), manganese (Mn), and zinc (Zn). Minerals were determined by atomic absorption spectrophotometry (AAnalyst 800, Perkin Elmer, Shelton, CT, USA) after nitric acid (HNO
3)—perchloric acid (HClO
4) digestion [
20]. The chemical properties of the soil in the experimental area are shown in
Table 1. The particle sizes were 84.3% sand, 12.1% clay, and 3.6% silt (sand soil).
The experimental area was previously prepared through ploughing, sorting, and liming, according to the soil analysis and indications for the crop [
21].
Four banana cultivars were evaluated, Prata Anã, SCS 451 Catarina, BRS FHIA Maravilha, and BRS Pacoua, which are resistant to the main banana diseases and have the potential to contribute to the diversification of cultivars.
The seedlings of the banana cultivars were produced by tissue culture in a biofactory located in the city of Cruz das Almas, state of Bahia, Brazil. The seedlings were planted in 1 L plastic bags filled with Carolina Soil® substrate (Santa Cruz do Sul, RS, Brazil) and were kept in a mini-tunnel before being transplanted into the field. The seedlings were transplanted to the field in November 2020, with a spacing of 3.0 m between rows and 2.5 m between plants, in a dryland system.
The lemongrass seedlings were obtained from the medicinal plant garden and grown in a nursery at Fazenda Experimental de São Manuel (UNESP), São Manuel city, São Paulo state, Brazil. The seedlings were transplanted to the field in February 2021, employing a spacing of 60 cm between plants in the banana row, with a total cultivation density of two lemongrass plants between banana trees in the row.
Weed control, tiller thinning, pest and disease control, male inflorescence elimination, and pistil removal were performed according to recommended practices for the crop [
21].
2.2. Treatments and Experimental Design
The experimental design used randomized blocks in a split plot arrangement (4 × 2), with the mathematical model based on the following formula:
yijk = the observation made in the k-th block, the i-th primary treatment, and the j-th secondary treatment;
µ = a constant common to all observations;
βk = the effect of the k-th block, for k = 1, …, r;
Ai = the effect of the i-th primary treatment, for i = 1, …, α.
The plots corresponded to the four banana cultivars (16 plants per cultivar), and the subplots corresponded to the treatments with and without lemongrass intercropping, totalling 32 plants. Replicates consisted of four plants per experimental plot. Each cropping season was analysed separately.
2.3. Harvest and Sample Preparation
A replicated trial was conducted during two consecutive cropping seasons. The first season occurred from November 2020 to May 2022 and the second from August 2022 to May 2023. The first cropping season corresponded to the period between planting and harvesting, whereas the second season occurred between inflorescence emissions and harvesting. The average periods of the first season for monocropping and intercropping were 595 and 550 days, respectively. In the second season, the periods were 145 and 140 days for monocropping and intercropping, respectively.
The banana bunches were harvested in the morning, when the central fruit of the second hand had reached a minimum calibre of 34 mm [
21]. The second hands of each bunch were separated, sanitized with chlorinated water, and the ripe and unripe fruit were evaluated for physicochemical characteristics. (
Figure 2).
2.4. Ripe Fruit Quality Evaluation
Aiming to reduce the heterogeneity of hand ripening between banana cultivars, a concentration of 4 mL/L of Etil
® concentrate (São Paulo, SP, Brazil) was sprayed directly onto the hands. The hands were then kept on benches at room temperature (median value 25 °C) until they were fully ripe. The fruits were analysed when they reached ripeness stage 6 (100% yellow skin), according to the Von Loesecke scale [
22]. The following analyses were carried out on the five central fruits of the second ripe hand in two harvest seasons.
- -
Firmness: obtained using a texturometer (TA. XT plus stable micro-systems, Surrey, UK). The measurement was performed using a probe TA-52 (2 mm) with the following operating conditions: penetration distance into the sample: 20 mm, pre-test speed: 1.0 mm/s, test speed into the sample: 2.0 mm/s, post-test speed: 10 mm/s. Readings were taken at two different central points of the fruit, with and without the peel, and the results were expressed in newton (N) units [
23].
- -
Fresh fruit mass and pulp/peel ratio: obtained by weighing the whole fruit (peel and pulp) on a digital scale (BEL, model RB 16001, Piracicaba, SP, Brazil) to obtain the fresh fruit mass and then weighing the pulp and peel of each fruit individually to obtain the pulp/peel ratio [
23].
- -
Titratable acidity: a total of 5.0 g of pulp, homogenized in a mixer, was added to 95 mL of distilled water, and the suspension was titrated with a standardized 0.1 N NaOH solution, using the phenolphthalein turning point as an indicator. The results were expressed as a percentage, corresponding to grams of malic acid in 100 g of pulp [
24].
- -
Soluble solids: determined on an aliquot of homogenized pulp by direct reading on a digital refractometer, with automatic temperature compensation (model Palette PR-32, ATAGO, Ribeirão Preto, SP, Brazil). The results were expressed as °Brix.
- -
Ripening index: obtained from the ratio between soluble solids content and titratable acidity.
- -
pH: measured in the homogenized fruit pulp using a digital potentiometer (model K38-1465, KASVI, Pinhais, PR, Brazil), calibrated with pH 7.0 and 4.0 buffers at 25 °C [
25].
- -
Moisture: determined according to method 934.06 of the Association of Official Analytical Chemists (AOAC) [
24]. The results were expressed as a percentage, based on the fresh matter.
- -
Total and reducing sugars: The total sugar content was determined using spectrophotometry, according to the methodology of Nelson [
26] and Somogyi [
27], employing the acid hydrolysis step for the determination of total sugars [
24]. The results were expressed as a percentage, based on the fresh matter.
2.5. Total Starch, Resistant Starch, and Mineral Content in Unripe Fruits
Ten fruits at stage 1 of ripeness (completely green peel) [
21] were randomly selected from the third and fourth hands of the bunches to analyse the total starch, resistant starch, and mineral content in the banana pulp. The fresh fruit pulp was sliced (6 mm thick) and dehydrated in an oven with air circulation (45 °C/48 h) to avoid gelatinization of the starch and changes in the resistant starch content [
28].
- -
Total starch: the total starch content of the dehydrated fruit was determined by enzymatic hydrolysis using α-amylase and amyloglucosidase (method 996.11) [
25].
- -
Resistant starch: resistant starch content was determined according to the method described by Goni et al. [
29]. Glucose content was determined using the glucose oxidase–peroxidase method, and resistant starch content was calculated using the following formula:
S1: content of reducing sugar in the sample;
S0: content of reducing sugar in the blank sample;
V0: constant volume;
V1: volume of liquid collected during measurement;
W: weight of the sample.
The results were expressed as g 100 g−1 (dry basis).
- -
Minerals: the content of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), and zinc (Zn) in the dehydrated samples of unripe banana pulp were determined according to the method described by Malavolta et al. [
20]. The N content was determined using sulfuric acid (H
2SO
4) digestion and quantified using the semi-micro Kjeldahl method. P, K, Ca, Mg, S, Cu, Fe, Mn, and Zn contents were determined by atomic absorption spectrophotometry (Analyst 800, Perkin Elmer, Shelton, CT, USA) after nitric acid (HNO
3)—perchloric acid (HClO
4) digestion. The results were expressed as mg 100 g
−1 (dry basis).
2.6. Data Analysis
Data were submitted to analysis of variance (ANOVA) and compared using Tukey’s test at the 5% significance level. The effects of the interaction between banana cultivars and cropping systems were assessed. When the interaction was significant, the data was analysed, and if it was not significant, the isolated effects of the treatments, cultivars, and cropping systems were analysed and discussed. The computer program R v.4.3.1 (R Core Ream, 2023) was used for all analysis [
30].
4. Discussion
This study demonstrated that intercropping banana crops with lemongrass did not interfere with the quality characteristics of the ripe fruit (
Table 2), a result which was also reported by Almeida et al. [
31] when they evaluated the chemical quality of the fruits of the second hand of banana cultivar D’ Angola in intercropping with açaí palm (
Euterpe oleracea Mart).
In the study by Botelho et al. [
32], only certain quality parameters of banana fruit grown via intercropping with tree species (eucalyptus Urocam VM01 (
Eucalyptus urophyla S.T. Blake ×
E. camaldulensis Dehnh.), casuarina (
Casuarina equisetifolia L.), and acacia (
Acacia mangium Willd.) were influenced by intercropping, with the mass and length of the fruit and the soluble solids content being lower than those of the banana fruit in monocropping, but with the fruits meeting the quality standards for commercialization.
In the first season, harvests occurred around 45 days earlier in the intercropping system. The main hypothesis for this result is that this cropping system probably utilized available resources more efficiently, a result which was not observed in the second harvest season.
The intercropping of fruit trees with other crops appears as a promising farming system for smallholders. Studies such as the one conducted by Andrade Neto et al. [
33] indicate that, when well planned, intercrops offer a series of significant advantages, representing a viable alternative to monocropping. Furthermore, intercropped crops provide an increase in cycling and efficiency in the use of nutrients, resulting in benefits for both the soil and the crops involved [
34]. This cultivation system can also play an important role in the recovery of degraded areas, contributing to environmental restoration and the conservation of natural resources [
8,
33,
34]. Therefore, the results of this study showed that intercropping with lemongrass can be an option for sustainable agricultural practices, without compromising the quality of banana fruits.
Differences were observed among cultivars for the quality parameters of both ripe and unripe fruits (
Table 3,
Table 4,
Table 5,
Table 6 and
Table 7), which can be attributed to factors such as genetic inheritance and adaptation to the environment, among others [
32].
The SCS451 Catarina cultivar stood out for its higher fruit mass and the BRS Pacoua cultivar for its lower pulp/peel ratio (
Table 3). The lower pulp/peel ratio of BRS Pacoua suggests that this cultivar is not adapted to the subtropical region of the study, emphasizing the importance of choosing cultivars adapted to the region’s soil and climate conditions and of proper banana management in order to increase fruit quality. Different percentages of pulp and peel in the ripe banana fruits of 15 banana genotypes were observed by Aquino et al. [
35], with a variation in the pulp/peel ratio from 1.43 to 4.18.
Pulp fresh mass (FM) and pulp/peel ratio (PPR) are important quality parameters for banana fruits, as they are related to pulp yield, which is a relevant criterion for the concentrated products industry, where cultivars with high pulp yield are selected in order to generate greater profitability [
36]. The PPR is a variable that tends to increase as the fruits ripen, due to the more accelerated dehydration of the peel compared to the pulp. In addition to losing water to the pulp, the banana peel loses water to the environment through transpiration, resulting in an increase in PPR during ripening [
37].
Fruit firmness is an important variable to determine post-harvest quality and the viability of fruit transportation. Damatto Júnior et al. [
38] characterized firmness during the fruit ripening stages of the Prata Anã and Prata-Zulu cultivars and observed mean values of 1.58 N for ripe fruits, which are close to those presented in this study (
Table 3). The ripening stage and genetic characteristics of the cultivars directly influence firmness, with firmness as a prime indicator of fruit durability during handling and transportation. Fruits with greater firmness are favoured, as they are less likely to fall, an occurrence that can compromise the integrity and commercial value of the fruit [
37,
39,
40,
41].
Titratable acidity values vary depending on the stage of fruit maturation, with a sharp decrease generally being observed when the fruits are close to the senescence stage [
42]. The mean titratable acidity of fruit pulps from all banana cultivars (
Table 4) was higher than the minimum of 0.20% established by Normative Instruction No. 37 of the Ministry of Agriculture, Livestock, and Food Supply (MAPA) for banana pulp [
43]. This characteristic highlights the quality and the potential of these cultivars to meet the standards established by national legislation.
The mean total solid soluble values (SS) (23.7 °Brix in the 1st season and 24.7 °Brix in the 2nd season) (
Table 4) exceeded the minimum value of 18 °Brix established for ripe banana pulp [
43]. The SS value tends to increase as the fruit ripens.
The ratio between soluble solids and titratable acidity (SS/TA) is indicative of the sensory quality of fruits, reflecting the balance between sweetness and acidity. This parameter is recognized as one of the most representative for evaluating fruit ripeness and palatability, as a higher ratio indicates an advanced stage of ripeness and a potentially more pleasant flavour due to the ideal balance between sugars and organic acids [
44,
45]. The results obtained in this study (
Table 4) are similar to those observed by Batista et al. [
42], who reported values for titratable acidity of 0.57 and 0.58% malic acid, 4.56 and 4.63 pH, 25.2 °Brix and 25.7 °Brix soluble solids, and an SS/AT ratio of 44.5 and 44.7, respectively, for the Prata Anã and SCS 451 Catarina cultivars.
The moisture content in the pulp of ripe banana fruit varies (
Table 5). Bezerra and Dias [
46] reported moisture contents in the fruits of banana genotypes grown in Brazil similar to those observed in this study, with variation from 73.68 to 75.61% in fruit pulps of the cultivars Caipira, Thap Maeo, PV03-44, FHIA-01, FHIA-18, and FHIA-21.
The carbohydrate composition of bananas changes considerably during ripening, with an increase in the percentage of sugars in the pulp during fruit ripening due to starch hydrolysis. All cultivars presented a higher percentage of reducing sugars than non-reducing sugars (
Table 5). In the study by Aquino et al. [
36] using fifteen banana cultivars, the authors also observed variations regarding sugars in the pulps of ripe fruits, with TSS ranging from 10.9 to 23.06%, and RSS ranging from 4.18 to 12.58%. Total soluble sugar contents can vary significantly, with levels ranging from 15 to 20%, with a direct relationship between the sugars present in the fruit and the flavour. Furthermore, sugars play a fundamental role in determining shelf life, so fruits with high sugar contents tend to have a longer shelf life [
44,
47,
48].
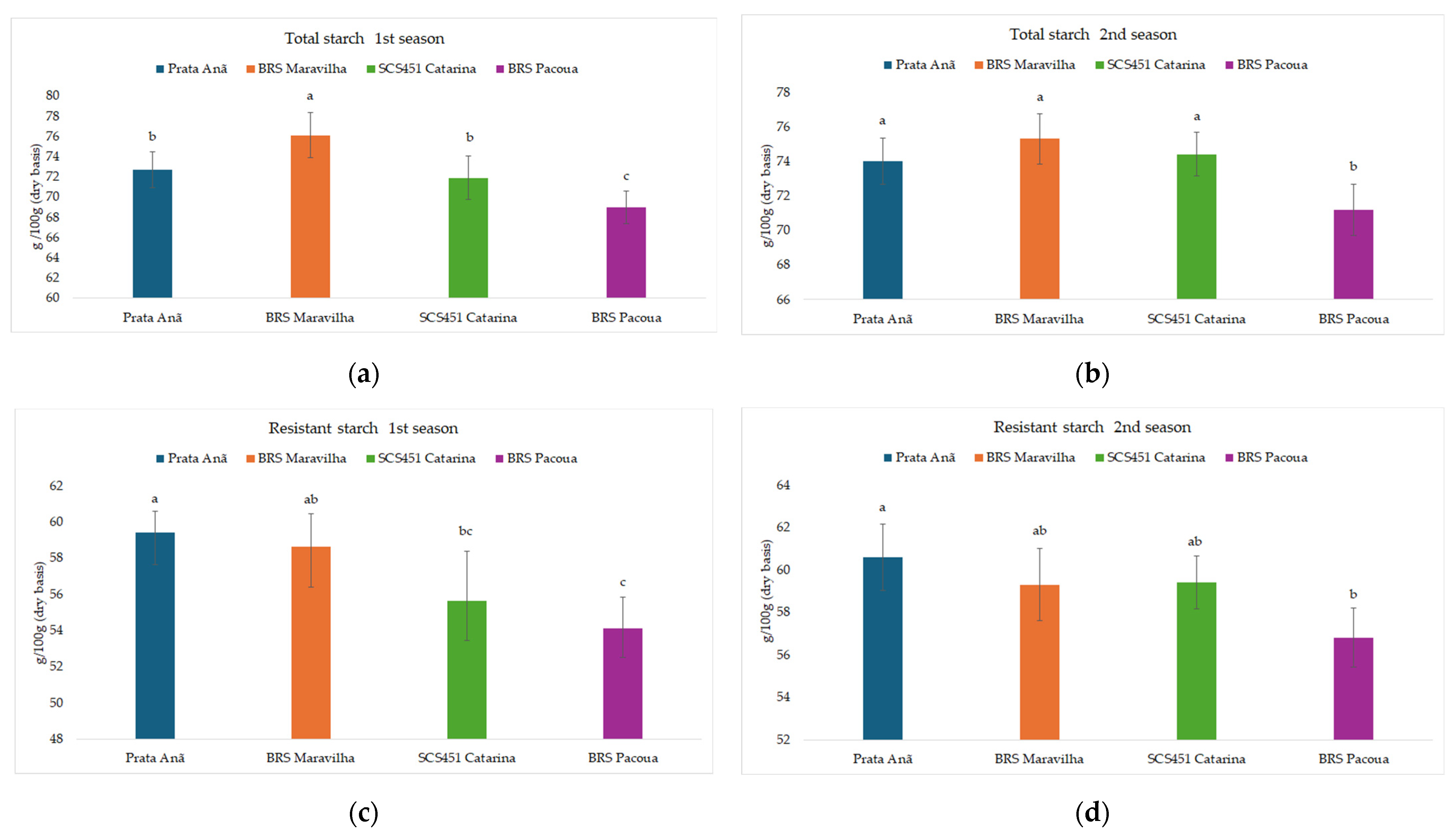
Unripe banana fruits exhibit high starch contents in dry matter, and this starch displays physicochemical and functional properties different from those of conventional starches, making this fruit a potential source of starch for industries [
49,
50]. Starch normally constitutes between 60% and 80% of the mass of dehydrated green banana pulp, reaching up to 90% under certain conditions [
50], in agreement with the levels found in the present study (
Figure 3).
Resistant starch (RS) has been defined as the starch fraction which escapes digestion in the small intestine of healthy individuals [
51,
52]. The resistant starch contents of unripe fruits (
Figure 3) are in accordance with the values reported by Reis et al. [
53], which showed average resistant starch contents of 58.3% in the Prata Anã cultivar.
Starch is an essential carbohydrate for food, as it provides energy and satiety. Nevertheless, its high digestion rate promotes a rapid rise in blood sugar levels, leading to inflammation, oxidative stress, and reduced insulin sensitivity, resulting in an increase in chronic diseases. Resistant starch is the portion of total starch that is partially or totally undigested and absorbed in the small intestine and is recognised as a functional food that promotes health benefits such as improving bacterial activity in the colon; reducing the risk of cancer; reducing the glycaemic index and cholesterol levels, contributing to a reduction in the incidence of type 2 diabetes and coronary heart disease [
54,
55,
56,
57,
58,
59].
The quantification of total starch and resistant starch content is of great relevance in the selection of banana cultivars for the starch industry, since total starch is directly related to the yield of the extraction process, while the content of resistant starch adds value to banana starch as a functional ingredient [
55,
56]. Therefore, these analyses are fundamental for the development of cropping and processing strategies that maximize the efficiency of starch production and meet market demands for functional foods [
56].
Variations in resistant starch content between different banana cultivars can be attributed to factors such as crystallinity, granule size, and amylose content. These factors can influence the digestibility of banana starches, which emphasizes the importance of genotype selection to improve the nutritional and functional properties of bananas [
52,
58]. Furthermore, high resistant starch contents can add value to banana cultivars, since this type of starch is known to improve the functional properties of foods, contributing to the availability of more nutritious food products with health benefits [
49].
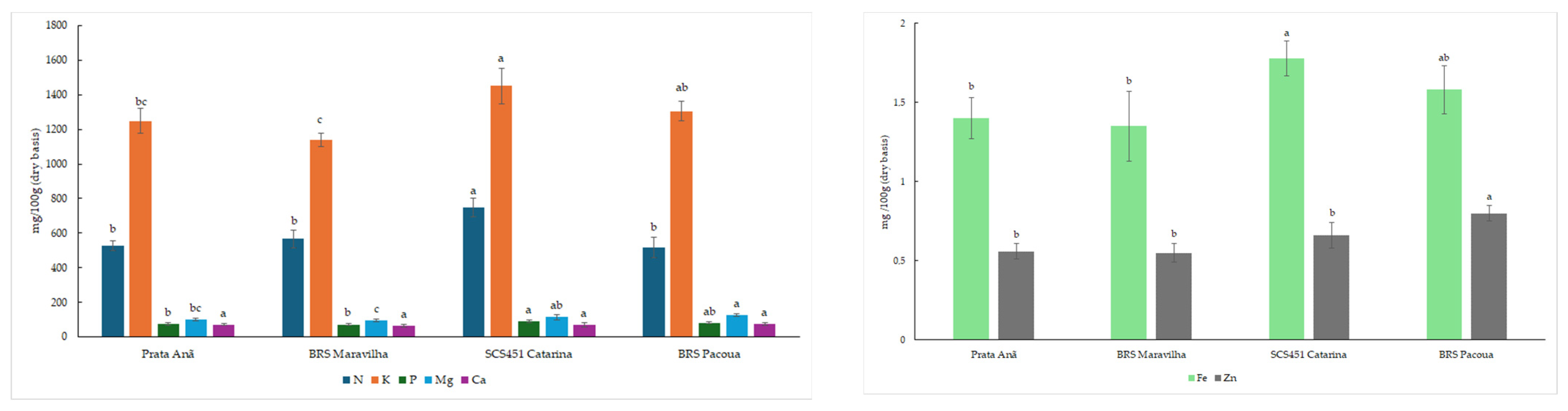
Minerals play an important role in maintaining proper function and good health in the human body, and bananas are a fruit with a considerable level of minerals. The results showed variations in mineral content for the banana cultivars (
Table 7,
Figure 4), with the SCS451 Catarina cultivar identified as exhibiting the highest total mineral content. The order of concentration of minerals in the green banana pulps for all cultivars was K > N > Mg > P > Ca > Fe > Zn. Zinc (Zn) is an important micronutrient, and the lower Zn content in unripe banana pulp can be attributed to a deficiency of this mineral in the soils used for cropping in Brazil [
59].
Variations in the mineral contents in banana fruit can be explained by cultivars, crop management, and climate change [
60,
61]. Khoza et al. [
60] analysed the mineral content in immature fruit from five banana cultivars (Grande Naine, FHIA-01, Finger Rose, Pisang Awak, and Du Roi) and observed values ranging from: K, 290.95 to 1033.25 mg/100 g; P, 31.72 to 99.25 mg/100 g; Mg, 92.4 to 118.5 mg/100 g; Ca, 8.7 to 28.25 mg/100 g; Fe, 1.33 to 2.88 mg/100 g; and Zn, 0.18 to 0.93 mg/100 g on a dry basis.
Huang et al. [
10], investigating changes in the physicochemical properties and nutritional composition of banana fruit (AAA Cavendish var. Pei Chiao) at ripening stages 1 to 9, observed variations in mineral content, with K varying from 887.76 to 933.57, Mg from 59.36 to 73.30, P from 57.81 to 64.71, Ca from 25.20 to 27.86, and Fe from 0.59 to 0.63 mg/100 g (d.b.).
It is important to highlight the nutritional relevance of green bananas as a raw material for obtaining flour, especially in the context of the recommended daily intake of minerals. Potassium helps control blood pressure and heart function, with a recommended daily intake of 4700 mg for adults. Magnesium plays essential roles in the cardiovascular system, glycaemic control, and bone and muscle health, with a recommended daily dosage of 420 mg for adult women and 450 mg for adult men. Phosphorus contributes to calcium absorption, as well as to bone and dental health, being essential for children and pregnant women, with recommended daily intake values varying between 250 and 1000 mg, depending on the age group. Calcium is essential for bone development, as well as muscle and nerve function, with a recommended daily dosage of 1000 mg for adults. Iron participates in the synthesis of haemoglobin, oxygen transport, and cell growth, being crucial for preventing anaemia, with recommended daily intake levels ranging between 14 and 18 mg. Zinc supports the immune system, wound healing, and sexual development, with a recommended daily dosage of 8 mg for adult women and 11 mg for adult men [
10,
61,
62,
63,
64].
The differences between the banana cultivars in the two harvest seasons highlighted genetic differences. In addition, the results emphasized the ability of the cultivars evaluated to adapt to the environmental conditions of the subtropical region studied, which are considered favourable for banana cultivation, if adapted cultivars are used. The relationship between banana genotypes, cropping systems, and the climatic conditions of the sites where the bananas are grown shows that these studies are important for crop management and the successful sustainability of orchards.
Bananas evolved to exploit the sunny spaces in the humid forests of Southeast Asia and have been part of the human diet for thousands of years [
65]. To solve phytosanitary and cultural problems, new banana cultivars have been developed over the years through genetic improvement. However, the creation of new banana cultivars is time-consuming and labour-intensive, largely due to hybrid sterility problems [
65]. Given the inherent vulnerability of monocultures to the combined threats of climate change, pests, and diseases, genotype diversification must be a priority. Diversification of banana genotypes is necessary for a sustainable and profitable crop [
2]. Therefore, the evaluation of new cultivars in the field is an urgent and necessary measure for the success of this crop. Further studies in this direction should be continually encouraged.
The use of aromatic species in intercropping can provide farmers with additional income. Lemongrass is widely cultivated for the commercial exploitation of its essential oil, which exhibits various medicinal properties, mainly in the form of leaf tea, with uses in the pharmaceutical, food, cosmetics, and perfumery industries, and as such, it could be a more profitable component crop in the system [
66,
67,
68]. The results obtained in this study are considered to be very favourable, as they can support the recommendation of banana and lemongrass intercropping, since the fruit quality indicators were not altered when banana and lemongrass were intercropped. Nevertheless, it is important to emphasize that the results suggest the need for ongoing evaluations including different climatic conditions, especially with regard to the soil’s physical, chemical, and biological indicators, considering that the main challenge in evaluating cropping systems is to quantify the agro-environmental benefits generated by intercropping systems. In addition, the farm income produced from intercropping also requires study.