Abstract
Red and blue light have significant effects on plant growth; however, most of the current studies have focused on common horticultural crops such as cucumber and tomato, and there are fewer studies on how red and blue light affect the growth of melon seedlings. Therefore, in this study, we used melon (Cucumis melo L.) as the experimental material to investigate the effects of red and blue light ratios on the photomorphogenesis and photosynthesis of melon seedlings. Five red and blue light ratios were set at a fixed light intensity 200 μmol·m−2·s−1, including R:B = 1:9, R:B = 3:7, R:B = 1:1, R:B = 7:3, and R:B = 9:1. The results showed that with the increase in red light ratios, melon seedling height, stem diameter, total leaf area and stomatal pore size of melon seedlings increased, while the upper epidermis, palisade tissue, spongy tissue, leaf thickness, and stomatal density showed a decreasing trend. Under the R:B = 7:3 treatment, melon seedlings were optimized in all morphological indexes and had higher photosynthetic efficiency; these results indicated that the growth of melon seedlings could be regulated by adjusting the ratio of red to blue light, thus promoting the morphogenesis of melon seedlings.
1. Introduction
Light is extremely important for plant growth, not only to provide energy for photosynthesis, but also as a signal of information about the external environment. The regulation of light on plants can be interpreted from three aspects: light quality, light intensity, and photoperiod. The spectral composition of sunlight is mostly in the range of 300 nm~2600 nm. Wavelengths of light in the range of 400 nm~700 nm can directly affect the photosynthesis of plants. Among various wavelengths, red light and blue light have the greatest impact on plants. Red light has the largest absorption value at 642–663 nm, which not only promotes the accumulation of soluble sugar, starch, and other substances in the plant, but also regulates the physiological and biochemical processes of the plant, thus promoting plant photosynthesis [1,2,3]. Blue light has the largest absorption value at 430–453 nm, which promotes the development of chloroplasts, increases the concentration of chlorophyll and nitrogen, and promotes the opening of stomata, thus improving photosynthesis [4,5,6].
Red and blue light have been widely studied as important light quality components in plant photosynthesis and light signaling, and are used in practical agricultural production. Previous studies have shown that monochromatic red or blue light cannot meet the requirements for plant growth [7,8]. Plants grown under monochromatic red light show severe red light syndrome, reduced photosynthetic capacity, and impaired chloroplast development, thus making them more susceptible to photodamage [9]. Blue light plays an important role in photosynthesis by mediating photosystem activity and photosynthesis, and it can alleviate the “Red Light Syndrome” by regulating the ultrastructure of chloroplasts, photosynthesis, and nutrient accumulation [10,11]. Research has found that when blue light is present alone, the stomatal response to blue light is almost invisible [12]. In addition, red and blue light affect photosynthetic rate and stomatal conductance [13]. Ren et al. [14] found that red and blue light could regulate photosynthesis genes and influence the activity of Rubisco, so it could influence the photosynthesis of rice seedlings. Nguyen et al. [15] found that the red–blue light with a 4:1 ratio treatment had the greatest effect on the growth parameters, photosynthetic pigments, leaf structural properties, and quality of spinach plants. Wang et al. [16] found that under the condition of red–blue light with a 9:1 ratio, the actual net photosynthesis rate, the height of the plant, and the area of the leaf were higher.
In recent years, light-emitting diodes (LEDs) have been widely used to modify the growth environment of vegetables to promote plant growth and increase yield. Light-emitting diode (LED) lighting systems offer several unique advantages over conventional light sources, including the ability to control spectral composition, having a small size, durability, and long lifetime [17,18,19]. Therefore, it is important to investigate how LED lights affect plant growth and development. In order to determine the optimal red and blue light ratios suitable for the growth of melon seedlings, this study used LEDs to produce various ratios of R/B light, and evaluated the growth and development of melon seedlings by measuring a series of physiological and biochemical parameters. This study aimed to provide a theoretical basis for the optimization of the R/B ratio during the cultivation of melon seedlings.
2. Materials and Methods
2.1. Test Materials
This experiment was conducted in 2022 at the Laboratory of Biological and Environmental Engineering for the Agriculture Facility, College of Horticulture, Northwest Agriculture and Forestry University. The test material, ‘Emerald’ melon (Cucumis melo L.), was purchased from Shandong Zibo Harvest Seed Industry Technology Co. (Zibo, China).
Seeds were germinated and sown in seedling trays and grown in an artificial climatic chamber. The environment setting parameters are as follows: the light intensity was set at 150 μmol·m−2·s−1, the day and night temperatures were 25 °C/20 °C, the photoperiod was 12 h/12 h, and the relative humidity was 60%. When cotyledons were fully expanded, the seedlings were transplanted into 10 cm × 10 cm nutrient pots, placed in a cultivation frame equipped with LED light sources, and watered with 1/4 Hoagland melon nutrient solution, where the day and night temperatures were 28 °C/25 °C, the relative humidity was kept at 40–50%, and the relevant indexes were measured when the seedlings grew to about 3 leaves and 1 heart.
2.2. Experimental Design
The light board and LED control system V1.0, produced by Xi’an InChange Photoelectric Technology Co. (Xi’an, China), was be used to regulate the LED light quality and light intensity. The photosynthetically active radiation intensity was set at 200 μmol·m−2·s−1, the photoperiod was set at 12 h/12 h, and the light intensity of each treatment was set as shown in Table 1.

Table 1.
Intensity of red and blue light under different treatments (μmol·m−2·s−1).
2.3. Measurement Items and Methods
2.3.1. Morphological Indicators
Six plants were randomly collected when the melon seedlings had grown to 3 leaves and 1 heart. The aboveground height was measured using a scale; the stem thickness was measured using digital vernier calipers; the total leaf area was measured using a leaf area meter (YaXin-1241 Portable Leaf Area Meter, Beijing, China). The aboveground and underground parts of the plant were separated, the aboveground fresh weight was measured using an electronic balance, it was put in an oven, and the dry weight was measured by drying at 60 °C to a constant weight after a short period of greening.
2.3.2. Stomatal Characteristics
Three seedlings were randomly collected for each treatment, then the third real leaf was taken to make a stomata slide. The selected leaves were placed on transparent adhesive tape, pressed gently to tighten the combination, and then then a razor blade was used to scrape off the chloroplastic tissues; then, the tape was pasted onto slides, and the slices were examined under a microscope (Olympus BX63 (Tokyo, Japan)), and the density of the stomata was measured at 20×. The stomatal length and width and the pore length and width were were examined at 40×; ten fields of view were selected for each slide, and 20 stomata were selected for each field of view. Pore area = π × pore length/2 × pore width/2.
2.3.3. Determination of Leaf Structure
Three seedlings were chosen at random for each treatment, and the third true leaf was chosen for paraffin sections. The leaves of 10 mm × 10 mm were collected from the same leaf location of the main vein and put into the FAA fixation solution. Then, the paraffin sections were prepared with ethanol dehydration, xylene transparency, Safrin-solid green staining and paraffin embedding sections, and were then observed under a microscope (Olympus BX63, Japan). Ten fields of view were selected for each slice. The leaf thickness, upper and lower epidermal thickness, palisade tissue, and spongy tissue thickness were measured by ImageJ V1.8 software.
2.3.4. Measurement of Photosynthetic Properties
Three seedlings were randomly collected from each treatment. Choosing the first real leaf, we measured the transpiration rate, net photosynthetic rate, intercellular CO2 concentration, and stomatal conductance using a plant photosynthetic tester 6800 (LI-6800, Lincoln, NE, USA). The relevant parameters are set as follows: the leaf chamber temperature was 25 °C, the CO2 concentration was 400 µmol/mol, the relative humidity was 60%, the light intensity was 1000 μmol·m−2·s−1, and the light source was R90B10.
2.3.5. Measurement of Rubisco Enzyme Activity
Rubisco enzyme activity was measured in each treatment using the Plant 1,5-Bisphosphate Ribulose Carboxylase ELISA Assay Kit (Enzyme Immunity Industries, Nanjing, China).
2.3.6. Determination of Chlorophyll Fluorescence
Chlorophyll fluorescence-related parameters were determined using a dual-channel modulated chlorophyll fluorometer (DUAL-PAM-100, Nuremberg, BA, Germany). Three seedlings were randomly collected and the third true leaf was chosen for measurement. The leaves were dark-adapted for 30 min before measurement, and then the measurement light was turned on to record the minimum fluorescence, Fo, after dark-adaptation; immediately after that, a saturating pulse of light with a duration of 0.3 s was turned on to measure and record the maximum fluorescence, Fm, after dark-adaptation; the fluorescence returned to the vicinity of Fo rapidly after the saturating pulse was turned off, and then the photochemical light was turned on to record the chlorophyll fluorescence response from darkness to light; the fluorescence curve reached a steady state and then the photochemical light was turned off to record the response of chlorophyll fluorescence from darkness to light. When the fluorescence curve reached a steady state, the light was turned off to end the whole measurement process, and the fluorescence data were recorded and saved.
2.3.7. Determination of Carbohydrates and Soluble Protein
Three seedlings were randomly selected for each treatment, and a total of three true leaves were taken from the first to the third measurement of relevant indexes after full grinding. The soluble protein concentration was measured by the Caulmer’s Coomassie Brilliant Blue G-250 staining method [20], and the starch concentration was measured by the starch concentration detection kit (Solepol, Beijing, China). The samples were briefly killed and dried at 60 °C until a constant weight was reached, then ground and passed through a 0.15 mm sieve for the determination of a soluble sugar mass fraction and sucrose concentration. The mass fraction of soluble sugars was measured by the anthrone colorimetric method [21], and the sucrose concentration was measured by the method of Zhang et al. [22].
2.3.8. Real-Time Quantitative qRT-PCR Assay
The primers were designed by Primer Premier 5.0, and the primer sequences are shown in Table 2. Total RNA was extracted from leaves using the SPARKeasy Plant RNA Kit (SparkJade, Qingdao, China) in accordance with a standard kit, and the extracted RNA was reverse transcribed into cDNA using a Reverse Transcription Kit (Cofitt, Chengdu, China). The reaction was carried out according to the 2× qPCR SmArt Mix (SYBR) kit (Dr. Di, Shanghai, China), the reaction system was 20 μL, using the 2−ΔΔCt analysis method.

Table 2.
Primers for real-time qRT-PCR detection.
3. Statistical Analysis
Data were collated by Microsoft office Excel 2020, graphed by Graphpad Pism9.5, to be read with ANOVA and have the means separated by Duncan’s Multipole Rage test in IBM SPSS Statistics 25 (p < 0.05).
4. Results
4.1. Effect on Morphological Indexes of Melon Seedlings
As shown in Table 3, with the increase in the ratio of red light under a certain range, except for stem thickness, melon seedlings’ plant height, dry weight, fresh weight, and leaf area showed a trend of first increasing, then decreasing, which reached the maximum under R:B = 7:3 treatment. Increases of 87.14%, 45.33%, 72.36%, and 29.09% were achieved, respectively, compared with R:B = 1:9. The stem diameter of R:B = 9:1 was the largest, which was significantly increased, by 42.51%, compared with R:B = 1:9.

Table 3.
Effects on various ratios of red to blue light on morphological indices of melon seedlings.
4.2. Effects on Stomatal Characteristics of Melon Seedlings
Leaf stomatal density of melon seedlings grown under low red light was significantly higher than that of high red light treatment, in which the stomatal density under R:B = 1:9 treatment was significantly increased by 38.39%, 41.98%, and 76.41%, compared with R:B = 1:1, R:B = 7:3, and R:B = 9:1 treatments (Table 4). However, the pore width and length and pore area of stomata in the low R:B treatment were less than those in the high R:B treatment. The pore area in the R:B = 7:3 treatment was increased by 14.18%, 15.58%, and 15.85% compared with R:B = 1:9, R:B = 3:7, and R:B = 1:1 treatments.

Table 4.
Effects on various ratios of red to blue light on stomatal characteristics of melon seedlings.
4.3. Effect on Leaf Structure of Melon Seedlings
The upper epidermis, palisade tissues, spongy tissues, and leaf thickness of melon seedlings showed a decreasing trend with the increased ratio of red light (Table 5). It can be seen that, except for the lower epidermis, after the proportion of red light exceeded 50%, the leaf structure-related indexes were significantly different from those of the treatments with low red light proportion, and there was no significant difference between the upper epidermis, palisade tissues, spongy tissues, or leaf thickness of R:B = 7:3 and R:B = 9:1, but they were significantly lower than those with a high ratio of blue light.

Table 5.
Effects on various ratios of red to blue light on structural parameters of melon seedling leaves (μm).
4.4. Effect on Photosynthetic Characteristics of Melon Seedlings
Different red and blue light ratios had significant effects on the photosynthesis characteristics of melon seedlings, and the Pn, E, and Gsw of melon seedlings under R:B = 7:3 treatment were significantly higher than those under the other treatments (Figure 1). Among them, Pn, E, and Gsw were significantly increased by 104.46%, 360.00%, and 457.63%, respectively, under R:B = 7:3 treatment as compared to R:B = 1:9 treatment.
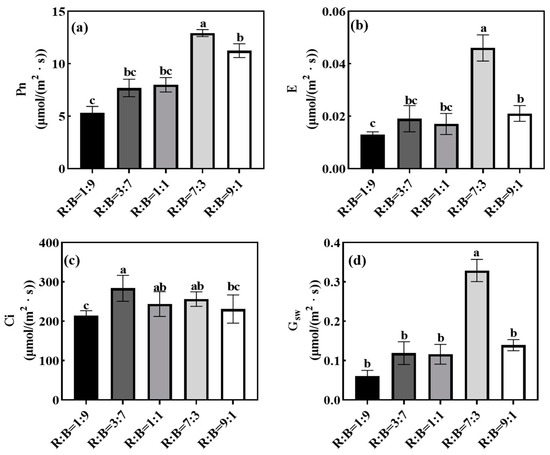
Figure 1.
Effects on various ratios of red to blue light on photosynthetic properties of melon seedlings. (a) Effect on net photosynthetic rate (Pn); (b) effect on transpiration rate (E); (c) effect on intercellular CO2 (Ci); (d) effect on stomatal conductance (Gsw). Note: Data in the same column with different letters indicate significant differences (p < 0.05).
The ΦPSII (PSII actual photochemical quantum yield), ETR (PSII photosynthetic electron transfer rate), and NPQ (Non-photochemical quenching) of melon seedlings under different treatments differed significantly and decreased with the increase in the red light ratio, in which the ΦPSII and ETR of R:B = 9:1 treatment decreased significantly, by 42.91%, 42.80%, and 19.47%, respectively, compared with that of R:B = 1:9 treatment (Table 6). With the increase in red light ratio, the ΦNO (PSII quantum yield of unregulated energy dissipation) value of melon seedlings showed a tendency to increase, and the R:B = 9:1 treatment significantly increased the ΦNO value by 80.30% compared with the R:B = 1:91 treatment.

Table 6.
Effects on various ratios of red to blue light on chlorophyll fluorescence parameters of melon seedlings.
4.5. Effect on Rubisco Enzyme Activity in Melon Seedlings
The effects of the ratio of red and blue light on the activity of the Rubisco enzyme was obvious, which showed a tendency of increasing and then decreasing, and the activity of the Rubisco enzyme was highest under the treatment of R:B = 7:3 (Figure 2).
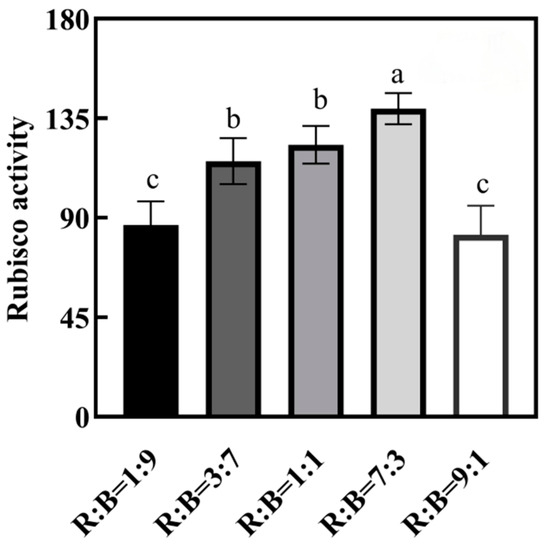
Figure 2.
Effects on various ratios of red to blue light on Rubisco enzyme activities of melon seedlings. Note: Data in the same column with different letters indicate significant differences (p < 0.05).
4.6. Effect on Carbohydrates and Soluble Protein of Melon Seedlings
With the increase in red light ratio (Figure 3), the soluble sugar and soluble protein concentrations showed a tendency of increasing and then decreasing, and the soluble sugar concentration reached its maximum value under R:B = 1:1 treatment, while the soluble protein reached its maximum value under R:B = 7:3 treatment. Sucrose concentration of melon seedlings showed a tendency to increase with the increase in red light ratio, and a sucrose concentration of R:B = 9:1 treatment significantly increased by 66.91% compared with that of R:B = 1:9 treatment. There was no significant effect of different red and blue light ratios on starch concentration.
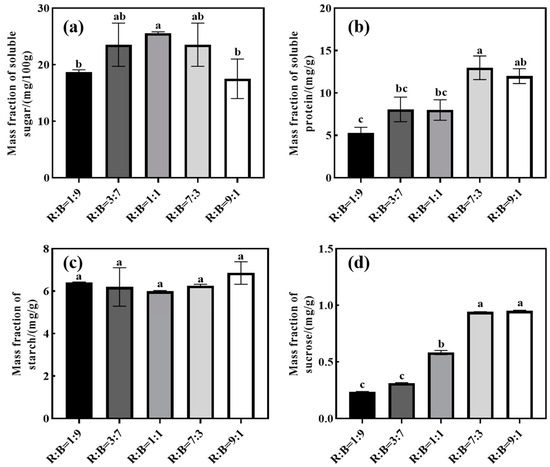
Figure 3.
Effects on various ratios of red to blue light on carbohydrates of melon seedlings. (a) Effect on soluble sugar concentration; (b) effect on soluble protein concentration; (c) effect on starch concentration; (d) effect on sucrose concentration. Note: Data in the same column with different letters indicate significant differences (p < 0.05).
4.7. Effects on the Relative Expression of Key Genes for Stomatal Development in Melon Seedlings
The stomatal development genes of melon seedlings were quantitatively analyzed under different treatments, and the results are shown in Figure 4. The expression of EPF1 and SLAC1 in melon seedlings varied significantly among different treatments, but there was no significant effect on MUTE and FAMA. The expression of EPF1 increased with the increase in red light ratio, and the relative expression of EPF1 was the highest in the treatment of R:B = 9:1, which was significantly increased by 31.67% compared with that in the treatment of R:B = 9:1. The expression of SLAC1 decreased with the increase in red light ratio, and the relative expression of SLAC1 was the highest in the treatment of R:B = 1:9. The expression of SLAC1 decreased with the increase in the red light ratio, and the relative expression of SLAC1 was the highest under R:B = 1:9 treatment, which decreased by 26.39% under R:B = 9:1 compared with the R:B = 1:9 treatment.
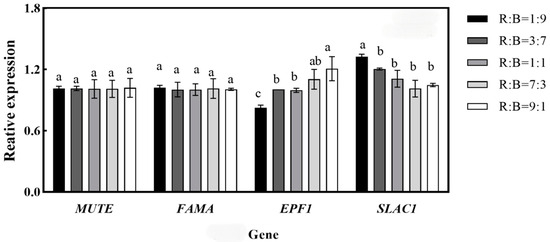
Figure 4.
Effects on various ratios of red to blue light on stomatal development genes. Note: Data in the same column with different letters indicate significant differences (p < 0.05).
5. Discussion
5.1. Effect on Morphological Indexes of Melon Seedlings
Light is a necessary element for the growth and development of plants. During the growth of plants, light not only acts as a source of energy, but also as a signal for the regulation of plant growth. LEDs provide a variety of spectral possibilities for plant growth, which can be adjusted to a specific spectrum according to the needs of different crops. It has been shown that the combination of red and blue LEDs can meet the growth needs of plants and favor the accumulation of plant biomass [23,24]. However, different plants have different needs for R:B ratios, depending on species differences or intraspecific genotype differences. Adding appropriate blue light to red light can effectively promote secondary metabolites and biomass accumulation, thus improving crop yield and quality [25,26,27]. The results of this experiment showed that within a certain extent, with the increases in red light ratio, melon seedlings’ plant height, fresh weight, total leaf area, and dry weight increased to an optimal point at R:B = 7:3 (Table 3).
5.2. Effect on Cell Morphology of Melon Seedlings
The leaf is a major photosynthetic organ of plant, and its structure has a direct influence on the photosynthesis of plants. Palisade tissue helps sunlight to better penetrate the chloroplasts to the interior of the leaf, and spongy tissue improves the ability of plant leaves to capture light [28,29]. Macedo et al. [30] used red and blue light to cultivate Amaranthus plants in vitro and found that the thickness of the distal axial surface epidermis and spongy thin-walled tissue of the plant was significantly reduced in the red-light-grown plants, and the thickness of the palisade thin-walled tissue and the upper epidermis of the plants grown under blue light was increased significantly. As the percentage of blue light increased, the leaf blade of oilseed rape changed from a wrinkled blade with an under-rolled margin to a flattened blade with a slightly up-rolled margin, and the compactness of the palisade tissue increased [31]. Gautier et al. [32] demonstrated that decreasing the percentage of blue light promoted an increase in petiole length, and the leaf blade of clover becoming larger and thinner, which proved that blue light has an inhibitory effect on growth. It indicates that different plants show different responses to the percentage of blue light under red and blue compound light. The results of this experiment indicated that leaf palisade tissue and leaf thickness were significantly higher in the low R:B treatment than in the high R:B treatment (Table 5), and the palisade tissue thickness of melon seedlings was reduced by 33.67% in the R:B = 9:1 compared with the R:B = 1:9 treatment. Phy (phytochrome) molecules absorb red light and convert the inactive Pr (red light-absorbing from) form into the active Pfr (far red light-absorbing form), whereas phy binds to PIF3 only in the active form. The binding of phy to PIF3 occurs specifically and reversibly only in the active Pfr form [33], and PIF3 overexpression negatively regulates plant photomorphogenesis, which in turn affects leaf growth [34]. Therefore, the leaf thickness of melon seedlings showed a decreasing trend as the R:B ratio decreased.
Plant stomata consist of two guard cells, and light waves are key exogenous signals that influence stomatal movement. Blue light-activated phototropin induces H—ATP enzyme activation via BLUS1. Red light-driven photosynthesis reduces intercellular CO2 concentration, thereby inhibiting S-type anion channels via CBC (convergence of blue light and CO2). In addition, phototropin inhibits anion channels through CBC-mediated signaling pathways. both activation of H—ATPase and inactivation of anion channels may lead to hyperpolarization of the plasma membrane, which prompts the k+ endocytosis channel to enter the guard cells, thereby promoting stomatal opening [35]. In this study, we found the stomatal pore area decreased as the blue light percentage decreased. Barillot et al. [36] found that blue light irradiation led to an increase in stomatal pore density in oxalis leaves. The results showed that with the increase in the red light ratio, the stomatal density of melon seedling leaves showed a significant decrease (Table 4), and the stomatal density of melon seedling R:B = 9:1 was significantly decreased by 43.31% compared with that of R:B = 1:9 treatment. EPF1 encodes a small, secreted peptide, which is expressed in stomata cells and precursors, and regulates stomata development by controlling asymmetrical cell division. There is a negative correlation between EPF1 and stomatal density. The expression level of EPF1 was negatively correlated with stomatal density [37] and the highest expression of EPF1 was found at R:B = 9:1 (Figure 4).
5.3. Effect on Photosynthesis Related Parameters and Chlorophyll Fluorescence Parameters of Melon Seedlings
Light is the driving force for photosynthesis in plants. Plants grown under red and blue composite light have higher photosynthetic capacity compared to monochromatic red or blue light [38]. The quantum yield of red light is higher than that of blue light, and therefore, when measured with the same light source, a higher R:B ratio leads to greater net photosynthesis [39]. The Pn, E, and Gsw of melon seedlings under R:B = 7:3 treatment in this experiment were significantly higher than the rest of the treatments. Jin et al. found that the Pn of a higher amount of red light was better than that of higher blue light [40].
The photosynthetic electron transport chain is located in the cystoid membrane and includes a series of electron transport carriers consisting of different pigment protein complexes. Chlorophyll fluorescence can be used to study the effects of environmental changes on the photosynthetic structure of PSII in plants. Izzo et al. [41] found that the blue wavelength was more important than the red one, since it stimulated the photosynthetic electron transport ability more, and the ETRmax was higher in the blue light. The data showed a tendency of decreasing ΦPSII, ETR, and NPQ with an increasing percentage of red light (Table 6). Similarly, Miao et al. [10] treated cucumber leaves with four light treatments and found that the photochemical efficiency was reduced under red light, which limited the rate of electron transport in PSII and PSI and increased the non-photochemical burst.
5.4. Effects on Carbon Assimilation Capacity and Carbohydrates of Cucumber and Melon Seedlings
Rubisco enzyme activity was affected by the light environment of the plant, and it increased with the increase in red light ratio, reaching a maximum at R:B = 7:3 in melon seedlings, and then decreased with the increase in red light ratio after reaching the maximum (Figure 2). Because the appropriate ratio of red and blue light makes plants have higher photochemical efficiency and a higher level of Rubisco binding capacity, and thus higher photosynthetic capacity, it ultimately leads to higher CO2 assimilation rate. Although blue light can have a positive effect on Rubisco expression [42], without the “mitigating effect” of red light, pure blue light, even if it induces the highest level of Rubisco expression, does not lead to higher photochemical efficiency [39].
Light quality regulates carbohydrate metabolism in higher plants [43]. Carbohydrate is the end product of photosynthesis and an important indicator of the photosynthetic capacity of plants [44]. The light quality of the combined red and blue ratio contributes to the carbon and nitrogen metabolism of seedlings [45]. Some studies have shown that red light is the most effective light source for the accumulation of soluble carbohydrates [46]; this study showed that it had a promotional effect on the accumulation of soluble proteins and sucrose in seedlings when increasing the ratio of red light up to a certain extent, but too high a ratio of red light treatment appeared to reduce the concentration of carbohydrates (Figure 3).
6. Conclusions
Different red and blue light treatments had significant effects on the leaf structure and stomatal density of melon seedlings, and melon seedlings had greater leaf thicknesses and stomatal density under low R:B treatment. The appropriate red and blue light ratio can make melon seedlings have more efficient photochemical efficiency and photosynthetic capacity, which can promote the accumulation of seedling biomass and carbohydrates. The results of this experiment showed that melon seedlings had better growth characteristics and higher photosynthetic efficiency under the R:B = 7:3 treatment.
Author Contributions
Methodology, X.L.; Data curation, S.Z. and X.L.; Writing—original draft, S.Z.; Writing—review and editing, Y.W. and Z.Y.; Visualization, Y.K. and Y.L.; Funding acquisition, Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shaanxi Provincial Technological Innovation Guiding Special Project (2021QFY08-02); Shaanxi Province 100 Billion Facility Agriculture Special Project in 2021 (K3030821094); Key Technological Innovation and Integration of Facility Vegetables in the Tibetan Plateau (XZ202202YD0002C); Introduction of Famous Varieties of Facility Vegetables, Melons and Fruits and Construction of Standardised Demonstration Bases (QYXTZX-AL2023-07).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mei, Z.; Li, Z.; Lu, X.; Zhang, S.; Liu, W.; Zou, Q.; Yu, L.; Fang, H.; Zhang, Z.; Mao, Z.; et al. Supplementation of natural light duration promotes accumulation of sugar and anthocyanins in apple (Malus domestica Borkh.) fruit. Environ. Exp. Bot. 2023, 205, 105133. [Google Scholar] [CrossRef]
- Urbonavičiūtė, A.; Pinho, P.; Samuolienė, G.; Duchovskis, P.; Vitta, P.; Stonkus, A.; Tamulaitis, G.; Žukauskas, A.; Halonen, L. Effect of short-wavelength light on lettuce growth and nutritional quality. Sodininkystė Daržininkystė 2007, 26, 157–165. [Google Scholar]
- Lawson, T.; Oxborough, K.; Morison, J.I.; Baker, N.R. The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J. Exp. Bot. 2003, 54, 1743–1752. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Cheng, R.; Kaiser, E.; Yang, Q.; Li, T. Acclimating cucumber plants to blue supplemental light promotes growth in full sunlight. Front. Plant Sci. 2021, 12, 782465. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Yanagi, T.; Takita, S.; Tanaka, M.; Higuchi, T.; Ushida, Y.; Watanabe, H. Development of plant growth apparatus using blue and red LED as artificial light source. In International Symposium on Plant Production in Closed Ecosystems; International Society for Horticultural Science: Leuven, Belgium, 1996; Volume 1. [Google Scholar]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched led lights in a plant factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Ieperen, W.V.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; Qu, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Lin, A.; Yang, Y.; Zhang, G.; Xu, P.; Wu, Y.; Yang, Z. Effect of different ratios of red and blue light on maximum stomatal conductance and response rate of cucumber seedling leaves. Agronomy 2023, 13, 1941. [Google Scholar] [CrossRef]
- Ren, M.; Liu, S.; Tang, C.; Mao, G.; Gai, P.; Guo, X.; Zheng, H.; Tang, Q. Photomorphogenesis and photosynthetic traits changes in rice seedlings responding to red and blue light. Int. J. Mol. Sci. 2023, 24, 11333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.D.; Jang, D.C.; Tran, T.T.H.; Nguyen, Q.T.; Kim, I.S.; Hoang, T.L.H.; Vu, N.T. Influence of green light added with red and blue LEDs on the growth, leaf microstructure and quality of spinach (Spinacia oleracea L.). Agronomy 2021, 11, 1724. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Q.; Zheng, Y.; Xu, Y.; Liu, B.; Li, Q. Effects of Red and Blue Light on the Growth, Photosynthesis, and Subsequent Growth under Fluctuating Light of Cucumber Seedlings. Plants 2024, 13, 1668. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Qu, W.J. Experimental Instruction of Plant Physiology; Higher Education Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Darko, E.; Hamow, K.A.; Marček, T.; Dernovics, M.; Ahres, M.; Galiba, G. Modulated light dependence of growth, flowering, and the accumulation of secondary metabolites in chilli. Front. Plant Sci. 2022, 13, 801656. [Google Scholar] [CrossRef] [PubMed]
- Utasi, L.; Kovács, V.; Gulyás, Z.; Marcek, T.; Janda, T.; Darko, E. Threshold or not: Spectral composition and light-intensity dependence of growth and metabolism in tomato seedlings. Sci. Hortic. 2023, 313, 111946. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Bhandari, S.R.; Shin, Y.K.; Lee, J.G. The influence of red and blue light ratios on growth performance, secondary metabolites, and antioxidant activities of Centella asiatica (L.) Urban. Horticulturae 2022, 8, 601. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Gonçalves, B.; Correia, C.M.; Silva, A.P.; Bacelar, E.A.; Santos, A.; Moutinho-Pereira, J.M. Leaf structure and function of sweet cherry tree (Prunus avium L.) cultivars with open and dense canopies. Sci. Hortic. 2008, 116, 381–387. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Chang, S.; Li, C.; Yao, X.; Song, C.; Jiao, X.; Liu, X.; Xu, Z.; Guan, R. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar]
- Gautier, H.; Varlet-Grancher, C.; Baudry, N. Effects of blue light on the vertical colonization of space by white clover and their consequences for dry matter distribution. Ann. Bot. 1997, 80, 665–671. [Google Scholar] [CrossRef]
- Ni, M.; Tepperman, J.M.; Quail, P.H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 1998, 95, 657–667. [Google Scholar] [CrossRef]
- Monte, E.; Tepperman, J.M.; Al-Sady, B.; Kaczorowski, K.A.; Alonso, J.M.; Ecker, J.R.; Li, X.; Zhang, Y.; Quail, P.H. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 2004, 101, 16091–16098. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, S.; Yamauchi, S.; Kobayashi, H.; Fuji, S.; Koya, S.; Shimazaki, K.I.; Takemiya, A. A BLUS1 kinase signal and a decrease in intercellular CO2 concentration are necessary for stomatal opening in response to blue light. Plant Cell 2021, 33, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Barillot, R.; Frak, E.; Combes, D.; Durand, J.L.; Escobar-Gutiérrez, A.J. What determines the complex kinetics of stomatal conductance under blueless PAR in Festuca arundinacea? Subsequent effects on leaf transpiration. J. Exp. Bot. 2010, 61, 2795–2806. [Google Scholar] [CrossRef]
- Hara, K.; Kajita, R.; Torii, K.U.; Bergmann, D.C.; Kakimoto, T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007, 21, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Sawbridge, T.I.; López-Juez, E.; Knight, M.R.; Jenkins, G.I. A blue-light photoreceptor mediates the fluence rate-dependant control of rbcS gene expression in light grown Phaseolus vulgaris primary leaves. Planta 1994, 192, 1–8. [Google Scholar]
- Dong, F.; Wang, C.; Sun, X.; Bao, Z.; Dong, C.; Sun, C.; Ren, Y.; Liu, S. Sugar metabolic changes in protein expression associated with different light quality combinations in tomato fruit. Plant Growth Regul. 2019, 88, 267–282. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Di, Q.; Li, J.; Du, Y.; Wei, M.; Shi, Q.; Li, Y.; Yang, F. Combination of red and blue lights improved the growth and development of eggplant (Solanum melongena L.) seedlings by regulating photosynthesis. J. Plant Growth Regul. 2021, 40, 1477–1492. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).