Abstract
Lavender (Lavandula angustifolia Mill.) is a valuable crop with diverse applications, but its in vitro rooting can be hindered by its sensitivity to natural auxins and it often fails to root due to callus formation. The current study investigates the effects of light spectra emitted by LEDs and the application of trans-cinnamic acid (t-CA) on the morphology, propagation, and rooting of lavender shoots in vitro. Initially, the influence of different concentrations of t-CA (0, 1.25, 2.5, or 5 µM) was evaluated under fluorescent light. The application of 1.25 µM t-CA was found to be the most effective in promoting root development while minimizing callus formation. Subsequently, the impact of different light spectra (fluorescent light and combinations of blue, red, and far-red monochromatic LED light) was explored. The combination of red and far-red (RFR) light significantly accelerated rooting and resulted in the most substantial increase in root number and length. Finally, the combined effects of 1.25 µM t-CA and RFR light were assessed. This combination produced the most favorable overall results, notably, a 4.3-fold increase in lateral root number compared to RFR light alone. These findings underscore the potential of optimizing both t-CA concentration and light spectra to enhance the in vitro propagation of lavender.
1. Introduction
Lavandula angustifolia Mill., a valuable aromatic shrub cultivated worldwide for its essential oil, is traditionally propagated through cuttings. However, this method faces challenges, such as inconsistent rooting success and the risk of virus transmission [1]. In vitro micropropagation techniques offer a promising solution by enabling the production of disease-free, genetically uniform plant material [2]. Although many publications discuss lavender micropropagation [2,3], the consequences of basal callus production on the critical root–stem connection are often neglected.
Light is a crucial environmental factor influencing plant growth and development, affecting processes such as photosynthesis, morphogenesis, and root formation [4]. Light-emitting diodes (LEDs) have emerged as a valuable tool in plant tissue culture, allowing for precise control over light spectra and intensity. Previous research has demonstrated the impact of specific light wavelengths on various aspects of plant growth, including shoot elongation, chlorophyll synthesis, and root development [5,6].
The ratio of red (R) to far-red (FR) light, in particular, has been shown to influence plant morphology and development through its effects on phytochrome, a light-sensing pigment involved in various signaling pathways [7]. The type of light influences plant rooting by modulating auxin homeostasis, a key hormone for growth and development [8,9]. In Arabidopsis, red light encourages an auxin flux from leaves to roots, possibly through the transcriptional regulation of the PIN-FORMED 1 (PIN1), PIN-FORMED 3 (PIN3), and PIN-FORMED 4 (PIN4) genes, which promotes root elongation and nutrient uptake [8]. Conversely, exposure to white light supplemented with far-red light reduces lateral root density in Arabidopsis. Blue light, through phototropins PHOT1 and PHOT2, enhances adventitious root formation in Arabidopsis, increasing the root absorption area [8]. Red light is also known to stimulate adventitious rooting in recalcitrant species [10]. Allalaq et al. [11] suggested that red light promotes adventitious root induction in Norway spruce by reducing jasmonate (JA) and JA-isoleucine biosynthesis and repressing the accumulation of isopentyl-adenine-type cytokinins.
In addition to light, plant growth regulators play a significant role in in vitro propagation. Auxins, such as indole-3-butyric acid (IBA) and 1-naphthaleneacetic acid (NAA), are commonly used to stimulate root formation. However, these auxins can also induce excessive callus formation, hindering successful plant acclimatization and the overall success of propagation [12]. Trans-cinnamic acid (t-CA), a naturally occurring compound found in various plant species, has been investigated as an alternative rooting agent due to its potential to promote lateral root development after photo-isomerization into cis-cinnamic acid (c-CA) [13].
This study aimed to investigate the effects of various treatments on L. angustifolia. Specifically, we examined the influence of trans-cinnamic acid (t-CA) concentrations on root induction, explored the impact of monochromatic light colors (red, blue, far-red) and their combinations on shoot and root development, and assessed the combined effects of t-CA and red + far-red light spectra. We hypothesized that t-CA could act as an effective rooting agent, potentially minimizing the callus formation often associated with traditional auxins [14]. Additionally, we hypothesized that specific light spectra would influence shoot and root development. By combining optimized light spectra and t-CA application, we developed an enhanced protocol for in vitro lavender propagation.
2. Materials and Methods
2.1. Propagating In Vitro Shoot Cultures
Foliated nodal segments (approximately 1.5 cm) of L. angustifolia ‘Hidcote’ were micropropagated on a modified Murashige and Skoog (MSm) [15] medium with half a concentration of NH4NO3 and KNO3, supplemented with 3% sucrose and 0.7% plant agar, to which 0.74 mg/L of meta-Topolin Riboside (mTR) was added before adjusting the pH to 5.8 and autoclaving for 20 min at 121 °C. The cultures were maintained at 25 ± 1 °C under a 16/8 h light/dark photoperiod, and they were subcultured every six weeks.
2.2. Root Induction under Fluorescent Light
Internodal segments (approximately 1.5 cm) excised from the shoot multiplication cultures were inoculated, in groups of five, into 380 mL glass containers, each containing 100 mL of MSm medium. The medium was supplemented with 0, 1.25, 2.5, or 5 µM of trans-cinnamic acid (t-CA) after autoclaving. This experiment was carried out using a completely randomized design, consisting of four treatments, with three containers per treatment, and the experimental unit was the in vitro shoot (five shoots per container). The experiment was repeated three times. The cultures were kept under controlled environmental conditions of 25 ± 1 °C and a 16/8 h light/dark photoperiod. After three weeks of incubation, the following parameters were recorded: number of adventitious and lateral roots, average adventitious root length, number of shoots, average shoot length, and callus weight.
2.3. Light Spectrum Experiment
Five foliated nodal explants, each measuring 1.5 cm in length, were inoculated into 380 mL glass vessels containing 100 mL of auxin-free MSm medium. Seven light treatments were applied, with a control group under cool white fluorescent light (FL) and six experimental groups under different LED light combinations: 100% blue (B), 100% red (R), 100% far-red (FR), 50% blue + 50% red (BR), 50% red + 50% far-red (RFR), and 50% blue + 50% far-red (BFR). The photosynthetic photon flux density (PPFD) for all LED treatments was maintained at 40 µmol m−2 s−1 under a 16/8 h light/dark photoperiod. Cultures were incubated at 25 ± 1 °C. Individual light treatments were administered using separate light-proof boxes (57 cm × 40 cm × 37.5 cm) constructed on culture racks and equipped with Philips Green Power LED research modules, as described by [14]. Each treatment group consisted of three inoculated glass vessels. After one week, the number of primary roots was recorded; this was continued weekly until week 8. After three weeks, the number of adventitious and lateral roots, average adventitious root length, number of shoots per explant, and average shoot length were determined.
After three weeks of culture, chlorophyll was extracted using an 80:20 (v/v) mixture of acetone and water. Fresh lavender leaves (100 mg) were crushed in 10 mL of 80% acetone for 5 min using a mortar and pestle. After filtration, the homogenate was transferred to a 15 mL Falcon tube and centrifuged at 3000× g for 15 min. Following centrifugation, the supernatant was carefully transferred to a new 15 mL Falcon tube. The filtrate was kept in the dark to prevent the oxidation of the chlorophyll by light.
The absorbance of the extract was measured using a spectrophotometer set at specific wavelengths to determine its <chlorophyll a (645 nm), chlorophyll b (663 nm), and total carotenoids (470 nm). The determination of chlorophyll a, chlorophyll b, and total chlorophyll was performed according to the methods of Arnon et al. [16]. Total carotenoids were determined according to Lichtenthaler et al. [17]. The photosynthetic pigment content, expressed as µg/mL of fresh weight (FW) tissue, was calculated using the following formulas:
Chlorophyll a (mg g−1 FW) = 12.7 × A663 − 2.69 × A645
Chlorophyll b (mg g−1 FW) = 22.9 × A645 − 4.68 × A663
Chlorophyll a + b (mg g−1 FW) = 8.02 × A663 + 20.2 × A645
Carotenoids (mg g−1 FW) = (1000 × A470 − 1.82 × Chl a − 85.02 × Chl b)/198
2.4. Combining t-CA and RFR Light
Foliated internodal segments of approximately 1.5 cm were excised from shoot multiplication cultures and inoculated, at five segments per 380 mL glass vessel, in 100 mL of MSm medium supplemented with 0, 1.25, 2.5, or 5 µM of t-CA following autoclaving, and the cultures were subjected to RFR light treatment. Each treatment group consisted of three inoculated glass vessels, and the total PPFD was maintained at 40 µmol m−2 s−1. After a four-week incubation period, the following parameters were recorded: the adventitious and lateral root number, average adventitious root length, number of shoots per explant, average shoot length, and callus weight.
2.5. Statistical Analysis
Each experiment was repeated three times. Statistical analysis was conducted using IBM SPSS Statistics for Windows, version 29 (IBM Corp, North Castle, NY, USA) with a significance level of 5% (α = 0.05). Data normality was assessed using the Kolmogorov–Smirnov test. For all data, the non-parametric Kruskal–Wallis test was employed (α = 0.05) to evaluate the differences between groups. In this case, the alternative hypothesis (H1) was retained.
3. Results
3.1. Root Induction of Trans-Cinnamic Acid under Fluorescent Light
Figure 1, Figure 2 and Figure 3 show that 1.25 µM of trans-cinnamic acid (t-CA) significantly increased the adventitious and lateral root number, average adventitious root length, and average shoot length and number compared to the control and other suboptimal concentrations. Additionally, 1.25 µM of t-CA induced limited callus formation.
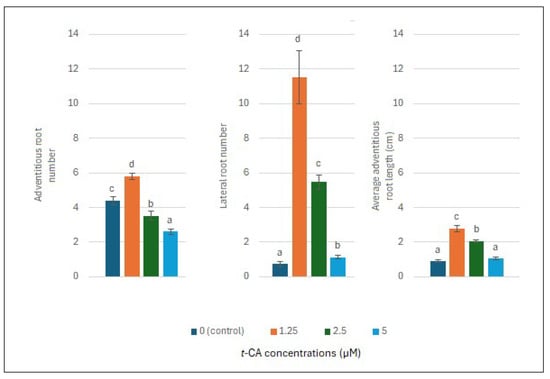
Figure 1.
Effect of trans-cinnamic acid (t-CA) concentration on the root parameters of L. angustifolia after three weeks of in vitro culture. Data represent the mean ± standard error (SE) of 3 × 3 × 5 plants per treatment. Bars with the same letter are not significantly different from each other (Kruskal–Wallis test, p < 0.05).
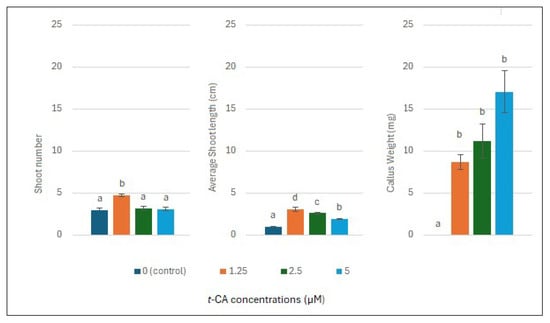
Figure 2.
Effect of trans-cinnamic acid (t-CA) concentration on the callus formation and shoot growth parameters of L. angustifolia after three weeks of in vitro culture. Data represent the mean ± standard error (SE) of 3 × 3 × 5 plants per treatment. Bars with the same letter are not significantly different from each other (Kruskal–Wallis test, p < 0.05).

Figure 3.
Influence of trans-cinnamic acid (t-CA) treatments on rooting and shoot growth in L. angustifolia after three weeks of in vitro culture. Treatments include (a) control (hormone-free medium); (b) 1 µM trans-cinnamic acid (t-CA); (c) 2.5 µM trans-cinnamic acid (t-CA); and (d) 5 µM trans-cinnamic acid (t-CA).
3.2. Light Spectrum Experiment
Different light spectra significantly impacted both the rooting speed and the overall morphology of lavender grown in vitro. The fastest root development was observed under combined red + far-red light (RFR), with roots appearing within the first week. This was followed by the red (R), blue + red (BR), and blue (B), and light combinations, where roots appeared in the third week. Root formation was considerably delayed under the far-red (FR) and blue + far-red (BFR) light conditions. After eight weeks, the RFR group showed a remarkably high root count of 34, while the red light group averaged 18 roots. In comparison, the control group grown under white light only produced 9 roots. Far-red light alone had a negative impact, resulting in poor root development, with only a single root observed after eight weeks (Figure 4).
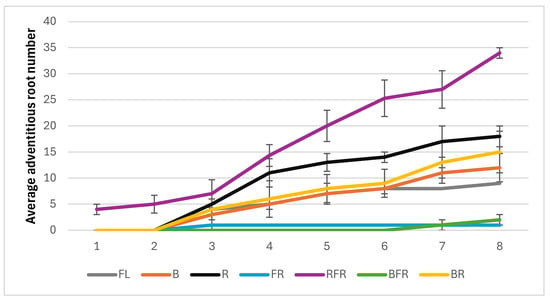
Figure 4.
Effect of light spectra on root development, in auxin-free medium, of L. angustifolia cultured in vitro for eight weeks. The number of roots per plant was measured over time under different light spectra: (FL) fluorescent light (control); (B) blue; (R) red; (FR) far-red; (RFR) red + far-red; (BFR) blue + far-red; (BR) blue + red. Data are presented as mean ± standard deviation (SD) of 3 × 3 × 5 plants per treatment.
Table 1 and Figure 5 detail the effects of different light spectra on L. angustifolia growth after three weeks. Red + far-red (RFR) light induced the highest number of adventitious roots (6.67) and lateral roots (6.91), with an average length of 2.72 cm. Conversely, far-red (FR) and blue + far-red (BFR) light inhibited root development, yielding only 1.06 and 0.13 roots per plant, respectively. The R and RFR light treatments promoted the highest number of shoots, while BFR light resulted in the greatest average stem length of 6.04 cm. BFR light produced excessively elongated plants. Far-red light exposure led to weakened plants with yellowing shoots. Notably, callus formation was not observed under any light treatment.

Table 1.
Shoot and root development on auxin-free medium of in vitro-grown L. angustifolia after three weeks of exposure to different light spectra: (FL) fluorescent light; (B) blue; (R) red; (FR) far-red; (RFR) red + far-red; (BFR) blue + far-red; (BR) blue + red.

Figure 5.
Effect of light-emitting diode (LED) spectra on shoot development and rooting in L. angustifolia after three weeks. (FL) fluorescent light; (R) red; (RFR) red + far-red; (FR) far-red; (BR) blue + red; (B) blue; (BFR) blue + far-red, (bar = 1 cm).
Table 2 summarizes the chlorophyll and carotenoid content in leaves under different light spectra. Blue + red (BR) light induced the highest levels of chlorophyll a and total chlorophyll, followed by the blue (B) and red + far-red (RFR) treatments. On the contrary, far-red (FR) and blue + far-red (BFR) light resulted in significantly lower chlorophyll concentrations, leading to a visible yellowing of leaves (Figure 5). Carotenoid accumulation was greatest under the red + far-red (RFR) and red (R) conditions, with blue light treatments also promoting substantial levels compared to fluorescent light. Notably, FR light yielded the lowest carotenoid concentrations.

Table 2.
Effect of light spectra on chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content (mg/g fresh weight) in leaves of L. angustifolia cultured in vitro for four weeks: (FL) fluorescent light; (B) blue; (R) red; (FR) far-red; (RFR) red + far-red; (BFR) blue + far-red; (BR) blue + red.
3.3. Combining Trans-Cinnamic Acid and RFR Light
Table 3 and Figure 6 show that the addition of t-CA to the culture medium under red + far-red (RFR) light significantly increased lateral root formation in L. angustifolia. The highest number of lateral roots was observed with 1.25 µM of t-CA, with a decrease at higher concentrations. Additionally, 1.25 µM of t-CA resulted in the greatest average root length and nearly eliminated callus formation. RFR light alone produced the greatest number of adventitious roots. Notably, callus formation was not observed during RFR light exposure with the different concentrations of t-CA.

Table 3.
Effect of t-CA and red + far-red (RFR) light on adventitious and lateral root number, average adventitious root length, shoot number, average shoot length, and callus weight of in vitro-grown L. angustifolia after four weeks.
Table 3.
Effect of t-CA and red + far-red (RFR) light on adventitious and lateral root number, average adventitious root length, shoot number, average shoot length, and callus weight of in vitro-grown L. angustifolia after four weeks.
| t-CA (µM) under RFR | Adventitious Root Number | Lateral Root Number | Average Adventitious Root Length (cm) | Shoot Number | Average Shoot Length (cm) | Callus Weight (mg) |
|---|---|---|---|---|---|---|
| 0 | 12.6 ± 0.48 c | 7.36 ± 0.52 a | 2.58 ± 0.14 b | 4.27 ± 0.29 b | 2.63 ± 0.27 b | 0 |
| 1.25 | 9.1 ± 0.3 b | 31.87 ± 1.16 ab | 3.82 ± 0.17 c | 5.1 ± 0.23 c | 3.33 ± 0.21 c | 0 |
| 2.5 | 5.27 ± 0.23 a | 24.13 ± 0.94 ab | 2.89 ± 0.14 b | 4.13 ± 0.23 b | 3.05 ± 0.16 c | 0 |
| 5 | 5.53 ± 0.27 a | 5.02 ± 0.5 b | 1.29 ± 0.12 a | 3.33 ± 0.25 a | 1.51 ± 0.14 a | 0 |
Averages ± SE followed by different letters in the same column are significantly different at p < 0.05 according to the Kruskal–Wallis test.

Figure 6.
Effect of varying trans-cinnamic acid (t-CA) concentrations on in vitro root and shoot morphology of L. angustifolia under red + far-red (RFR) light after four weeks, (bar = 1 cm).
4. Discussion
In our study, 1.25 µM of trans-cinnamic acid (t-CA) significantly increased the adventitious and lateral root number and the average adventitious root length, but there was still callus formation. Higher t-CA concentrations elicited a decline in those root parameters, coupled with an increased callus weight, suggesting a concentration-dependent biphasic response. This observation aligns with Lupini et al. [18]. Furthermore, our results corroborate Salvador et al. [19], who demonstrated that increasing concentrations of t-CA enhance indole-3-acetic acid (IAA) oxidase and cinnamate 4-hydroxylase (C4H) activities. Elevated C4H activity, in turn, increased the lignin content, which, combined with heightened IAA oxidase activity, curtailed root growth in soybean. Interestingly, 1.25 µM of t-CA induced abundant lateral root formation in lavender, an effect that diminished with increasing t-CA concentrations. This aligns with Steenackers et al. [13], who proposed that t-CA can influence the root architecture of Arabidopsis through its conversion to c-CA under UV-B light. They hypothesized that c-CA inhibits auxin efflux, leading to localized auxin accumulation behind the root tip and triggering lateral root initiation.
Despite its positive effects on root development, our experiments revealed that t-CA in the culture medium also led to callus formation at the base of lavender stems. This callus formation can obstruct the vascular connection between developing roots and shoots, thereby impeding the efficient uptake of water and nutrients [20,21]. Souza et al. [22] further noted a negative correlation between callus formation and plant survival during acclimatization, and Arab et al. [21] observed similar issues with the in vitro rooting of Prunus using IBA. This highlights the pressing need to develop rooting protocols that minimize or eliminate callus formation [12,23].
Under natural conditions, R and FR light serve as a crucial environmental signal, indicating the proximity of neighboring vegetation and triggering shade avoidance responses. The responses to FR light include physiological and morphological adaptations, such as stem elongation and decreased branching, which enhance the plant’s ability to compete for light resources. The influence of R and B light on in vitro shoot growth is species-specific and has been extensively documented in recent decades. While R light generally promotes shoot elongation and B light encourages compactness and branching [24], lavender species exhibit a contrasting response, producing a greater number of shorter shoots under R light compared to B. Our experiments confirm that light wavelength significantly impacts both growth and rooting in L. angustifolia.
Is a combination of far-red (FR) and red (R) light necessary for stimulating root development? Treatment with RFR light led to enhanced rooting, as evidenced by faster root development, a greater number of roots, and an increased average adventitious root length. In vivo, Christiaens et al. [25] corroborated these findings, demonstrating that supplementing the culture medium with FR light in Chrysanthemum morifolium resulted in accelerated rooting, increased root dry mass, and a greater number of primary roots. In our experimental setup, the RFR light condition meant that the plant received an equivalent irradiance of red and far-red light (R:FR = 1). This specific light environment simulates the spectral distribution observed beneath a dense canopy, where upper leaves preferentially absorb red light, resulting in an increased proportion of far-red light reaching the lower foliage. The balanced R:FR ratio in our experiment would establish a dynamic equilibrium between the Pr and Pfr conformations of phytochrome, precluding a substantial accumulation of either form and maintaining a relatively stable Pfr/Pr ratio. Consequently, downstream signaling pathways associated with both Pr and Pfr would likely experience moderate activation, potentially leading to a balanced response in growth and developmental processes [26]. Our results are consistent with the findings of Holalu et al. [27], who observed an inverse relationship between auxin levels and the red-to-far-red light ratio (R:FR), a key environmental signal influencing shoot branching. It is proposed that the perception of far-red light by phytochrome B (PHYB) promotes the accumulation of phytochrome-interacting factors (PIFs), which in turn enhance auxin biosynthesis and stimulate growth responses [28,29,30].
Given that photosynthesis is not possible under FR light alone and in vitro plants obtain energy solely from sucrose, our observations indicate that FR light negatively impacts in vitro lavender growth. This is evident in lavender in reduced root development, pale green to yellow stems, and decreased chlorophyll a + b and carotenoid content, suggesting impaired chlorophyll biosynthesis. These findings align with [14], who reported no improvement in shoot quality under FR conditions but instead observed hyperhydricity, pale green shoots, and narrow leaves, which are also associated with reduced chlorophyll a + b and carotenoid content. Similarly, Werbrouck et al. [31] documented the detrimental effects of FR light on the in vitro biomass production of Ficus benjamina, noting reductions in total shoot number, shoot cluster weight, and callus weight.
The ratio of red (R) to far-red (FR) light influences several parameters, including leaf thickness, the number of palisade mesophyll cells, stomatal regulation, chloroplast development, and thylakoid structure [26]. R combined with FR light was not optimal for chlorophyll content. As anticipated, in this study, blue + red (BR) light resulted in the highest levels of chlorophyll a, chlorophyll b, and total chlorophyll compared to B and other light treatments. This effect has been demonstrated in numerous studies [24]. Carotenoids are essential components of the reaction center complex and act as accessory pigments in antenna systems, performing functions such as harvesting light energy and enhancing photosynthetic efficiency [32]. In our study, RFR light yielded the highest carotenoid content. However, there are conflicting results in the literature. Zhao et al. [33] found that white (W) and red + blue (RB) light resulted in the highest total carotenoid content in seedling leaves. Lotfi et al. [14] reported that Pyrus communis grown under BR light exhibited the highest carotenoid content, while Johkan et al. [34] and Schagerl et al. [35] demonstrated that blue fluorescent light positively influenced carotenoid accumulation. Therefore, it can be concluded that the addition of red (R) or blue (B) light is essential for chlorophyll to capture energy.
While R combined with FR light is optimal for root development under our conditions, the combination of B and FR is not, despite B light promoting rooting. To a somewhat lesser degree, R light also stimulated both the adventitious root number and the average adventitious root length. These results align with Christiaens et al. [36], who reported enhanced root formation under 100% R light in L. angustifolia. Similarly, in Jatropha, Daud et al. (2013) [37] observed no rooting under BR light conditions, while R light improved root formation. In cherry, Iacona et al. [38] reported the highest number of roots per microcutting under BR light, with R light subsequently proving more effective for root elongation than B light. Li et al. [39] demonstrated that red LEDs appeared to be the most suitable for root growth in upland cotton plantlets. Numerous other examples are reviewed by Fan et al. [24].
Our study investigated the combined effects of t-CA concentration and red + far-red (RFR) light on L. angustifolia rooting and production. We found that supplementing the culture medium with 1.25 µM of t-CA improved the shoot number, average shoot and adventitious root length, and, most notably, lateral root number under RFR light. This combined treatment leveraged the positive effects of both t-CA and RFR light. Additionally, the absence of callus formation even with t-CA suggests that RFR light alleviated plant stress and t-CA did not directly promote callus formation. The surprising outcome of using t-CA and RFR light together suggests a potential method for preventing the unwanted basal callus growth that often occurs with auxin treatments.
5. Conclusions
In conclusion, our study reveals that the combination of 1.25 µM of trans-cinnamic acid (t-CA) and red + far-red (RFR) light provides an optimal environment for promoting root development in L. angustifolia, enhancing various root parameters without inducing callus formation. This combined treatment demonstrates the synergistic benefits of t-CA and RFR light, suggesting a promising strategy for improving rooting efficiency in lavender micropropagation. Furthermore, our findings highlight the critical role of light quality in influencing plant growth and development in vitro, with specific light wavelengths differentially affecting various physiological and morphological parameters. Understanding these intricate interactions between light and plant hormones can facilitate the development of more effective and sustainable micropropagation protocols for lavender and other economically important plant species.
Author Contributions
Conceptualization, S.P.O.W. and H.D.; methodology, H.D.; software, H.D.; validation, H.D.; formal analysis, H.D.; investigation, H.D. resources, S.P.O.W.; data curation, H.D.; writing—original draft preparation, S.P.O.W. and H.D.; writing—review and editing, S.P.O.W. and H.D.; visualization, H.D.; supervision, S.P.O.W.; project administration, S.P.O.W.; funding acquisition, S.P.O.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Upson, T.; Andrews, S.; Harriott, G. The Genus Lavandula; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Babanina, S.S.; Yegorova, N.A.; Stavtseva, I.V.; Abdurashitov, S.F. Genetic Stability of Lavender (Lavandula angustifolia Mill.) Plants Obtained During Long-Term Clonal Micropropagation. Russ. Agric. Sci. 2023, 49, 132–139. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Dalda Şekerci, A.; Isak, M.A.; Bulut, F.; İzgü, T.; Tütüncü, M.; Dönmez, D. Optimizing Micropropagation and Rooting Protocols for Diverse Lavender Genotypes: A Synergistic Approach Integrating Machine Learning Techniques. Horticulturae 2024, 10, 52. [Google Scholar] [CrossRef]
- Okamoto, K.; Yanagi, T.; Takita, S. Light quality and photomorphogenesis in tissue culture. In Vitro Cell. Dev. Biol. Plant 1997, 33, 155–160. [Google Scholar]
- Lin, Y.; Li, J.; Li, B.; He, T.; Chun, Z. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 105, 329–335. [Google Scholar] [CrossRef]
- Shin, S.K.; Murthy, N.H.; Heo, W.J.; Hahn, J.E.; Paek, Y.K. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plantae 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Sakr, S. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Yun, F.; Liu, H.; Deng, Y.; Hou, X.; Liao, W. The role of light-regulated auxin signaling in root development. Int. J. Mol. Sci. 2023, 24, 5253. [Google Scholar] [CrossRef]
- Alabadí, D.; Blázquez, M.A. Molecular interactions between light and hormone signaling to control plant growth. Plant Mol. Biol. 2009, 69, 409–417. [Google Scholar] [CrossRef]
- Christiaens, A.; Gobin, B.; Van Labeke, M.C. Light quality and adventitious rooting: A mini-review. In Proceedings of the VIII Int. Symp. on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016; Currey, C.J., Lopez, R.G., Runkle, E.S., Eds.; Acta Horticulturae 1134; International Society for Horticultural Science: Leuven, Belgium, 2016. [Google Scholar] [CrossRef]
- Alallaq, S.; Ranjan, A.; Brunoni, F.; Novák, O.; Lakehal, A.; Bellini, C. Red Light Controls Adventitious Root Regeneration by Modulating Hormone Homeostasis in Picea abies Seedlings. Front. Plant Sci. 2020, 11, 586140. [Google Scholar] [CrossRef]
- Welander, M.; Geier, T.; Smolka, A.; Ahlman, A.; Fan, J.; Zhu, L.H. Origin, Timing, and Gene Expression Profile of Adventitious Rooting in Arabidopsis Hypocotyls and Stems. Am. J. Bot. 2014, 101, 255–266. [Google Scholar] [CrossRef]
- Steenackers, W.; Klíma, P.; Quareshy, M.; Cesarino, I.; Kumpf, R.P.; Corneillie, S.; Araújo, P.; Viaene, T.; Goeminne, G.; Nowack, M.K.; et al. Cis-cinnamic acid is a novel, natural auxin efflux inhibitor that promotes lateral root formation. Plant Physiol. 2017, 173, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Mars, M.; Werbrouck, S. Optimizing pear micropropagation and rooting with light emitting diodes and trans-cinnamic acid. Plant Growth Regul. 2019, 88, 173–180. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–603. [Google Scholar] [CrossRef]
- Lupini, A.; Sorgonà, A.; Princi, M.P.; Sunseri, F.; Abenavoli, M.R. Morphological and physiological effects of trans-cinnamic acid and its hydroxylated derivatives on maize root types. Plant Growth Regul. 2016, 78, 263–273. [Google Scholar] [CrossRef]
- Salvador, V.H.; Lima, R.B.; dos Santos, W.D.; Soares, A.R.; Böhm, P.A.F.; Marchiosi, R.; Ferrarese-Filho, O. Cinnamic acid increases lignin production and inhibits soybean root growth. PLoS ONE 2013, 8, e69105. [Google Scholar] [CrossRef] [PubMed]
- Radmann, E.B.; Fachinello, J.C.; Peters, J.A. Effect of auxin and cultivation conditions in in vitro rooting of rootstock of apple ‘M-9’. Rev. Bras. Frutic. 2002, 24, 624–628. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Eftekhari, M.; Ahmadi, H.; Akbari, M.; Khorami, S.S. Modeling and optimizing a new culture medium for in vitro rooting of G×N15 Prunus rootstock using artificial neural network-genetic algorithm. Sci. Rep. 2018, 8, 7930. [Google Scholar] [CrossRef]
- Souza, J.A.; Bettoni, J.C.; Costa, M.D.; Baldissera, T.C.; Passos, J.F.M.D.; Primieri, S. In vitro rooting and acclimatization of ‘Marubakaido’ apple rootstock using indole-3-acetic acid from rhizobacteria. Commun. Plant Sci. 2022, 12, 16–23. [Google Scholar] [CrossRef]
- Srikanth, S.; Choong, T.W.; Yan, A.; He, J.; Chen, Z. An efficient method for adventitious root induction from stem segments of Brassica species. Front. Plant Sci. 2016, 7, 943. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, C.; Manivannan, A.; Wei, H. Light Quality-Mediated Influence of Morphogenesis in Micropropagated Horticultural Crops: A Comprehensive Overview. BioMed Res. Int. 2022, 2022, 4615079. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, A.; Gobin, B.; Van Huylenbroeck, J.; Van Labeke, M.C. Adventitious rooting of Chrysanthemum is stimulated by a low red: Far-red ratio. J. Plant Physiol. 2019, 236, 117–123. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Holalu, S.V.; Finlayson, S.A. The ratio of red light to far-red light alters Arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. J. Exp. Bot. 2017, 68, 943–952. [Google Scholar]
- Li, L.; Ljung, K.; Breton, G.; Schmitz, R.J.; Pruneda-Paz, J.; Cowing-Zitron, C.; Chory, J. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012, 26, 785–790. [Google Scholar] [CrossRef]
- Pantazopoulou, C.K.; Bongers, F.J.; Küpers, J.J.; Reinen, E.; Das, D.; Evers, J.B.; Anten, N.P.; Pierik, R. Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proc. Natl. Acad. Sci. USA 2017, 114, 7450–7455. [Google Scholar] [CrossRef]
- Courbier, S.; Grevink, S.; Sluijs, E.; Bonhomme, P.O.; Kajala, K.; Van Wees, S.C.; Pierik, R. Far-red light promotes Botrytis cinerea disease development in tomato leaves via jasmonate-dependent modulation of soluble sugars. Plant Cell Environ. 2020, 43, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Werbrouck, S.; Buyle, H.; Geelen, D.; Van Labeke, M.C. Effect of red-, far-red-and blue-light-emitting diodes on in vitro growth of Ficus benjamina. Acta Hortic. 2012, 961, 533–538. [Google Scholar] [CrossRef]
- Goodwin, T.W.; Britton, G. Distribution and analysis of carotenoids. In Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1988; pp. 61–132. [Google Scholar]
- Zhao, J.; Thi, L.T.; Park, Y.G.; Jeong, B.R. Light quality affects growth and physiology of Carpesium triste Maxim. cultured in vitro. Agriculture 2020, 10, 258. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue Light-Emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth After Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Schagerl, M.; Muller, B. Acclimation of chlorophyll a and carotenoid levels to different irradiance in four freshwater cyanobacteria. J. Plant Physiol. 2006, 163, 709–716. [Google Scholar] [CrossRef]
- Christiaens, A.; Van Labeke, M.C.; Gobin, B.; Van Huylenbroeck, J. Rooting of ornamental cuttings affected by spectral light quality. Acta Hortic. 2015, 1104, 219–224. [Google Scholar] [CrossRef]
- Daud, N.; Faizal, A.; Geelen, D. Adventitious rooting of Jatropha curcas L. is stimulated by phloroglucinol and by red LED light. In Vitro Cell. Dev. Biol. Plant 2013, 49, 183–190. [Google Scholar] [CrossRef]
- Iacona, C.; Muleo, R. Light quality affects in vitro adventitious rooting and ex vitro performance of cherry rootstock Colt. Sci. Hortic. 2010, 125, 630–636. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Tang, C. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tiss. Org. Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).