Phenotypic and Biochemical Trait Improvement in Husk Tomatoes (Physalis sp.) through EMS-Induced Mutagenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Mutagenesis
2.2. Morphological Attributes
2.3. Biochemical Analysis
2.3.1. Measurement of Total Phenol
2.3.2. Measurement of Antioxidant Activity
2.3.3. Determination of Chlorophyll a and b
2.3.4. Mineral Determination
2.4. Data Collection and Analysis
3. Results
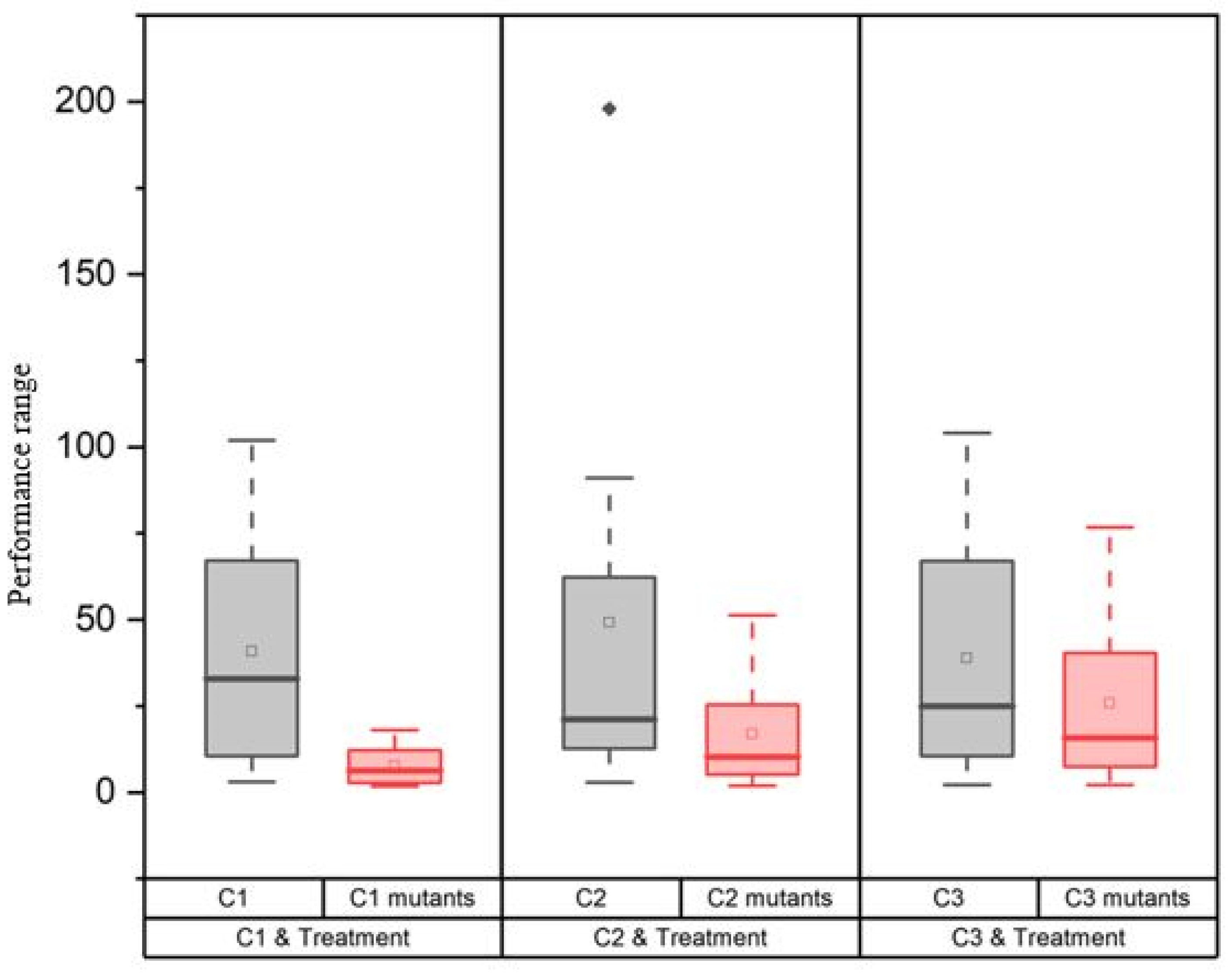
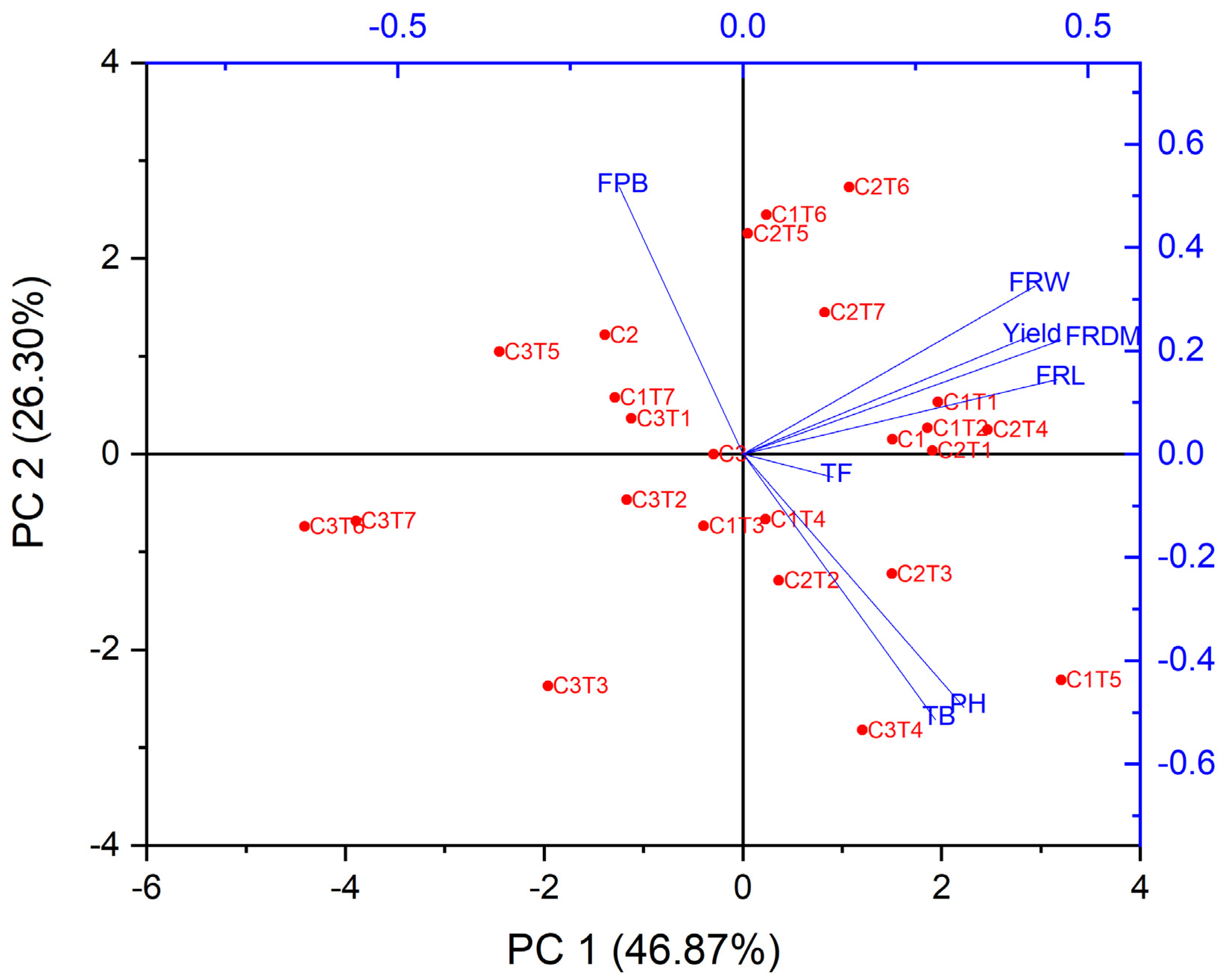
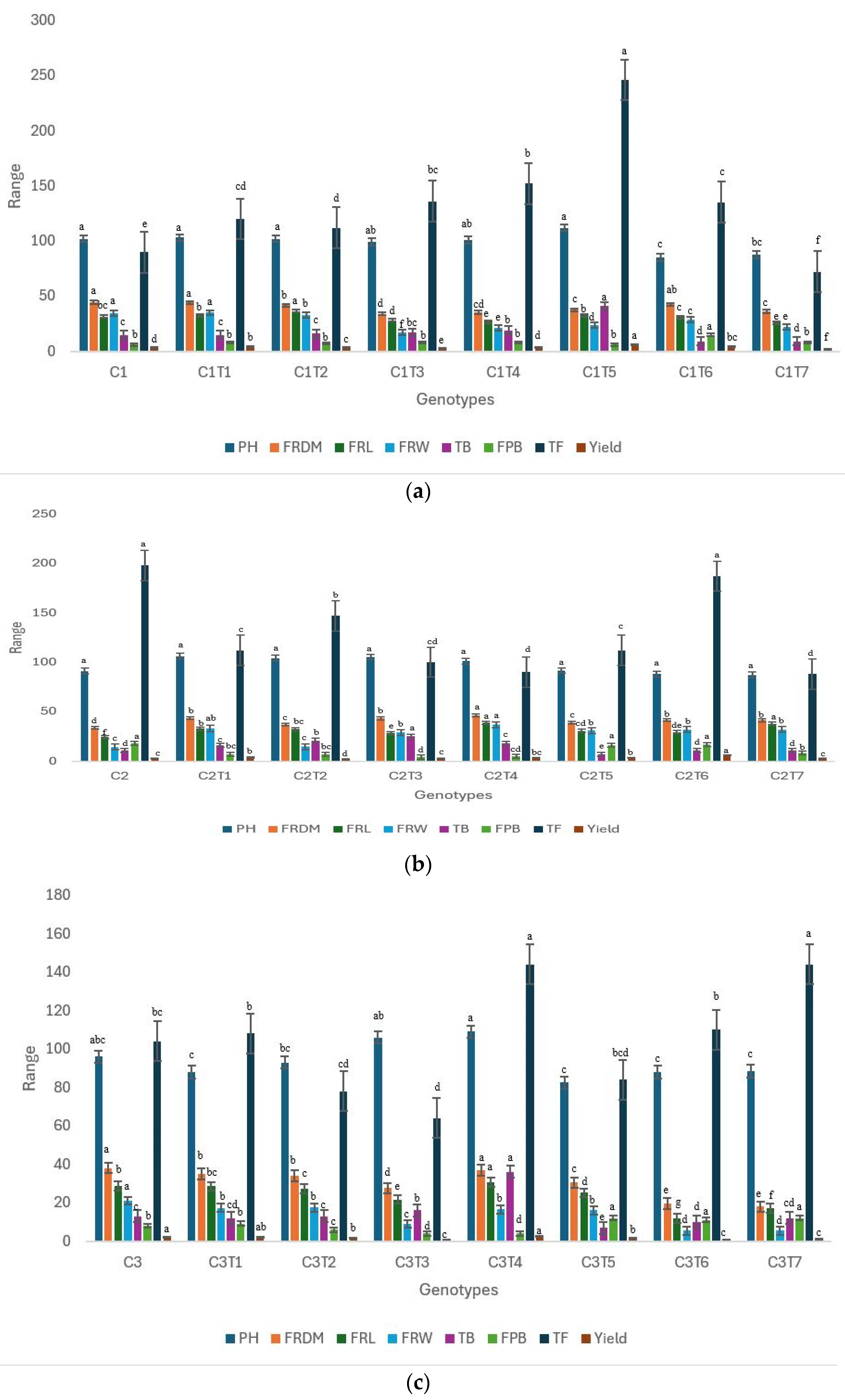
3.1. Morphological Changes
3.2. Biochemical Changes
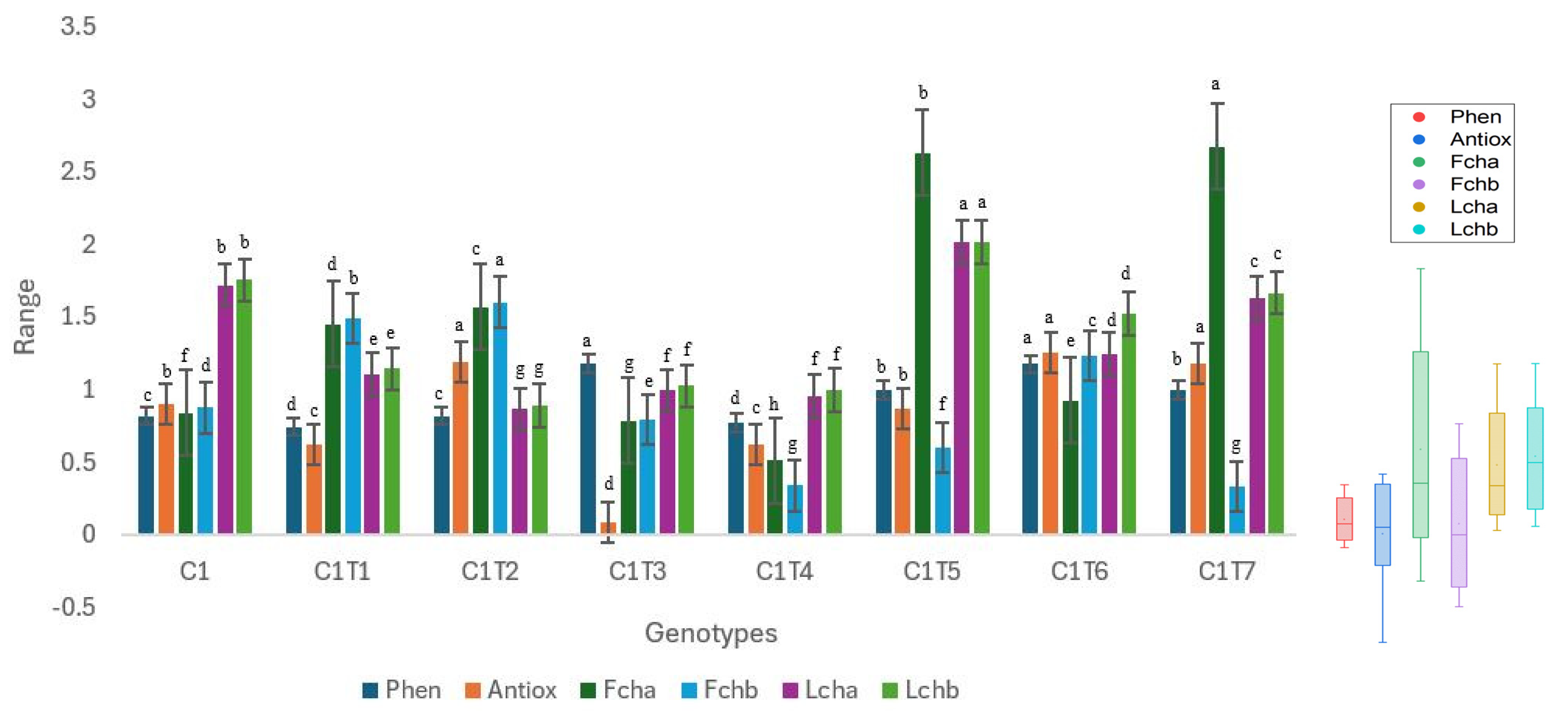
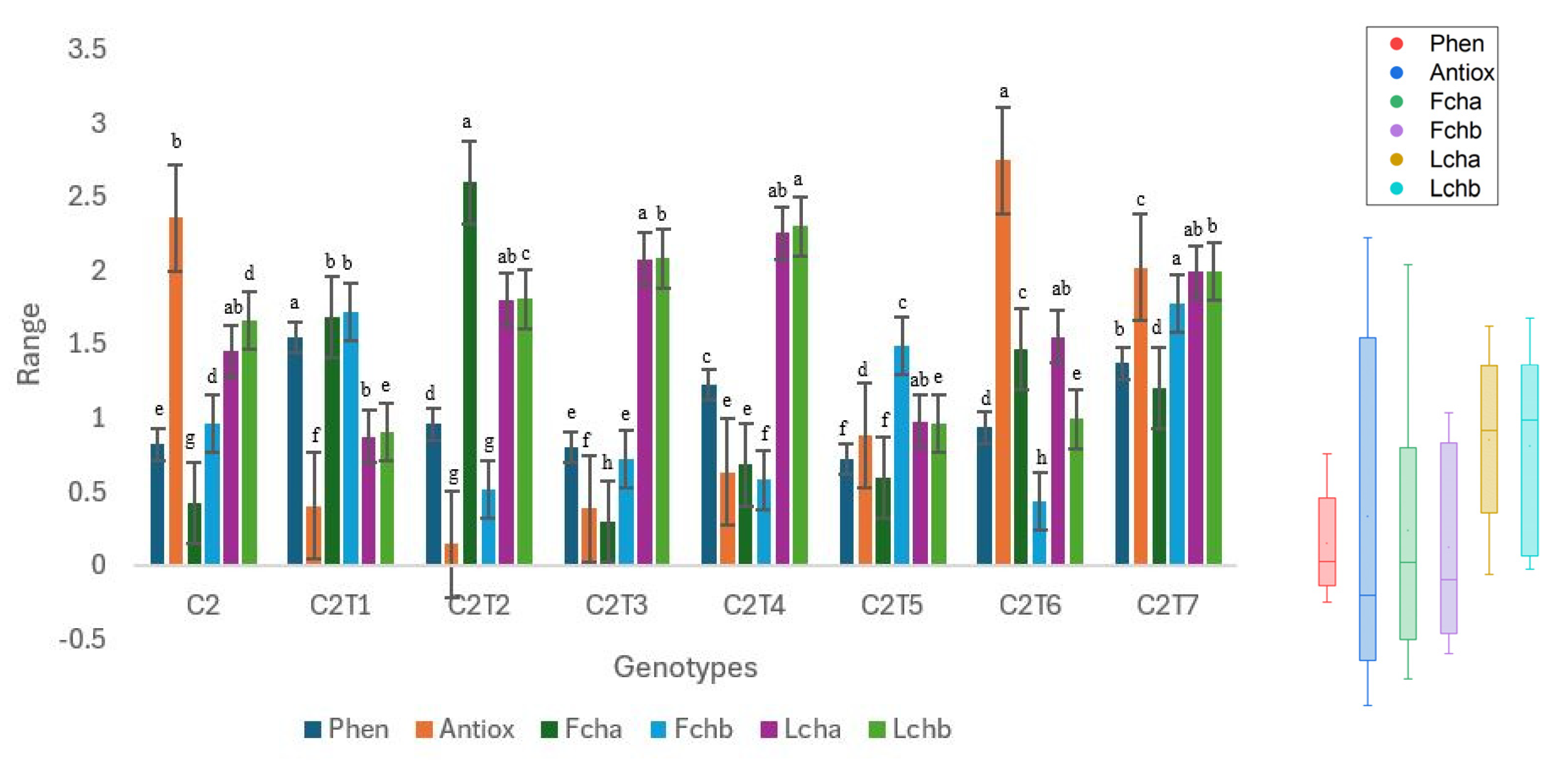
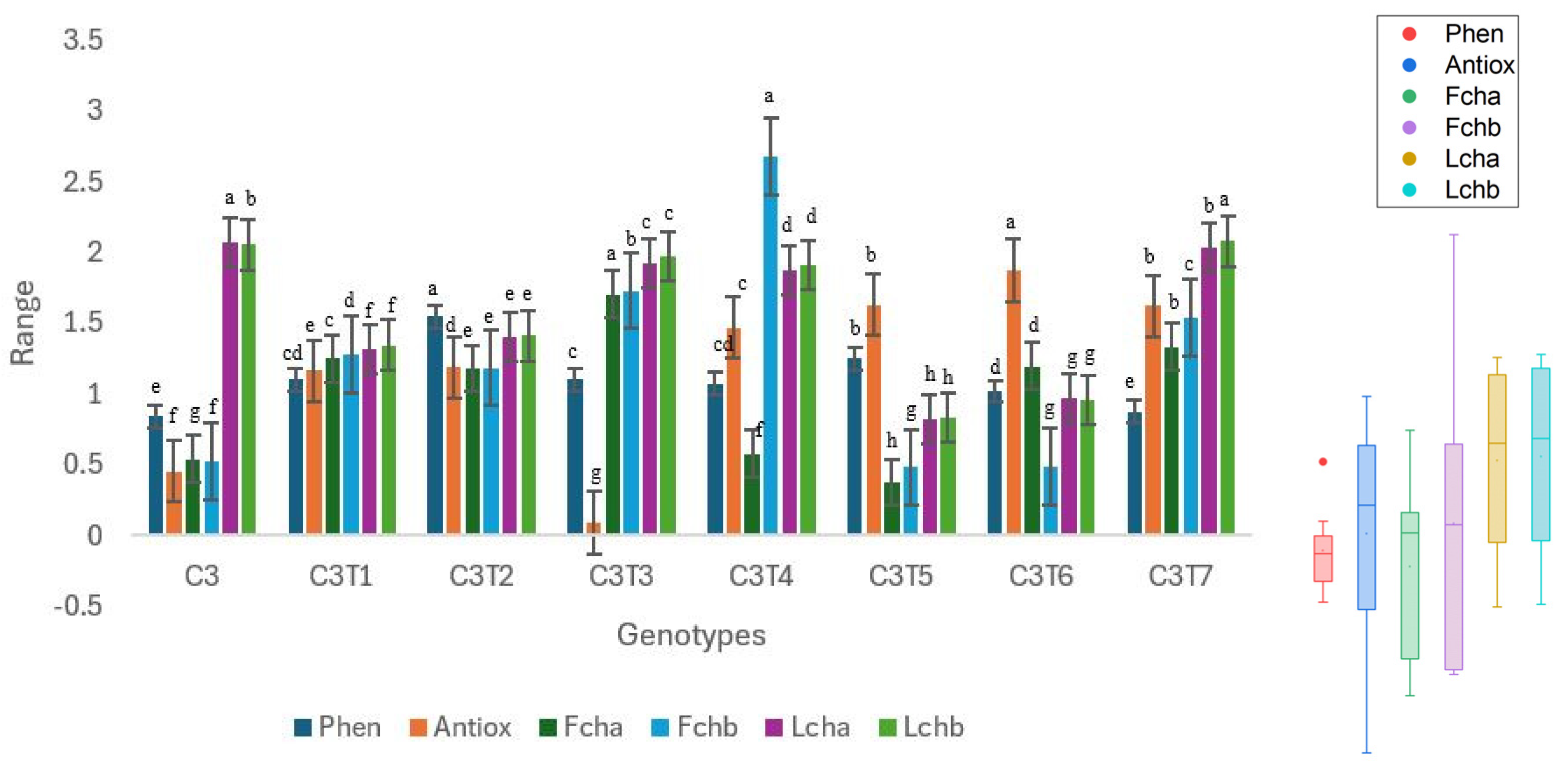
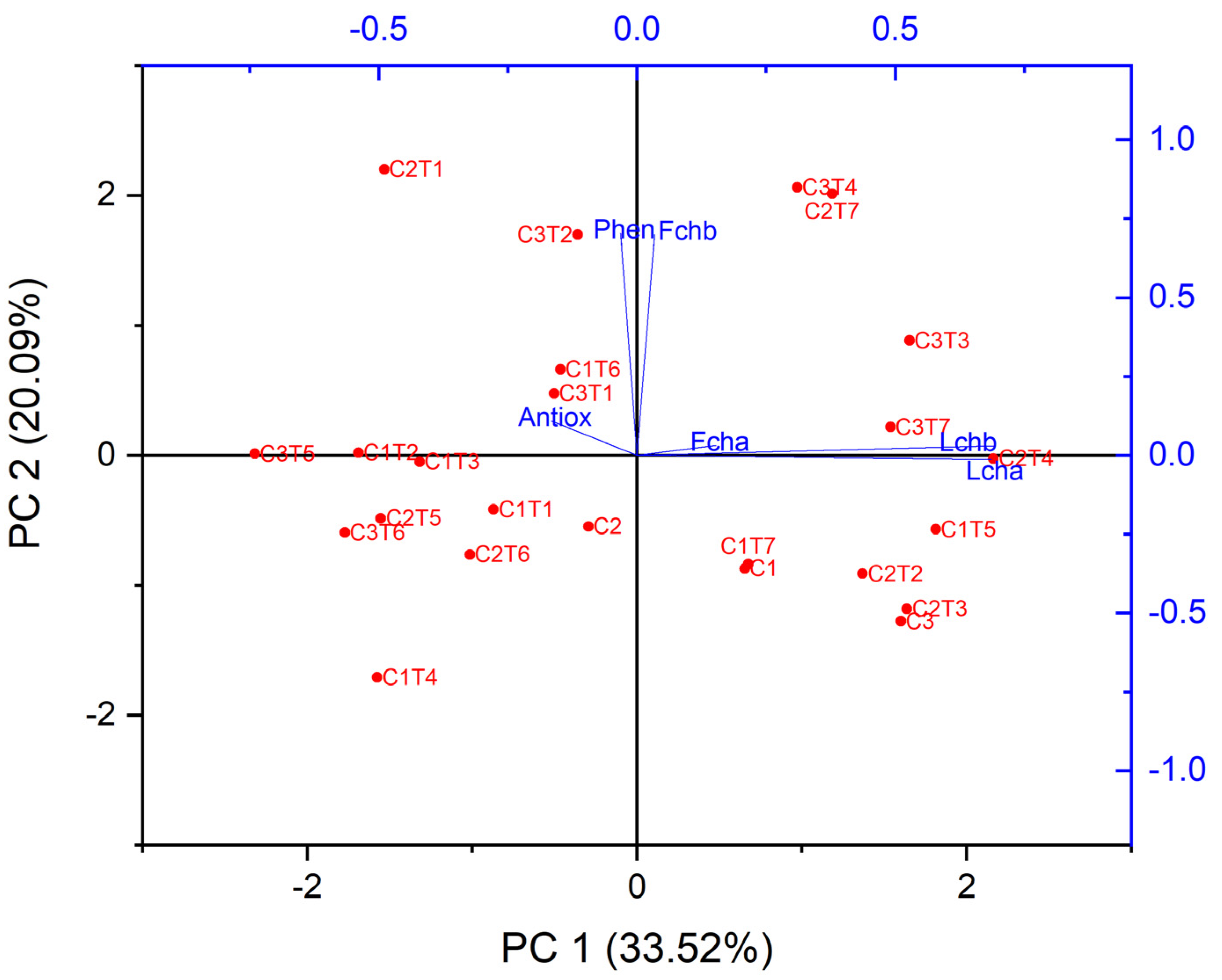
3.2.1. Changes in Total Phenol, Antioxidant Activity, and Chlorophyll Content
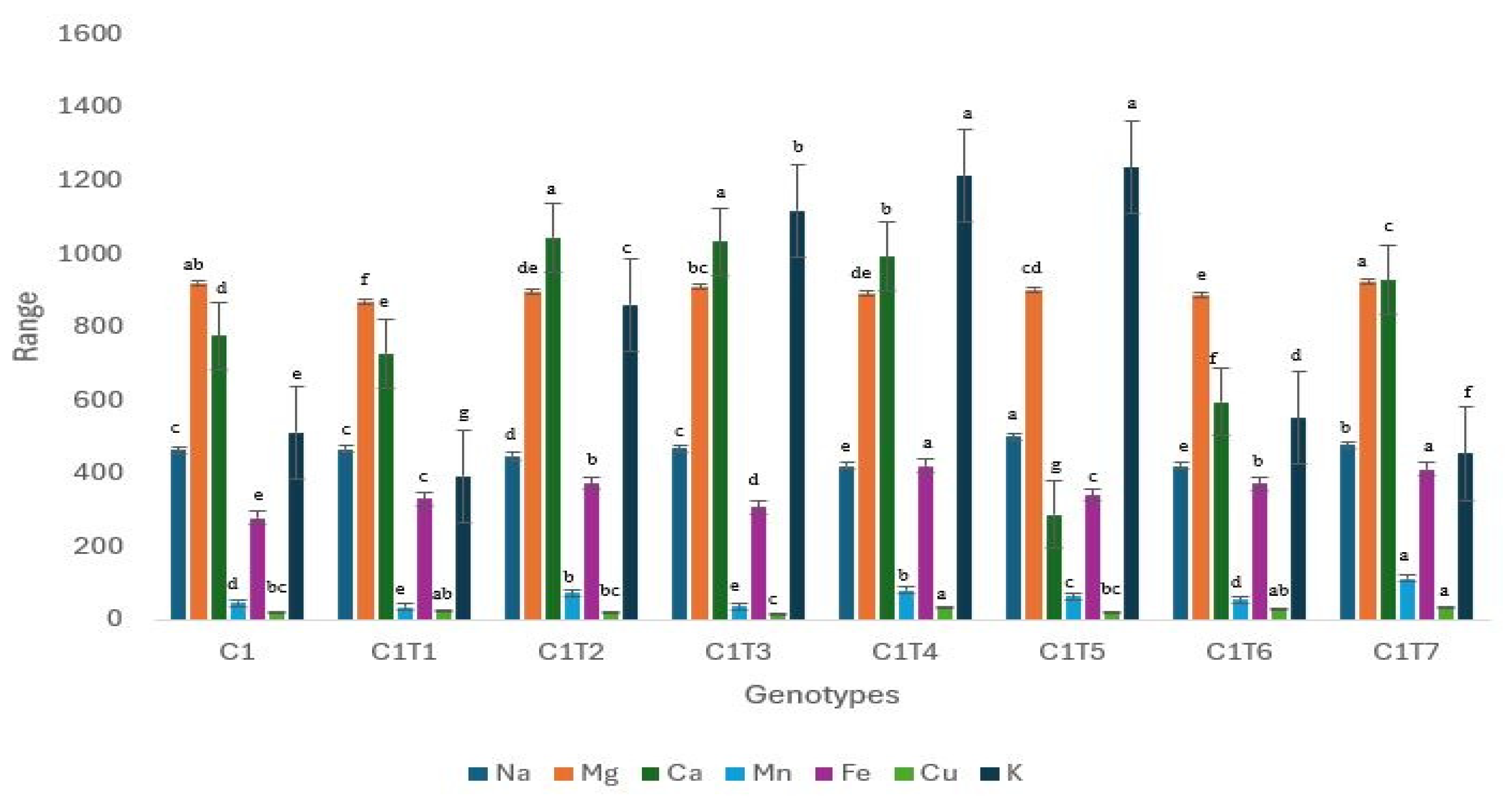
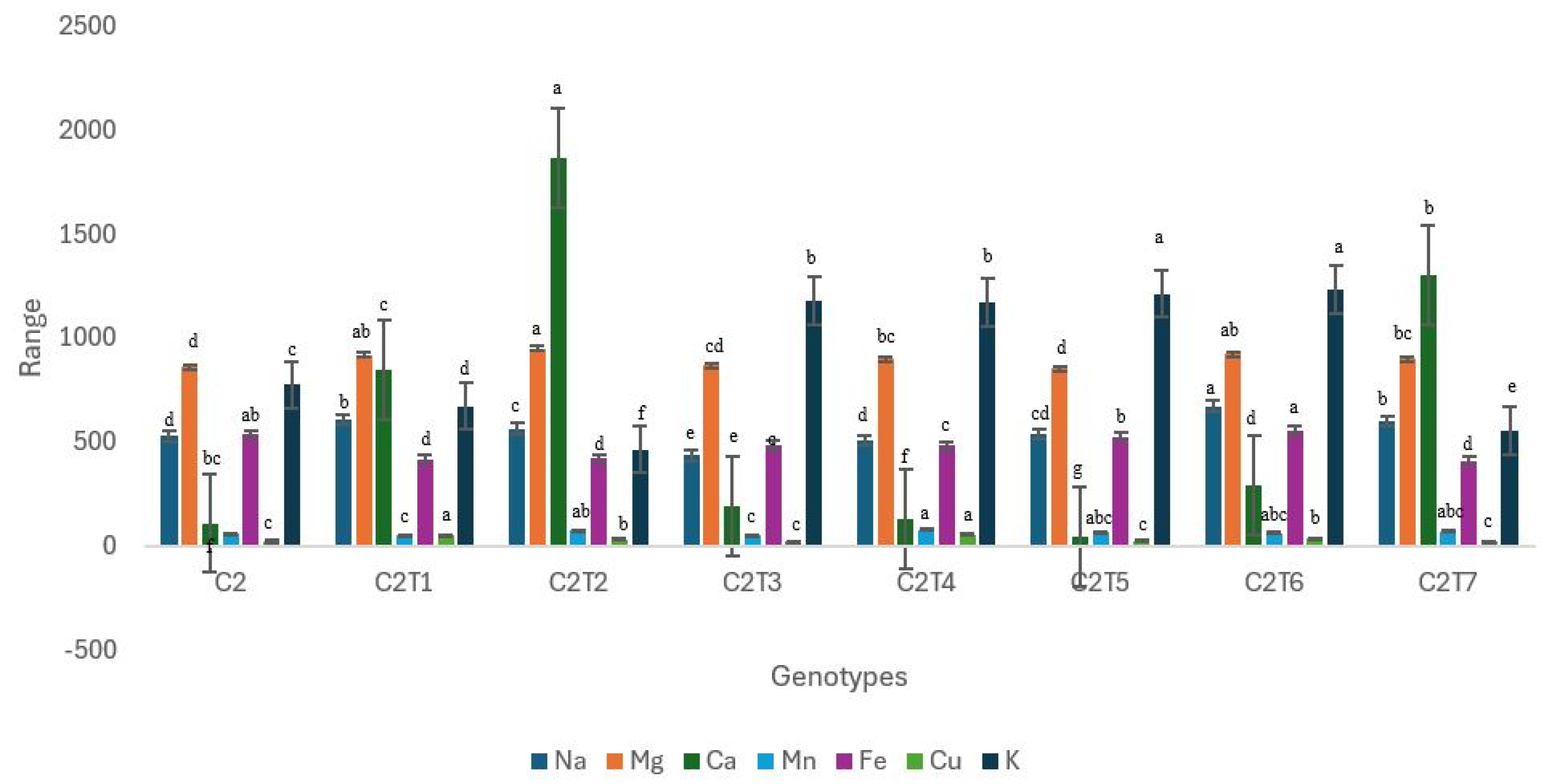
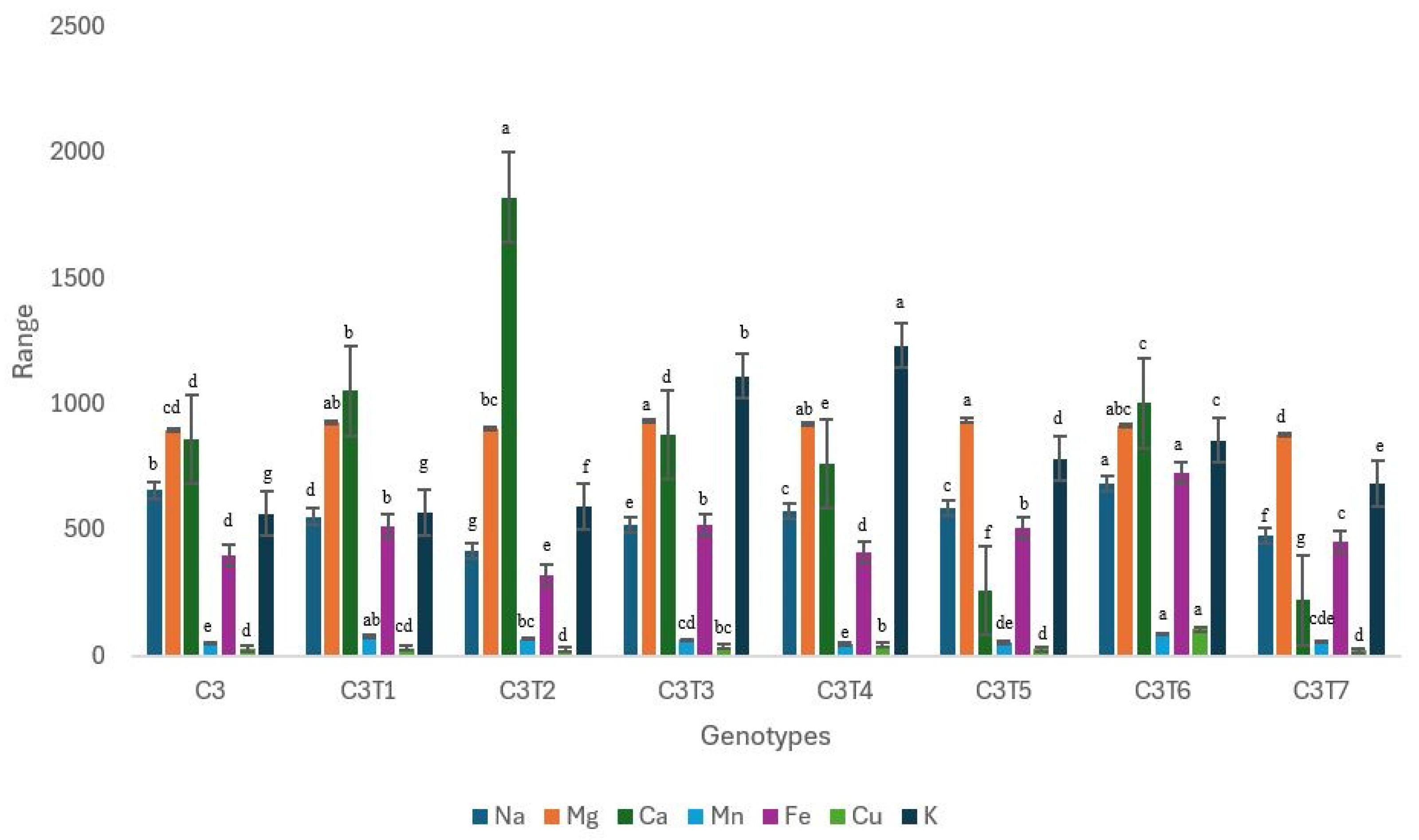
3.2.2. Changes in Mineral Contents
4. Discussion
4.1. Effect of EMS on Morphological Traits
4.2. Effect of EMS on Biochemical Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- González-Pérez, J.E.; Guerrero-Beltrán, J.Á. Tomatillo or husk tomato (Physalis philadelphica and Physalis ixocarpa): A review. Sci. Hortic. 2021, 288, 110306. [Google Scholar] [CrossRef]
- Ramírez-Cariño, H.F.; Ochoa-Velasco, C.E.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Calderón-García, C.; González-Terreros, E.; Escamirosa-Tinoco, C.; Morales, I.; Valadez-Blanco, R. Combined effect of the potassium dose and plant biofertilization by Acinetobacter calcoaceticus on the growth, mineral content, nutritional quality, antioxidant activity, and metabolomic features of tomatillo fruits (Physalis ixocarpa Brot.). Plants 2023, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Majkowska-Gadomska, J.; Mikulewicz, E.; Francke, A. Effects of plant covers and mulching on the biometric parameters, yield and nutritional value of tomatillos (Physalis ixocarpa Brot. Ex Hornem.). Agronomy 2021, 11, 1742. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Grimaldo-Juárez, O.; Soto-Ortiz, R.; Escoboza-Garcia, F.; Hernández, J.F.S. Evaluation of total phenolics, anthocyanins and antioxidant capacity in purple tomatillo (Physalis ixocarpa) genotypes. Afr. J. Biotechnol. 2010, 9, 5173–5176. [Google Scholar]
- Robledo-Torres, V.; Ramírez-Godina, F.; Foroughbakhch-Pournavab, R.; Benavides-Mendoza, A.; Hernández-Guzmán, G.; Reyes-Valdés, M.H. Development of Physalis philadelphica (Physalis ixocarpa Brot.) autotetraploids and their chromosome and phenotypic characterization. Breed. Sci. 2011, 61, 288–293. [Google Scholar] [CrossRef]
- Peña-Lomelí, A.; Ríos-Hernández, N.E.; Santos-Moreno, O.; Magaña-Lira, N. Genetic parameters of the Gema population of husk tomato (Physalis ixocarpa Brot. ex Horm.). Rev. Chapingo Ser. Hort. 2020, 26, 83–94. [Google Scholar]
- Shamsolshoara, N.; Miri, S.M.; Badi, H.N. Arginine and Folic Acid Improve Metabolites Content of Tomatillo (Physalis philadelphica) Fruit. Russ. J. Plant Physiol. 2023, 70, 211. [Google Scholar] [CrossRef]
- Anand, A.; Subramanian, M.; Kar, D. Breeding techniques to dispense higher genetic gains. Front. Plant Sci. 2023, 13, 1076094. [Google Scholar] [CrossRef]
- Chen, L.; Duan, L.; Sun, M.; Yang, Z.; Li, H.; Hu, K.; Yang, H.; Liu, L. Current trends and insights on EMS mutagenesis application to studies on plant abiotic stress tolerance and development. Front. Plant Sci. 2023, 13, 1052569. [Google Scholar] [CrossRef]
- Islam, M.A.; Bin Mohi Uddin, M.M.; Rasul, M.G.; Haque Swapon, M.A.; Ahmed, M.; Hasan, M. In vitro screening and field performance of EMS-treated eggplants for the selection of shoot and fruit borer-resistant plants. Agronomy 2022, 12, 1832. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Haliloğlu, K.; Tosun, M.; Bujak, H.; Eren, B.; Demirel, F.; Szulc, P.; Karagöz, H.; Selwet, M.; Özkan, G.; et al. Ethyl methanesulfonate (EMS) mutagen toxicity-induced DNA damage, cytosine methylation alteration, and iPBS-retrotransposon polymorphisms in wheat (Triticum aestivum L.). Agronomy 2023, 13, 1767. [Google Scholar] [CrossRef]
- Fonseca, R.; Capel, C.; Nieto-Canseco, R.; Ortiz-Atienza, A.; Bretones, S.; López-Fábregas, J.D.; Quevedo-Colmena, A.S.; Lebrón, R.; Barragán-Lozano, T.; Villalobos-Ramírez, V.; et al. A tomato EMS-mutagenized population provides new valuable resources for gene discovery and breeding of developmental traits. Plants 2022, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.I.; Back, S.; Lee, J.H.; Jo, J.; Jang, S.; Han, K.; Venkatesh, J.; Kwon, J.K.; Jo, Y.D.; Kang, B.C. Development and characterization of an ethyl methane sulfonate (EMS) induced mutant population in Capsicum annuum L. Plants 2020, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Puripunyavanich, V.; Chanchula, N.; Maikaeo, L.; Limtiyayothin, M.; Orpong, P.; Tamman, A.; Piriyaphattarakit, A. Effects of Ethyl Methanesulfonate on Mutation Induction in Chrysanthemum spp. TiS 2023, 20, 6904. [Google Scholar] [CrossRef]
- Chaudhary, J.; Alisha, A.; Bhatt, V.; Chandanshive, S.; Kumar, N.; Mir, Z.; Kumar, A.; Yadav, S.K.; Shivaraj, S.M.; Sonah, H.; et al. Mutation breeding in tomato: Advances, applicability and challenges. Plants 2019, 8, 128. [Google Scholar] [CrossRef]
- Jabeen, A.; Mir, J.I.; Malik, G.; Yasmeen, S.; Ganie, S.A.; Rasool, R.; Hakeem, K.R. Biotechnological interventions of improvement in cabbage (Brassica oleracea var. capitata L.). Sci. Hortic. 2024, 329, 112966. [Google Scholar] [CrossRef]
- Subramaniam, R.; Kumar, V.S. Ethyl methanesulphonate (EMS)-mediated mutagenesis induces genetic and morphological variations in eggplant (Solanum melongena L.). Int. J. Plant Biol. 2023, 14, 714–728. [Google Scholar] [CrossRef]
- Benzie, I.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Yan, W.; Frégeau-Reid, J. Genotype by yield* trait (GYT) biplot: A novel approach for genotype selection based on multiple traits. Sci. Rep. 2018, 8, 8242. [Google Scholar] [CrossRef]
- Luo, S.; Wang, K.; Li, Z.; Li, H.; Shao, J.; Zhu, X. Salicylic acid enhances cadmium tolerance and reduces its shoot accumulation in Fagopyrum tataricum seedlings by promoting root cadmium retention and mitigating oxidative stress. Int. J. Mol. Sci. 2022, 23, 14746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Y.; Ning, S.; Xia, L.; Zhan, J.; Yang, Z.; Cheng, C.; Lou, Q.; Li, J.; Chen, J. QTL mapping for ovary-and fruit-related traits in Cucumis sativus-c. hystrix introgression line IL. Genes 2023, 14, 1133. [Google Scholar] [CrossRef]
- Arashi, M.; Roozbeh, M.; Hamzah, N.A.; Gasparini, M. Ridge regression and its applications in genetic studies. PLoS ONE 2021, 16, e0245376. [Google Scholar] [CrossRef]
- Páez, A.; Boisjoly, G. Exploratory Data Analysis. In Discrete Choice Analysis with R; Springer International Publishing: Cham, Switzerland; Singapore, 2023; pp. 25–64. [Google Scholar]
- Cicevan, R.; Sestras, A.F.; Plazas, M.; Boscaiu, M.; Vilanova, S.; Gramazio, P.; Vicente, O.; Prohens, J.; Sestras, R.E. Biological traits and genetic relationships amongst cultivars of three species of tagetes (Asteraceae). Plants 2022, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Dostalíková, L.; Hlásná Čepková, P.; Janovská, D.; Svoboda, P.; Jágr, M.; Dvořáček, V.; Viehmannová, I. Nutritional evaluation of quinoa genetic resources growing in the climatic conditions of central Europe. Foods 2023, 12, 1440. [Google Scholar] [CrossRef]
- Vescio, R.; Abenavoli, M.R.; Araniti, F.; Musarella, C.M.; Sofo, A.; Laface, V.L.A.; Spampinato, G.; Sorgonà, A. The assessment and the within-plant variation of the morpho-physiological traits and vocs profile in endemic and rare salvia ceratophylloides ard (lamiaceae). Plants 2021, 10, 474. [Google Scholar] [CrossRef]
- Santos, K.S.D.; Passos, A.R.; Silva, L.C.C.; Silva, A.L.D.; Tanan, T.T. Genetic variability of Physalis ixocarpa and P. philadelphica from physicochemical fruit traits. Pesqui. Agropecu. Bras. 2022, 56, e01534. [Google Scholar] [CrossRef]
- Trevisani, N.; Schmit, R.; Beck, M.; Guidolin, A.F.; Coimbra, J.L.M. Selection of fisális populations for hybridization, based on fruit traits. Rev. Bras. Frutic. 2016, 38, e-568. [Google Scholar] [CrossRef]
- Antúnez-Ocampo, O.M.; Serafín, C.I.; Mendoza-Onofre, L.E.; Sandoval-Villa, M.; Santacruz-Varela, A.; De La Cruz-Torres, E.; Aureliano, P.L. Growth dynamics of morphological and reproductive traits of Physalis peruviana L. M1 plants obtained from seeds irradiated with gamma rays. Not. Bot. Horti. Agrobot. 2020, 48, 200–209. [Google Scholar] [CrossRef]
- Khan, M.H.; Tyagi, S.D. Studies on effectiveness and efficiency of gamma rays, EMS and their combination in soybean (Glycine max (L.) Merrill). J. Plant Breed. Crop Sci. 2010, 2, 55–58. [Google Scholar]
- Thakur, G.; Paul, S.; Kumar, A. Mutagenic effectiveness and efficiency of ethyl methane sulphonate (EMS) mutagen in linseed (Linum usitatissimum L.). J. Oilseeds Res. 2020, 37, 260–266. [Google Scholar]
- Wani, M.R.; Khan, S.; Kozgar, M.I. Induced chlorophyll mutations. I. Mutagenic effectiveness and efficiency of EMS, HZ and SA in mungbean. Front. Agric. China 2011, 5, 514–518. [Google Scholar] [CrossRef]
- Shenstone, E.; Lippman, Z.; Van Eck, J. A review of nutritional properties and health benefits of Physalis species. Plant Foods Hum. Nutr. 2020, 75, 316–325. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, M.R.A.; Khalifa, A.H.; Abo Zeiad, F.K.; Mohamed, M.I.A. Nutrient and Phytochemical Compounds of Persimmon and Husk Tomato. Assiut J. Agric. Sci. 2017, 48, 102–112. [Google Scholar]
- Cagnato, C. Shedding light on the nightshades (solanaceae) used by the ancient Maya: A review of existing data, and new archeobotanical (macro-and microbotanical) evidence from archeological sites in Guatemala. Econ. Bot. 2018, 72, 180–195. [Google Scholar] [CrossRef]
- Leung, C.C.; Tarté, D.A.; Oliver, L.S.; Wang, Q.; Gendron, J.M. Systematic characterization of photoperiodic gene expression patterns reveals diverse seasonal transcriptional systems in Arabidopsis. PLoS Biol. 2023, 21, e3002283. [Google Scholar] [CrossRef]
- Schroeder, L.; Robles, V.; Jara-Arancio, P.; Lapadat, C.; Hobbie, S.E.; Arroyo, M.T.; Cavender-Bares, J. Drivers of plant diversity, community composition, functional traits, and soil processes along an alpine gradient in the central Chilean Andes. Ecol. Evol. 2024, 14, e10888. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, E.M.; Ali, A.O.; El-Sayed, H.S.; Kassem, J.M. Quality properties of husk tomato juice and its impact in stirred probiotic yogurt. Asian Food Sci. J. 2019, 7, 1–10. [Google Scholar] [CrossRef]


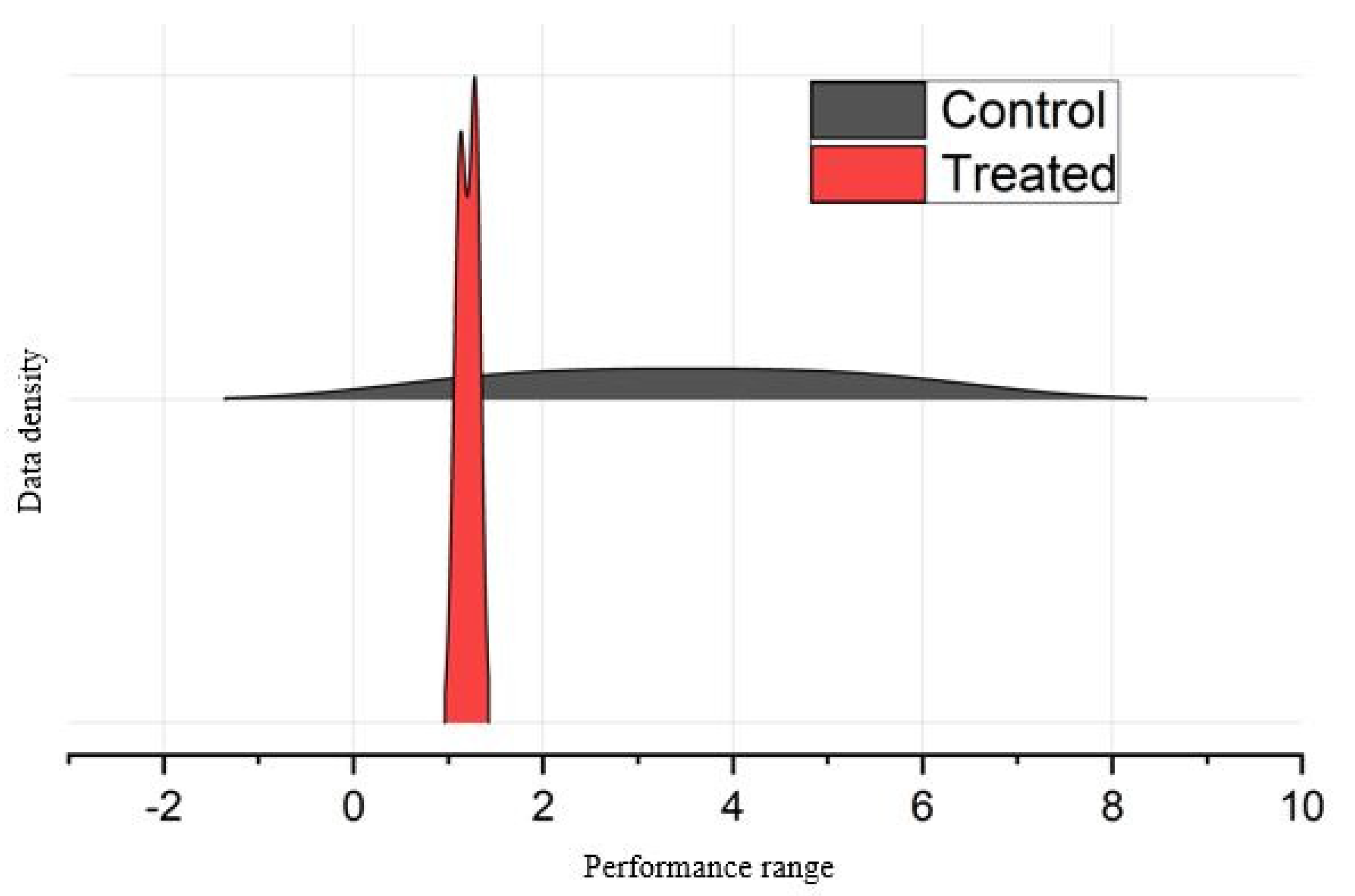
| Control | Mutants | |
|---|---|---|
| C1 | C1T1 | Mutant of C1 |
| C1T2 | ||
| C1T3 | ||
| C1T4 | ||
| C1T5 | ||
| C1T6 | ||
| C1T7 | ||
| C2 | C2T1 | Mutant of C2 |
| C2T2 | ||
| C2T3 | ||
| C2T4 | ||
| C2T5 | ||
| C2T6 | ||
| C2T7 | ||
| C3 | C3T1 | Mutant of C3 |
| C3T2 | ||
| C3T3 | ||
| C3T4 | ||
| C3T5 | ||
| C3T6 | ||
| C3T7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Tarannum, F.; Dina, A.H.; Ahmed, M.; Haque, M.A.; Ercişli, S.; Rasul, M.G.; Simsek, D.; Hasan, M. Phenotypic and Biochemical Trait Improvement in Husk Tomatoes (Physalis sp.) through EMS-Induced Mutagenesis. Horticulturae 2024, 10, 913. https://doi.org/10.3390/horticulturae10090913
Islam MA, Tarannum F, Dina AH, Ahmed M, Haque MA, Ercişli S, Rasul MG, Simsek D, Hasan M. Phenotypic and Biochemical Trait Improvement in Husk Tomatoes (Physalis sp.) through EMS-Induced Mutagenesis. Horticulturae. 2024; 10(9):913. https://doi.org/10.3390/horticulturae10090913
Chicago/Turabian StyleIslam, Md Ashraful, Fabeeha Tarannum, Afsana Hossain Dina, Minhaz Ahmed, Md Ahsanul Haque, Sezai Ercişli, Md Golam Rasul, Duran Simsek, and Mehfuz Hasan. 2024. "Phenotypic and Biochemical Trait Improvement in Husk Tomatoes (Physalis sp.) through EMS-Induced Mutagenesis" Horticulturae 10, no. 9: 913. https://doi.org/10.3390/horticulturae10090913
APA StyleIslam, M. A., Tarannum, F., Dina, A. H., Ahmed, M., Haque, M. A., Ercişli, S., Rasul, M. G., Simsek, D., & Hasan, M. (2024). Phenotypic and Biochemical Trait Improvement in Husk Tomatoes (Physalis sp.) through EMS-Induced Mutagenesis. Horticulturae, 10(9), 913. https://doi.org/10.3390/horticulturae10090913








