Quality Parameters of Plum Orchard Subjected to Conventional and Ecological Management Systems in Temperate Production Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area Description
2.2. Experimental Setup and Tillage Management Practices
2.3. Sample Collection
Methodology of Determining Soil Chemical Parameters
2.4. Physical–Chemical and Phytochemical Determinations for Plum Quality Parameters
2.4.1. Preparation of Extracts
Reagents and Chemicals
Plum Powder Preparation
Extraction of Bioactive Compounds from Plum Powders
2.4.2. Extract Characterization
Determination of Total Anthocyanin Content
Determination of Total Flavonoid Content
Determination of Total Polyphenol Content
Determination of Antioxidant Activity
2.4.3. Colorimetric Analysis
2.4.4. Measuring Heavy Metals in Samples
2.5. Assessment of Health Risks in Orchard Plums
2.5.1. Factors of Heavy Metal Transfer (MTF)
2.5.2. Health Risk Assessment of Heavy Metals in Plum Consumption
2.6. Data Analysis
2.6.1. Analysis of Bivariate Correlation
2.6.2. Analytical Statistics
3. Results and Discussion
3.1. Soil Chemical Parameters
3.2. Physical–Chemical Plum Quality Parameters
3.3. Phytochemical Characterization of Plum Powders
3.4. Color Evaluation of Plum Powders
3.5. Pearson Correlation Analysis of Plum Fruit Characteristics
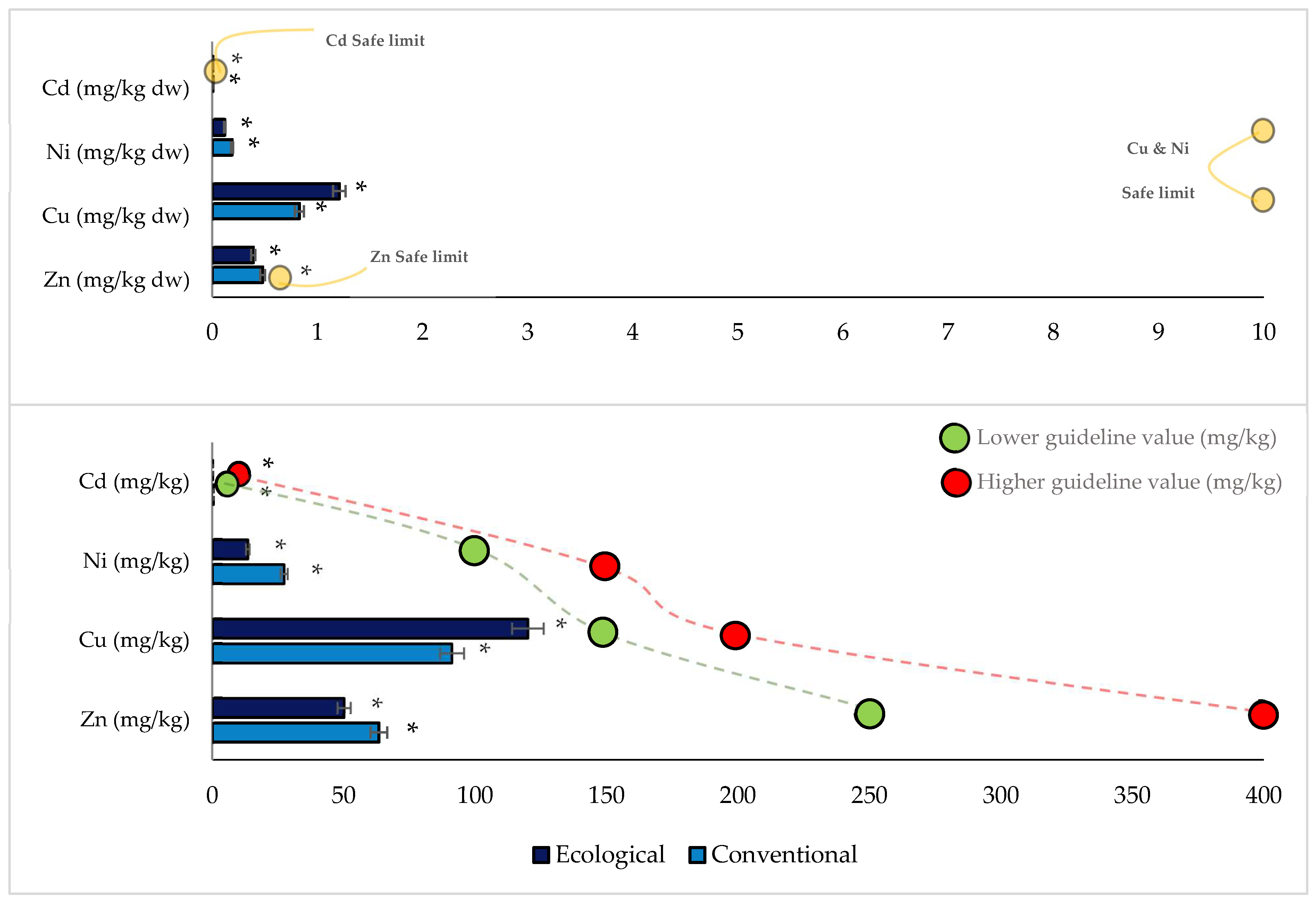
3.6. Concentrations of Heavy Metals in Orchard Plums
3.7. Pearson Correlation Analysis of Heavy Metals in Plum Orchard
Metal Transfer Factor
3.8. Assessment of Health Risks of Metals in Plum Fruits
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, H.; Long, X.; Wang, X.; Wang, Y.; Pang, C.; Xia, H.; Liang, D.; Zhang, H.; Luo, X.; Wang, J.; et al. Comparative analysis of carotenoid profiles and biosynthetic gene expressions among ten plum cultivars. Plants 2023, 12, 2711. [Google Scholar] [CrossRef] [PubMed]
- Matłok, N.; Piechowiak, T.; Krempa, A.; Puchalski, C.; Balawejder, M. Cyclic storage chamber ozonation as a method to inhibit ethylene generation during plum fruit storage. Agriculture 2023, 13, 2274. [Google Scholar] [CrossRef]
- Vaško, Ž.; Cvetković, M. Processing of plums into brandy—A matter of the economic feasibility of growing plum (Prunus domestica) in Bosnia and Herzegovina. Acta Hortic. 2017, 1175, 9–14. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in phenolic compounds and antioxidant activity during development of ‘Qiangcuili’ and ‘Cuihongli’ fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Q.Y.; Venkitasamy, C.; Chai, H.; Gao, H.; Cheng, N.; Cao, W.; Lv, X.; Pan, Z. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller.) during three edible maturity stages. LWT Food Sci. Technol. 2016, 66, 56–62. [Google Scholar] [CrossRef]
- Kim, H.J.; Yu, M.H.; Lee, I.S. Inhibitory effects of methanol extract of plum (Prunus salicina L., cv. ‘Soldam’) fruits against benzo (α) pyrene−induced toxicity in mice. Food Chem. Toxicol. 2008, 46, 3407–3413. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Kalt, W.; Carey, A.N.; Vinqvist-Tymchuk, M.; McDonald, J.; Joseph, J.A. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition 2009, 25, 567–573. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Cho, S.M.; Chun, H.K.; Park, H.J.; Lim, C.I.; Lillehoj, E.P. Immunostimulatory effects of oriental plum (Prunus salicina Lindl). Comp. Immunol. Microbiol. Infect. Dis 2009, 32, 407–417. [Google Scholar]
- Sahamishirazi, S.; Moehring, J.; Claupein, W.; Graeff-Hoenninger, S. Quality assessment of 178 cultivars of plum regarding phenolic, anthocyanin and sugar content. Food Chem. 2017, 214, 694–701. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R. Cisneros-Zevallos Selecting new peach and plum genotypes rich in phenolics compounds and enhanced functional properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Rop, O.; Jurikova, T.; Mlcek, J.; Kramarova, D. Sengee Antioxidant activity and selected nutritional values of plums (Prunus domestica L.) typical of the White Carpathian Mountains. Sci. Hortic. 2009, 122, 545–549. [Google Scholar] [CrossRef]
- Kayano, S.-I.; Kikuzaki, H.; Fukutsuka, N.; Mitani, T.; Nakatani, N. Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. J. Agric. Food Chem. 2002, 50, 3708. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical composition and potential health effects of prunes: A functional food? Crit. Rev. Food Sci. Nutr. 2001, 41, 251–286. [Google Scholar] [CrossRef]
- Bošković-Rakočević, L.; Milivojević, J.; Milošević, T.; Paunović, G. Heavy metal content of soils and plum orchards in an uncontaminated area. Water Air Soil Pollut. 2014, 225, 11. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Herencia, J.F.; Pérez-Romero, L.F.; Daza, A.; Arroyo, F.T. Chemical and biological indicators of soil quality in organic and conventional Japanese plum orchards. Biol. Agric. Hortic. 2020, 37, 71–90. [Google Scholar] [CrossRef]
- Shannon, D.; Sen, A.M.; Johnson, D.B. A comparative study of the microbiology of soils managed under organic and conventional regimes. Soil Use Manag. 2002, 18, 274–283. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. Adv. Agron. 2013, 120, 275–379. [Google Scholar]
- Rusu, M.; Cara, I.G.; Filip, M.; Calistru, A.E.; Topa, D.; Jitareanu, G. Transfer of heavy metals in soil in-plum cultivation: A field study in adamachi Iasi, Romania. J. Appl. Life Sci. Environ. 2023, 56, 59–74. [Google Scholar] [CrossRef]
- Singh, N.; Meena, R.; Meena, K.; Patle, N.; Meena, R.K.; Srivastava, R.; Singh, P. Soil profile studies under different orchard management system in Chhindwara District of Madhya Pradesh, India. Int. J. Plant Soil Sci. 2022, 34, 711–717. [Google Scholar] [CrossRef]
- Aguirre, J. The Kjeldahl method. In The Kjeldahl Method: 140 Years; Springer Nature: Cham, Switzerland, 2023; pp. 53–78. [Google Scholar]
- Brown, J.R. Soil Testing: Sampling, Correlation, Calibration, and Interpretation; SSSA Special Publications: Madison, WI, USA, 1987. [Google Scholar]
- Legua, P.; Modica, G.; Porras, I.; Conesa, A.; Continella, A. Bioactive compounds, antioxidant activity and fruit quality evaluation of eleven blood orange cultivars. J. Sci. Food Agric. 2022, 102, 2960–2971. [Google Scholar] [CrossRef]
- Liu, Z.L.; Bai, J.W.; Yang, W.X.; Wang, J.; Deng, L.Z.; Yu, X.L.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Effect of high-humidity hot air impingement blanching (HHAIB) and drying parameters on drying characteristics and quality of broccoli florets. Dry. Technol. 2019, 37, 1251–1264. [Google Scholar] [CrossRef]
- Vanek, A.; Komarek, M.; Chrastny, V.; Becka, D.; Mihaljevic, M.; Sebek, O.; Panuskova, G.; Schusterova, Z. Thallium uptake by white mustard (Sinapis alba L.) grown on moderately contaminated soils-Agro-environmental implications. J. Hazard. Mater. 2010, 182, 303–308. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barbosa-Cánovas, G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. J. Food Eng. 2015, 166, 268–275. [Google Scholar] [CrossRef]
- Dumitriu, G.D.; Teodosiu, C.; Morosanu, I.; Plavan, O.; Gabur, I.; Cotea, V.V. Heavy metals assessment in the major stages of winemaking: Chemometric analysis and impacts on human health and environment. J. Food Comp. Anal. 2021, 100, 103935. [Google Scholar] [CrossRef]
- Tamene, B.; Kindnew, D.; Israel, L. Levels and health risk assessment of trace metals in honey from different districts of Bench Sheko Zone; Southwest Ethiopia. Heliyon 2022, 8, 10535. [Google Scholar]
- Saif, U.D.; Ali, S.; Hussain, S.; Hussain, J.; Luqman, M.; Hussain, J.; Ali, S. Evaluation of heavy metal contamination in indigenous fruits and associated human health risk: Evidence from Fuzzy-TOPSIS approach. Glob. NEST J. 2022, 24, 435–444. [Google Scholar]
- Zunaidi, A.A.; Lim, L.H.; Metali, F. Assessments of heavy metals in commercially available fertilizers in Brunei Darussalam. Agric. Res. 2021, 10, 234–242. [Google Scholar] [CrossRef]
- Ezez, D.; Belew, M. Analysis of physicochemical attributes, contamination level of trace metals and assessment of health risk in mango fruits from Southern region Ethiopia. Toxicol. Rep. 2023, 10, 124–132. [Google Scholar] [CrossRef] [PubMed]
- ICPA. Available online: http://Icpa.ro (accessed on 12 June 2024).
- Butac, M.; Chitu, E.; Militaru, M.; Sumedrea, M.; Sumedrea, D.; Plopa, C. Orchard performance of some Romanian plum cultivars grafted on two rootstocks. Agric. Agric. Sci. Procedia 2015, 6, 118–123. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, D.D.; Sharma, D.P.; Shylla, B.; Singh, U.; Verma, P.; Mushtaq, M.; Parihar, N.S.; Wani, O.A.; Casini, R.; et al. Integrated nutrient management as a low cost and Eco-friendly strategy for sustainable fruit production in apricot (Prunus armeniaca L.). Int. J. Fruit Sci. 2024, 24, 18–32. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, H.; Tu, C.; Li, L.; Liu, X.; Luo, Y. Spatial interpolation of orchard soil pH using soil type and planting duration as auxiliary information. Pedosphere 2020, 30, 628–637. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Y.; Xie, X.; Li, X.; Zhang, X.; Shen, X. Effect of annual variation in soil pH on available soil nutrients in pear orchards. Sheng Tai Xue Bao 2011, 31, 212–216. [Google Scholar] [CrossRef]
- Pathania, S.; Kumari, N.; Sharma, A. Comparative effect of natural and chemical farming systems of apple production on soil physio-chemical properties, leaf mineral content and fruit quality. Int. J. Plant Soil Sci. 2023, 35, 48–59. [Google Scholar] [CrossRef]
- Kai, T.; Mukai, M.; Araki, K.S.; Adhikari, D.; Kubo, M. Analysis of chemical and biological soil properties in organically and conventionally fertilized apple orchards. J. Agric. Chem. Environ. 2016, 5, 92–99. [Google Scholar] [CrossRef][Green Version]
- Kai, T.; Kubo, M. Chemical and biological properties of apple orchard soils under natural, organic, hybrid, and conventional farming methods. J. Agric. Chem. Environ. 2020, 9, 134–146. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E. Chemical properties of soil in four-field crop rotations under organic and conventional farming systems. Agronomy 2020, 10, 1045. [Google Scholar] [CrossRef]
- Maucieri, C.; Tolomio, M.; Raimondi, G.; Toffanin, A.; Morari, F.; Berti, A.; Borin, M. Organic versus conventional farming: Medium-term evaluation of soil chemical properties. Ital. J. Agron. 2022, 17. [Google Scholar] [CrossRef]
- Razanov, S.; Melnyk, V. Agrochemical evaluation of soils under horticulture on the suitability of their usage for the main agricultural crops growing. Agric. For. 2022, 171–181. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and enzymatic soil properties influenced by cropping of primary wheat under organic and conventional farming systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
- Kim, J.W.; Hong, Y.K.; Lee, C.R.; Kim, S.C. Comparison of physicochemical and biological soil properties in organic and conventional upland fields. Hanguk Toyang Piryo Hakhoe Chi 2023, 56, 77–89. [Google Scholar] [CrossRef]
- Potor, D.C.; Dobrin, A.; Georgescu, M.I.; Hoza, D. Physical and chemical parameters of the fruit in four Prunus domestica local populations from Buzău county. Sci. Pap. Ser. B Hortic. 2018, 62, 65–69. [Google Scholar]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M.A. Review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valero, D.; Serrano, M. Preharvest application of methyl jasmonate (MeJA) in two plum cultivars. 1. Improvement of fruit growth and quality attributes at harvest. Postharvest Biol. Technol. 2014, 98, 98–105. [Google Scholar] [CrossRef]
- Saridaş, M.A.; Kafkas, E.; Zarifikhosroshahi, M.; Bozhaydar, O.; Kargi, P. Quality traits of green plums (Prunus cerasifera Ehrh.) at different maturity stages. Turk. J. Agric. For. 2016, 40, 655–663. [Google Scholar]
- Ayanoğlu, S.; Bayazit, G.; İnan, M.; Bakır, A.E.; Akpınar, K.; Kazan, A.; Ergül, A. AFLP analysis of genetic diversity in Turkish green plum accessions (Prunus cerasifera L.) adapted to the Mediterranean region. Sci. Hortic. Amst. 2007, 114, 263–267. [Google Scholar] [CrossRef]
- da Silva, N.K.V.; de Sabino, L.B.S.; de Oliveira, L.S.; de Torres, L.B.V.; de Sousa, P.H.M. Effect of food additives on the antioxidant properties and microbiological quality of red guava juice. Rev. Cienc. Agron. 2016, 47, 77–85. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Zhao, J.; Liu, Y. The effect of non-covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of in vitro protein digests. Food Chem. 2018, 268, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Nergiz, C. Yıldız Research on chemical composition of some varieties of european plums (Prunus domestica) adapted to the aegean district of Turkey. J. Agric. Food Chem. 1997, 45, 2820–2823. [Google Scholar] [CrossRef]
- Sikora, E.; Bieniek, M.I.; Borczak, B. Composition and antioxidant properties of fresh and frozen stored blackthorn fruits (Prunus spinosa L). Acta Sci. Pol. Hortic. 2013, 12, 365–372. [Google Scholar]
- US Department of Agriculture. Agricultural Research Service; USDA: Washington, DC, USA, 2018. [Google Scholar]
- Brown, S.K.; Bourne, M.C. Assessment of components of fruit firmness in selected sweet cherry genotypes. Hortic. Sci. 1988, 23, 902–904. [Google Scholar] [CrossRef]
- Goosen, N.J.; Oosthuizen, D.; Stander, M.A.; Dabai, A.I.; Pedavoah, M.M.; Usman, G.O. Phenolics, organic acids and minerals in the fruit juice of the indigenous African sourplum (Ximenia caffra, Olacaceae). S. Afr. J. Bot. 2018, 119, 11–16. [Google Scholar] [CrossRef]
- Marakoğlu, T.; Arslan, D.; Özcan, M.; Hacıseferoğullari, H. Proximate composition and technological properties of fresh blackthorn (Prunus spinosa L. subsp dasyphylla (Schur.)) fruits. J. Food Eng. 2005, 68, 137–142. [Google Scholar] [CrossRef]
- Çalısır, S.; Hacıseferoğullari, H.; Özcan, M.; Arslan, D. Some nutritional and technological properties of wild plum (Prunus spp.) fruits in Turkey. J. Food Eng. 2005, 66, 233–237. [Google Scholar] [CrossRef]
- Tounsi, L.; Karra, S.; Kechaou, H.; Kechaou, N. Processing, physico-chemical and functional properties of carob molasses and powders. J. Food Meas. Charact. 2017, 11, 1440–1448. [Google Scholar] [CrossRef]
- İncedayi, B.; Tamer, C.E.; Çopur, Ö.U. A research on the composition of pomegranate molasses. J. Agric. Fac. Uludag Univ. 2010, 24, 37–47. [Google Scholar]
- Leterme, P.; Buldgen, A.; Estrada, F.; Londoño, A.M. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chem. 2006, 95, 644–652. [Google Scholar] [CrossRef]
- Karunasena, G.; Chandrajith, V.; Nawaratne, S.B. Physicochemical characteristics of pea nut butter fruit (Bunchosia armeniaca). Int. J. Food Sci. Nutr. 2018, 3, 46–51. [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Li, Q.; Chang, X.X.; Wang, H.; Brennan, C.S.; Guo, X.B. Phytochemicals accumulation in sanhua plum (Prunus salicina L.) during fruit development and their potential use as antioxidants. J. Agric. Food Chem. 2019, 67, 2459–2466. [Google Scholar] [CrossRef]
- Mertoğlu, K. Investıgatıon of genetıc parameters and phytochemıcal characterıstıcs ın plum under altıtude change. Genetika 2022, 54, 73–89. [Google Scholar] [CrossRef]
- Sanchi, M.; Soni, N.; Satpathy, G.; Rajinder, K. Evaluation of nutritional, phytochemical, antioxidant and antibacterial activity of dried plum (Prunus domestica). J. Pharmacogn. Phytochem. 2014, 3, 166–171. [Google Scholar]
- Kaur, N.; Aggarwal, P.; Kumar, V.; Kaur, S. Ultrasound-assisted extraction of phytochemicals from java plum (Syzygium cumini L.) pomace: Process optimization, phytochemical characterization using HPLC, FTIR, SEM and mineral profiling. Waste Biomass Valorization 2022, 14, 949–961. [Google Scholar] [CrossRef]
- Najafabad, A.M.; Jamei, R. Free radical scavenging capacity and antioxidant activity of methanolic and ethanolic extracts of plum (Prunus domestica L.) in both fresh and dried samples. Avicenna J. Phytomed. 2014, 4, 343–353. [Google Scholar]
- Vasantha-Rupasinghe, H.P.; Jayasankar, S. Lay Variation in total phenolics and antioxidant capacity among European plum genotypes. Sci. Hortic. 2006, 108, 243–246. [Google Scholar] [CrossRef]
- Vizzotto, M.; Cisneros-Zevallos, L.; Byrne, D.H.; Ramming, D.W.; Okie, W.R. Large variation found in the phytochemical and antioxidant activity of peach and plum germplasm. J. Am. Soc. Hortic. Sci. 2007, 132, 334–340. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Gündüz, K.; Özbay, H. The effects of genotype and altitude of the growing location on physical, chemical, and phytochemical properties of strawberry. Turk. J. Agric. For. 2018, 42, 145–153. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Ortega-Regules, A.E.; Lozada-Ramírez, J.D.; Pérez-Pérez, M.C.I.; Vernon-Carter, E.J.; Welti-Chanes, J. Color and chemical stability of spray-dried blueberry extract using mesquite gum as wall material. J. Food Compos. Anal. 2011, 24, 889–894. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Reguła, J.; Łysiak, G. Physico-chemical properties and antioxidant activity of selected plum cultivars fruit. Acta Sci. Pol. Technol. Aliment. 2008, 7, 15–22. [Google Scholar]
- Poposka, H.; Mukaetov, D.; Cvetkovic, J.; Andreevski, M.; Tasevska, M.G.; Bandjo, K.O.; Nedelkovski, D.; Gjoljevska, R.M. Copper monitoring in vineyard soils of the Tikvesh region, North Macedonia. Агрoзнање 2022, 23, 183–197. [Google Scholar] [CrossRef]
- Jez, E.; Pellegrini, E.; Contin, M. Copper bioavailability and leaching in conventional and organic viticulture under environmental stress. Appl. Sci. 2023, 13, 2595. [Google Scholar] [CrossRef]
- Kir, A.; Cetinel, B.; Sevim, D.; Gungor, F.O.; Rayns, F.; Touliatos, D.; Schmutz, U. Agroecological screening of copper alternatives for the conservation of soil health in organic Olive production. Agronomy 2022, 12, 1712. [Google Scholar] [CrossRef]
- Ameen, F.; Mumtaz, S.; Ali, B.; Hussain, I.; Hafeez, A.; Gul, A.; Elsharkawy, M.M.; Hashim, T.A.; Yasin, G.; Khan, M.N.; et al. The impact of Cu-polluted and organic soil on the fibrous plant; insights into plant growth promotion, antioxidant defences system, and oxidative stress. Funct. Plant Biol. 2023, 50, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Katsoulas, N.; Løes, A.K.; Andrivon, D.; Cirvilleri, G.; de Cara, M.; Kir, A.; Knebl, L.; Malińska, K.; Oudshoorn, F.W.; Willer, H.; et al. Current use of copper, mineral oils and sulphur for plant protection in organic horticultural crops across 10 European countries. Org. Agric. 2020, 10, 159–171. [Google Scholar] [CrossRef]
- Radwan, M.A.; Salama, A.K. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem. Toxicol. 2006, 44, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Onianwa, P.C.; Lawal, J.A.; Ogunkeye, A.A.; Orejimi, B.M. Cadmium and Nickel composition of some Nigerian Foods. J. Food. Anal. 2000, 13, 961–969. [Google Scholar] [CrossRef]
- Alcalá Jáuregui, J.A.; Rodríguez Ortiz, J.C.; Filippini, M.F.; Martínez Carretero, E.; Hernández Montoya, A.; Rojas Velázquez, Á.N.; Méndez Cortés, H.; Beltrán Morales, F.A. Metallic elements in foliar material and fruits of three tree species as bioindicators. Rev. Fac. Cienc. Agrar. 2022, 54, 61–72. [Google Scholar] [CrossRef]
- Uzakov, Z.Z.; Karimov, K.N.; Uzakov, Z.U.; Eshonkulov, R.A. Accumulation of heavy metals in agricultural crops and ecological series of crops placement. Baghdad Sci. J. 2023, 20, 58–73. [Google Scholar] [CrossRef]
- Obi-Iyeke, G.E. Heavy metal concentrations in street-vended fruits and vegetables in Warri, Delta State, Nigeria. J. Appl. Sci. Environ. Manag. 2019, 23, 443. [Google Scholar] [CrossRef]
- Vincent, O.; Samuel, E.A.; Iloba, B.N.; Friday, O. Occurrence and Concentration of Heavy Metals in Garden Egg, Tomatoes, Cucumber and Watermelon from Two Major Markets in Edo State, Nigeria. Food and Public Health 2019, 10, 63–67. [Google Scholar]
- Taşpinar, K.; Ateş, Ö.; Yalçin, G.; Kizilaslan, F.; Pinar, M.Ö. Soil contamination and healthy risk assessment of peach orchards soil of Bilecik Province Turkey. Int. J. Environ. Health Res. 2022, 32, 1915–1924. [Google Scholar] [CrossRef]
- Hou, D. Sustainable soil management for food security. Soil Use Manag. 2023, 39, 1–7. [Google Scholar] [CrossRef]
- Nițu, M.; Pruteanu, A.; Găgeanu, I. Research on the accumulation and transfer of heavy metals from the soil to berries (blueberries—Vaccinium myrtillus L. and raspberries—Rubus idaeus). INAMTEH-Agric. Eng. 2022, 39, 722–728. [Google Scholar] [CrossRef]
- Pruteanu, A.; Voicea, I.; Fatu, V. Accumulation of copper in vegetables and fruits. Eng. Rural Dev. 2022. [Google Scholar] [CrossRef]
- Waida, J.; Ibrahim, U.; Goki, N.G.; Yusuf, S.D.; Usman, R.L. Transfer factor of heavy metals due to mining activities in plateau state, Nigeria (health implications on the inhabitants). J. Oncol. Res. 2022, 4, 13–26. [Google Scholar] [CrossRef]
- de Sousa, F.F.; do Carmo, M.G.F.; Lima, E.S.A.; da Costa Barros de Souza, C.; do Amaral Sobrinho, N.M.B. Lead and cadmium transfer factors and the contamination of tomato fruits (Solanum lycopersicum) in a tropical mountain agroecosystem. Bull. Environ. Contam. Toxicol. 2020, 105, 325–331. [Google Scholar] [CrossRef] [PubMed]
- El-Saka, M.S.; El-Kholy, M.M.; Aiad, M.A. The contamination status of heavy metals associated with fruits and vegetables collected from some markets at El-gharbia governorate. J. Soil Sci. Agric. Eng. 2020, 11, 299–305. [Google Scholar] [CrossRef]
- Abbas, S.; Azhar, B.J.; Irfan, M.; Ahmad, S.; Ahmed, I.; Hussain, J.; Shakeel, S.N. Analysis of organoleptic parameters and heavy metals in artificially ripened mango fruits in Pakistan. J. Anim. Plant Sci. 2021, 31, 733–742. [Google Scholar]
- Bhatti, S.S.; Kumar, V.; Kumar, A.; Kirby, J.K.; Gouzos, J.; Correll, R.; Singh, V.; Sambyal, A.K. Nagpal, Potential carcinogenic and non-carcinogenic health hazards of metal (loid)s in food grains. Environ. Sci. Pollut. Res. 2020, 27, 17032–17042. [Google Scholar] [CrossRef]
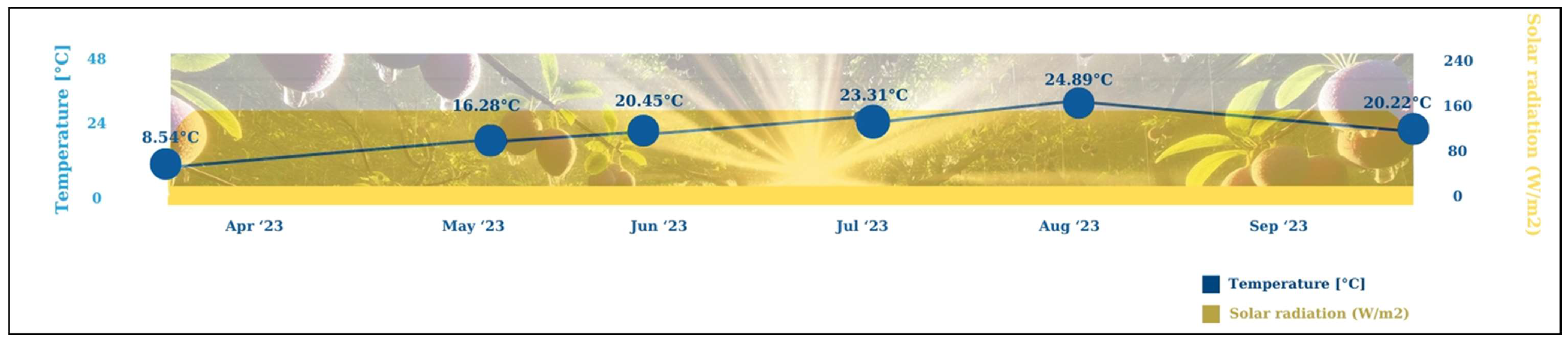
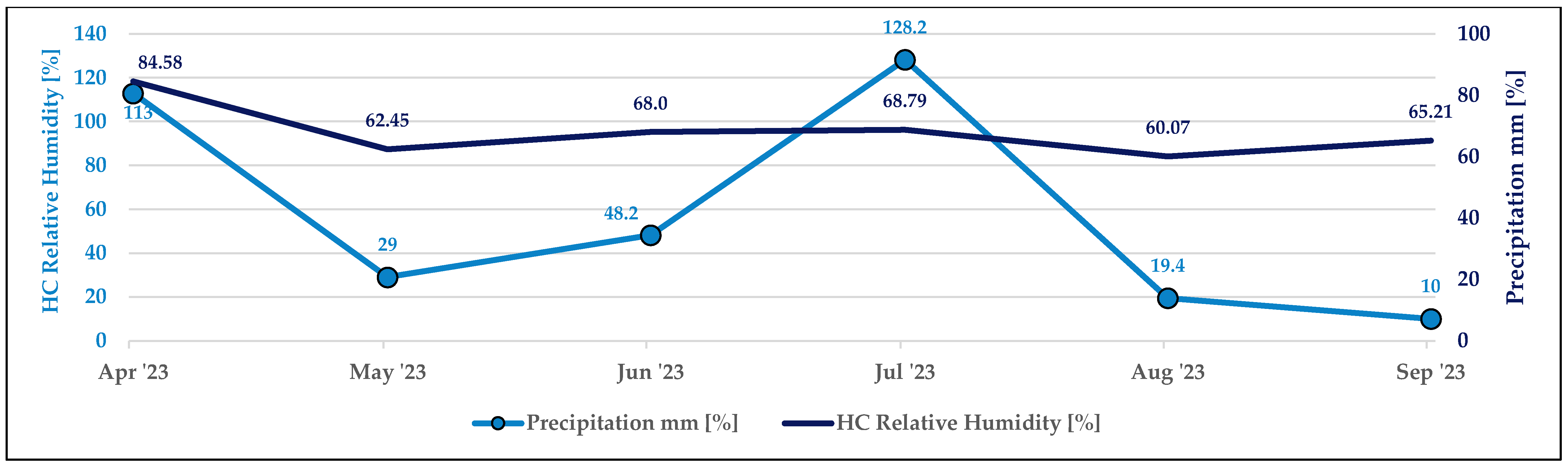
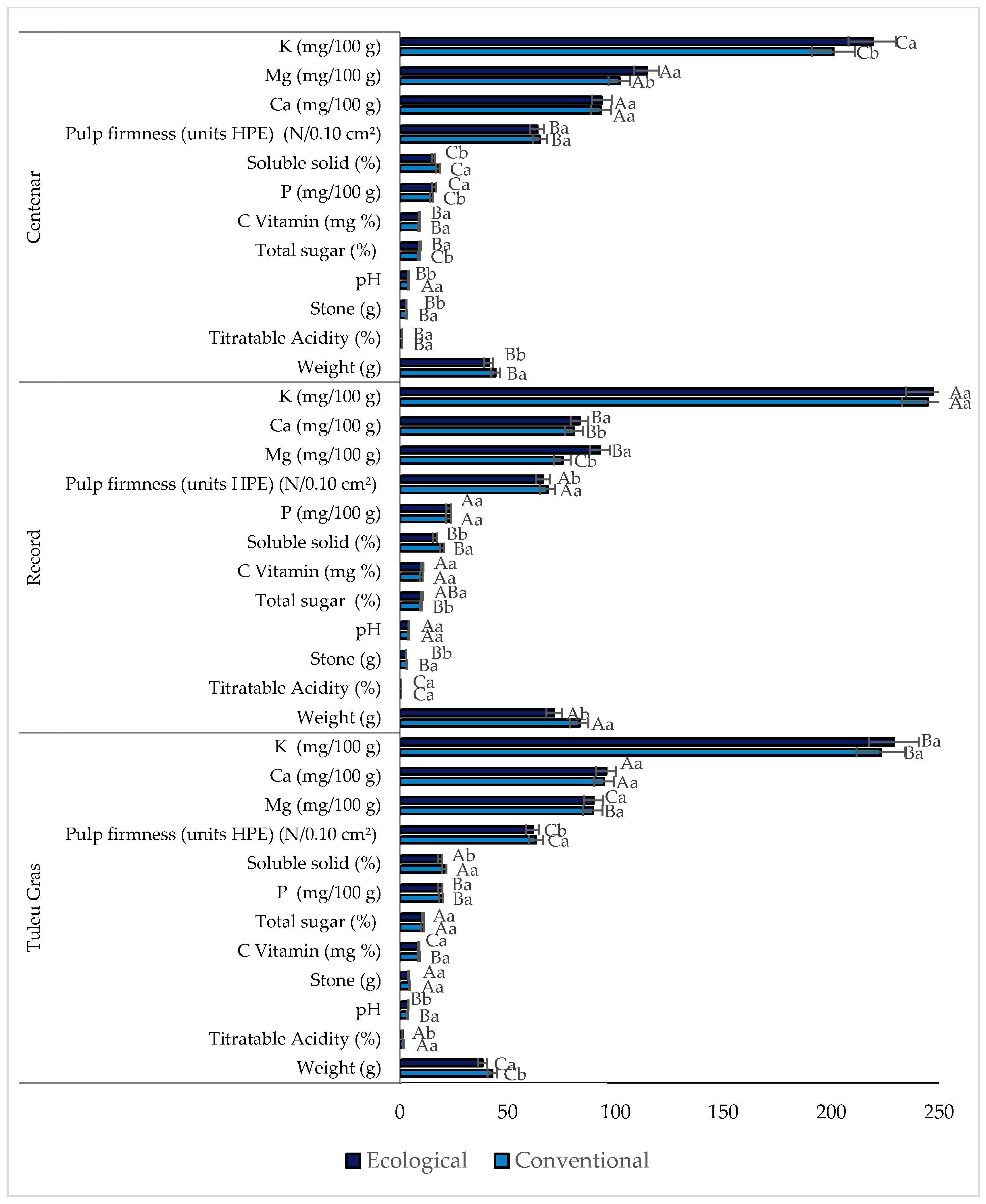
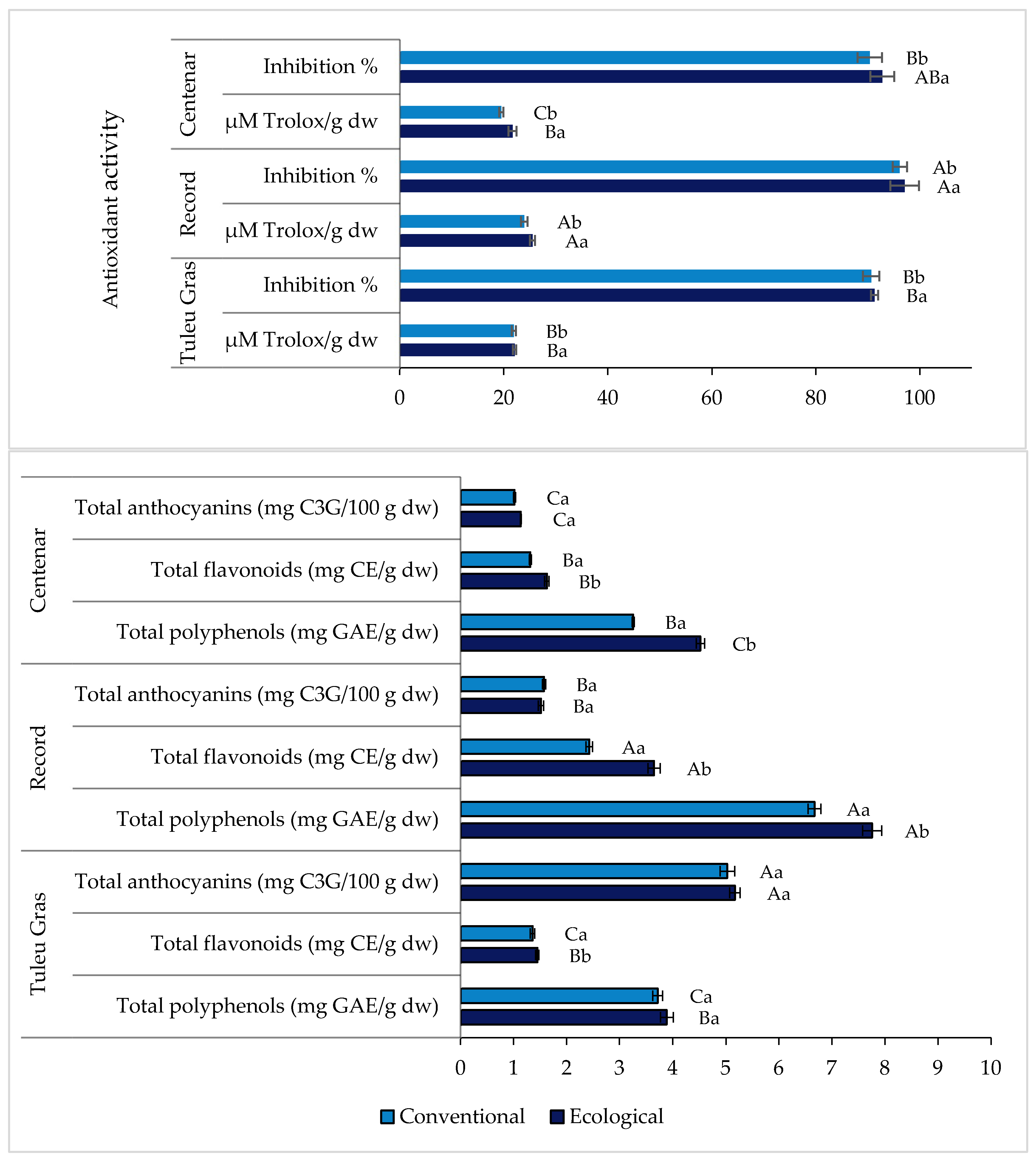

| Parameters | Setting |
|---|---|
| Elements | Cu, Ni, Zn, and Cd |
| Relative sensitivity | 100% |
| Measurement | Absorbance |
| Flame | C2H2 |
| C2H2 flow rate | 250 L h−1 |
| Burner height | 5–9 nm |
| Measurement time | 3 s |
| Management System | Cv | Eco |
|---|---|---|
| pH | 7.11 ± 0.17 | 7.17 ± 0.11 |
| ns | ||
| Nt (%) | 0.178 ± 0.00 | 0.206 ± 0.00 |
| * | ||
| P (mg/kg) | 98.60 ± 1.88 | 89.70 ± 2.41 |
| * | ||
| K (mg/kg) | 413.20 ± 14.68 | 406.87 ± 8.32 |
| ns | ||
| Ca2+sch (me/100 g sol) | 13.54 ± 0.10 | 14.07 ± 0.25 |
| ns | ||
| Mg2+ sch (me/100 g sol) | 7.62 ± 0.22 | 7.84 ± 0.11 |
| ns | ||
| Corg (%) | 2.04 ± 0.05 | 2.18 ± 0.06 |
| ns | ||
| Humus (%) | 3.47 ± 0.11 | 3.68 ± 0.09 |
| ns | ||
| Varieties | Management System | L* | a* | b* | ΔE (Total Color Difference) | C* (Chroma) | h* (°) Dual Color Appearance (Hue Angle) | BI | YI |
|---|---|---|---|---|---|---|---|---|---|
| Tuleu Gras | Eco | 53.36 ± 0.80 Ca | 10.27 ± 0.08 Aa | 16.47 ± 0.52 Ba | 56.78 ± 1.08 Ca | 19.41 ± 0.28 Ba | 1.01 ± 0.03 Aa | 50.46 ± 1.35 Ba | 44.09 ± 1.08 Ba |
| Cv | 52.21 ± 0.95 Cb | 9.14 ± 0.16 Ab | 15.33 ± 0.37 Bb | 55.17 ± 1.48 Bb | 17.85 ± 0.44 Bb | 1.03 ± 0.02 Aa | 47.06 ± 1.67 Bb | 41.95 ± 1.09 Bb | |
| Record | Eco | 56.89 ± 1.08 Aa | 8.33 ± 0.24 Ca | 11.34 ± 0.26 Ca | 58.94 ± 2.09 Ba | 14.08 ± 0.37 Ca | 0.94 ± 0.02 Aa | 32.54 ± 0.67 Ca | 28.48 ± 0.89 Ca |
| Cv | 56.49 ± 1.52 Aa | 7.56 ± 0.11 Cb | 10.86 ± 0.19 Cb | 58.03 ± 1.19 Aa | 13.23 ± 0.42 Ca | 0.96 ± 0.01 Aa | 30.75 ± 0.23 Cb | 27.46 ± 0.67 Ca | |
| Centenar | Eco | 54.15 ± 1.92 Ba | 9.26 ± 0.23 Ba | 26.14 ± 0.46 Aa | 60.83 ± 0.46 Aa | 27.73 ± 0.67 Aa | 1.23 ± 0.03 Aa | 76.70 ± 1.34 Aa | 68.96 ± 1.57 Aa |
| Cv | 53.48 ± 1.09 Bb | 8.67 ± 0.22 Bb | 25.02 ± 0.13 Ab | 59.68 ± 1.04 Aa | 26.49 ± 0.60 Ab | 1.24 ± 0.02 Aa | 70.58 ± 2.00 Ab | 66.83 ± 1.15 Ab |
| Pearson Correlation | L* | a* | b* | AA (Inhibition %) | TP | TF | TA |
|---|---|---|---|---|---|---|---|
| L* | 1 | ||||||
| a* | −0.705 | 1 | |||||
| b* | −0.527 | 0.390 | 1 | ||||
| (AA) (Inhibition %) | 0.992 ** | −0.809 | −0.515 | 1 | |||
| TP | 0.969 ** | −0.790 | −0.586 | 0.989 ** | 1 | ||
| TF | 0.897 * | −0.651 | −0.570 | 0.927 * | 0.967 ** | 1 | |
| TA | −0.781 | 0.669 | −0.100 | −0.781 | −0.711 | −0.615 | 1 |
| Pearson Correlation | Zn (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Cd (mg/kg) |
|---|---|---|---|---|
| Zn (mg/kg) | 1 | |||
| Cu (mg/kg) | 0.887 ** | 1 | ||
| Ni (mg/kg) | 0.982 ** | 0.873 ** | 1 | |
| Cd (mg/kg) | 0.913 ** | 0.786 * | 0.961 ** | 1 |
| Pearson Correlation | Soil Cv | Soil Eco | Fruit Cv | Fruit Eco |
|---|---|---|---|---|
| Soil Cv | 1 | |||
| Soil Eco | 0.951 * | 1 | ||
| Fruit Cv | 0.705 | 0.479 | 1 | |
| Fruit Eco | 0.918 | 0.995 ** | 0.963 * | 1 |
| Management System | Heavy Metals | Children | Adults | ||||
|---|---|---|---|---|---|---|---|
| EDI (mg/kg/Day) | THQ (mg/kg/Day) | HI = ∑THQ | EDI (mg/kg/Day) | THQ (mg/kg/Day) | HI = ∑THQ | ||
| Cv | Zn | 3.57 × 10−3 | 1.19 × 10−2 | 3.71 × 10−1 | 2.27 × 10−3 | 7.56 × 10−3 | 2.35 × 10−1 |
| Cu | 6.17 × 10−3 | 1.54 × 10−1 | 3.92 × 10−3 | 9.80 × 10−2 | |||
| Ni | 1.41 × 10−3 | 7.05 × 10−2 | 8.90 × 10−4 | 4.45 × 10−2 | |||
| Cd | 6.74 × 10−5 | 1.35 × 10−1 | 4.49 × 10−5 | 8.51 × 10−2 | |||
| Eco | Zn | 2.89 × 10−3 | 9.63 × 10−3 | 3.69 × 10−1 | 1.84 × 10−3 | 6.13 × 10−3 | 2.31 × 10−1 |
| Cu | 8.99 × 10−3 | 2.25 × 10−1 | 5.72 × 10−3 | 1.43 × 10−1 | |||
| Ni | 8.90 × 10−4 | 4.45 × 10−2 | 5.10 × 10−4 | 2.55 × 10−2 | |||
| Cd | 4.25 × 10−5 | 8.99 × 10−2 | 2.84 × 10−5 | 5.67 × 10−3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, M.; Cara, I.-G.; Stoica, F.; Țopa, D.; Jităreanu, G. Quality Parameters of Plum Orchard Subjected to Conventional and Ecological Management Systems in Temperate Production Area. Horticulturae 2024, 10, 907. https://doi.org/10.3390/horticulturae10090907
Rusu M, Cara I-G, Stoica F, Țopa D, Jităreanu G. Quality Parameters of Plum Orchard Subjected to Conventional and Ecological Management Systems in Temperate Production Area. Horticulturae. 2024; 10(9):907. https://doi.org/10.3390/horticulturae10090907
Chicago/Turabian StyleRusu, Mariana, Irina-Gabriela Cara, Florina Stoica, Denis Țopa, and Gerard Jităreanu. 2024. "Quality Parameters of Plum Orchard Subjected to Conventional and Ecological Management Systems in Temperate Production Area" Horticulturae 10, no. 9: 907. https://doi.org/10.3390/horticulturae10090907
APA StyleRusu, M., Cara, I.-G., Stoica, F., Țopa, D., & Jităreanu, G. (2024). Quality Parameters of Plum Orchard Subjected to Conventional and Ecological Management Systems in Temperate Production Area. Horticulturae, 10(9), 907. https://doi.org/10.3390/horticulturae10090907









