Integrating Spectral Sensing and Systems Biology for Precision Viticulture: Effects of Shade Nets on Grapevine Leaves
Abstract
1. Introduction
1.1. Grapevine Biology Modulation under Light Stress
1.2. Systems Biology to Unravel Grape Physiology under Light Stress
1.3. Integrating Plant Spectral Sensing and Systems Biology
2. Materials and Methods
2.1. Test Site and Sampling
2.2. Phenotype Characterisation
2.2.1. Hyperspectral Data
2.2.2. Water Status
2.3. Biochemical Analysis
2.3.1. Pigments Analysis
2.3.2. Superoxide () Quantification for ROS Assessment
2.4. Spectral Biochemical Modelling Approach
2.5. In Silico Simulations
3. Results and Discussion
3.1. Phenotype Characterisation
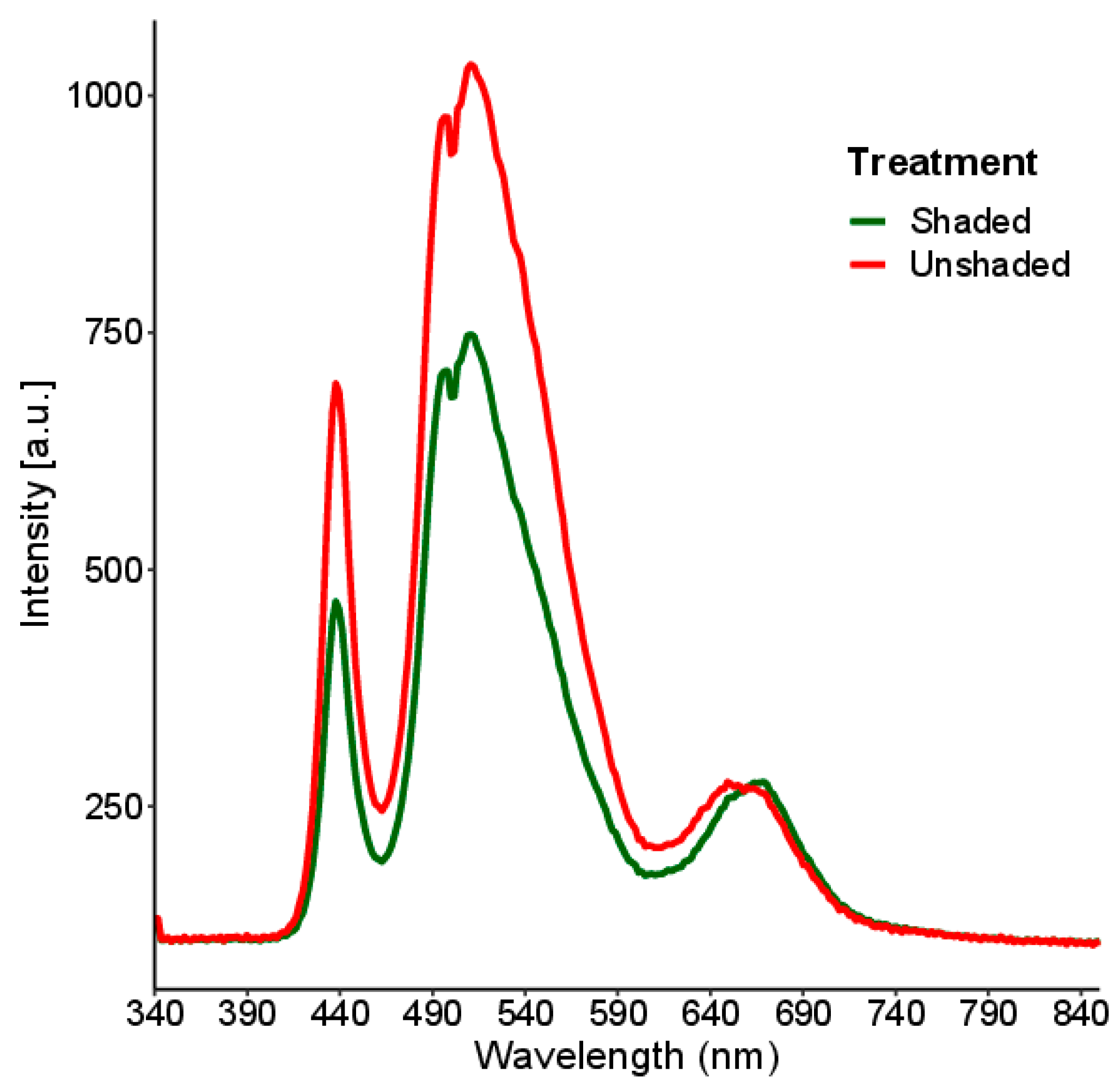
3.1.1. Hyperspectral Signatures
3.1.2. Water Status
3.1.3. Pigment and ROS Analysis
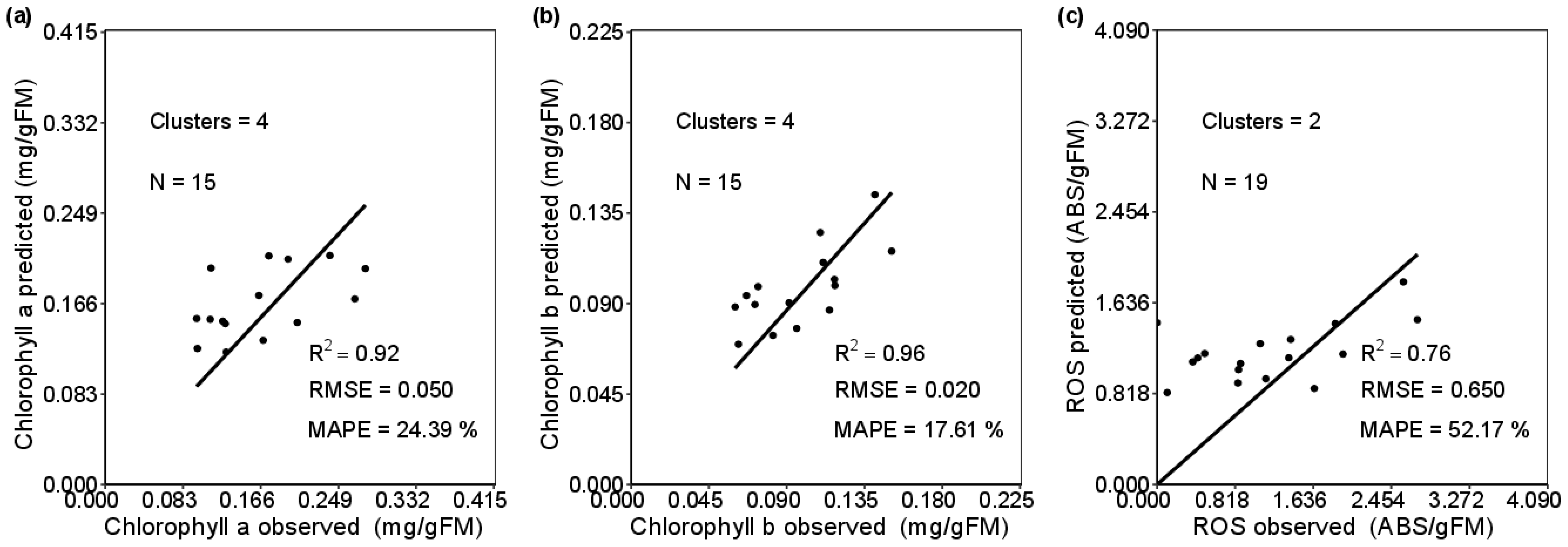
3.2. Modelling Biochemical Variables
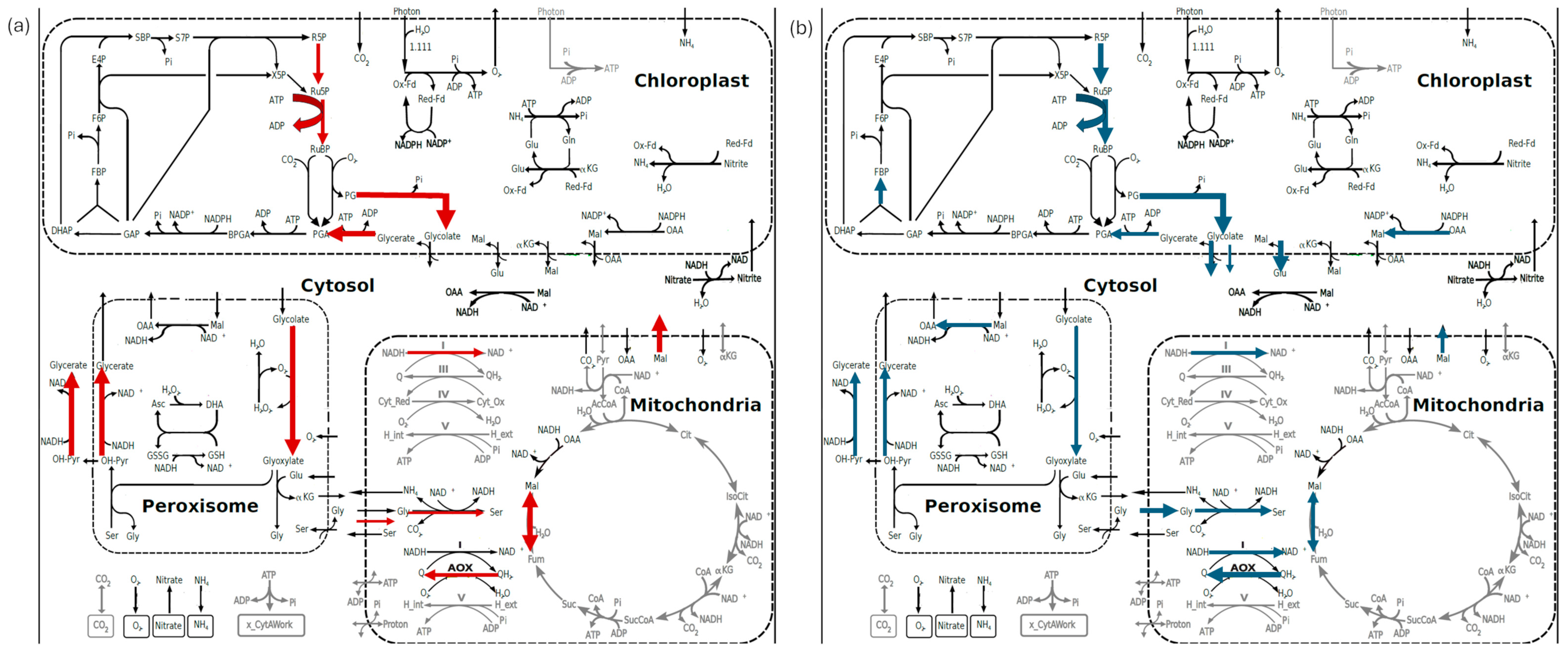
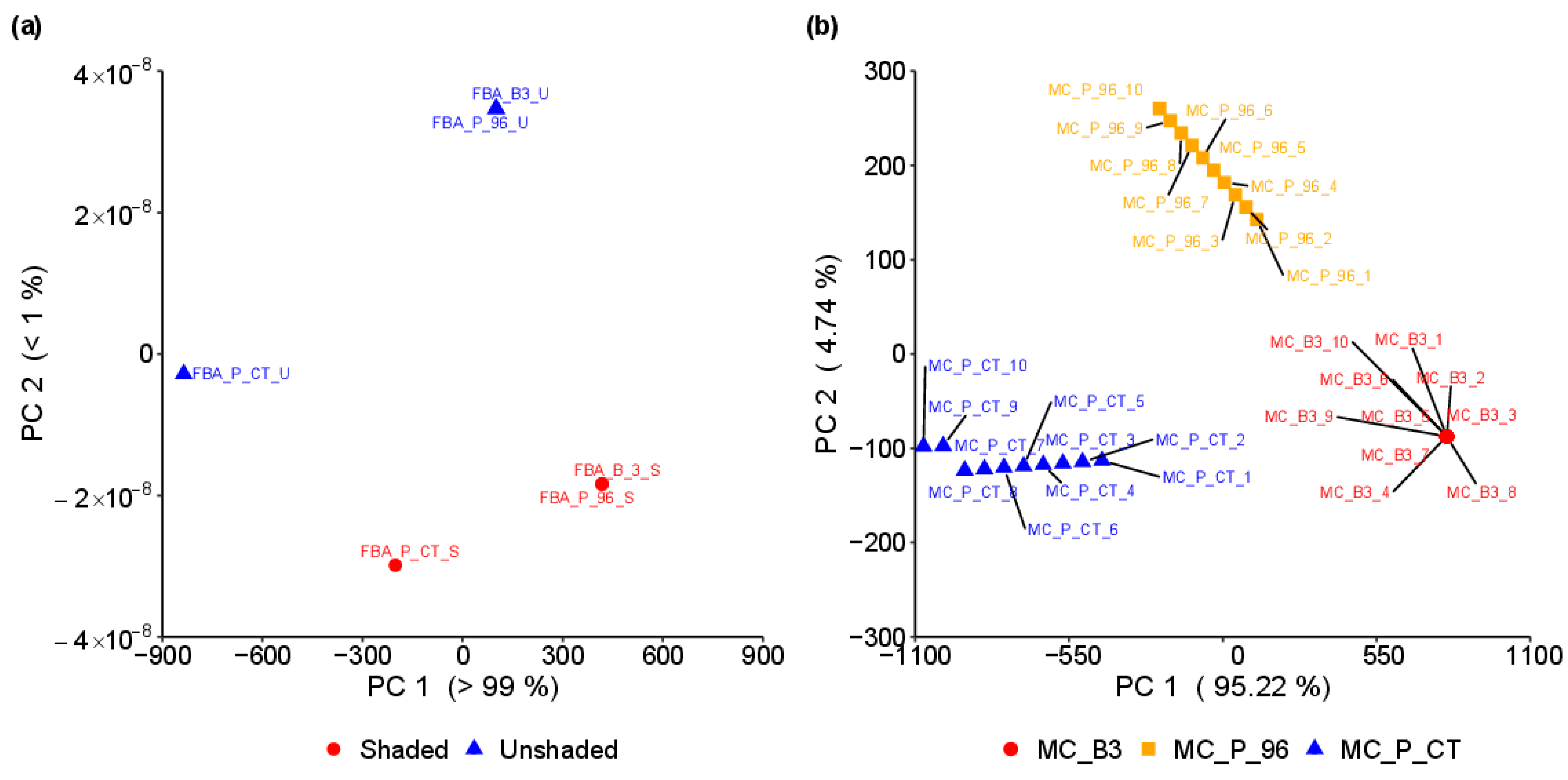
3.3. In Silico Simulations
3.4. Innovation for Precision Viticulture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pallas, B.; Christophe, A. Relationships between biomass allocation, axis organogenesis and organ expansion under shading and water deficit conditions in grapevine. Funct. Plant Biol. 2015, 42, 1116–1128. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Liu, Y.; Baby, T.; Xiao, Z. Impact of climate change on grape berry ripening: An assessment of adaptation strategies for the Australian vineyard. Front. Plant Sci. 2022, 13, 1094633. [Google Scholar] [CrossRef]
- Cunha, M.; Martins, R.; Neves dos Santos, F. Sustainable agriculture in the Era of field-omics. Let’s improve AgronOmics. INESC TEC Sci. Soc. 2022, 1. Available online: https://science-society.inesctec.pt/index.php/inesctecesociedade/article/view/93 (accessed on 15 August 2024).
- Adams, D.O. Phenolics and Ripening in Grape Berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Geros, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of Sunlight and Temperature Effects on the Composition of Vitis vinifera cv. Merlot Berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar] [CrossRef]
- Sternad Lemut, M.; Trost, K.; Sivilotti, P.; Vrhovsek, U. Pinot Noir grape colour related phenolics as affected by leaf removal treatments in the Vipava Valley. J. Food Compos. Anal. 2011, 24, 777–784. [Google Scholar] [CrossRef]
- Nilo-Poyanco, R.; Olivares, D.; Orellana, A.; Hinrichsen, P.; Pinto, M. Proteomic analysis of grapevine (Vitis vinifera L.) leaf changes induced by transition to autotrophy and exposure to high light irradiance. J. Proteom. 2013, 91, 309–330. [Google Scholar] [CrossRef]
- Sun, R.Z.; Cheng, G.; Li, Q.; He, Y.N.; Wang, Y.; Lan, Y.B.; Li, S.Y.; Zhu, Y.R.; Song, W.F.; Zhang, X.; et al. Light-induced Variation in Phenolic Compounds in Cabernet Sauvignon Grapes (Vitis vinifera L.) Involves Extensive Transcriptome Reprogramming of Biosynthetic Enzymes, Transcription Factors, and Phytohormonal Regulators. Front. Plant Sci. 2017, 8, 547. [Google Scholar] [CrossRef]
- Ayenew, B.; Degu, A.; Manela, N.; Perl, A.; Shamir, M.O.; Fait, A. Metabolite profiling and transcript analysis reveal specificities in the response of a berry derived cell culture to abiotic stresses. Front. Plant Sci. 2015, 6, 728. [Google Scholar] [CrossRef]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight Modulates Fruit Metabolic Profile and Shapes the Spatial Pattern of Compound Accumulation within the Grape Cluster. Front. Plant Sci. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, K.; Young, P.R.; Eyeghe-Bickong, H.A.; Vivier, M.A. The Transcriptional Responses and Metabolic Consequences of Acclimation to Elevated Light Exposure in Grapevine Berries. Front. Plant Sci. 2017, 8, 1261. [Google Scholar] [CrossRef]
- Pazhamala, L.T.; Kudapa, H.; Weckwerth, W.; Millar, A.H.; Varshney, R.K. Systems biology for crop improvement. Plant Genome 2021, 14, e20098. [Google Scholar] [CrossRef]
- Trewavas, A. A brief history of systems biology. Every object that biology studies is a system of systems. Francois Jacob (1974). Plant Cell 2006, 18, 2420–2430. [Google Scholar] [CrossRef]
- van Diepen, C.A.; Wolf, J.; van Keulen, H.; Rappoldt, C. WOFOST: A simulation model of crop production. Soil. Use Manag. 1989, 5, 16–24. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, R.K.; Gupta, S.M.; Gaur, V.S.; Pandey, D. Systems Biology for Smart Crops and Agricultural Innovation: Filling the Gaps between Genotype and Phenotype for Complex Traits Linked with Robust Agricultural Productivity and Sustainability. OMICS 2015, 19, 581–601. [Google Scholar] [CrossRef]
- Brisson, N.; Mary, B.; Ripoche, D.; Jeuffroy, M.H.; Ruget, F.; Nicoullaud, B.; Gate, P.; Devienne-Barret, F.; Antonioletti, R.; Durr, C.; et al. STICS: A generic model for the simulation of crops and their water and nitrogen balances. I. Theory and parameterization applied to wheat and corn. Agronomie 1998, 18, 311–346. [Google Scholar] [CrossRef]
- Lo Presti, D.; Di Tocco, J.; Massaroni, C.; Cimini, S.; De Gara, L.; Singh, S.; Raucci, A.; Manganiello, G.; Woo, S.L.; Schena, E.; et al. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens. Bioelectron. 2023, 222, 115005. [Google Scholar] [CrossRef]
- Li, C.; Donizelli, M.; Rodriguez, N.; Dharuri, H.; Endler, L.; Chelliah, V.; Li, L.; He, E.; Henry, A.; Stefan, M.I.; et al. BioModels Database: An enhanced, curated and annotated resource for published quantitative kinetic models. BMC Syst. Biol. 2010, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Thiele, I.; Palsson, B.O. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Yuan, H.; Cheung, C.Y.; Poolman, M.G.; Hilbers, P.A.; van Riel, N.A. A genome-scale metabolic network reconstruction of tomato (Solanum lycopersicum L.) and its application to photorespiratory metabolism. Plant J. 2016, 85, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Dal’Molin, C.G.; Quek, L.E.; Palfreyman, R.W.; Brumbley, S.M.; Nielsen, L.K. C4GEM, a genome-scale metabolic model to study C4 plant metabolism. Plant Physiol. 2010, 154, 1871–1885. [Google Scholar] [CrossRef]
- de Oliveira Dal’Molin, C.G.; Quek, L.E.; Palfreyman, R.W.; Brumbley, S.M.; Nielsen, L.K. AraGEM, a genome-scale reconstruction of the primary metabolic network in Arabidopsis. Plant Physiol. 2010, 152, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Gerlin, L.; Cottret, L.; Escourrou, A.; Genin, S.; Baroukh, C. A multi-organ metabolic model of tomato predicts plant responses to nutritional and genetic perturbations. Plant Physiol. 2022, 188, 1709–1723. [Google Scholar] [CrossRef]
- Rai, A.; Yamazaki, M.; Saito, K. A new era in plant functional genomics. Curr. Opin. Syst. Biol. 2019, 15, 58–67. [Google Scholar] [CrossRef]
- Tosin, R.; Monteiro-Silva, F.; Martins, R.; Cunha, M. A New Approach for Element Characterization of Grapevine Tissue with Laser-Induced Breakdown Spectroscopy. Horticulturae 2024, 10, 82. [Google Scholar] [CrossRef]
- D’Agostino, N.; Tripodi, P. NGS-Based Genotyping, High-Throughput Phenotyping and Genome-Wide Association Studies Laid the Foundations for Next-Generation Breeding in Horticultural Crops. Diversity 2017, 9, 38. [Google Scholar] [CrossRef]
- Tosin, R.; Pôças, I.; Novo, H.; Teixeira, J.; Fontes, N.; Graça, A.; Cunha, M. Assessing predawn leaf water potential based on hyperspectral data and pigment’s concentration of Vitis vinifera L. in the Douro Wine Region. Sci. Hortic. 2021, 278, 109860. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Amin, E.; Pascual-Venteo, A.B.; Pérez-Suay, A.; Tenjo, C.; Sabater, N.; van der Tol, C.; Drusch, M.; Moreno, J. Retrieval of leaf-level fluorescence quantum efficiency and NPQ-related xanthophyll absorption through spectral unmixing strategies for future VIS-NIR imaging spectroscopy. Remote Sens. Environ. 2024, 300, 113879. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Shi, Z.; Chang, Q. Comparison of prediction power of three multivariate calibrations for estimation of leaf anthocyanin content with visible spectroscopy in Prunus cerasifera. PeerJ 2019, 7, e7997. [Google Scholar] [CrossRef]
- Falcioni, R.; Goncalves, J.V.F.; de Oliveira, K.M.; de Oliveira, C.A.; Reis, A.S.; Crusiol, L.G.T.; Furlanetto, R.H.; Antunes, W.C.; Cezar, E.; de Oliveira, R.B.; et al. Chemometric Analysis for the Prediction of Biochemical Compounds in Leaves Using UV-VIS-NIR-SWIR Hyperspectroscopy. Plants 2023, 12, 3424. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.; Zhang, Y.; Castranova, V.; Neal, S.L. Emerging technologies for optical spectral detection of reactive oxygen species. Anal. Bioanal. Chem. 2018, 410, 6079–6095. [Google Scholar] [CrossRef] [PubMed]
- Tomar, N.; De, R.K. Comparing methods for metabolic network analysis and an application to metabolic engineering. Gene 2013, 521, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Ribeiro, H.; Abreu, I. Pollen-based predictive modelling of wine production: Application to an arid region. Eur. J. Agron. 2016, 73, 42–54. [Google Scholar] [CrossRef]
- Cunha, M.; Richter, C. The impact of climate change on the winegrape vineyards of the Portuguese Douro region. Clim. Change 2016, 138, 239–251. [Google Scholar] [CrossRef]
- Martins, R.C.; Magalhães, S.; Jorge, P.; Barroso, T.; Santos, F. Metbots: Metabolomics Robots for Precision Viticulture. In Proceedings of the Progress in Artificial Intelligence, Vila Real, Portugal, 3–6 September 2019; pp. 156–166. [Google Scholar]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activitiesin wheat leaves. BioMetals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Costa-Santos, M.; Mariz-Ponte, N.; Dias, M.C.; Moura, L.; Marques, G.; Santos, C. Effect of Bacillus spp. and Brevibacillus sp. on the Photosynthesis and Redox Status of Solanum lycopersicum. Horticulturae 2021, 7, 24. [Google Scholar] [CrossRef]
- Martins, R.C.; Barroso, T.G.; Jorge, P.; Cunha, M.; Santos, F. Unscrambling spectral interference and matrix effects in Vitis vinifera Vis-NIR spectroscopy: Towards analytical grade ‘in vivo’ sugars and acids quantification. Comput. Electron. Agric. 2022, 194, 106710. [Google Scholar] [CrossRef]
- Martins, R. Big Data Self-Learning Methodology for the Accurate Quantification and Classification of Spectral Information under Complex Variability and Multi-Scale Interference. WO Patent 2018060967, 5 April 2018. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018060967 (accessed on 15 August 2024).
- Tosin, R.; Martins, R.; Pôças, I.; Cunha, M. Canopy VIS-NIR spectroscopy and self-learning artificial intelligence for a generalised model of predawn leaf water potential in Vitis vinifera. Biosyst. Eng. 2022, 219, 235–258. [Google Scholar] [CrossRef]
- Tosin, R.; Monteiro-Silva, F.; Martins, R.; Cunha, M. Precision maturation assessment of grape tissues: Hyperspectral bi-directional reconstruction using tomography-like based on multi-block hierarchical principal component analysis. Biosyst. Eng. 2023, 236, 147–159. [Google Scholar] [CrossRef]
- Huma, B.; Kundu, S.; Poolman, M.G.; Kruger, N.J.; Fell, D.A. Stoichiometric analysis of the energetics and metabolic impact of photorespiration in C3 plants. Plant J. 2018, 96, 1228–1241. [Google Scholar] [CrossRef]
- Adams, W.W.; Stewart, J.J.; Demmig-Adams, B. Photosynthetic Modulation in Response to Plant Activity and Environment. In The Leaf: A Platform for Performing Photosynthesis; Adams Iii, W.W., Terashima, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 493–563. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Light Adaptation and Senescence of the Photosynthetic Apparatus. Changes in Pigment Composition, Chlorophyll Fluorescence Parameters and Photosynthetic Activity. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 713–736. [Google Scholar] [CrossRef]
- Palliotti, A.; Poni, S. Grapevine under light and heat stresses. In Grapevine in a Changing Environment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Chapter 7; pp. 148–178. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, T.; Jin, H.; Pei, D.; Pervaiz, T.; Ren, Y.; Jia, H.; Fang, J. Analysis of photosynthetic ability and related physiological traits in nodal leaves of grape. Sci. Hortic. 2022, 304, 111251. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yue, X.F.; Zhao, X.F.; Zhao, H.; Fang, Y.L. Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem. 2018, 130, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Hörtensteiner, S. Mechanism and Significance of Chlorophyll Breakdown. J. Plant Growth Regul. 2013, 33, 4–20. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Li, R.X.; Jiang, Q.S.; Bai, R.; Duan, D. Changes in the chlorophyll content of grape leaves could provide a physiological index for responses and adaptation to UV-C radiation. Nord. J. Bot. 2019, 37. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vidigal, P.; Amâncio, S. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front. Environ. Sci. 2015, 3, 20. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; del Río, L.A. Role of Peroxisomes as a Source of Reactive Oxygen Species (ROS) Signaling Molecules. In Peroxisomes and Their Key Role in Cellular Signaling and Metabolism; del Río, L.A., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 231–255. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd Allah, E.F.; Ansari, M.I. Plant Defense Responses to Biotic Stress and Its Interplay With Fluctuating Dark/Light Conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- van Wyngaard, E.; Blancquaert, E.; Nieuwoudt, H.; Aleixandre-Tudo, J.L. Infrared Spectroscopy and Chemometric Applications for the Qualitative and Quantitative Investigation of Grapevine Organs. Front. Plant Sci. 2021, 12, 723247. [Google Scholar] [CrossRef]
- Carmo de Carvalho e Martins, M.d.; da Silva Santos Oliveira, A.S.; da Silva, L.A.A.; Primo, M.G.S.; de Carvalho Lira, V.B. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. In Biomarkers in Nutrition; Springer: Cham, Switzerland, 2022; pp. 833–856. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Geigenberger, P.; Fernie, A.R. Metabolic Control of Redox and Redox Control of Metabolism in Plants. Antioxid. Redox Signal. 2014, 21, 1389–1421. [Google Scholar] [CrossRef]
- Tomaz, T.; Bagard, M.; Pracharoenwattana, I.; Lindén, P.; Lee, C.P.; Carroll, A.J.; Ströher, E.; Smith, S.M.; Gardeström, P.; Millar, A.H. Mitochondrial Malate Dehydrogenase Lowers Leaf Respiration and Alters Photorespiration and Plant Growth in Arabidopsis. Plant Physiol. 2010, 154, 1143–1157. [Google Scholar] [CrossRef]
- Zakhartsev, M.; Medvedeva, I.; Orlov, Y.; Akberdin, I.; Krebs, O.; Schulze, W.X. Metabolic model of central carbon and energy metabolisms of growing Arabidopsis thaliana in relation to sucrose translocation. BMC Plant Biol. 2016, 16, 262. [Google Scholar] [CrossRef]
- Burbidge, C.A.; Ford, C.M.; Melino, V.J.; Wong, D.C.J.; Jia, Y.; Jenkins, C.L.D.; Soole, K.L.; Castellarin, S.D.; Darriet, P.; Rienth, M.; et al. Biosynthesis and Cellular Functions of Tartaric Acid in Grapevines. Front. Plant Sci. 2021, 12, 643024. [Google Scholar] [CrossRef] [PubMed]
- Trost, P.; Fermani, S.; Marri, L.; Zaffagnini, M.; Falini, G.; Scagliarini, S.; Pupillo, P.; Sparla, F. Thioredoxin-dependent regulation of photosynthetic glyceraldehyde-3-phosphate dehydrogenase: Autonomous vs. CP12-dependent mechanisms. Photosynth. Res. 2006, 89, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Vélez, S.; Barajas, E.; Rubio, J.A.; Pereira-Obaya, D.; Rodríguez-Pérez, J.R. Field-Deployed Spectroscopy from 350 to 2500 nm: A Promising Technique for Early Identification of Powdery Mildew Disease (Erysiphe necator) in Vineyards. Agronomy 2024, 14, 634. [Google Scholar] [CrossRef]


| Enzymes and Compounds | Pathway | Expression | Function | Reference |
|---|---|---|---|---|
| Unshaded | ||||
| hydroxycinnamic acids (HCAs) | Phenolic acids | Up | UV protection, pigmentation, cell defense | [4,5] |
| hydroxybenzoic acids (HBAs) | Phenolic acids | Up | UV protection, pigmentation, cell defense | [4,5] |
| Resveratrol | Non-flavonoid polyphenols | Up | UV protection, pigmentation, cell defense | [6,7] |
| Quercetin | Polyphenol | Up | UV protection | [6,7] |
| Kaempferol | Polyphenol | Up | UV protection | [6,7] |
| Myricetin | Polyphenol | Up | UV protection | [6,7] |
| Lipocalin (s240) | Oxidative stress defense | Up | Cell defense | [8] |
| quinone oxidoreductase-like protein (s472) | Oxidative stress defense | Up | Cell defense | [8] |
| ascorbate peroxidase2 (APX2) | Oxidative stress defense | Up | Cell defense | [8] |
| peroxiredoxin (PRX) | Oxidative stress defense | Up | Cell defense | [8] |
| glutathione-s-transferase (GST) | Oxidative stress defense | Up | Cell defense | [8] |
| catalase (CAT) | Oxidative stress defense | Up | Cell defense | [8] |
| isoflavone-reductase-like protein (IRL) | Oxidative stress defense | Up | Cell defense | [8] |
| nucleoside diphosphate kinase2 (NDPK2) | Oxidative stress defense | Up | Cell defense | [8] |
| Auxin (AUX) | Hormones | UP | Hormone signal | [9] |
| Xylose | Cell membrane | Down | Cell membrane | [10] |
| Xylobiose | Cell membrane | Down | Cell membrane | [10] |
| Phenylalanine | Amino acid pathway | Up | UV protection, pigmentation, cell defense | [11] |
| Light-inducible protein (ELIP1) | Chlorophyll biosynthesis | Up | Regulates the chlorophyll biosynthesis | [12] |
| photosystem II PsbO protein | Photosynthesis | Down (final stage) | Photosynthesis | [12] |
| LHB1B1 light-harvesting protein | Photosynthesis | Down (final stage) | Photosynthesis | [12] |
| polyphenol oxidase chloroplast precursor | Photosynthesis | Down (final stage) | Photosynthesis | [12] |
| Shade | ||||
| blue light receptor cryptochrome 2 (CRY2) | Photoreceptors | Down | Light receptors | [9] |
| HY5 | Photoreceptor regulator | Down | Light receptors | [9] |
| HY5- homolog (HYH | Photoreceptor regulator | Down | Light receptors | [9] |
| cytokinin (CTK) | Hormones | Up | Hormone signal | [9] |
| brassinosteroid (BR) | Hormones | Up | Hormone signal | [9] |
| pyrabactin resistance 1/PYR1-like (PYR) | Hormones | Up | Hormone signal | [9] |
| ABA-responsive (element binding factor) | Hormones | Up | Hormone signal | [9] |
| Maleate | Maleic acid | Up | UV protection, pigmentation, cell defense | [11] |
| beta-alanine | Amino acid pathway | Up | UV protection, pigmentation, cell defense | [11] |
| Citrate | Amino acid pathway | Up | UV protection, pigmentation, cell defense | [11] |
| Aspartate | Amino acid pathway | Up | UV protection, pigmentation, cell defense | [11] |
| procyanidin B1 | Polyphenol | Up | UV protection, pigmentation, cell defense | [11] |
| Epigallocatechin | Polyphenol | Up | UV protection, pigmentation, cell defense | [11] |
| Catechin | Polyphenol | Up | UV protection, pigmentation, cell defense | [11] |
| Raffinose | Sugars; Carbon metabolism | Up | Carbon metabolism | [11] |
| Biochemical Analytes | Shaded | Unshaded | p-Value | Variation % |
|---|---|---|---|---|
| Chlorophyll a (mg/gFM) | 0.19 | 0.12 | 0.001 * | 158.33 |
| Chlorophyll b (mg/gFM) | 0.08 | 0.11 | 0.006 * | 71.03 |
| Chlorophyll a + b (mg/gFM) | 0.27 | 0.23 | 0.037 * | 117.39 |
| ROS O2.− (ABS/gMF) | 0.99 | 1.90 | 0.019 * | 52.10 |
| Top 10 Reactions | % | FBA_P_CT_U | FBA_P_CT_S | FBA_P_96_U | FBA_P_96_S | FBA_B3_U | FBA_B_3_S |
|---|---|---|---|---|---|---|---|
| Chl_FerredoxinReductase | 7.39% | −793.91 | −525 | −396.95 | −262.5 | −396.95 | −262.5 |
| Chl_G3Pdh | 7.04% | 756.10 | 500 | 378.05 | 250 | 378.05 | 250 |
| Chl_PGK | 7.04% | 756.10 | 500 | 378.05 | 250 | 378.05 | 250 |
| Chl_Ru5Pk | 4.22% | 453.66 | 300 | 226.83 | 150 | 226.83 | 150 |
| Chl_PGlyPase | 2.81% | 302.44 | 200 | 151.22 | 100 | 151.22 | 100 |
| Chl_RuBPOxid | 2.81% | 302.44 | 200 | 151.22 | 100 | 151.22 | 100 |
| Chl_TPI | 2.81% | 302.44 | 200 | 151.22 | 100 | 151.22 | 100 |
| Chl_X5Piso | 2.81% | 302.44 | 200 | 151.22 | 100 | 151.22 | 100 |
| Mit_Gly_tx | 2.81% | 302.44 | 200 | 151.22 | 100 | 151.22 | 100 |
| Per_Glycolate_tx | 2.81% | 302.44 | 200 | 151.22 | 100 | 151.22 | 100 |
| Total | 42.57% | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosin, R.; Portis, I.; Rodrigues, L.; Gonçalves, I.; Barbosa, C.; Teixeira, J.; Mendes, R.J.; Santos, F.; Santos, C.; Martins, R.; et al. Integrating Spectral Sensing and Systems Biology for Precision Viticulture: Effects of Shade Nets on Grapevine Leaves. Horticulturae 2024, 10, 873. https://doi.org/10.3390/horticulturae10080873
Tosin R, Portis I, Rodrigues L, Gonçalves I, Barbosa C, Teixeira J, Mendes RJ, Santos F, Santos C, Martins R, et al. Integrating Spectral Sensing and Systems Biology for Precision Viticulture: Effects of Shade Nets on Grapevine Leaves. Horticulturae. 2024; 10(8):873. https://doi.org/10.3390/horticulturae10080873
Chicago/Turabian StyleTosin, Renan, Igor Portis, Leandro Rodrigues, Igor Gonçalves, Catarina Barbosa, Jorge Teixeira, Rafael J. Mendes, Filipe Santos, Conceição Santos, Rui Martins, and et al. 2024. "Integrating Spectral Sensing and Systems Biology for Precision Viticulture: Effects of Shade Nets on Grapevine Leaves" Horticulturae 10, no. 8: 873. https://doi.org/10.3390/horticulturae10080873
APA StyleTosin, R., Portis, I., Rodrigues, L., Gonçalves, I., Barbosa, C., Teixeira, J., Mendes, R. J., Santos, F., Santos, C., Martins, R., & Cunha, M. (2024). Integrating Spectral Sensing and Systems Biology for Precision Viticulture: Effects of Shade Nets on Grapevine Leaves. Horticulturae, 10(8), 873. https://doi.org/10.3390/horticulturae10080873














