1. Introduction
In the field of agrotechnology, there is a rising demand and increasing acceptance of optical measurement techniques for evaluating the internal quality attributes of agricultural products. This is true for pears, with sweet, juicy, and nutritious flesh, making them one of the most popular fruits among consumers. Nevertheless, a fundamental concern lies in comprehending the extent to which the optical properties are associated with specific internal quality attributes. For instance, the soluble solids content (SSC) and flesh firmness are crucial parameters for assessing quality as they can reflect the taste of the fruit. These two indices have a direct impact on the commercial value of pears and consumer satisfaction. Therefore, there is a necessity for a more profound comprehension of the correlation between optical properties and the quality attributes of fruits.
The transmission of light in a turbid medium such as fruit tissues can be described by two fundamental mechanisms, namely absorption and scattering [
1]. Absorption is primarily dictated by the chemical composition of the tissue (including water, sugars, acids, etc.), whereas scattering is contingent upon the density and structure of the tissue (such as cell size and cell distribution). Therefore, measuring these two optical properties can offer more accurate insights about the physical structure and composition characteristics of the samples. Absorption and scattering are typically quantified using the absorption coefficient
and the reduced scattering coefficient
. Assessing these two optical properties can offer more accurate insights into the physical structure and composition traits of the samples. Absorption and scattering are typically quantified by the absorption coefficient (
) and the reduced scattering coefficient (
).
At present, various methods have been employed to assess the optical properties of fruit tissues, including integrating sphere (IS) [
2], spatial resolved (SR) [
3], time resolved (TR) [
4], and spatial frequency domain imaging (SFDI) [
5]. The correlation of the optical properties obtained using these techniques with chemical substances and tissue structure was investigated.
Fang et al. utilized an automated single integrating sphere (SIS) system to determine the absorption and reduced scattering characteristics of ‘Korla’ pears. They examined the relationship between various physical and chemical parameters and
and
, as well as their principal component (PC1) scores within the 600–1500 nm wavelength range. This indicated that the
and
have the potential to present the characteristics of these indices of pears [
6]. Tian et al. estimated the optical properties of kiwifruit using a SIS system and explored the connection between the absorption, reduced scattering properties, SSC, and flesh firmness. It shows that the
spectra had a superior capability in determining SSC, while
spectra were better in predicting the firmness of kiwifruit [
7]. Nicolai et al. measured the TR near-infrared reflectance of pears and used the spectral data to construct calibration models of SSC and firmness. The results showed that reasonable SSC models can be obtained when using
in the range of 780–1700 nm. In this study, they found that despite the nonlinear correlation between
and firmness at 900 nm,
was not able to develop an effective firmness calibration model [
8]. In 2020, Vanoli et al. compared the performance of TR and SR spectra of ‘Braeburn’ apple by analyzing the ripening process over a period of 21 days. They analyzed the relationship between a range of internal and external indicators of apple and optical properties. Their research revealed that alterations in pigments within the flesh and skin influenced absorption, while scattering reflected changes in flesh texture [
9]. Joseph et al. employed SR to characterize the
and
of pears and investigated the relationship between fruit porosity and light scattering. Their study revealed a linear correlation between tissue porosity from the fruit skin to a depth of 3 mm with
spectra at 760 nm and 835 nm [
10]. But the three techniques have their drawbacks. The IS technique is destructive as the detected samples need to be cut into thin sheets. The SR technique is limited to detecting only a small area due to the point light source. And the TR technique usually needs expensive equipment.
In contrast, SFDI uses spatially modulated structured light illumination to characterize the optical properties of the examined samples. Compared with other optical detection techniques, it replaces the point light source with a regional illumination of different frequencies and combines with specific light transmission models to evaluate the absorption and reduced scattering properties of biological tissues to obtain depth resolution information, such as the chemical composition and microstructure of biological tissues; it has a broad field of view, non-contact operation, depth discrimination in imaging, and effective signal enhancement [
11].
SFDI technique was first proposed by Dognitz and Wagnieres et al. in 1998 [
12]; then, a research group at the University of California carried out further research from 2005 [
13]. Since then, many research teams have begun to improve the demodulation, inversion, and other related algorithms, and apply them for practical application in the biomedical field. In terms of agricultural science, there has been a delay in starting and developing the SFDI technique; thus, there are fewer related studies. Anderson et al. were the first to utilize the SFDI technique in agricultural product detection research, employing SFDI to identify hidden damage beneath the surface of apples [
14]. In 2016, the first SFDI system in the agricultural field in China was built, which characterized the
and
of apple slice tissue through the way of damage detection. Since then, more and more scientific research teams at home and abroad have started to apply SFDI technique to the detection of agricultural products [
15].
Regarding quality detection, Hu et al. put forward an improved stepwise method to optimize the spatial frequency interval, and used it to evaluate the optical transmission properties of milk samples and four kinds of apple tissues. This enhancement led to improved accuracy in estimating the optical transmission properties [
16]. After that, they characterized the optical transmission properties of the fruit skin and flesh tissue of various fruits, such as apple, mango, and kiwifruit, respectively. The results showed that the absorption coefficient curves of the skin and flesh tissues could reflect the absorption peaks of pigments and other components at specific wavelengths, and the absorption coefficient and reduced scattering coefficient of the skin tissues were typically elevated compared to those of the flesh tissues [
17,
18]. Fu et al. detected the optical properties of tissues from different parts of pork, beef, and chicken, and classified them using a support vector machine (SVM) and the K-nearest neighbors (KNN) algorithm [
19]. The study revealed significant variations in the optical properties of various types of meat, with the classification accuracy being higher when using
and
as features compared to using reflectance as the feature. Lohner et al. conducted a long-time and large-batch experiment on four varieties of apples, and used SFDI to extract images of optical properties to further show the different morphological characteristics inside the apple. It demonstrated that the
of apples in the ripening stage varied with the cell gap and starch content; it also examined the radial trend of
and
from the apple core to the apple skin, which illustrated the radial dependence of these two optical properties. It provided numerous novel perspectives for investigating the complex correlation between optical properties and physiological processes throughout the ripening stage [
20].
In terms of damage detection, He et al. used the least squares support vector regression (LSSVR) method to effectively fit the fuzzy nonlinear relationship between the (
,
) vector and
in high dimensional space. The image of
calculated using this method can clearly show the invisible damage on the surface of crown pear. By comparing the coefficient of variation (CV) at 527 nm, the damaged pears could be identified with 98.33% accuracy, and by comparing the average
ratio of the damaged area to the normal area, the damage of grade 1 (the lightest degree) could be distinguished from the damage of grade 2 and 3 (two more serious damage degrees). The results indicated that SFDI has the latent ability to detect the invisible sub-pericarp damage of crown pear [
21]. Sun et al. combined SFDI and an artificial neural network (ANN) model to accelerate the high precision mapping of optical properties, which can measure the
and
of Golden Delicious apples efficiently and detect the early invisible damage of apples through
mapping [
22]. Luo et al. used the independently developed SFDI system to detect pears with different bruising types, taking the image as the input to distinguish different damage types of crown pears via linear discriminant analysis (LDA) pattern recognition method [
23].
It has been proved by previous studies that the SFDI technique can effectively characterize the optical properties of agricultural products, but there still exist some problems and difficulties to be solved. For example, the SFDI systems adopted in the previous studies generally used filters to select several individual wavelengths for detection. The hyperspectral spatial frequency domain imaging (HSFDI) system has since been developed, which can obtain continuous wave spectra of optical properties in the region of 360–1000 nm. The HSFDI system was applied for measuring the
and
of milk at continuous bands, which validated the association between the protein contents and
as well as the fat contents and
[
23,
24]. In order to realize practical applications such as quality analysis and grade sorting of fruits, this study intended to use the HSFDI system to obtain HSFDI data. Chemometric methods were used to establish quantitative models through diffuse reflectance
and absorption coefficient
and reduced scattering coefficient
in the range of 360–1000 nm for predicting the soluble solid content and firmness of pear. The particular aims of this research were as follows:
- (1)
To comprehensively describe the optical properties of pears at different wavelengths and provide basic data for subsequent analysis, obtaining HSFDI images of pears at 360–1000 nm and calculating the , , and of pears, respectively, based on three-phase demodulation and partial least squares (PLS) fitting;
- (2)
To reveal how the optical properties reflect the internal quality of pears and to clarify the relationship between the spectral parameters and the internal quality parameters so as to provide a theoretical basis for non-destructive testing, discussing the correlation between the spectra of these optical parameters and the internal quality parameters of pears, including SSC and firmness;
- (3)
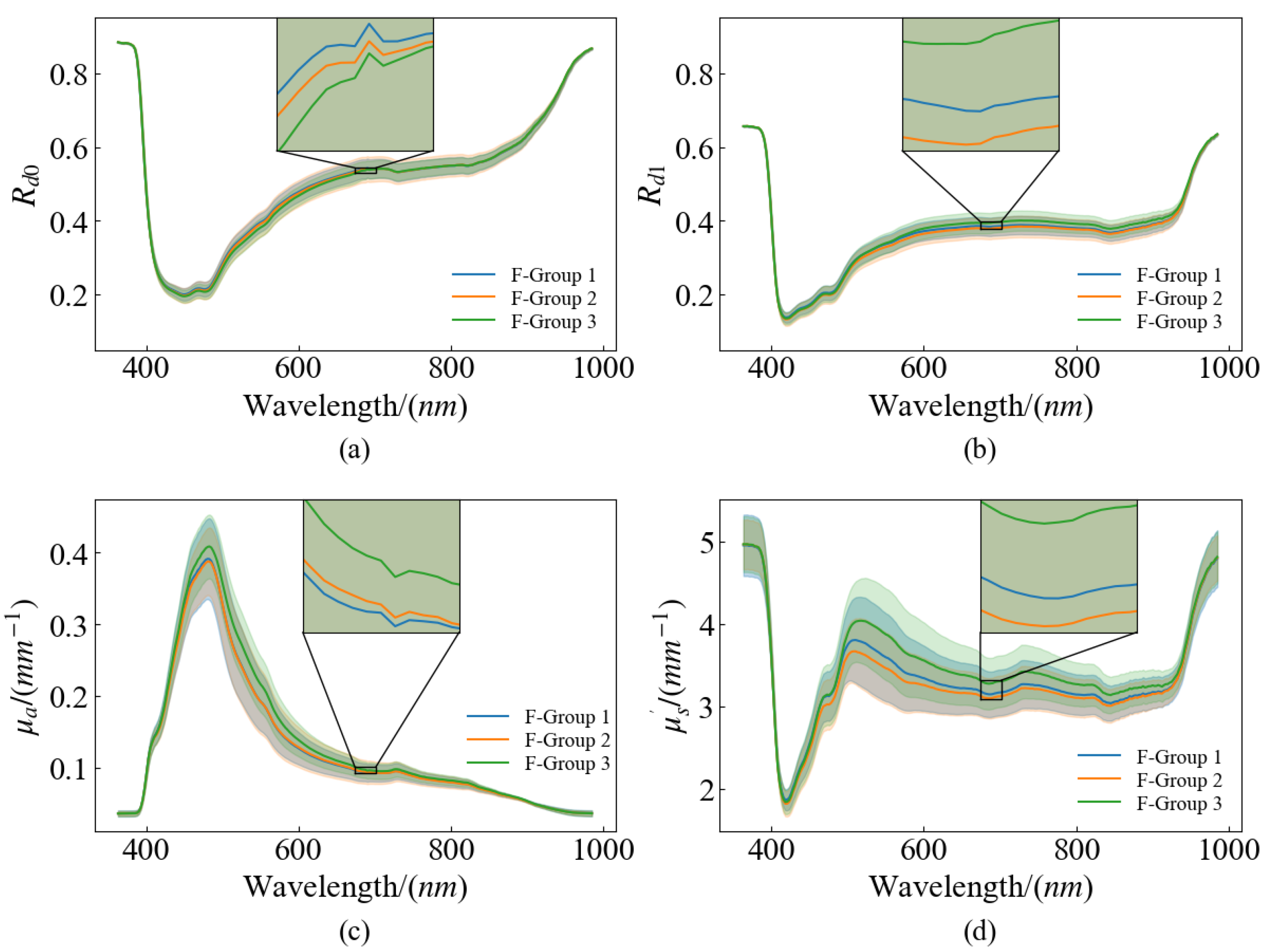
To improve the accuracy and reliability of non-destructive testing, comparing different models to find the optimal combination of optical parameters, which includes the SSC and firmness prediction models built by optical properties (, reflectance at spatial frequencies of 0 ; , reflectance at spatial frequencies of 0.2 ; , and ) and their specific combinations (, reflectance at two spatial frequencies; and ), respectively.
2. Materials and Methods
2.1. Samples
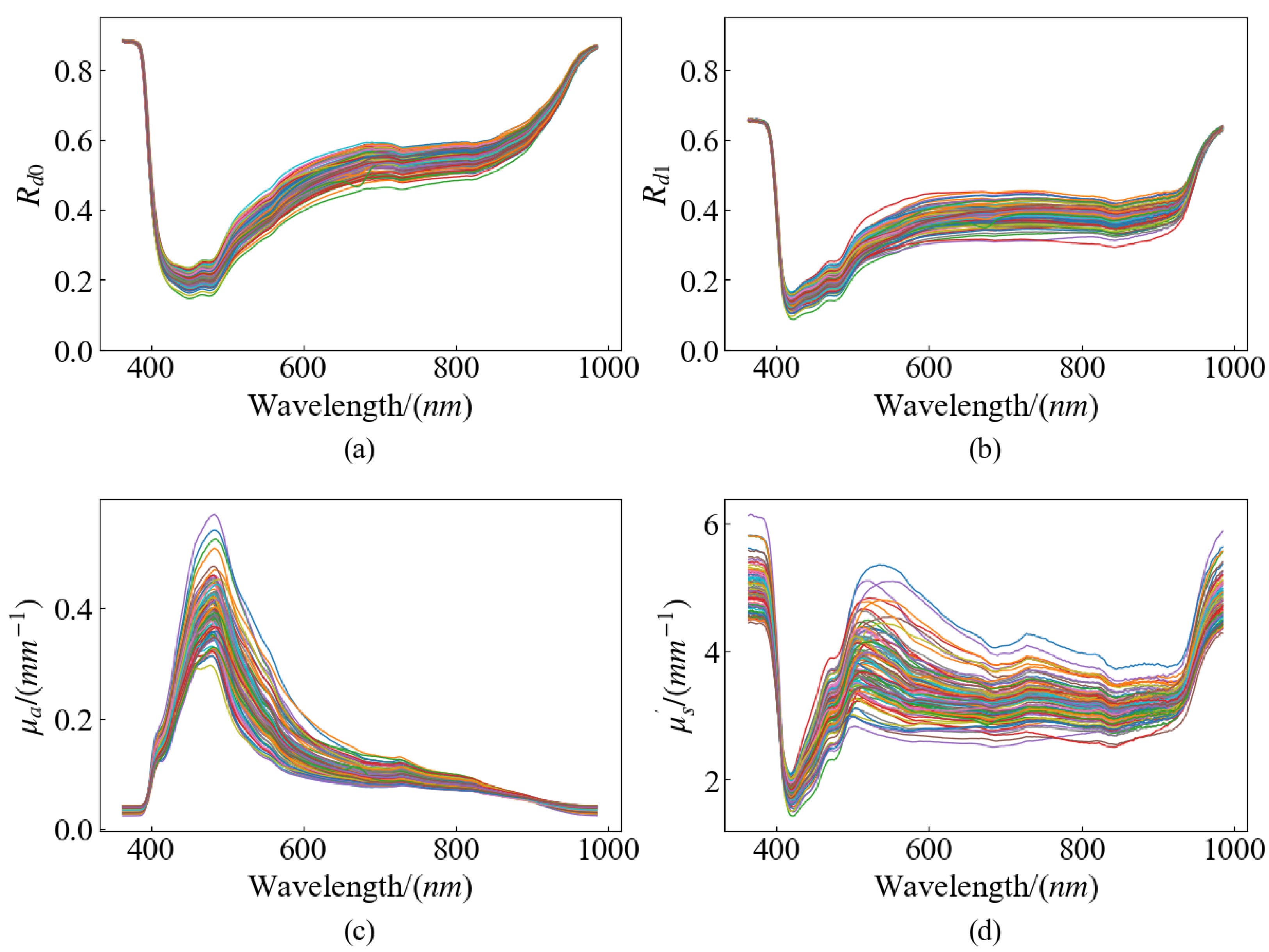
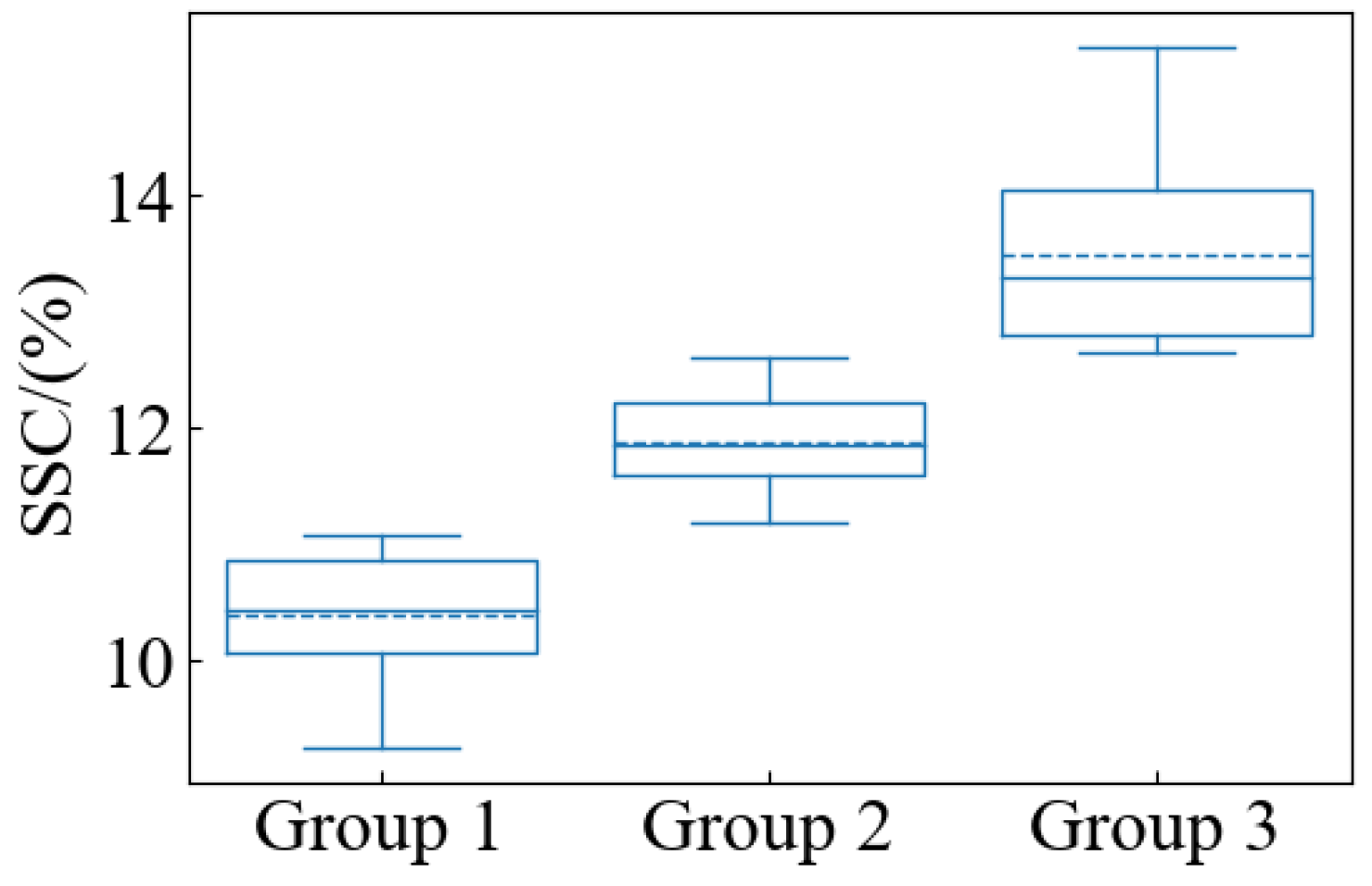
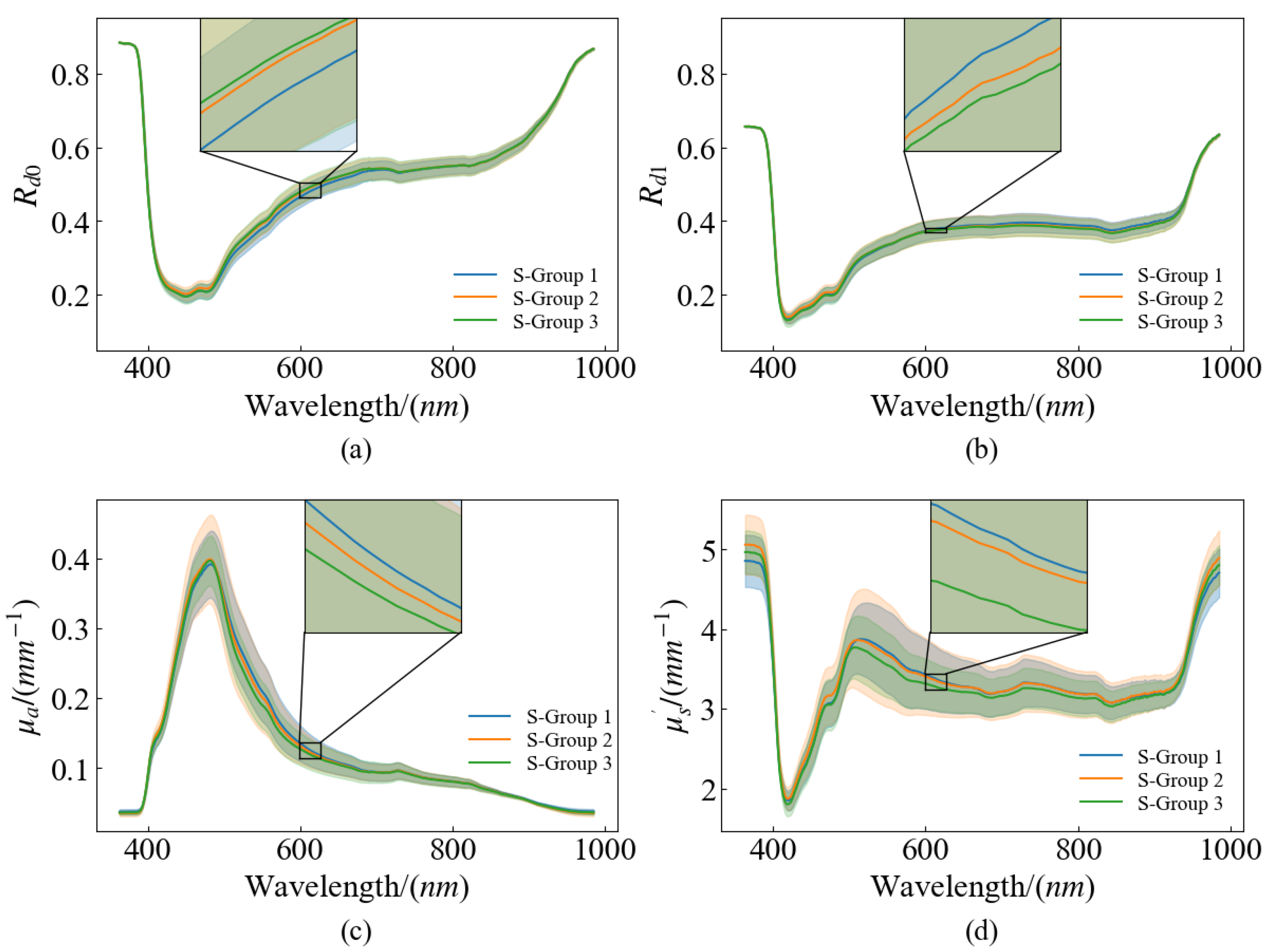
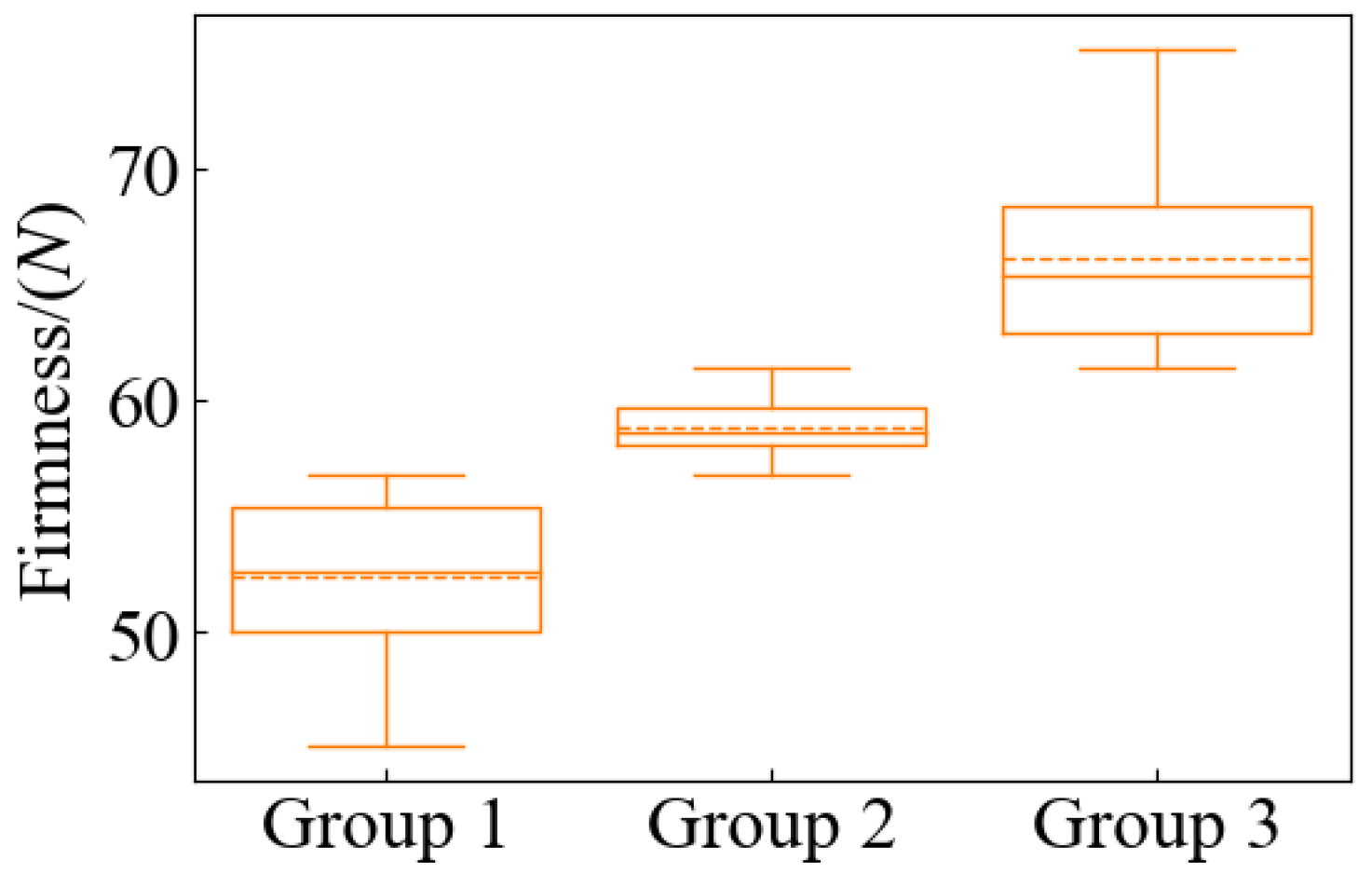
Pears that are similar in color and uniform in size were selected to avoid any differences in these factors affecting SSC and firmness measurements. The selected samples were intact in appearance and free of cracks and pests to ensure that the measurements were not affected by external damage. One hundred ‘Gong’ pears were acquired from a local wholesale market in Hangzhou, Zhejiang Province. To make the collected data closer to the actual situation, the samples with no obvious defects and uniform shapes were selected. After arrival at the laboratory, the samples were deposited at a temperature of 4 °C to ensure the stability of the same batch and to minimize experimental errors. The experiments were conducted for 18 days, and the samples to be tested were removed from the incubator before each experiment and placed in the laboratory environment (temperature ~24 °C, relative humidity ~65%) for 12 h. After that, the HSFDI data were collected and the quality parameters of SSC and firmness were measured. The temperature and humidity during measurement were kept consistent to avoid the influence of environmental factors on the results. After removing the pears that broke down in storage and the invalid data that were damaged during collection, finally, 96 valid samples were used. The pear samples were randomly split into two groups at the ratio of 3:1, with 72 samples allocated to the calibration set and the remaining 24 samples assigned to the prediction set. The range of the two data subsets were checked to ensure the calibration set contained the maximum and minimum values of the internal quality parameters.
2.2. Measurement of Raw HSFDI Data
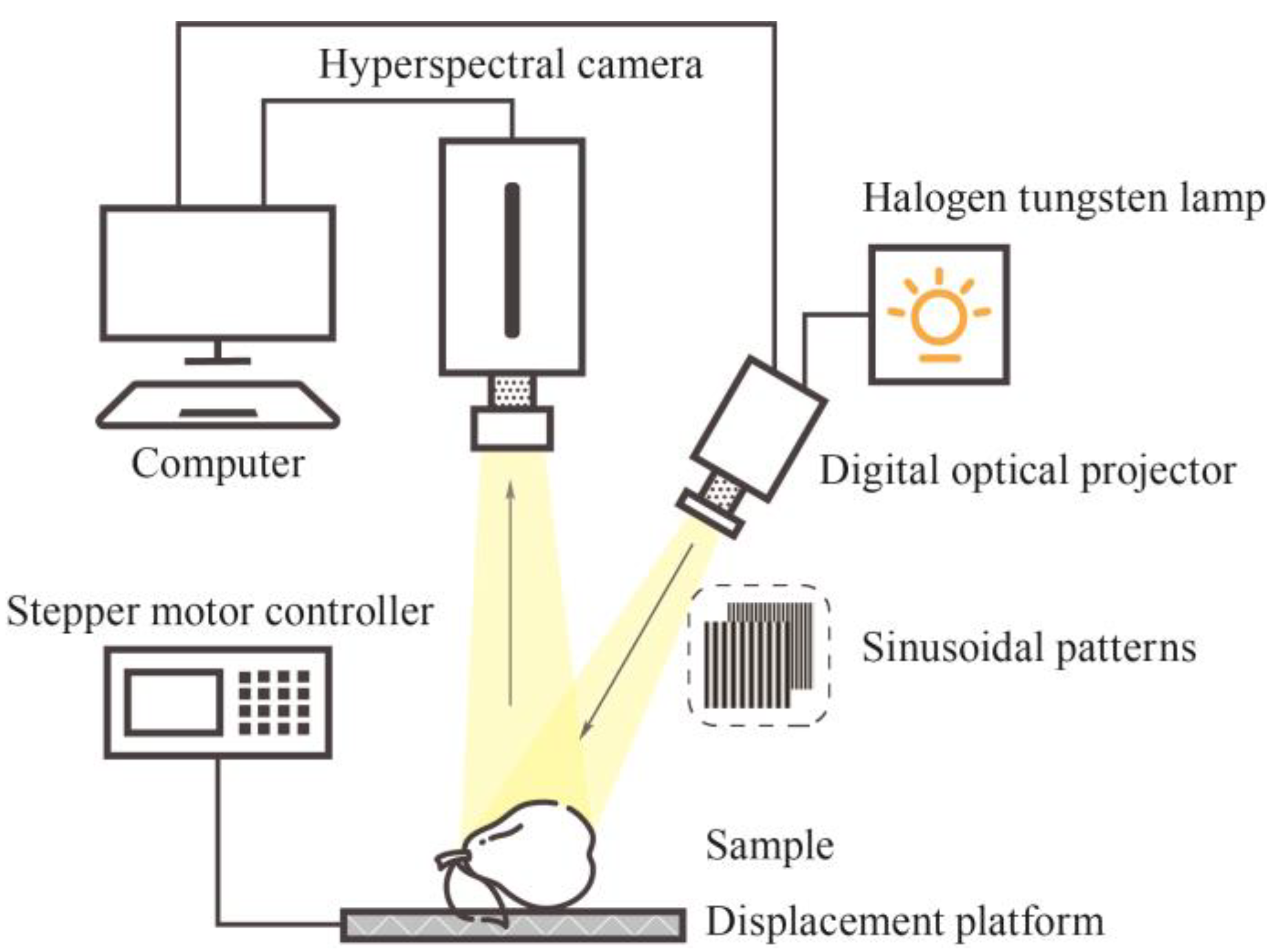
Hyperspectral spatial frequency domain images were acquired using the HSFDI system in the range of 360–1000 nm, which mainly consists of a halogen tungsten lamp and a light source power controller (150 W, 400–1200 nm, Changchun Ocean Electro-Optics Co., Ltd., Changchun, China), a digital optical projector (DLi CEL5500, Texas Instruments, Dallas, TX, USA), a line-scan hyperspectral camera with an optical fiber (GaiaField-V10E-AZ4, Dualix Spectral Imaging Technology Co., Ltd., Chengdu, China), a set of linear polarizers (OCZ203, BOCIC Co., Ltd., Beijing, China), an electric displacement platform for sample height adjustment (MTS303S, BOCIC Co., Ltd., Beijing, China), and a stepper motor controller (SC102S, BOCIC Co., Ltd., Beijing, China). The polarizers are placed before the hyperspectral camera and the DLP, respectively, to reduce direct reflections on the surface of fruit. The camera is directly above the platform, and the projector is in the top right corner of the sample at a 15-degree angle to the vertical line. The camera, projector, and platform are positioned within a black box to prevent interference from ambient light during the experiment. The 8-bit sinusoidal fringe patterns were projected using the CEL conductor control software accompanying the DLP projector, while the HSFDI images were captured with ‘Specview’ software (V1.0, Dualix Spectral Image Technology Co., Ltd., Chengdu, China) accompanying the hyperspectral camera. The specific construction, calibration, and verification process of this hyperspectral spatial frequency domain imaging joint system have been described in detail in the previous study [
24].
To ensure a stable light source for the HSFDI system, the halogen tungsten lamp needed to be warmed up for 10 min. The system frequency was adjusted in synchronization with the camera calibration. The sinusoidal waveforms captured using the line-scan camera were filtered. The linearity of the system response was calibrated using a linear fit of the measured diffuse reflectance to a reference value. The constructed HSFDI system was subjected to validation experiments with the phantom of known optical parameters to ensure its measurement accuracy. In HSFDI experiments, there is a trade-off between spectral and spatial resolution. High spectral resolution provides fine spectral information but slows down imaging speed, while high spatial resolution improves image quality but increases the data processing load. Increasing both parameters at the same time will significantly increase the data volume, and the solution is that a reasonable balance needs to be found when setting the parameters to optimize the data volume and improve the processing efficiency. The spectral resolution in this study was set to 2.4 nm with 250 wavelengths while the spatial resolution was .
In the case of SFDI, the spatial frequency of structured light directly affects the depth of light penetration into fruit tissues. In other words, in order to precisely detect the intrinsic quality of pears, it is essential to select the applicable spatial frequency of structured light. Light of different spatial frequencies penetrates biological tissues with different depths; the penetration depth decreases with increasing spatial frequency. Conversely, the resolution and contrast of the captured images exhibit an inverse relationship. Therefore, based on the pre-experiment, the selected spatial frequencies were 0 and 0.2 . Only one image is required when is 0 , and three images with different phases (0, , ) are required when is 0.2 . For each sample, four images were taken in total.
Figure 1 illustrates the schematic diagram of the HSFDI image system. Before the acquisition of all the data, the tungsten halogen lamp was turned on to preheat for 10 min, and then the two-dimensional grayscale sinusoidal patterns of various spatial frequencies were illuminated on the measured object, which was placed on the displacement platform. The hyperspectral images of the standard reflector (with 0.99 reflectivity) and the samples were required in sequence. During the collection process, the stepper motor was controlled to adjust the displacement platform so that the highest point of all the measured objects remains remained at the same horizontal height. The square standard reflector should cover the full area of the sample; therefore, the standard reflector and all samples were placed in the fixed position displacement platform to reduce the error and facilitate the selection of the region of interest (ROI). After all the shots were completed, the light and projection were turned off, and the dark image was collected in the dark environment.
2.3. Extraction of Optical Properties
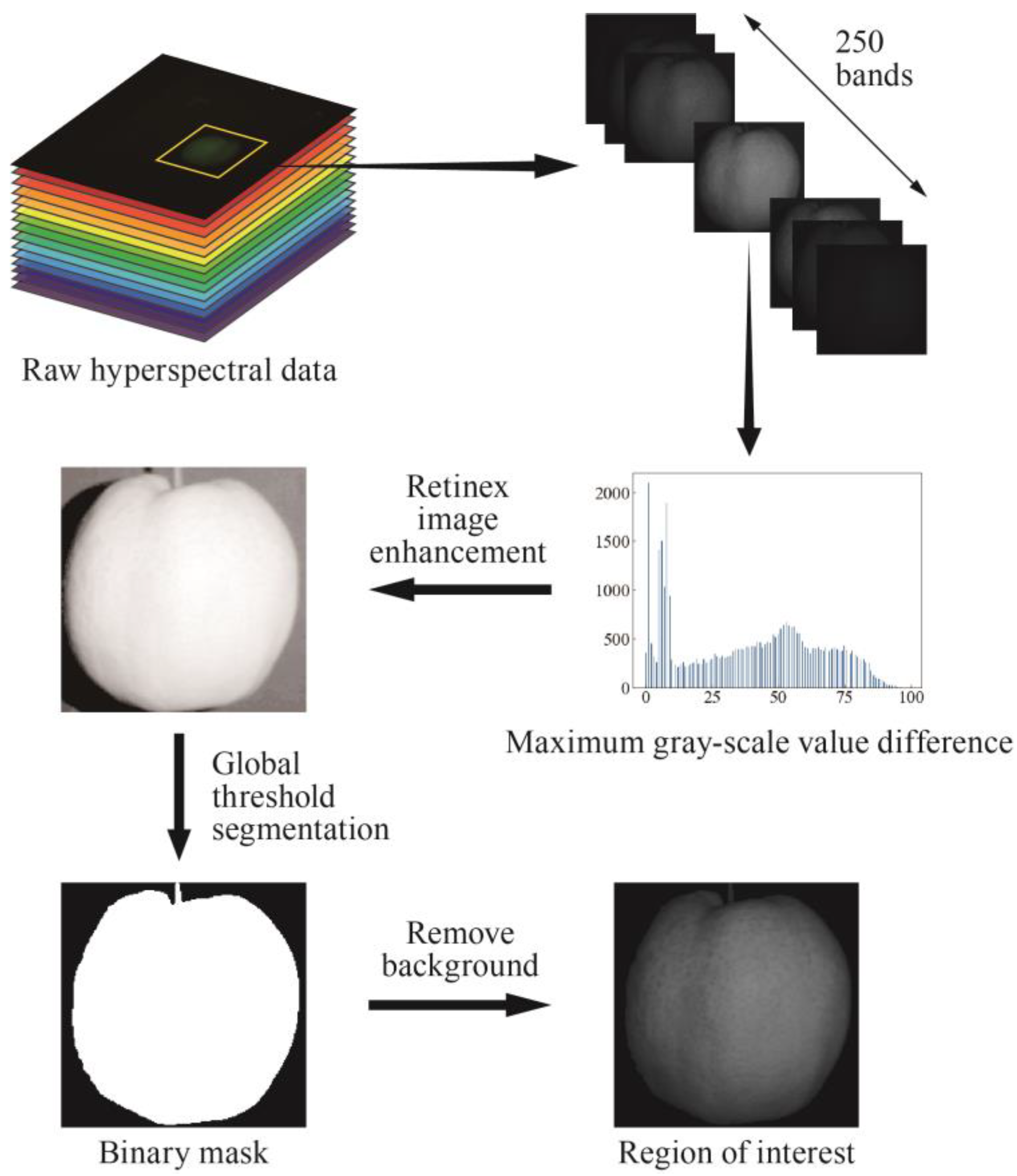
In an attempt to obtain the data of pears’ optical properties, a series of image processing was performed on the raw SFDI data. The image processing flow is shown in
Figure 2.
Firstly, single wavelength images were extracted from the acquired hyperspectral data, and the images of total 250 wavelengths were separated. After the sample image was cropped out in each band, all the single wavelength images were traversed. The image with the largest gray difference between the fruit and the background was found at 636.3 nm, and the single scale Retinex image enhancement was performed for the image at this wavelength. The enhanced image was segmented by the global threshold, and the binary mask was obtained according to the optimal threshold value of the binarized sample image. Then, the region of each pear was segmented from the single wavelength image by the mask, and it was selected as the ROI.
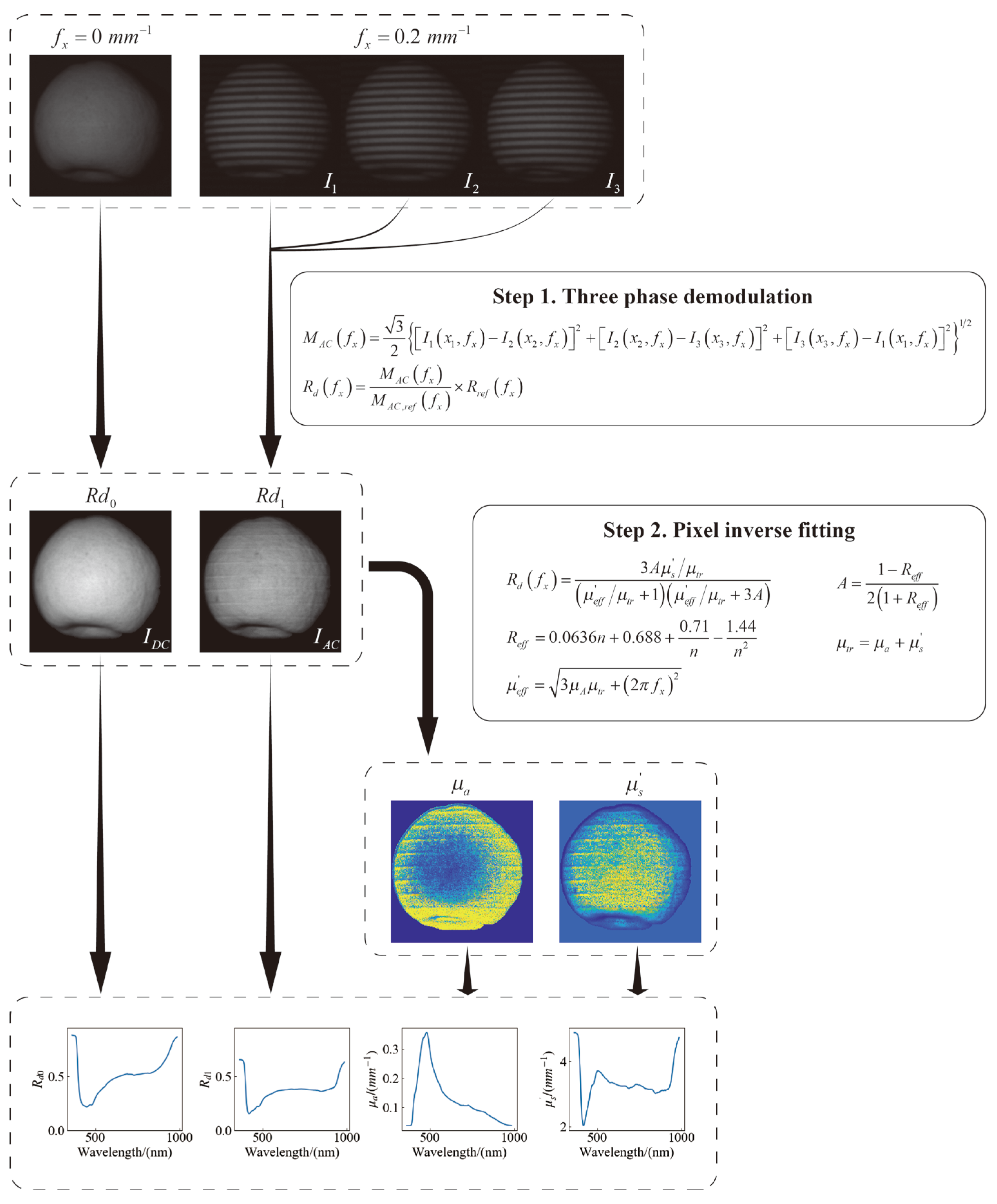
The process of extracting the diffuse reflectance
, absorption coefficient
and the reduced scattering coefficient
from the raw data is shown in
Figure 3. The first step is to demodulate the single wavelength images. According to the three-phase demodulation (TPD) method proposed by Cuccia et al. [
25], the illumination intensity of the structured light can be decomposed into a DC (plane) part
and an AC (space) part
. The DC image corresponds to the image captured with uniform illumination, while the AC image contains depth information specific to the spatial frequency of the sinusoidal illumination pattern. With the initial SFDI images at 0,
, and
phases (i.e.,
,
and
), the corresponding diffuse reflectance
is calculated to obtain the amplitude envelope
of the diffuse reflectivity photon density at that frequency
. The diffuse reflectance of the target samples is then calibrated with the known reflectance of the standard reflector plate
and the diffuse reflectance intensity amplitude envelope of the standard reflector plate
to minimize the systematic error.
The diffuse reflection image and reflectance can directly detect the surface defects and internal quality of agricultural products, but the further processing of diffuse images is required to obtain the optical transmission properties parameters that are more directly related to the pear fruit and its quality parameters. So, the second step is reverse inversion to solve the absorption coefficient
and reduced scattering coefficient
. The scattering of most of the agricultural products’ tissues are much larger than absorption, which is just in line with the constraints of the diffusion approximation equation (DAE) [
26]. Therefore, DAE is the most commonly used light transmission model in the inversion for detection agricultural products. The parameters in DAE are described as follows:
is the effective reflection coefficient;
n is the tissue refractive index;
A is a proportional constant;
is the full attenuation coefficient;
is the approximate attenuation coefficient. The reciprocal of
is the effective penetration depth of photons in the spatial frequency domain, and the intensity of the planar-structured light source
decays exponentially with the penetrated depth. Based on the diffuse reflectance at different spatial frequencies, the
and
of each pixel within ROI can be obtained via pixel-by-pixel fitting using nonlinear partial least squares (PLS).
2.4. Measurement of Internal Quality
Before the image acquisition, external quality parameters such as the range of equatorial diameter, weight, and height at the image acquisition of each fruit were first measured using the digital vernier caliper (CR2032, Jinhua Shijian Tool Co., Ltd., Jinhua, China) and the electronic scale (CH-305, Deqing Baijie Electric Appliance Co., Ltd., Huzhou, China), respectively.
After the image acquisition was completed, the firmness of the peeled flesh was measured with the firmness tester (GY-4, Dongguan three precision measuring instrument Co., Ltd., Dongguan, China) on three points distributed in the equator of the pear fruit corresponding to the image data collection. The measurement process was as follows: Before each measurement, ensure that the zero position of the firmness tester is accurate. Hold in the pear firmly in place to avoid movement during the measurement. Slowly press the 11.1 mm diameter probe into the flesh, making sure that the indenter of the durometer was perpendicular to the surface of the pear, and apply uniform pressure until the indenter is completely embedded in the surface of the pear to a maximum depth of 10 mm of the indenter. Hold the indenter in the measuring position for a few seconds and wait for the reading to stabilize before recording the firmness value (in units of N). After each measurement, wipe the indenter with a soft cloth to ensure that no residue remains. Avoid vibration and external interference during the measurement.
The SSC of the samples was then measured using a hand-held refractometer (PAL-1, Alto Scientific Instruments Ltd., Tokyo, Japan). Pieces of pulp (about 20 mm thick) were cut from the point of firmness measurement separately and the juice was obtained using a clean knife and a squeezer to avoid contamination. A small amount of pear juice was evenly dripped onto the prismatic surface of the refractometer to ensure that there were no air bubbles or impurities, and the reading was stabilized for a few seconds before taking the reading. Before each measurement, the refractometer was calibrated with distilled water to ensure that the zero point of the refractometer was accurate. The measurements were taken at a constant room temperature to avoid temperature fluctuations affecting the reading. After each measurement, the prism was wiped with distilled water to ensure that there was no residue.
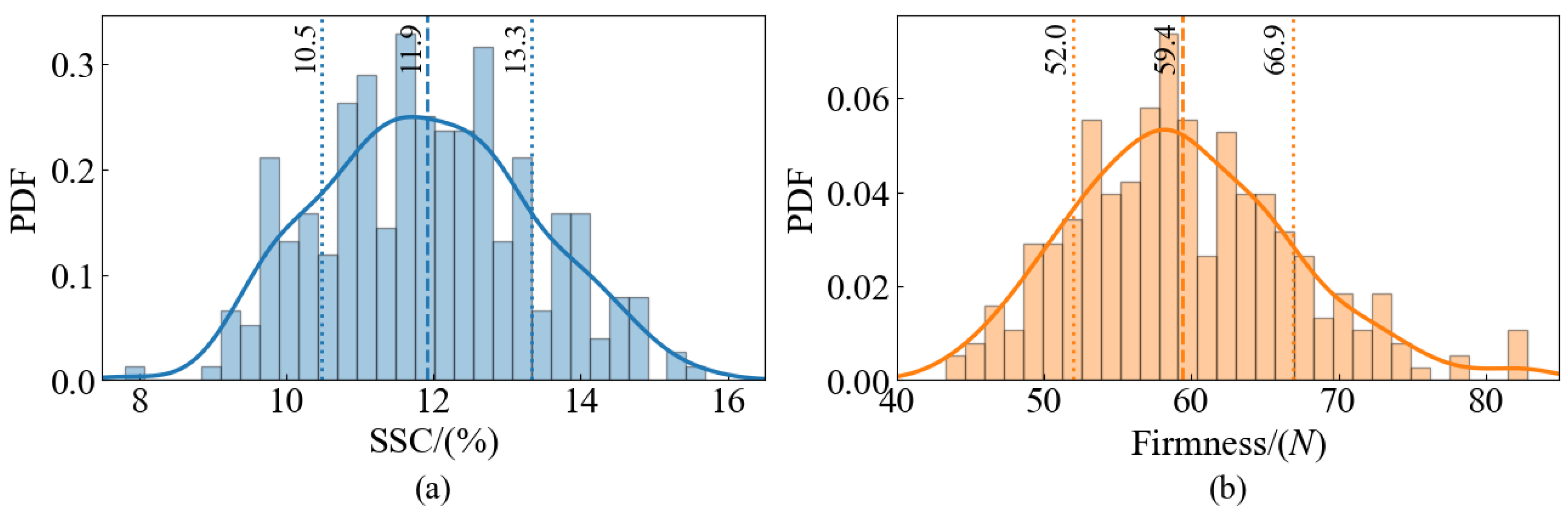
Once the measurements were completed, the mean and standard deviation (SD) of the quality parameters were calculated for each sample. Subsequent analyses will use the average of multiple measurements to increase the reliability of the data.
2.5. Data Analysis
2.5.1. Effective Wavelength Selection
A key step before establishing the physicochemical index prediction model based on optical properties is the feature wavelength extraction. Extracting the bands related to the sample properties is vital to establish the highly precise and stable models. Selecting appropriate features for the characteristic sample can significantly decrease the dimensionality and redundancy of the variables and enhance modeling efficiency. In this research, the competitive adaptive reweighted sampling algorithm (CARS) was employed to extract the effective wavelengths (EWs) of the preprocessed spectral data. The CARS algorithm can build a partial least squares model from the calibration set samples selected via Monte Carlo sampling and can also select the most competitive band combination using an adaptive reweighting sampling method [
27].
2.5.2. Prediction Model Development
Partial least squares regression (PLSR) is commonly utilized in chemometrics research because of its fast computational speed and efficient extraction of useful information from high-dimensional data. PLSR was utilized as a modeling algorithm to determine the optimal number of latent variables (LVs) by the minimum root-mean-square error of 10-fold cross-validation. The effectiveness of the PLSR model was assessed by comparing the determination coefficient and the root-mean-square error of the calibration set ( and ), as well as the determination coefficient and the root-mean-square error of the prediction set ( and ). This comparison was carried out to determine the optimal pretreatment method and bands. A model’s quality and predictive accuracy improve with higher values and lower RMSE values. Furthermore, the model’s relative percent deviation (RPD), calculated as the ratio of the standard deviation to the of pear samples, serves as an indicator of the model’s predictive capability. Therefore, an effective prediction model should exhibit low RMSE and high and RPD values.
2.6. Software
All the above image processing and optical properties data extraction and processing were completed by using PyCharm Community Edition (3.9.13, JetBrains s.r.o., Prague, Czech Republic) and MATLAB (R2021b, The Mathworks Inc., Natick, MA, USA).
4. Discussion
The present study provides a comprehensive analysis of the efficacy of various optical properties as input variables for predicting the SSC and firmness of pear flesh using PLSR. The results indicate that models based on reflectance at zero spatial frequency
achieved the best performance among those using raw input data for SSC prediction. This finding demonstrated that reflectance-based models could be employed due to their simplicity and effectiveness in non-destructive quality assessment of fruits. When the input data were subjected to CARS for feature selection, the combined reflectance spectra
significantly improved prediction accuracy, suggesting that high-frequency reflectance data provide additional valuable information, thus enhancing the model’s predictive capability. This observation supports the hypothesis that incorporating multiple spatial frequencies can capture more comprehensive spectral features, thereby improving SSC prediction. The prediction of SSC from optical property spectra measured through hyperspectral spatial frequency domain imaging in this study is comparable or better than those reported in previous studies using a single integrating sphere [
33,
35]. For example, Xia et al. reported a
and
of 0.7757 and 0.5321, respectively, for the SSC prediction model for tomato when using the CARS-PLSR model to calculate reflectance measured using a single integrating sphere system in the 900–1700 nm spectral range [
33].
Furthermore, the PLSR models for SSC prediction based on
yielded comparable and sometimes superior results to those using reflectance data alone. This is likely due to the absorption characteristics being directly influenced by the chemical composition of the pear, such as carbohydrates and water content, which are more directly related to SSC than scattering properties. The results based on absorption coefficient spectroscopy in predicting SSC in this study were superior to the prediction of the average
spectra of different parts of pear obtained by Liu et al. using the 500–1050 nm by integrating sphere measurements, with a
of 0.837 and
of 0.429 [
29].
In parallel, the above findings on pear firmness reveal significant insights into predictive modeling using various optical properties. The raw spectral data in the 360–1000 nm range did not provide reliable firmness predictions, highlighting a weak intrinsic relationship between the optical properties and firmness within these wavelengths. This indicates that raw spectral data often contains noise and irrelevant information, which can obscure meaningful relationships. After implementing CARS for band selection, a marked improvement in the firmness model performance was observed, underscoring the effectiveness of CARS in isolating relevant spectral bands and removing extraneous information. However, the
-based prediction of CARS-PLSR hardness in this study is worse than that reported by Fang et al. [
6], but better than the
results measured through Vis-NIR spatially resolved spectroscopy (
= 0.76,
= 1.06) by Ma et al. [
3].
Notably, reflectance models showed that outperformed , suggesting that high frequency reflectance has spectral features more related to tissue structure and is more effective for firmness prediction. Moreover, the model utilizing demonstrated superior predictive capability over that of . This reinforces the understanding that , being more indicative of the internal structure, correlates better with firmness. The optimal model, combining , achieved the best prediction accuracy, likely due to its ability to retain characteristic absorption peaks while scaling the scattering values appropriately, thus capturing structural and compositional nuances affecting firmness.
In contrast, the prediction results of quality indicators based on reflectance spectroscopy in this study were also superior to the results using only hyperspectral spectra [
36,
37]. For example, Xuan et al. developed a CARS-MLR model to predict the SSC of peaches, which resulted in
= 0.841,
= 0.546, and RPD = 2.51 [
37]. For the same spatial frequency domain imaging technique, the prediction of fruit quality indexes based on continuous and high-resolution spectroscopy in this study is also superior to that of previous studies. He et al. detected the SSC and flesh hardness of pears using the optical property of multiwavelength SFDI measurements, with the results of
being 0.468 and 0.588 and
being 0.644 and 1.169, respectively [
30]. It is demonstrated that the combination of hyperspectral and spatial frequency domain imaging techniques improves the accuracy of fruit quality detection to a great extent, while the wavelength selection in modeling may be another key factor in obtaining the satisfactory prediction accuracy of physicochemical indexes.
This study concentrates on how SSC and pear fruit firmness affect the characterization of spectral optical properties in the 360 to 1000 nm band. It is important to note that the percentage of moisture within the pear is equally important in the spectral information. In order to construct a model for spectral analysis that is less affected by changes in moisture content, the CARS algorithm was used in this study to screen out key spectral bands that are closely related to SSC and hardness, respectively, and the corresponding calibration models were created based on these bands. The next research work can evaluate in detail how moisture content specifically affects spectral properties by measuring the moisture content of pears.
Future research could also optimize the spatial frequency to improve detection accuracy for different fruits and their detection needs. Combining information from different spatial frequencies, the quality of fruits can be assessed more comprehensively. Capturing the shallow details of the fruit through high spatial frequencies while utilizing low spatial frequencies to obtain deeper information about the internal structure provides a comprehensive assessment of the fruit’s appearance and internal quality. In addition, exploring other fruits and expanding the dataset to include different varieties and growing conditions to build comprehensive assessment models that include multiple detection parameters such as sugar, acidity, hardness, and ripeness can provide valuable insights into the generalization of these models to provide a comprehensive fruit quality assessment system.