A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives
Abstract
1. Introduction
2. Biocontrol as a Viable Approach for Controlling Disease in Horticulture Crops
3. Factors Affecting the BCAs Activities and Strategies to Overcome Limitations of BCA Effectiveness
4. Fungal Species as BCAs: Mechanisms and Applications
Mechanism of Action
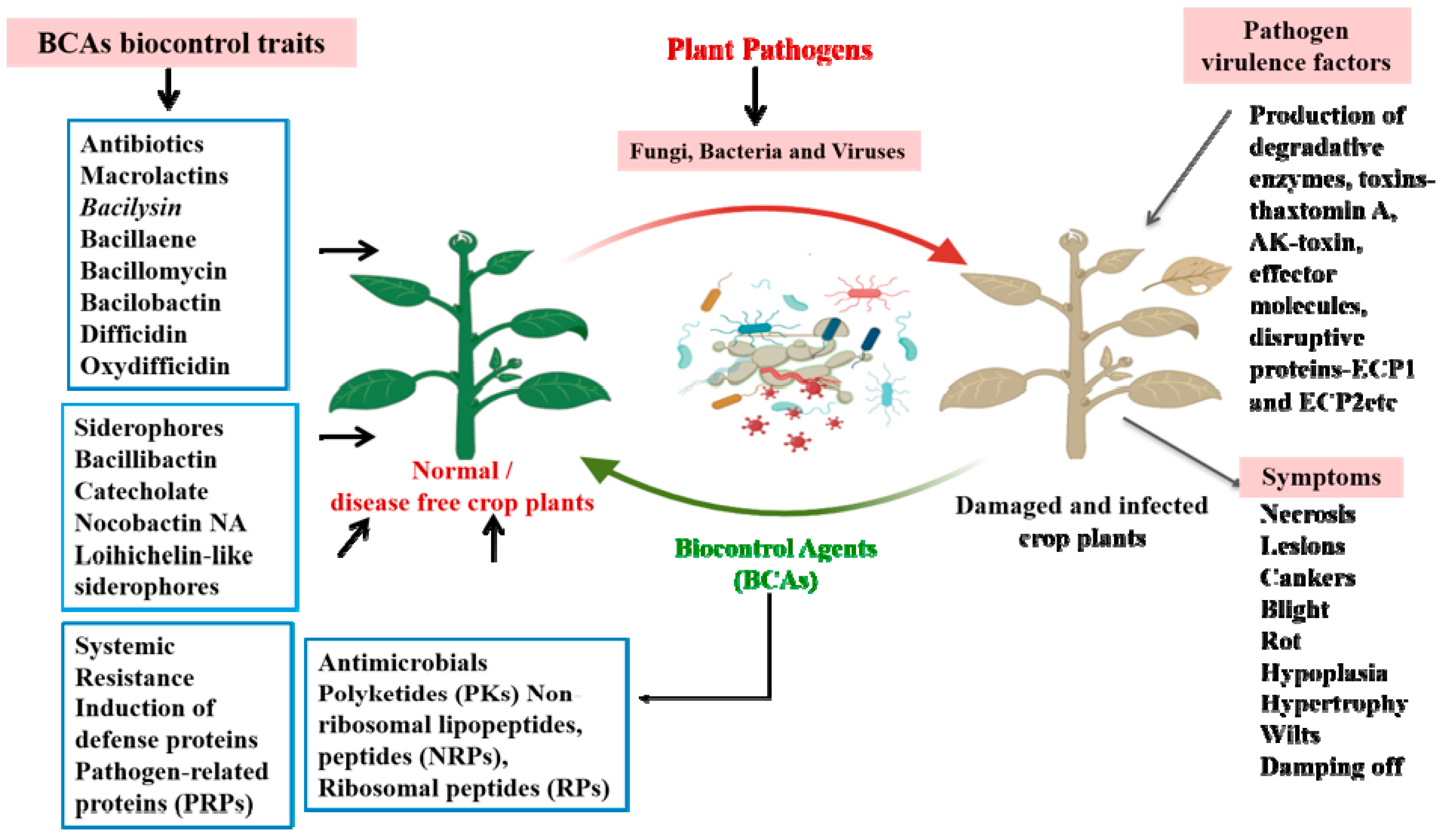
5. Bacteria as BCAs for Plant Disease Management
6. Viruses as Biocontrol Agents
6.1. Biocontrol Potential of Mycoviruses against Fungal Plant Pathogens
6.2. Phage-Based Biobactericides
6.3. Cross-Protection
7. Biocontrol Agents’ Production and Formulations
7.1. Production of BCAs
7.2. BCA Formulation
7.2.1. Liquid Formulation
7.2.2. Solid Formulation
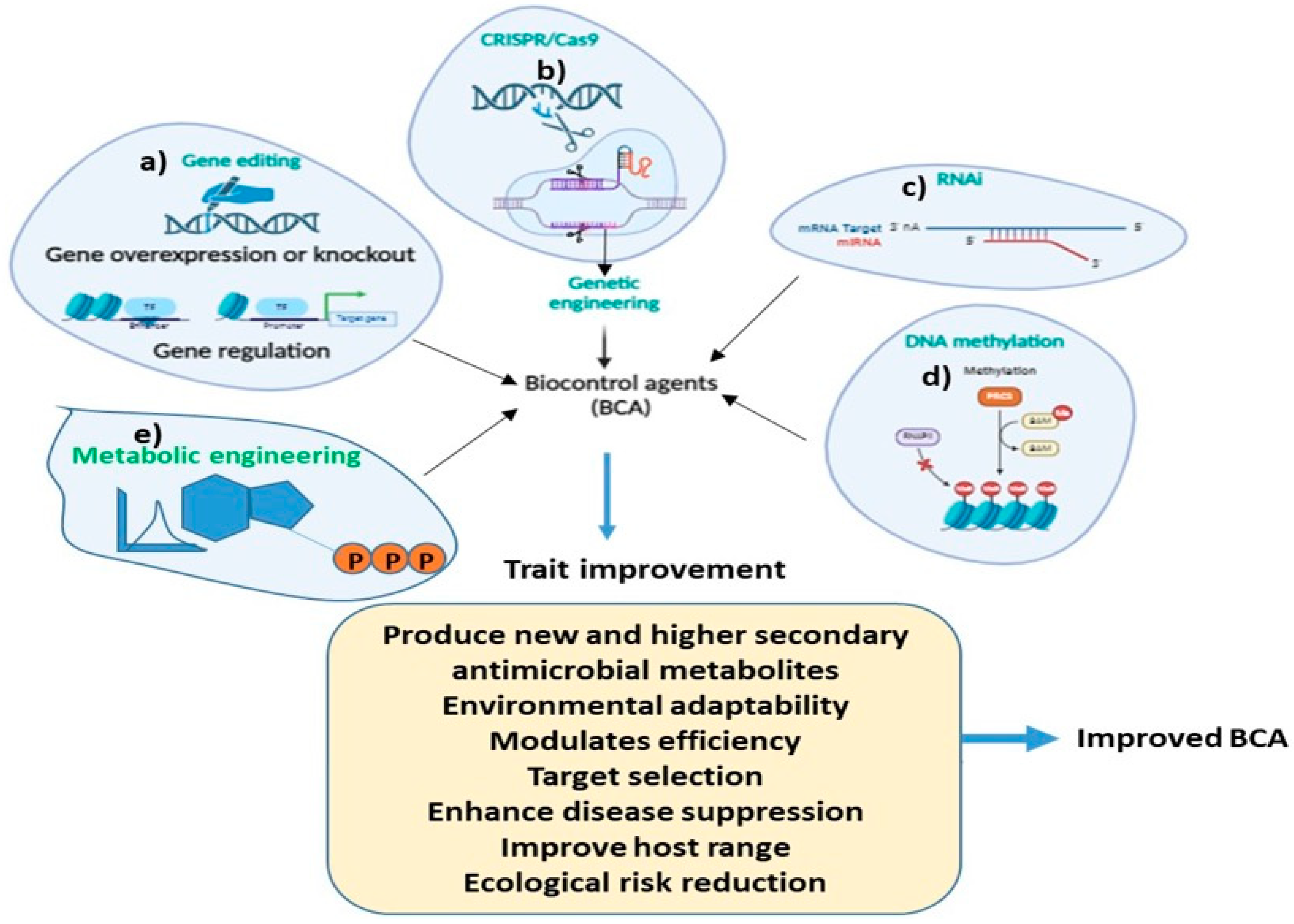
8. Role of Genetic Engineering for Improving BCAs Traits
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mall, M.; Kumar, R.; Akhtar, M.Q. Horticultural crops and abiotic stress challenges. In Stress Tolerance in Horticultural Crops; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–19. [Google Scholar]
- Xu, J.; Zhang, N.; Wang, K.; Xian, Q.; Dong, J.; Chen, X. Exploring new strategies in diseases resistance of horticultural crops. Front. Sustain. Food Syst. 2022, 6, 1021350. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Rajarammohan, S.; Mir, Z.A.; Bae, H. Revisiting Alternaria-host interactions: New insights on its pathogenesis, defense mechanisms and control strategies. Sci. Hortic. 2023, 322, 112424. [Google Scholar] [CrossRef]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Utkhede, R.S.; Mathur, S. Preventive and curative biological treatments for control of Botrytis cinerea stem canker of greenhouse tomatoes. BioControl 2006, 51, 363–373. [Google Scholar] [CrossRef]
- Charoenporn, C.; Kanokmedhakul, S.; Lin, F.C.; Poeaim, S.; Soytong, K. Evaluation of bio-agent formulations to control Fusarium wilt of tomato. Afr. J. Biotechnol. 2010, 9, 5836–5844. [Google Scholar]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Khodadadi, F.; González, J.B.; Martin, P.L.; Giroux, E.; Bilodeau, G.J.; Peter, K.A.; Doyle, V.P.; Aćimović, S.G. Identification and characterization of Colletotrichum species causing apple bitter rot in New York and description of C. noveboracense sp. nov. Sci. Rep. 2020, 10, 11043. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General Characteristics of Pathogenicity and Methods of Control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Tripathi, A.N.; Singh, B.P. Evaluation of seed health of seeds of different vegetable crops. Veg. News Letter. 2022, 9, 7. [Google Scholar]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 25, 161–180. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Martín-Gil, J.; Lorenzo-Vidal, B.; Palacio-Bielsa, A.; Martín-Ramos, P. Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae 2023, 9, 201. [Google Scholar] [CrossRef]
- Hull, R. Symptoms and Host Range. In Plant Virology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 145–198. [Google Scholar]
- Mehetre, G.T.; Leo, V.V.; Singh, G.; Sorokan, A.; Maksimov, I.; Yadav, M.K.; Upadhyaya, K.; Hashem, A.; Alsaleh, A.N.; Dawoud, T.M.; et al. Current Developments and Challenges in Plant Viral Diagnostics: A Systematic Review. Viruses 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water—A review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, E.; Gurr, S. Address the growing urgency of fungal disease in crops. Nature 2023, 617, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M.P.; Mahillon, J.; Bragard, C. On the use of antibiotics to control plant pathogenic bacteria: A genetic and genomic perspective. Front. Microbiol. 2023, 14, 1221478. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, A.D.; Sarkale, P.S.; Patil, P.A. Transgenic Plants and Animals in Agriculture: Assessing the Risks and Benefits. Nat. Camp. 2024, 28, 95–102. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant Soil and Microbes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 253–269. [Google Scholar]
- Sandy, Y.A.; Zahro, F.A.; Rizky, D.R.; Fajarwati, S.K.; Effendi, M. Knowledge Level of Farmers regarding the Use of Pesticide for Pest and Disease Control. J. Agrinika J. Agroteknologi Agribisnis 2024, 8, 12–22. [Google Scholar]
- Dalavayi, H.M.; Bala, S.; Choudhury, D. Eco-friendly plant based on botanical pesticides. Plant Arch. 2021, 21, 2197–2204. [Google Scholar]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.K.; Han, J.A.; Lee, H.S. Exploiting bacterial genera as biocontrol agents: Mechanisms, interactions and applications in sustainable agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Theresa, M.; Radhakrishnan, E.K. Microbial biocontrol formulations for commercial applications. In Microbiome Stimulants for Crops; Woodhead Publishing: Cambridge, UK, 2021; pp. 179–192. [Google Scholar]
- Galli, M.; Feldmann, F.; Vogler, U.K.; Kogel, K.-H. Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J. Plant Dis. Prot. 2024, 131, 265–291. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Funck Jensen, D.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys rosea for the control of plant diseases. In Microbial Bioprotectants for Plant Disease Management; BDS Publishing: Cambridge, UK, 2021; pp. 429–471. [Google Scholar]
- Köhl, J.; Kolnaar, R.; Ravensberg, J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kamle, M.; Borah, R.; Mahato, D.K.; Sharma, B. Bacillus thuringiensis as microbial biopesticide: Uses and application for sustainable agriculture. Egypt. J. Biol. Pest Control 2021, 31, 95. [Google Scholar] [CrossRef]
- Salazar, B.; Ortiz, A.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Singh, S.P.; Rekadwad, B.; Borriss, R.; Jain, A.; Singh, H.B.; et al. Bacillus spp. as bio-factories for antifungal secondary metabolites: Innovation beyond whole organism formulations. Microb. Ecol. 2023, 86, 1–24. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Shao, J.; Xu, Z.; Liu, Y.; Xun, W.; Miao, Y.; Shen, Q.; Zhang, R. Biocontrol mechanisms of Bacillus: Improving the efficiency of green agriculture. Microb. Biotechnol. 2023, 16, 2250–2263. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd_Allah, E.F. Bacillus subtilis: A plant-growth-promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting Rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus Species: Excellent Biocontrol Agents against Tomato Diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Berdi, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Müller, A.; Miess, H.; Gross, H. Cyclic lipopeptides as antibacterial agents-Potent antibiotic activity mediated by intriguing mode of actions. Int. J. Med. Microbiol. 2014, 304, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Helmy, N.M.; Parang, K. Cyclic peptides with antifungal properties derived from bacteria, fungi, plants, and synthetic sources. Pharmaceuticals 2023, 16, 892. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. BioMed Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Tai, B.; Ali, M.; Jahan, I.; Sakina, S.; Wang, G.; Zhang, X.; Yin, Y.; Xing, F. Antifungal potential of lipopeptides produced by the Bacillus siamensis Sh420 strain against Fusarium graminearum. Microbiol. Spectr. 2024, 12, 4008–4023. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.H.; Lu, Y.Q.; Ye, Z.W.; Zheng, Q.W.; Wei, T.; Lin, J.F.; Guo, L.Q. Genomics. guided discovery and structure identification of cyclic lipopeptides from the Bacillus siamensis JFL15. PLoS ONE 2018, 13, e0202893. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Pan, Y.; Qi, D.; Zhou, D.; Chen, Y.; Feng, J.; Wei, Y.; Zhao, Y.; Li, K.; et al. Biocontrol mechanism of Bacillus siamensis sp. QN2MO.1 against tomato fusarium wilt disease during fruit postharvest and planting. Microbiol. Res. 2024, 283, 127694. [Google Scholar] [CrossRef]
- Epparti, P.; Eligar, S.M.; Sattur, A.; Kumar, B.S.G.; Halami, P.M. Characterization of dual bacteriocins producing Bacillus subtilis SC3.7 isolated from fermented food. Food Sci. Technol. 2022, 154, 112854. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Benjamin, C.K.; Margaret, A.R. Colicins and Microcins: The next generation antimicrobials. Adv. Appl. Microbiol. 2004, 54, 129–146. [Google Scholar] [PubMed]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Im, S.M.; Yu, N.H.; Joen, H.W.; Kim, S.O.; Park, H.W.; Park, A.R.; Kim, J.C. Biological control of tomato bacterial wilt by oxydifficidin and difficidin-producing Bacillus methylotrophicus DR-08. Pestic. Biochem. Physiol. 2020, 163, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Devappa, V.; Yadav, D.K. Suppression of tomato bacterial wilt incited by Ralstonia pseudosolanacearum using polyketide antibiotic-producing Bacillus spp. isolated from rhizospheric soil. Agriculture 2022, 12, 2009. [Google Scholar] [CrossRef]
- Chen, Q.; Qiu, Y.; Yuan, Y.; Wang, K.; Wang, H. Biocontrol activity and action mechanism of Bacillus velezensis strain SDTB038 against Fusarium crown and root rot of tomato. Front. Microbiol. 2022, 13, 994716. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases-A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, L.; Wang, X.; Li, D.; Yin, Z.; Zhang, J.; Ding, G.; Chen, L. Structures and biological activities of secondary metabolites from the Trichoderma genus (covering 2018–2022). J. Agric. Food Chem. 2023, 71, 13612–13632. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Manganiello, G.; Nicastro, N.; Ortenzi, L.; Pallottino, F.; Costa, C.; Pane, C. Trichoderma Biocontrol Performances against Baby-Lettuce Fusarium Wilt Surveyed by Hyperspectral Imaging-Based Machine Learning and Infrared Thermography. Agriculture 2024, 14, 307. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef]
- Timper, P. Conserving and Enhancing Biological Control of Nematodes. J. Nematol. 2014, 46, 75–89. [Google Scholar]
- Umer, M.; Mubeen, M.; Iftikhar, Y.; Shad, M.A.; Usman, H.M.; Sohail, M.A.; Atiq, M.N.; Abbas, A.; Ateeq, M. Role of Rhizobacteria on Plants Growth and Biological Control of Plant Diseases: A Review. Plant Prot. 2021, 5, 59–73. [Google Scholar]
- Matsumoto, A.; Takahashi, Y. Endophytic Actinomycetes: Promising Source of Novel Bioactive Compounds. J. Antibiot. 2017, 70, 514–519. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the Induction of Systemic Resistance and Regulation of Antioxidant Pathways in Tomato Using Fengycin Produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Ezrari, S.; Mhidra, O.; Radouane, N.; Tahiri, A.; Polizzi, G.; Lazraq, A.; Lahlali, R. Potential Role of Rhizobacteria Isolated from Citrus Rhizosphere for Biological Control of Citrus Dry Root Rot. Plants 2021, 10, 872. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic Screening and Expression Analysis of Psychrophilic Bacillus spp. Reveal Their Potential to Alleviate Cold Stress and Modulate Phytohormones in Wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef]
- Sutthisa, W.; Hompana, W.; Yutthasin, R. Enhancing Biocontrol Potential: Development and Efficacy Assessment of a Liquid Formulation of Trichoderma Asperellum MSU007 against Sclerotium Rrolfsii. Trends Sci. 2024, 21, 7550. [Google Scholar] [CrossRef]
- Hjeljord, L.; Tronsmo, A. Trichoderma and Gliocladium in biological control: An overview. In Trichoderma & Gliocladium—Enzymes, Biological Control and Commercial Applications; Harman, G.E., Kubicek, C.P., Eds.; Taylor & Francis Ltd.: London, UK, 1998; pp. 131–151. [Google Scholar]
- Minuto, A.; Migheli, Q.; Garibaldi, A. Evaluation of antagonistic strains of Fusarium spp. in the biological and integrated control of Fusarium wilt of cyclamen. Crop Prot. 1995, 14, 221–226. [Google Scholar] [CrossRef]
- Vehapi, M.; İnan, B.; Kayacan-Cakmakoglu, S.; Sagdic, O.; Özçimen, D.B. Preparation of Bacillus pumilus loaded electrosprayed nanoparticles as a plant protective against postharvest fungal decay. Eur. J. Plant Pathol. 2024, 168, 121–136. [Google Scholar] [CrossRef]
- Goulet, F.; Aulagnier, A.; Fouilleux, E. Moving beyond pesticides: Exploring alternatives for a changing food system. Environ. Sci. Policy 2023, 147, 177–187. [Google Scholar] [CrossRef]
- Galluzzo, N. How does eliminating the use of pesticides affect technical efficiency in Italian farms? Bulg. J. Agric. Sci. 2023, 29, 14–23. [Google Scholar]
- Palmisano, T. Narratives and practices of pesticide removal in the Andean valleys of Chile and Argentina. Environ. Sci. Policy 2023, 139, 149–156. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Sánchez-Cruz, R.; Mehta, R.; Atriztán-Hernández, K.; Martínez-Villamil, O.; del Rayo Sánchez-Carbente, M.; Sánchez-Reyes, A.; Folch-Mallol, J.L. Effects on Capsicum annuum plants colonized with Trichoderma atroviride P. karst strains genetically modified in Taswo1, a gene coding for a protein with Expansin-like activity. Plants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Liu, P.; Luo, L.; Long, C.A. Characterization of competition for nutrients in the biocontrol of Penicillium italicum by Kloeckera apiculata. Biol. Control 2013, 67, 157–162. [Google Scholar] [CrossRef]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Altomare, C. Potential of Trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Zhou, L. Trichoderma spp. genes involved in the biocontrol activity against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar] [CrossRef] [PubMed]
- Manzar, N.; Singh, Y.; Kashyap, A.S.; Sahu, P.K.; Rajawat, M.V.S.; Bhowmik, A.; Saxena, A.K. Biocontrol potential of native Trichoderma spp. against anthracnose of great millet (Sorghum bicolor L.) from Tarai and hill regions of India. Biol. Control 2021, 152, 104474. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Garrido-Jurado, I.; Yousef-Yousef, M.; González-Mas, N. Multitrophic Interactions of Entomopathogenic Fungi. Biol. Control 2022, 67, 457–472. [Google Scholar]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Persoon, C.H. Disposita methodical fungorum. Romers. Neues. Mag. Bot. 1794, 1, 81–128. [Google Scholar]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schone, J.; Buchenauer, H. Detection of viridiofungin A and another antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur. J. Plant Pathol. 2009, 124, 457–470. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Manzar, N.; Rajawat, M.V.S.; Kesharwani, A.K.; Singh, R.P.; Dubey, S.C.; Pattanayak, D.; Dhar, S.; Lal, S.K.; Singh, D. Screening and Biocontrol Potential of Rhizobacteria Native to Gangetic Plains and Hilly Regions to Induce Systemic Resistance and Promote Plant Growth in Chilli against Bacterial Wilt Disease. Plants 2021, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- del Carmen, H.; Rodríguez, M.; Evans, H.C.; de Abreu, L.M.; de Macedo, D.M.; Ndacnou, M.K.; Bekele, K.B.; Barreto, R.W. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci. Rep. 2021, 11, 5671. [Google Scholar] [CrossRef]
- Sundheim, L. Control of Cucumber Powdery Mildew by the Hyperparasite Ampelomyces quisqualis and Fungicides. Plant Pathol. 1982, 31, 209–214. [Google Scholar] [CrossRef]
- Gurung, M. Evaluation of Trichoderma asperellum as Biocontrol Agent for Phytophthora Foot and Root Rot of Citrus. Master’s Thesis, Texas A&M University, Kingsville, TX, USA, 2018. [Google Scholar]
- Contina, J.B. Biological Control of Fusarium solani, Rhizoctonia solani and the Pale Cyst Nematode Globodera pallida with Trichoderma harzianum ThzID1; University of Idaho: Pocatello, ID, USA, 2016. [Google Scholar]
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Jaihan, P.; Sangdee, K.; Sangdee, A. Disease suppressive activity of extracts from entomopathogenic fungus Ophiocordyceps sobolifera against chili anthracnose fungi Colletotrichum spp. in a pot experiment. J. Gen. Plant Pathol. 2018, 84, 237–242. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Abdel-Hafez, S.I.; Abdel-Rahim, I.R. Isolation of Trichoderma and evaluation of their antagonistic potential against Alternaria porri. J. Phytopathol. 2014, 162, 567–574. [Google Scholar] [CrossRef]
- Kalimutu, P.K.; Mahardika, I.B.K.; Sagung, P.R.A.A.A. Antagonism Test of Trichoderma atroviride and Gliocladium sp. Bali Local Isolates As a Disease Control of Blendok Disease (Botryodiplodia theobromae) in Grapefruit (Citrus grandis L. Osbeck). Sustain. Environ. Agric. Sci. 2020, 4, 102–110. [Google Scholar] [CrossRef]
- Sherkhane, P.D.; Bansal, R.; Banerjee, K.; Chatterjee, S.; Oulkar, D.; Jain, P.; Mukherjee, P.K. Genomics-Driven Discovery of the Gliovirin Biosynthesis Gene Cluster in the Plant Beneficial Fungus Trichoderma Virens. ChemSelect 2017, 2, 3347–3352. [Google Scholar] [CrossRef]
- Hua, L.; Zeng, H.; He, L.; Jiang, Q.; Ye, P.; Liu, Y.; Zhang, M. Gliotoxin Is an Important Secondary Metabolite Involved in Suppression of Sclerotium rolfsii of Trichoderma virens T23. Phytopathology 2021, 111, 1720–1725. [Google Scholar] [CrossRef]
- Boureghda, H.; Bouznad, Z. Biological control of Fusarium wilt of chickpea using isolates of Trichoderma atroviride, T. harzianum and T. longibrachiatum. Acta Phytopathol. Entomol. Hung. 2009, 44, 25–38. [Google Scholar] [CrossRef]
- Tsai, C.C.; Tzeng, D.S.; Hsieh, S.P.Y. Biological control of Fusarium stem rot of Anoectochilus formosanus Hayata by Trichoderma asperellum TA strain. Plant Pathol. Bull. 2008, 17, 243–254. [Google Scholar]
- Schubert, M.; Fink, S.; Schwarze, F.W.M.R. Field experiments to evaluate the application of Trichoderma strain (T.15603.1) for biological control of wood decay fungi in trees. Part II. Arboric. J. 2008, 31, 249–268. [Google Scholar] [CrossRef]
- Waqas, M.; Khana, A.L.; Hamayuna, M.; Shahzad, R.; Kang, S.M.; Kim, J.G.; Lee, I.J. Endophytic Fungi Promote Plant Growth and Mitigate the Adverse Effects of Stem Rot: An Example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Gressel, J. Four pillars are required to support a successful biocontrol fungus. Pest Manag. Sci. 2024, 80, 5–39. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, P.; Fernández-González, R.A.; Sanz-Ros, A.V.; Pando, V.; Diez, J.J. Two fungal endophytes reduce the severity of pitch canker disease in Pinus radiata seedlings. Biol. Control 2016, 94, 1–10. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; AbuQamar, S.F. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Kumari, N.; Srividhya, S. Secondary Metabolites and Lytic Tool Box of Trichoderma and Their Role in Plant Health. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 305–320. [Google Scholar]
- Segarra, G.; Casanova, E.; Avilés, M.; Trillas, I. Trichoderma asperellum Strain T34 Controls Fusarium Wilt Disease in Tomato Plants in Soilless Culture through Competition for Iron. Microb. Ecol. 2010, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Lorito, M. Harzianic Acid: A Novel Siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef]
- Yan, L.; Khan, R.A.A. Biological Control of Bacterial Wilt in Tomato through the Metabolites Produced by the Biocontrol Fungus, Trichoderma harzianum. Egypt. J. Biol. Pest Control 2021, 31, 1–9. [Google Scholar]
- Konappa, N.; Krishnamurthy, S.; Siddaiah, C.N.; Ramachandrappa, N.S.; Chowdappa, S. Evaluation of biological efficacy of Trichoderma asperellum against tomato bacterial wilt caused by Ralstonia solanacearum. Egypt J. Biol. Pest Control 2018, 28, 63. [Google Scholar] [CrossRef]
- Choi, G.J.; Kim, J.C.; Jang, K.S.; Cho, K.Y.; Kim, H.T. Mycoparasitism of Acremonium strictum BCP on Botrytis cinerea, the Gray Mold Pathogen. J. Microbiol. Biotechnol. 2008, 18, 167–170. [Google Scholar] [PubMed]
- Fuchs, J.G.; Moënne-Loccoz, Y.; Défago, G. Nonpathogenic Fusarium oxysporum strain Fo47 induces resistance to Fusarium wilt in tomato. Plant Dis. 1997, 81, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Y.; Sariah, M.; Ismail, M.R.; Ali, A. Trichoderma.fortified compost extracts for the control of choanephora wet rot in okra production. Crop Prot. 2008, 27, 385–390. [Google Scholar] [CrossRef]
- Kowalska, J. Effects of Trichoderma asperellum [T1] on Botrytis cinerea [Pers.: Fr.], growth and yield of organic strawberry. Acta Scientiarum Polonorum. Hortorum Cultus 2011, 10, 107–114. [Google Scholar]
- Djonovic, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007, 145, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.M.; Al-Masri, M.I. Effect of Trichoderma harzianum in combination with fungicides in controlling gray mould disease (Botrytis cinerea) of strawberry. Am. J. Plant Sci. 2017, 8, 651–665. [Google Scholar] [CrossRef][Green Version]
- Sallam, N.; Eraky, A.M.I.; Sallam, A. Effect of Trichoderma spp. on Fusarium wilt disease of tomato. Mol. Biol. Rep. 2019, 46, 4463–4470. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Urbano, M.; Goicoechea, N.; Velasco, P.; Poveda, J. Development of agricultural bio-inoculants based on mycorrhizal fungi and endophytic filamentous fungi: Co-inoculants for improve plant-physiological responses in sustainable agriculture. Biol. Control 2023, 182, 105223. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J. Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plants. J. Basic Microbiol. 2014, 54, S115–S124. [Google Scholar] [CrossRef]
- Půža, V.; Tarasco, E. Interactions between entomopathogenic fungi and entomopathogenic nematodes. Microorganisms 2023, 11, 163. [Google Scholar] [CrossRef]
- Intana, W.; Wonglom, P.; Suwannarach, N.; Sunpapao, A. Trichoderma asperelloides PSU-P1 Induced Expression of Pathogenesis-Related Protein Genes against Gummy Stem Blight of Muskmelon (Cucumis melo) in Field Evaluation. J. Fungi 2022, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Gezgin, Y.; Maral Gül, D.; Sözer Şenşatar, S.; Kara, C.U.; Sargın, S.; Sukan, F.V.; Eltem, R. Evaluation of Trichoderma atroviride and Trichoderma citrinoviride growth profiles and their potentials as biocontrol agent and biofertilizer. Turk. J. Biochem. 2020, 45, 163–175. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, H.; Zhang, F.; Guo, N.; Wang, Y.; Chen, L.; Li, C. Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Biochem. 2016, 100, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Javaid, A. In Vitro Biocontrol Potential of Trichoderma pseudokoningii against Macrophomina phaseolina. Int. J. Agric. Biol. 2020, 24, 730–736. [Google Scholar]
- Chen, K.; Zhuang, W.Y. Three new soil-inhabiting species of Trichoderma in the Stromaticum clade with test of their antagonism to pathogens. Curr. Microbiol. 2017, 74, 1049–1060. [Google Scholar] [CrossRef]
- Degani, O.; Rabinovitz, O.; Becher, P.; Gordani, A.; Chen, A. Trichoderma longibrachiatum and Trichoderma asperellum confer growth promotion and protection against late wilt disease in the field. J. Fungi 2021, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ma, Y.; Guo, Q.; Xu, B. Biological weed control using Trichoderma polysporum strain HZ-31. Crop Prot. 2020, 134, 105161. [Google Scholar] [CrossRef]
- Hinterdobler, W.; Li, G.; Spiegel, K.; Basyouni-Khamis, S.; Gorfer, M.; Schmoll, M. Trichoderma reesei Isolated from Austrian Soil with High Potential for Biotechnological Application. Front. Microbiol. 2021, 12, 552301. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.; Inam-ul-Haq, M.; Bibi, S.; Humayun, A.; Ghuffar, S.; Iqbal, S. Novel Potential of Trichoderma spp. as Biocontrol Agent. J. Entomol. Zool. Stud. 2017, 5, 214–222. [Google Scholar]
- Kumar, P.; Misra, A.K.; Modi, D.R.; Gupta, V.K. Biocontrol Potential of Trichoderma Species against Mango Malformation Pathogens. Arch. Phytopathol. Plant Prot. 2012, 45, 1237–1245. [Google Scholar] [CrossRef]
- El-Shennawy, M.Z.; Khalifa, E.Z.; Ammar, M.M.; Mousa, E.M.; Hafez, S.L. Biological Control of the Disease Complex on Potato Caused by Root-Knot Nematode and Fusarium Wilt Fungus. Nematol. Mediterr. 2012, 40, 169–172. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 2: Microbial Interactions and Agro-Ecological Impacts; Springer: Singapore, 2017; pp. 135–191. [Google Scholar]
- Patel, Z.M.; Mahapatra, R.; Jampala, S.S.M. Role of Fungal Elicitors in Plant Defense Mechanism. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 143–158. [Google Scholar]
- Elsharkawy, M.M. Suppression of Potato Virus Y Infection in Tobacco by Plant Growth Promoting Fungi. Egypt. J. Biol. Pest Control 2016, 26, 695–700. [Google Scholar]
- De Meyer, G.; Bigirimana, J.; Elad, Y.; Hofte, M. Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. Eur. J. Plant Pathol. 1998, 104, 279–286. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- El-Hasan, A.; Ngatia, G.; Link, T.I.; Voegele, R.T. Isolation, Identification, and Biocontrol Potential of Root Fungal Endophytes Associated with Solanaceous Plants against Potato Late Blight (Phytophthora infestans). Plants 2022, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Malviya, D.; Thosar, R.; Kokare, N.; Pawar, S.; Singh, U.B.; Saha, S.; Rai, J.P.; Singh, H.V.; Somkuwar, R.G.; Saxena, A.K. A comparative analysis of microbe-based technologies developed at ICAR-NBAIM against Erysiphe necator causing powdery mildew disease in grapes (Vitis vinifera L.). Front. Microbiol. 2022, 13, 871901. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Sánchez, C.P.; Candela, M.E. Evaluation of Induction of Systemic Resistance in Pepper Plants (Capsicum annuum) to Phytophthora capsici using Trichoderma harzianum and its Relation with Capsidiol Accumulation. Eur. J. Plant Pathol. 2000, 106, 817–824. [Google Scholar] [CrossRef]
- Santos, M.; Diánez, F.; Sánchez-Montesinos, B.; Huertas, V.; Moreno-Gavira, A.; Esteban García, B.; Garrido-Cárdenas, J.A.; Gea, F.J. Biocontrol of Diseases Caused by Phytophthora capsici and P. parasitica in Pepper Plants. J. Fungi 2023, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Avilés, M.; Casanova, E.; Borrero, A.; Trillas, I. Effectiveness of biological control of Phytophthora capsici in pepper by Trichoderma asperellum strain T34. Phytopathol. Mediterr. 2013, 52, 77–83. [Google Scholar]
- Adikaram, N.K.; Joyce, D.C.; Terryc, L.A. Biocontrol activity and induced resistance as a possible mode of action for Aureobasidium pullulans against grey mould of strawberry fruit. Australas. Plant Pathol. 2002, 31, 223–229. [Google Scholar] [CrossRef]
- Iqbal, M.; Andreasson, E.; Stenberg, J.A. Biological control of strawberry diseases by Aureobasidium pullulans and sugar beet extract under field conditions. J. Plant Pathol. 2023, 105, 933–941. [Google Scholar] [CrossRef]
- Ippolito, A.; Nigro, F.; Romanazzi, G.; Campanella, V. Field application of Aureobasidium pullulans against Botrytis storage rot of strawberry. In Non Conventional Methods for the Control of Post-Harvest Disease and Microbiological Spoilage; Workshop Proceedings; COST: Brussels, Belgium, 1997; pp. 127–133. [Google Scholar]
- Iqbal, M.; Jamshaid, M.; Zahid, M.A.; Andreasson, E.; Vetukuri, R.R.; Stenberg, J.A. Biological control of strawberry crown rot, root rot and grey mould by the beneficial fungus Aureobasidium pullulans. BioControl 2021, 66, 535–545. [Google Scholar] [CrossRef]
- Arras, G. Mode of action of an isolate of Candida famata in biological control of Penicillium digitatum in orange fruits. Postharvest Biol. Technol. 1996, 8, 191–198. [Google Scholar] [CrossRef]
- Droby, S.; Hofstein, R.; Wilson, C.L.; Wisniewski, M.; Fridlender, B.; Cohen, L.; Weiss, B.; Daus, A.; Timar, D.; Chalutz, E. Pilot Testing of Pichia guilliermondii: A Biocontrol Agent of Postharvest Diseases of Citrus Fruit. Biol. Control 1993, 3, 47–52. [Google Scholar] [CrossRef]
- Lahlali, R.; Hamadi, Y.; El Guilli, M.; Jijakli, M.H. Efficacy assessment of Pichia guilliermondii strain Z1, a new biocontrol agent, against citrus blue mould in Morocco under the influence of temperature and relative humidity. Biol. Control 2011, 56, 217–224. [Google Scholar] [CrossRef]
- Torres, D.E.; Rojas-Martínez, R.I.; Zavaleta-Mejía, E.; Guevara-Fefer, P.; Márquez-Guzmán, G.J.; Pérez-Martínez, C. Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PLoS ONE 2017, 12, 0170782. [Google Scholar] [CrossRef] [PubMed]
- Demirci, F. Effects of Pseudomonas fluorescens and Candida famata on blue mould of citrus caused by Penicillium italicum. Aust. J. Crop Sci. 2011, 5, 341–346. [Google Scholar]
- Bandara, A.Y.; Kang, S. Trichoderma application methods differentially affect the tomato growth, rhizomicrobiome, and rhizosphere soil suppressiveness against Fusarium oxysporum. Front. Microbiol. 2024, 15, 1366690. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Ucko, O.; Chet, I. Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field conditions. Plant Dis. 1987, 71, 587–592. [Google Scholar] [CrossRef]
- Kexiang, G.; Xiaoguang, L.; Yonghong, L.; Tianbo, Z.; Shuliang, W. Potential of Trichoderma harzianum and T. atroviride to control Botryosphaeria berengeriana f. sp. piricola, the cause of apple ring rot. J. Phytopathol. 2002, 150, 271–276. [Google Scholar] [CrossRef]
- Bunker, R.N.; Kusum Mathur, K.M. Antagonism of local biocontrol agents to Rhizoctonia solani inciting dry root rot of chilli. J. Mycol. Plant Pathol. 2001, 31, 50–53. [Google Scholar]
- Trutmann, P.; Keane, P.J. Trichoderma koningii as a biological control agent for Sclerotinia sclerotiorum in Southern Australia. Soil Biol. Biochem. 1990, 22, 43–50. [Google Scholar] [CrossRef]
- Camacho-Luna, V.; Pizar-Quiroz, A.M.; Rodríguez-Hernández, A.A.; Rodríguez-Monroy, M.; Sepúlveda-Jiménez, G. Trichoderma longibrachiatum, a biological control agent of Sclerotium cepivorum on onion plants under salt stress. Biol. Control 2023, 180, 105168. [Google Scholar] [CrossRef]
- El-Komy, M.H.; Al-Qahtani, R.M.; Ibrahim, Y.E.; Almasrahi, A.A.; Al-Saleh, M.A. Soil application of Trichoderma asperellum strains significantly improves Fusarium root and stem rot disease management and promotes growth in cucumbers in semi-arid regions. Eur. J. Plant Pathol. 2022, 162, 637–653. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, R.; Duan, Y.N.; Jiang, W.T.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. The endophytic strain Trichoderma asperellum 6S-2: An efficient biocontrol agent against apple replant disease in China and a potential plant-growth-promoting fungus. J. Fungi 2021, 7, 1050. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Tyagi, R.D.; Prévost, D.; Brar, S.K.; Pouleur, S.; Surampalli, R.Y. Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot. 2010, 29, 1452–1459. [Google Scholar] [CrossRef]
- Zegeye, E.D.; Santhanam, A.; Gorfu, D.; Tessera, M.; Kassa, B. Biocontrol activity of Trichoderma viride and Pseudomonas fluorescens against Phytophthora infestans under greenhouse conditions. J. Agric. Technol. 2011, 7, 1589–1602. [Google Scholar]
- Zaharia, R.; Petrisor, C.; Fatu, V.; Leveanu, I.; Botea, M.C.; Chireceanu, C. Biocontrol potential of Trichoderma viride against main phytopathogenic fungi associated with Capsicum peppers cultivated in IPM system. In Proceedings of the IX South-Eastern Europe Symposium on Vegetables and Potatoes, Bucharest, Romania, 5–9 September 2023; ISHS: Leuven, Belgium, 2023; Volume 1391, pp. 401–406. [Google Scholar]
- Djonovic, S.; Pozo, M.J.; Kenerley, C.M. Tvbgn3, a β-1, 6-glucanase from the biocontrol fungus Trichoderma virens, is involved in mycoparasitism and control of Pythium ultimum. Appl. Environ. Microbiol. 2006, 72, 7661–7670. [Google Scholar] [CrossRef]
- Etebarian, H.R.; Scott, E.S.; Wicks, T.J. Trichoderma harzianum T39 and T. virens DAR 74290 as potential biological control agents for Phytophthora erythroseptica. Eur. J. Plant Pathol. 2000, 106, 329–337. [Google Scholar] [CrossRef]
- Galli, V.; Romboli, Y.; Barbato, D.; Mari, E.; Venturi, M.; Guerrini, S.; Granchi, L. Indigenous Aureobasidium pullulans Strains as Biocontrol Agents of Botrytis cinerea on Grape Berries. Sustainability 2021, 13, 9389. [Google Scholar] [CrossRef]
- Taping, J.M.F.; Borja, B.T.; Bretaña, B.L.P.; Tanabe, M.E.N.; Cabasan, M.T.N. Fungal endophytes as potential biocontrol agent of Panama disease of banana. Egypt. J. Biol. Pest Control 2023, 33, 84. [Google Scholar] [CrossRef]
- Aggarwal, R. Chaetomium globosum: A potential biocontrol agent and its mechanism of action. Indian Phytopathol. 2015, 68, 8–24. [Google Scholar]
- Shanthiyaa, V.; Saravanakumar, D.; Rajendran, L.; Karthikeyan, G.; Prabakar, K.; Raguchander, T. Use of Chaetomium globosum for biocontrol of potato late blight disease. Crop Prot. 2013, 52, 33–38. [Google Scholar] [CrossRef]
- Di Pietro, A.; Gut-Rella, M.; Pachlatko, J.P.; Schwinn, F.J. Role of antibiotics produced by Chaetomium globosum in biocontrol of Pythium ultimum, a causal agent of damping-off. Phytopathology 1992, 82, 131–135. [Google Scholar] [CrossRef]
- Alexander, B.J.R.; Stewart, A. Glasshouse screening for biological control agents of Phytophthora cactorum on apple (Malus domestica). N. Z. J. Crop Hortic. Sci. 2001, 29, 159–169. [Google Scholar] [CrossRef]
- Utkhede, R.S. Biological Control of Apple Crown Rot and Replant Disease. In Biological Control of Plant Diseases; Tjamos, E.C., Papavizas, G.C., Cook, R.J., Eds.; NATO ASI Series; Springer: Boston, MA, USA, 1992; Volume 230, pp. 410–415. [Google Scholar]
- Nakayama, T.; Sayama, M. Suppression of potato powdery scab caused by Spongospora subterranea using an antagonistic fungus Aspergillus versicolor isolated from potato roots [Conference poster]. In Proceedings of the Ninth Symposium of the International Working Group on Plant Viruses with Fungal Vectors, Obihiro, Japan, 19–22 August 2013. [Google Scholar]
- Chin-A-Woeng, T.F.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [PubMed]
- Mouloud, G.; Daoud, H.; Bassem, J.; Laribi Atef, I.; Hani, B. New bacteriocin from Bacillus clausii strainGM17: Purification, characterization, and biological activity. Appl. Biochem. Biotechnol. 2013, 171, 2186–2200. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of action of microbial biocontrol in the phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Savini, V.; Fazii, P. Bacillus thuringiensis insecticide properties. In The Diverse Faces of Bacillus Cereus; Academic Press: Cambridge, MA, USA, 2016; pp. 139–155. [Google Scholar]
- Lobanovska, M.; Pilla, G. Focus: Drug development: Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J. Biol. Med. 2017, 90, 135. [Google Scholar]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Farag, M.A.; Zhang, H.; Ryu, C.M. Dynamic chemical communication between plants and bacteria through airborne signals: Induced resistance by bacterial volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Liu, D.; Wei, Z.; Huang, Q.; Shen, Q. Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol. Res. 2016, 192, 103–113. [Google Scholar] [CrossRef]
- Kamilova, F.; Validov, S.; Azarova, T.; Mulders, I.; Lugtenberg, B. Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ. Microbiol. 2005, 7, 1809–1817. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tan, Y.; Peng, Q.; Xiao, Y.; Xie, J.; Li, Z.; Ding, H.; Pan, H.; Wei, L. Biocontrol potential of endophytic bacterium Bacillus altitudinis GS-16 against tea anthracnose caused by Colletotrichum gloeosporioides. Peer J. 2024, 12, 16761. [Google Scholar] [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Matveev, S.; Rav-David, D.; Shulhani, R.; Elad, Y.; Ment, D.; Bar, M. Bacillus thuringiensis promotes systemic immunity in tomato, controlling pests and pathogens and promoting yield. Food Secur. 2024, 16, 675–690. [Google Scholar] [CrossRef]
- Heo, Y.; Lee, Y.; Balaraju, K.; Jeon, Y. Characterization and evaluation of Bacillus subtilis GYUN-2311 as a biocontrol agent against Colletotrichum spp. on apple and hot pepper in Korea. Front Microbiol. 2024, 14, 1322641. [Google Scholar] [CrossRef]
- Wockenfuss, A.; Chan, K.; Cooper, J.G.; Chaya, T.; Mauriello, M.A.; Yannarell, S.M.; Maresca, J.A.; Donofrio, N.M. A Bacillus velezensis strain shows antimicrobial activity against soilborne and foliar fungi and oomycetes. Front Fung Biol. 2024, 5, 1332755. [Google Scholar] [CrossRef]
- Sumera Yasmin, S.Y.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Muhammad Zubair, M.Z.; Mazhar Iqbal, M.I. Biocontrol of Bacterial Leaf Blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar]
- Xie, J.; Singh, P.; Qi, Y.; Singh, R.K.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. Pseudomonas aeruginosa Strain 91: A Multifaceted Biocontrol Agent against Banana Fusarium Wilt. J. Fungi 2023, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Cai, Z.; Guo, L.; Chen, X.; Chen, X.; Liu, J.; Feng, M.; Qiu, Y.; Zhang, Y.; et al. A biocontrol strain of Pseudomonas aeruginosa CQ-40 promote growth and control Botrytis cinerea in tomato. Pathogens 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Amaradasa, B.S.; Mei, C.; He, Y.; Chretien, R.L.; Doss, M.; Durham, T.; Lowman, S. Biocontrol potential of endophytic Pseudomonas strain IALR1619 against two Pythium species in cucumber and hydroponic lettuce. PLoS ONE 2024, 19, 0298514. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Yi, Y.; Luan, P.; Liu, S.; Shan, Y.; Hou, Z.; Zhao, S.; Jia, S.; Li, R. Efficacy of Bacillus subtilis XZ18-3 as a Biocontrol Agent against Rhizoctonia cerealis on Wheat. Agriculture 2022, 12, 258. [Google Scholar] [CrossRef]
- Myers, J.M.; James, T.Y. Mycoviruses. Curr. Biol. 2022, 32, 150–155. [Google Scholar] [CrossRef]
- Di Giallonardo, F.; Holmes, E.C. Viral biocontrol: Grand experiments in disease emergence and evolution. Trends Microbiol. 2015, 23, 83–90. [Google Scholar] [CrossRef]
- Wagemans, J.; Holtappels, D.; Vainio, E.; Rabiey, M.; Marzachì, C.; Herrero, S.; Turina, M. Going viral: Virus-based biological control agents for plant protection. Ann. Rev. Phytopathol. 2022, 60, 21–42. [Google Scholar] [CrossRef]
- Mallmann, W.; Hemstreet, C. Isolation of an inhibitory substance from plants. Agric. Res. 1924, 28, 599–602. [Google Scholar]
- Nakayinga, R.; Makumi, A.; Tumuhaise, V.; Tinzaara, W. Xanthomonas bacteriophages: A review of their biology and biocontrol applications in agriculture. BMC Microbiol. 2021, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M. Check for Mycoviruses Jillian M. Myers and Timothy Y. James. Evol. Fungi Fungal-Like Org. 2023, 14, 151. [Google Scholar]
- Moscardi, F. Assessment of the application of baculoviruses for control of Lepidoptera. Annu. Rev. Entomol. 1999, 44, 257–289. [Google Scholar] [CrossRef]
- Pechinger, K.; Choo, K.M.; MacDiarmid, R.M.; Harper, S.J.; Ziebell, H. A new era for mild strain crossprotection. Viruses 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Padilla, A.; Rodriguez-Romero, J.; Gomez-Cid, I.; Pacifico, D.; Ayllón, M.A. Novel mycoviruses discovered in the mycovirome of a necrotrophic fungus. mBio 2021, 12, 3705–3720. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hantula, J. Fungal viruses. In Viruses of Microorganisms; Hyman, P., Abedon, S.T., Eds.; Caster Acad.: Norfolk, UK, 2018; pp. 193–209. [Google Scholar]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef]
- Chen, B.; Chen, C.H.; Bowman, B.H.; Nuss, D.L. Phenotypic changes associated with wild-type and mutant hypovirus RNA transfection of plant pathogenic fungi phylogenetically related to Cryphonectria parasitica. Phytopathology 1996, 86, 301–310. [Google Scholar] [CrossRef]
- van Heerden, S.W.; Geletka, L.M.; Preisig, O.; Nuss, D.L.; Wingfield, B.D.; Wingfield, M.J. Characterization of South African Cryphonectria cubensis isolates infected with a C. parasitica hypovirus. Phytopathology 2001, 91, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Onoue, M.; Kanematsu, S.; Suzaki, K.; Miyanishi, M.; Suzuki, N.; Nuss, D.L.; Yoshida, K. Extending chestnut blight hypovirus host range within diaporthales by biolistic delivery of viral cDNA. Mol. Plant. Microbe Interact. 2002, 15, 780–789. [Google Scholar] [CrossRef]
- Kanematsu, S.; Sasaki, A.; Onoue, M.; Oikawa, Y.; Ito, T. Extending the fungal host range of a partitivirus and a mycoreovirus from Rosellinia necatrix by inoculation of protoplasts with virus particles. Phytopathology 2010, 100, 922–930. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, J.; Tang, J.; Fu, Y.; Jiang, D.; Baker, T.S.; Ghabrial, S.A.; Xie, J. A novel partitivirus that confers hypovirulence on plant pathogenic fungi. J. Virol. 2014, 88, 10120–10133. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Yu, J.; Son, M.; Lee, Y.W.; Kim, K.H. Transmission of Fusarium boothii mycovirus via protoplast fusion causes hypovirulence in other phytopathogenic fungi. PLoS ONE 2011, 6, e21629. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Haque, M.A.; Barai, H.R.; Istiaq, A.; Kim, J.J. Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative. Plants 2024, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.T.; Oh, C.S. Bacteriophage usage for bacterial disease management and diagnosis in plants. Plant Pathol. J. 2020, 36, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Balogh, B.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Obradovic, A. Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato. Plant Dis. 2003, 87, 949–954. [Google Scholar] [CrossRef]
- Balogh, B.; Canteros, B.I.; Stall, K.E.; Jones, J.B. Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Dis. 2008, 92, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Meczker, K.; Doemoetoer, D.; Vass, J.; Rakhely, G.; Schneider, G.; Kovacs, T. The genome of the Erwinia amylovora phage PhiEaH1 reveals greater diversity and broadens the applicability of phages for the treatment of fire blight. FEMS Microbiol. Lett. 2014, 350, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- Ahern, S.J.; Das, M.; Bhowmick, T.S.; Young, R.; Gonzalez, C.F. Characterization of novel virulent broad.host.range phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2014, 196, 459–471. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Askora, A.; Kawasaki, T.; Fujie, M.; Yamada, T. The filamentous phage XacF1 causes loss of virulence in Xanthomonas axonopodis pv. citri, the causative agent of citrus canker disease. Front. Microbiol. 2014, 5, 321. [Google Scholar] [CrossRef]
- Álvarez, B.; López, M.M.; Biosca, E.G. Biocontrol of the major plant pathogen Ralstonia solanacearum in irrigation water and host plants by novel waterborne lytic bacteriophages. Front. Microbiol. 2019, 10, 2813. [Google Scholar] [CrossRef]
- Doemoetoer, D.; Becsagh, P.; Rakhely, G.; Schneider, G.; Kovacs, T. Complete genomic sequence of Erwinia amylovora phage PhiEaH2. J. Virol. 2012, 86, 10899. [Google Scholar] [CrossRef] [PubMed]
- Mckinney, H.H. Mosaic diseases in the Canary Islands, West Africa, and Gibraltar. J. Agric. Res. 1929, 39, 557–578. [Google Scholar]
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Gal On, A.; Shiboleth, Y.M. Cross protection. In Natural Resistance Mechanisms of Plants to Viruses; Loebenstein, G., Carr, J.P., Eds.; Springer: Berlin, Germany, 2006; pp. 261–288. [Google Scholar]
- Tomitaka, Y.; Shimomoto, Y.; Ryang, B.S.; Hayashi, K.; Oki, T.; Matsuyama, M.; Sekine, K.T. Development and Application of Attenuated Plant Viruses as Biological Control Agents in Japan. Viruses 2024, 16, 517. [Google Scholar] [CrossRef]
- Salaman, R.N. Protective inoculation against a plant virus. Nature 1933, 131, 468. [Google Scholar] [CrossRef]
- Holmes, F.O. A masked strain of tobacco mosaic virus. Phytopathology 1934, 24, 845–873. [Google Scholar]
- Grant, T.J.; Costa, A.S. A mild strain of tristeza virus of citurs. Phytopathology 1951, 41, 114–122. [Google Scholar]
- Posnette, A.F.; Todd, J.M. Viruse diseases of Cacao in West Africa IX. Strain variation and interference in virus 1A. Ann. Appl. Biol. 1955, 43, 433–453. [Google Scholar] [CrossRef]
- Aguero, J.; Gomez-Aix, C.; Sempere, R.N.; Garcia-Villalba, J.; Garcia-Nunez, J. Stable and broad spectrum cross-protection against Pepino mosaic virus attained by mixed infection. Front. Plant Sci. 2018, 9, 1810. [Google Scholar] [CrossRef]
- Yeh, S.D.; Gonsalves, D.; Wang, H.L.; Namba, R.; Chiu, R.J. Control of papaya ringspot virus by cross protection. Plant Dis. 1988, 72, 375–380. [Google Scholar] [CrossRef]
- Folimonova, S.Y. Developing an understanding of cross-protection by Citrus tristeza virus. Front. Microbiol. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Breytenbach, J.H.; Steyn, C.; de Bruyn, R.; van Vuuren, S.P.; Burger, J.T.; Maree, H.J. Grapefruit field trial evaluation of Citrus tristeza virus T68-strain sources. Plant Dis. 2021, 105, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R.; Rhodes, D.J.; Larkin, R.P. Production and commercialization of biocontrol products. In Integrated Pest and Disease Management in Greenhouse Crops; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 365–376. [Google Scholar]
- Nunes, C. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Whipps, J.M. Developments in the biological control for soilborne plant pathogens. Adv. Bot. Res. 1997, 26, 1–134. [Google Scholar]
- Larena, I.; De Cal, A.; Melgarejo, P. Solid substrate production of Epicoccum nigrum conidia for biological control of brown rot on stone fruits. Int. J. Food Microbiol. 2004, 94, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.D.; Rangeshwaran, R.; Anuroop, C.P.; Phanikumar, P.R. Bioefficacy and shelf life of conidial and chlamydospore formulations of Trichoderma harzianum Rifai. J. Biol. Control 2002, 16, 145–148. [Google Scholar]
- Jeyarajan, R. Prospects of indigenous mass production and formulation of Trichoderma. In Current Status of Biological Control of Plant Diseases Using Antagonistic Organisms in India, Proceedings of the Group Meeting on Antagonistic Organisms in Plant Disease Management Held at Project Directorate of Biological Control, Bangalore, India, 10–11 July 2003; Project Directorate of Biological Control, Indian Council of Agricultural Research: Bangalore, India, 2006; pp. 10–11. [Google Scholar]
- Angeli, D.; Saharan, K.; Segarra, G.; Sicher, C.; Pertot, I. Production of Ampelomyces quisqualis conidia in submerged fermentation and improvements in the formualtion for increases shelf-lefe. Crop Prot. 2017, 97, 135–144. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Usall, J.; Ballesta, J.; Teixidó, N. Biocontrol potential of Ampelomyces quisqualis strain CPA-9 against powdery mildew: Conidia production in liquid medium and efficacy on zucchini leaves. Sci. Hortic. 2020, 267, 109337. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Usall, J.; Torres, R.; Abadias, M.; Teixidó, N. Formulation of the biocontrol agent Bacillus amyloliquefaciens CPA-8 using different approaches: Liquid, freeze-drying and fluid-bed spray-drying. BioControl 2017, 62, 545–555. [Google Scholar] [CrossRef]
- Keswani, C.; Bisen, K.; Singh, V.; Sarma, B.K.; Singh, H.B. Formulation technology of biocontrol agents: Present status and future prospects. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 35–52. [Google Scholar]
- Fravel, D.R.; Connick, W.J.; Lewis, J.A. Formulation of microorganisms to control plant diseases. In Formulation of Microbial Biopesticides; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 187–202. [Google Scholar]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Usall, J.; Torres, R.; Teixidó, N. Biological control of postharvest diseases on fruit: A suitable alternative. Curr. Opin. Food Sci. 2016, 11, 51–55. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of microbial biocontrol agents for a sustainable agriculture. In How Research Can Stimulate the Development of Commercial Biological Control against Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2020; pp. 275–293. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.G.; Valéro, J.R. Recent advances in dowstream processing and formulations of Bacillus thuringiensis based biopestices. Process Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Batta, Y.A. Postharvest biological control of apple gray mold by Trichoderma harzianum Rifai formulated in an invert emulsion. Crop Prot. 2004, 23, 19–26. [Google Scholar] [CrossRef]
- Nandhini, M.; Harish, S.; Aiyanathan, K.E.A.; Durgadevi, D.; Beaulah, A. Glycerol-based liquid formulation of the epiphytic yeast Hanseniaspora guilliermondii isolate YBB3 with multiple modes of action controls postharvest Aspergillus rot in grapes. J. Plant Pathol. 2021, 103, 1253–1264. [Google Scholar] [CrossRef]
- Abadias, M.; Teixidó, N.; Usall, J.; Solsona, C.; Viñas, I. Survival of the postharvest biocontrol yeast Candida sake CPA-1 after dehydration by spray-drying. Biocontrol Sci. Technol. 2005, 15, 835–846. [Google Scholar] [CrossRef]
- Adams, G. The principles of Freeze-Drying. In Cryopreservation and Freeze-Drying Protocols, 2nd ed.; Day, J.G., Stacey, G.N., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 15–38. [Google Scholar]
- Prakash, O.; Nimonkar, Y.; Shouche, Y.S. Practice and prospects of microbial preservation. FEMS Microbiol. Lett. 2013, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Machado, B.A.S.; Martin, A.R.; Bagnara, F.; Ragadalli, S.A.; Alves, A.R.C. Drying by spray drying in the food industry:Micro-encapsulation, process parameters and main carriers used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Cañamás, T.; Teixidó, N. Endospore production allows using spray-drying as a possible formulation system of the biocontrol agent Bacillus subtilis CPA-8. Biotechnol. Lett. 2012, 34, 729–735. [Google Scholar] [CrossRef]
- Strasser, S. Innovative Product Formulations Applying the Fluidised Bed Technology. Ph.D. Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, 2008. [Google Scholar]
- Larena, I.; De Cal, A.; Linan, M.; Melgarejo, P. Drying of Epicoccum nigrum conidia for obtaining a shelf-stable biological product against brown rot disease. J. Appl. Microbiol. 2003, 94, 508–514. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- González, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, R.D.; Walter, J.F. Development of the biocontrol fungus Gliocladium virens: Risk assessment and approval for horticultural use. In Biological Control: Benefits and Risks; Hokkanen, H.M.T., Lynch, J.M., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 263–269. [Google Scholar]
- Kruitwagen, A.; Beukeboom, L.W.; Wertheim, B. Optimization of native biocontrol agents, with parasitoids of the invasive pest Drosophila suzukii as an example. Evol. Appl. 2018, 11, 1473–1497. [Google Scholar] [CrossRef]
- Bielza, P.; Balanza, V.; Cifuentes, D.; Mendoza, J.E. Challenges facing arthropod biological control: Identifying traits for genetic improvement of predators in protected crops. Pest Manag. Sci. 2020, 76, 3517–3526. [Google Scholar] [CrossRef]
- Leung, K.; Ras, E.; Ferguson, K.B.; Ariëns, S.; Babendreier, D.; Bijma, P.; Bourtzis, K.; Brodeur, J.; Bruins, M.A.; Centurión, A.; et al. Next-generation biological control: The need for integrating genetics and genomics. Biol. Rev. 2020, 95, 1838–1854. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic Gene Clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, T.; Botelho, A. Metagenomics and Other Omics Approaches to Bacterial Communities and Antimicrobial Resistance Assessment in Aquacultures. Antibiotics 2021, 10, 787. [Google Scholar] [CrossRef]
- Lahlali, R.; Ibrahim, D.S.S.; Belabess, Z.; Roni, M.Z.K.; Radouane, N.; Vicente, C.S.L.; Menendez, E.; Mokrini, F.; Barka, E.A.; de Melo e Mota, M.G.; et al. High-Throughput Molecular Technologies for Unraveling the Mystery of Soil Microbial Community: Challenges and Future Prospects. Heliyon 2021, 7, e08142. [Google Scholar] [CrossRef] [PubMed]
- Barahona, E.; Navazo, A.; Martínez-Granero, F.; Zea-Bonilla, T.; Pérez-Jiménez, R.M.; Martín, M.; Rivilla, R. Pseudomonas fluorescens F113 mutant with enhanced competitive colonization ability and improved biocontrol activity against fungal root pathogens. Appl. Environ. Microbiol. 2011, 77, 5412–5419. [Google Scholar] [CrossRef]
- Leclère, V.; Béchet, M.; Adam, A.; Guez, J.-S.; Wathelet, B.; Ongena, M.; Thonart, P.; Gancel, F.; Chollet-Imbert, M.; Jacques, P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 2005, 71, 4577–4584. [Google Scholar] [CrossRef]
- Okay, S.; Tefon, B.; Özkan, M.; Özcengiz, G. Expression of chitinase A (chiA) gene from a local isolate of Serratia marcescens in Coleoptera-specific Bacillus thuringiensis. J. Appl. Microbiol. 2008, 104, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.; Kempf, H.J.; van Pée, K.H. Natural product with antimicrobial activity from Pseudomonas biocontrol bacteria. In Pesticide Chemistry and Bioscience—The Food-Environment Challenge; Brooks, G.T., Roberts, T.R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1999; Volume 22, pp. 179–189. [Google Scholar]
- Kota, M.; Daniell, H.; Varma, S.; Garczynski, S.F.; Gould, F.; Moar, W.J. Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc. Natl. Acad. Sci. USA 1999, 96, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Schneider, K.; Vater, J.; Süssmuth, R.; Piel, J.; Borriss, R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009, 140, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Clermont, N.; Lerat, S.; Beaulieu, C. Genome shuffling enhances biocontrol abilities of Streptomyces strains against two potato pathogens. J. Appl. Microbiol. 2011, 111, 671–682. [Google Scholar] [CrossRef]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J. Basic Microbiol. 2014, 54, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Côrtes, M.V.; de Sousa Oliveira, M.I.; Mateus, J.R.; Seldin, L.; Silva-Lobo, V.L.; Freire, D.M. A pipeline for the genetic improvement of a biological control agent enhances its potential for controlling soil-borne plant pathogens. Biol. Control 2021, 152, 104460. [Google Scholar] [CrossRef]
- Downing, K.J.; Thomson, J.A. Introduction of the Serratia marcescens chiA gene into an endophytic Pseudomonas fluorescens for the biocontrol of phytopathogenic fungi. Can. J. Microbiol. 2000, 46, 363–369. [Google Scholar] [CrossRef]
- Baek, J.M.; Howell, C.R.; Kenerley, C.M. The role of an extracellular chitinase from Trichoderma virens Gv29-8 in the biocontrol of Rhizoctonia solani. Curr. Genet. 1999, 35, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.B.; Sun, M.H.; Zhou, M.; Li, S.D. Transformation of the endochitinase gene Chi67-1 in Clonostachys rosea 67-1 increases its biocontrol activity against Sclerotinia sclerotiorum. AMB Express 2017, 7, 1. [Google Scholar] [CrossRef]
- Bakker, P.A.; Glandorf, D.; Viebahn, M.; Ouwens, T.W.; Smit, E.; Leeflang, P.; van Loon, L.C. Effects of Pseudomonas putida modified to produce phenazine-1-carboxylic acid and 2, 4-diacetylphloroglucinol on the microflora of field grown wheat. Antonie Leeuwenhoek 2002, 81, 617–624. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Pozo, M.J.; Grzegorski, D.; Martínez, P.; García, J.M.; Olmedo-Monfil, V.; Cortés, C.; Kenerley, C.; Herrera-Estrella, A. Enhanced biocontrol activity of Trichoderma through inactivation of a mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 15965–15970. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Garneau, J.E.; Moineau, S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu. Rev. Microbiol. 2010, 64, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Ma, L.; Gong, M.; Wu, Y.; Bao, D.; Zou, G. Use of CRISPR-Cas tools to engineer Trichoderma species. Microb. Biotechnol. 2022, 10, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, X.; Zhou, H.; Sun, N.; Wang, T.; Bi, C.; Zhang, X. CRISPR-based metabolic pathway engineering. Metab. Eng. 2021, 63, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Mine, K.; Akiyama, K.; Ohki, S.; Hayashi, H. Anti-plant viral activity of peptaibols, trichorzins HA II, HA V, and HA VI, isolated from Trichoderma harzianum HK-61. J. Pestic. Sci. 2018, 43, 283–286. [Google Scholar] [CrossRef]
- van Bohemen, A.I.; Ruiz, N.; Zalouk-Vergnoux, A.; Michaud, A.; Robiou du Pont, T.; Druzhinina, I.; Atanasova, L.; Prado, S.; Bodo, B.; Pouchus, Y.F.; et al. Pentadecaibins I-V: 15-residue peptaibols produced by a marine-derived Trichoderma sp. of the harzianum clade. J. Nat. Prod. 2021, 84, 1271–1282. [Google Scholar] [CrossRef]


| Fungal Species | Mode of Action | Disease/Host | Reference |
|---|---|---|---|
| Ampelomyces quisqualis | Hyperparasitism | Effective against powdery mildew in grapes | [137] |
| Trichoderma harzianum | Induction of systemic resistance in pepper plants along with capsidol accumulation | Effective against blight and fruit rot of peppers (Phytophthora capsica) | [138,139,140] |
| Aureobasidium pullulans | Antagonism | Effective against gray mold of strawberry | [141,142,143,144] |
| Pichia guilliermondii | Antagonism | Manage blue mold (Penicillium italicum) on citrus fruits | [145,146,147] |
| Cladosporium cladosporioides Cladosporium pseudocladosporioides | Antagonism | Manage white rust disease in chrysanthemum | [148] |
| Candida famata | Antagonism | Manage Penicillium digitatum in orange fruits | [145,149] |
| T. harzianum | Antagonism | Fusarium crown and root rot (FCRR) of tomato | [150] |
| T. atroviride T95 | Mycoparasitism | Botryosphaeria berengeriana f. sp. piricola | [151] |
| T. harzianum (Th.J.89-2), T. viride (TV.J.92-1 and ITCC-1433), T. auxeoviride | Antagonism | Dry root rot chilli (R. solani) | [152] |
| T. koningii | Mycoparasitism | Sclerotinia sclerotiorum | [153] |
| Trichoderma longibrachiatum | Antagonism | Sclerotium cepivorum | [154] |
| Trichoderma asperellum | Antagonism | Fusarium root and stem rot disease Apple replant disease | [155,156] |
| T. viride | Mycoparasitism | F. oxysporum f. sp. adzuki and Pythium arrhenomanes | [157] |
| T. viride | Antagonism | Phytophthora infestans | [158] |
| T. viride | Antagonism | Alternaria alternata, Alternaria solani, Colletotrichum spp., Fusarium solani, Fusarium equiseti, and F. oxysporum | [159] |
| T. virens | Mycoparasitism | Pythium ultimum | [160] |
| T. virens | Mycoparasitism | Phytophthora erythroseptica | [161] |
| Aureobasidium pullulan | Cell wall-degrading enzymes, synthesis of antifungal compounds, and mycoparasitism | Potential to control Penicillium expansum, B. cinerea, Aspergillus niger, and Rhizopus stolonifer in grapes | [162,163] |
| C. gloeosporioides and R. vinctus | Panama wilt of banana | [164] | |
| Chaetomium globosum | Mycofungicide compound production | Seed rot and damping-off of several seed- and soilborne plant pathogens like P. infestans (late blight of potato), Pythium ultimum (Pythium damping-off of sugar beet), Alternaria raphani, and A. brassicola | [165,166,167] |
| Paecilomyces | Antagonism | Apple rot, apple collar rot, and crown rot (Phytophthora cactorum) | [168,169] |
| Aspergillius versicolor lm6–50 | Antagonism | Powdery scab in potato (Spongospora subterranea) | [170] |
| S. No. | Bacterial Strain | Pathogen against | Disease | Crop | Dose/CFU (g−1/mL−1) | Mode of Action | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Bacillus altitudinis GS-16 | Colletotrichum gloeosporioides | Anthracnose disease | Tea | 1 × 108 cfu/mL. | Damage cell permeability and integrity | [183] |
| 2. | Bacillus thuringiensis | B. cinerea | Grey mold | Bean, potato, and rapeseed | 106 cfu g−1 | Inhibition mycelium growth | [184] |
| 3. | Bacillus subtilis GYUN-2311 | Colletotrichum spp. | Anthracnose diseases | Apple and hot pepper | 108 cfu/mL | Inhibition mycelium growth | [185] |
| 4. | Bacillus velezensis S4 | Magnaporthe oryzae | Rice blight | Rice | 2 × 107 cfu·mL−1 | Inhibits fungal and oomycete hyphae growth and alters appressoria | [186] |
| 5. | Pseudomonas aeruginosa 91 | Fusarium oxysporum f. sp. cubense | Banana Fusarium wilt | Banana | ~107 | Interacts directly with host cells via flagella, pili and lipoproteins | [187] |
| 6. | Pseudomonas aeruginosa BRp3 | Xanthomonas oryzae pv. oryzae | Bacterial leaf blight | Rice | 109 cfu mL−1 | Induction of defense related enzymes, production of 4-hydroxy-2-alkylquinolines (HAQs) along with siderophores | [188] |
| 7. | Pseudomonas aeruginosa CQ-40 | Botrytis cinerea | Gray mold | Tomato | 2.5 × 108 cfu mL−1 | Type III secretion system | [189] |
| 8. | Pseudomonas strain IALR1619 | Pythium ultimum | Damping-off | Lettuce and Cucumber | 7.47 × 108 cfu | Inhibit mycelial growth | [190] |
| 9. | Bacillus velezensis | Ralstonia solanacearum | Bacterial wilt | Tomato | 1 × 108 cfu/mL | Downregulation of genes associated with spore germination and growth | [191] |
| 10. | Bacillus subtilis XZ18-3 | Rhizoctonia cerealis | Foliar blight | Wheat | 3.7 ± 0.2 × 1011 cfu/g | Inhibit Rhizoctonia cerealis mycelial growth and induce mycelial swelling and rupture. | [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyagi, A.; Lama Tamang, T.; Kashtoh, H.; Mir, R.A.; Mir, Z.A.; Manzoor, S.; Manzar, N.; Gani, G.; Vishwakarma, S.K.; Almalki, M.A.; et al. A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives. Horticulturae 2024, 10, 805. https://doi.org/10.3390/horticulturae10080805
Tyagi A, Lama Tamang T, Kashtoh H, Mir RA, Mir ZA, Manzoor S, Manzar N, Gani G, Vishwakarma SK, Almalki MA, et al. A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives. Horticulturae. 2024; 10(8):805. https://doi.org/10.3390/horticulturae10080805
Chicago/Turabian StyleTyagi, Anshika, Tensangmu Lama Tamang, Hamdy Kashtoh, Rakeeb Ahmad Mir, Zahoor Ahmad Mir, Subaya Manzoor, Nazia Manzar, Gousia Gani, Shailesh Kumar Vishwakarma, Mohammed A. Almalki, and et al. 2024. "A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives" Horticulturae 10, no. 8: 805. https://doi.org/10.3390/horticulturae10080805
APA StyleTyagi, A., Lama Tamang, T., Kashtoh, H., Mir, R. A., Mir, Z. A., Manzoor, S., Manzar, N., Gani, G., Vishwakarma, S. K., Almalki, M. A., & Ali, S. (2024). A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives. Horticulturae, 10(8), 805. https://doi.org/10.3390/horticulturae10080805










