Genome-Wide Analysis of the TIFY Gene Family in Three Cymbidium Species and Its Response to Heat Stress in Cymbidium goeringii
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Experimental Materials
2.3. Identification and Physicochemical Properties of the TIFY Proteins
2.4. Phylogenetic Analysis of TIFY Genes
2.5. Protein Conservative Domain and Gene Structure Analysis
2.6. Collinearity and Location Analysis on Chromosome
2.7. Cis-Acting Regulatory Element Analysis of TIFY Genes
2.8. GO Analysis
2.9. RT-qPCR Analysis
3. Results
3.1. Identification and Physicochemical Properties of the TIFYs
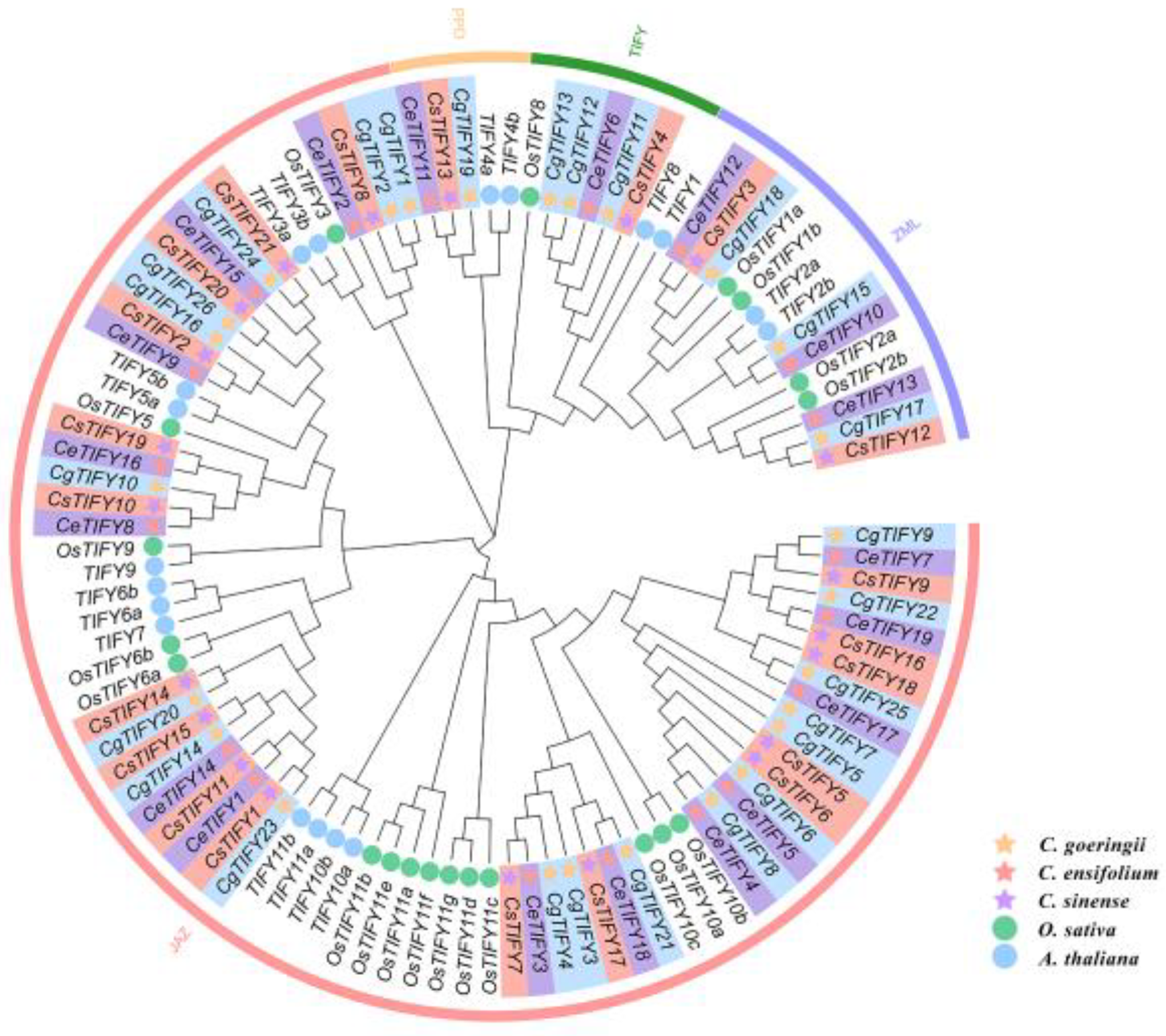
3.2. Phylogenetic Analysis of TIFY Genes
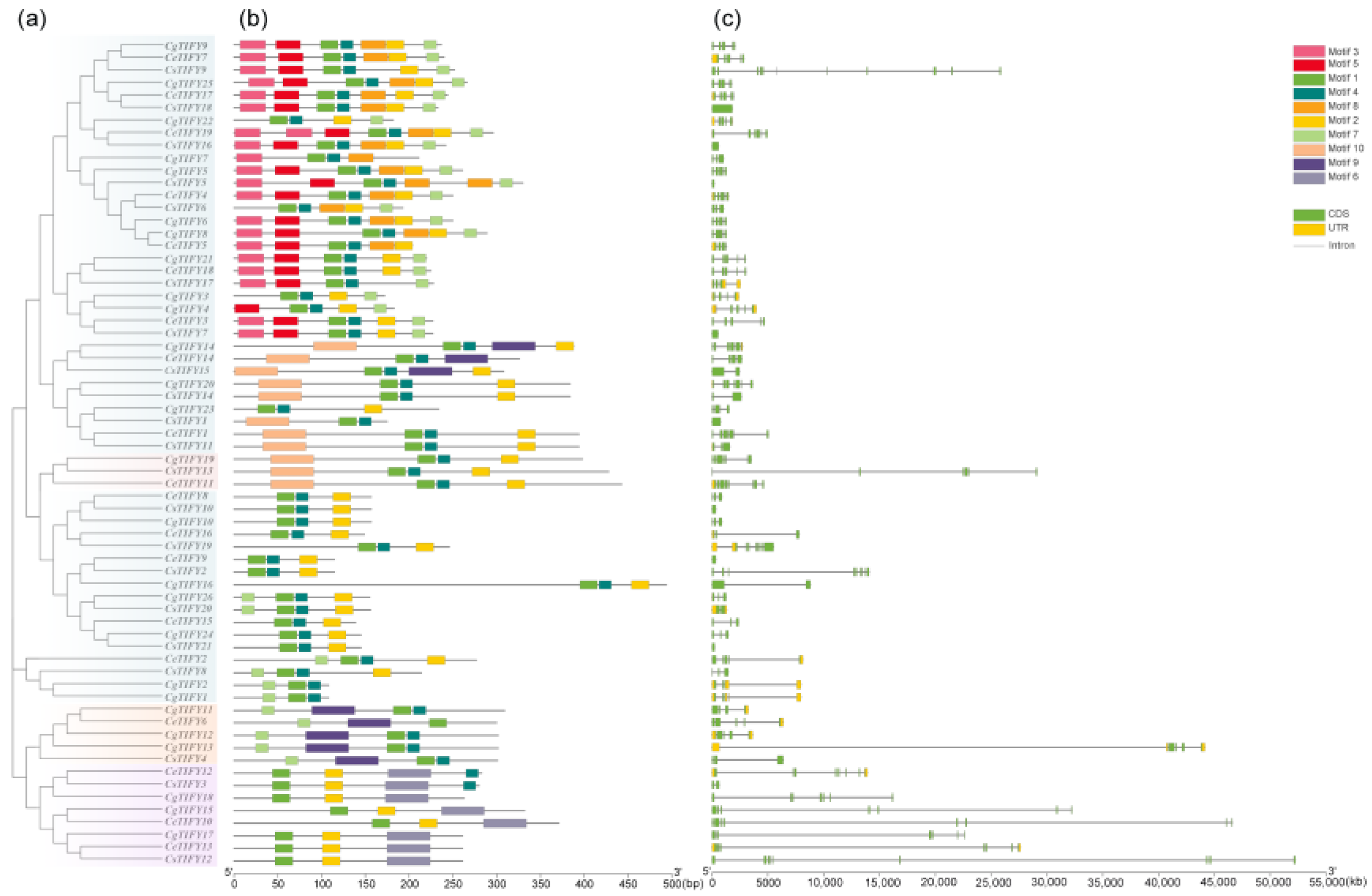
3.3. Gene Structure and Motif Analysis of TIFY Genes
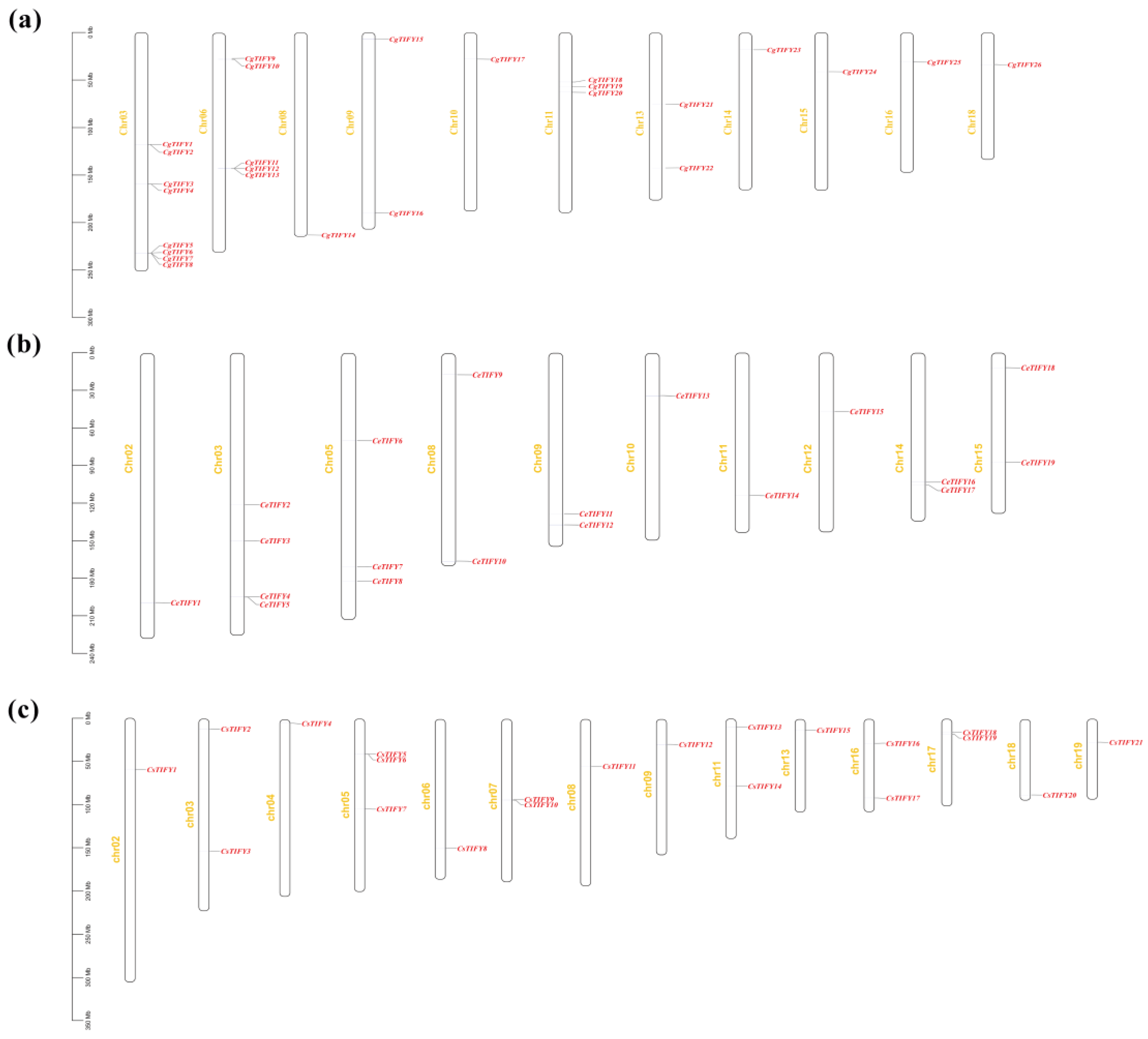
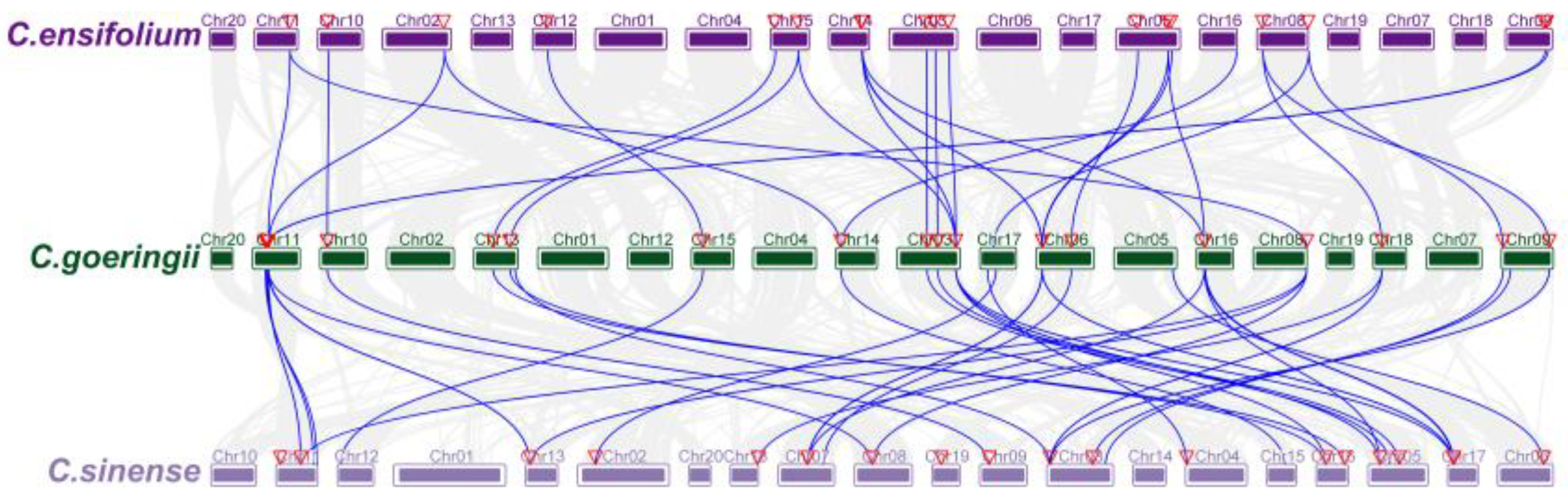
3.4. Collinearity and Location Analysis on Chromosome
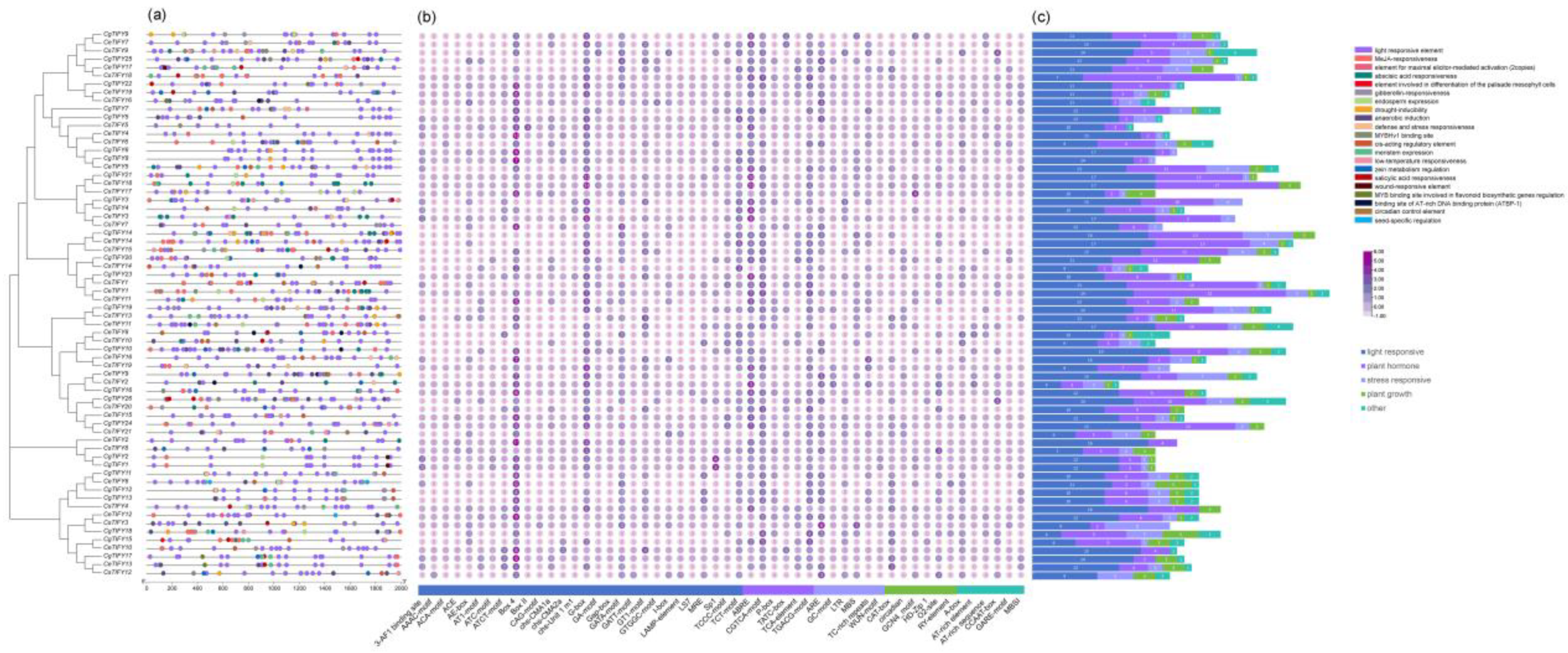
3.5. Cis-Element Analysis
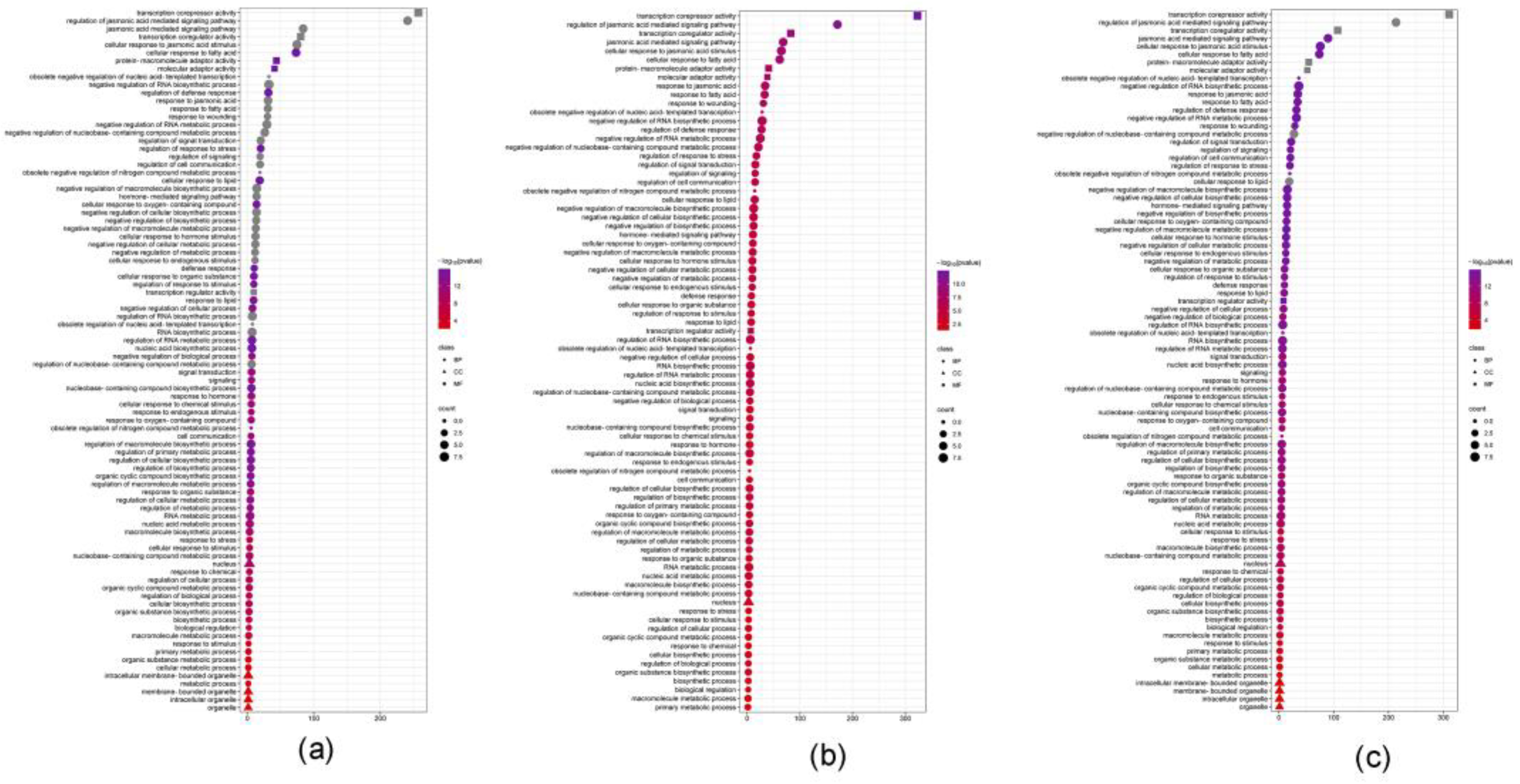
3.6. GO Analysis
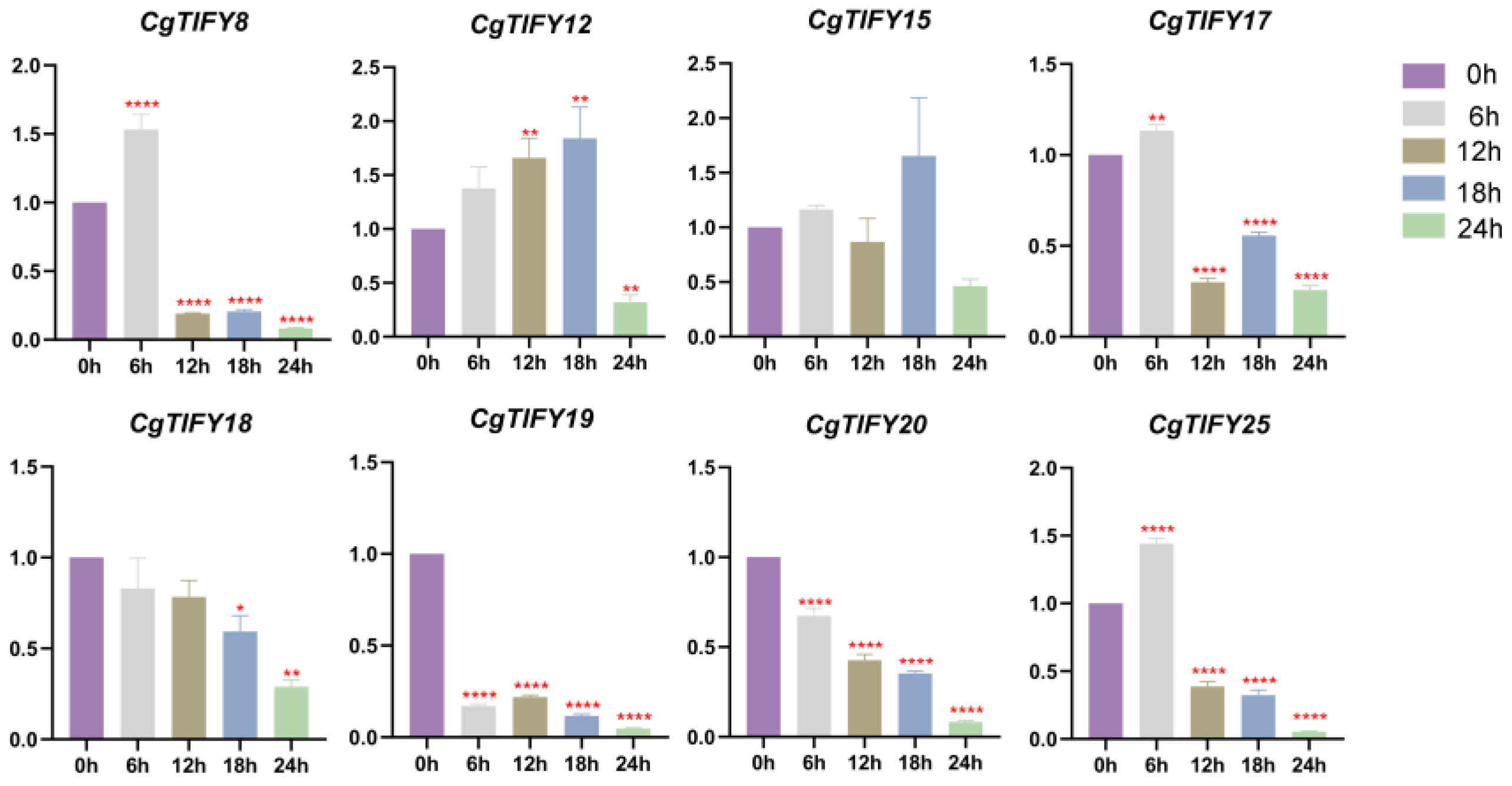
3.7. qRT-PCR Analysis of TIFYs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vanholme, B.; Grunewald, W.; Bateman, A.; Kohchi, T.; Gheysen, G. The Tify Family Previously Known as ZIM. Trends Plant Sci. 2007, 12, 239–244. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, Y.; Huang, D.; Qi, Y.; Chen, M. Origin and Evolutionary Analysis of the Plant-Specific TIFY Transcription Factor Family. Genomics 2011, 98, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Niu, Y.; Browse, J.; Howe, G.A. Top Hits in Contemporary JAZ: An Update on Jasmonate Signaling. Phytochemistry 2009, 70, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E. JAZing up Jasmonate Signaling. Trends Plant Sci. 2008, 13, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, P.; Browse, J. Male Sterility in A Rabidopsis Induced by Overexpression of a MYC 5-SRDX Chimeric Repressor. Plant J. 2015, 81, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J.; Chen, J.; Yan, J.; Chu, J.; Chen, K.; Sun, J. JAZ Proteins Modulate Seed Germination through Interaction with ABI 5 in Bread Wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Han, J.-A.; Lee, J.; Maeng, J.; Hur, Y. Gene Encoding PnFL-2 with TIFY and CCT Motifs May Control Floral Induction in Pharbitis Nil. Genes Genom. 2011, 33, 229–236. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Huang, H.; Ren, Q.; Wu, D.; Chang, C.; Peng, W.; Liu, Y.; Peng, J.; Xie, D. The Jasmonate-ZIM Domain Proteins Interact with the R2R3-MYB Transcription Factors MYB21 and MYB24 to Affect Jasmonate-Regulated Stamen Development in Arabidopsis. Plant Cell 2011, 23, 1000–1013. [Google Scholar] [CrossRef]
- Cai, Q.; Yuan, Z.; Chen, M.; Yin, C.; Luo, Z.; Zhao, X.; Liang, W.; Hu, J.; Zhang, D. Jasmonic Acid Regulates Spikelet Development in Rice. Nat. Commun. 2014, 5, 3476. [Google Scholar] [CrossRef]
- Tian, J.; Cao, L.; Chen, X.; Chen, M.; Zhang, P.; Cao, L.; Persson, S.; Zhang, D.; Yuan, Z. The OsJAZ1 Degron Modulates Jasmonate Signaling Sensitivity during Rice Development. Development 2019, 146, dev173419. [Google Scholar] [CrossRef]
- Zhang, X.; Ran, W.; Zhang, J.; Ye, M.; Lin, S.; Li, X.; Sultana, R.; Sun, X. Genome-Wide Identification of the Tify Gene Family and Their Expression Profiles in Response to Biotic and Abiotic Stresses in Tea Plants (Camellia sinensis). Int. J. Mol. Sci. 2020, 21, 8316. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, C.; Yang, L.; Zhuang, M.; Zhang, Y.; Wang, Y.; Fang, Z.; Lv, H. A Time-Resolved Dual Transcriptome Analysis Reveals the Molecular Regulating Network Underlying the Compatible/Incompatible Interactions between Cabbage (Brassica oleracea) and Fusarium oxysporum f. Sp. Conglutinans. Plant Soil 2020, 448, 455–478. [Google Scholar] [CrossRef]
- Shikata, M. Characterization of Arabidopsis ZIM, a Member of a Novel Plant-Specific GATA Factor Gene Family. J. Exp. Bot. 2004, 55, 631–639. [Google Scholar] [CrossRef] [PubMed]
- White, D.W.R. PEAPOD Regulates Lamina Size and Curvature in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 13238–13243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, G.; Yang, S.; Yu, D. Arabidopsis WRKY57 Functions as a Node of Convergence for Jasmonic Acid- and Auxin-Mediated Signaling in Jasmonic Acid–Induced Leaf Senescence. Plant Cell 2014, 26, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, T.; Geng, S.; Scott, P.B.; Li, H.; Chen, S. Comparative Proteomics and Metabolomics of JAZ7-Mediated Drought Tolerance in Arabidopsis. J. Proteom. 2019, 196, 81–91. [Google Scholar] [CrossRef]
- Ebel, C.; BenFeki, A.; Hanin, M.; Solano, R.; Chini, A. Characterization of Wheat (Triticum aestivum) TIFY Family and Role of Triticum Durum TdTIFY11a in Salt Stress Tolerance. PLoS ONE 2018, 13, e0200566. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ahammed, G.J.; Wan, C.; Liu, H.; Chen, R.; Zhou, Y. Comprehensive Analysis of TIFY Transcription Factors and Their Expression Profiles under Jasmonic Acid and Abiotic Stresses in Watermelon. Int. J. Genom. 2019, 2019, 6813086. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.-W.; Li, Y.-Y.; Ke, S.-J.; Yin, W.-L.; Lan, S.; Liu, Z.-J. Advances and Prospects of Orchid Research and Industrialization. Hortic. Res. 2022, 9, uhac220. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Sun, S.; Li, Y.; Jia, L.; Ye, S.; Yu, Y.; Dossa, K.; Luan, Y. Transcriptome Analysis Reveals the Key Pathways and Candidate Genes Involved in Salt Stress Responses in Cymbidium ensifolium Leaves. BMC Plant Biol 2023, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-L.; Jin, J.-P.; Liang, D.; Gao, J.; Li, J.; Xie, Q.; Lu, C.-Q.; Yang, F.-X.; Zhu, G.-F. Genome-Wide Identification of Cymbidium sinense WRKY Gene Family and the Importance of Its Group III Members in Response to Abiotic Stress. Front. Plant Sci. 2022, 13, 969010. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Jing, X.; Chen, Y.; Hu, F. Functional Analysis of CgWRKY57 from Cymbidium goeringii in ABA Response. PeerJ 2021, 9, e10982. [Google Scholar] [CrossRef]
- Lai, H.; Wang, M.; Yan, L.; Feng, C.; Tian, Y.; Tian, X.; Peng, D.; Lan, S.; Zhang, Y.; Ai, Y. Genome-Wide Identification of bZIP Transcription Factors in Cymbidium ensifolium and Analysis of Their Expression under Low-Temperature Stress. Plants 2024, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Chen, J.; Peng, Y.; Zhu, X.; Niu, M.; Chen, X.; Xie, K.; Huang, R.; Zhan, S.; Su, Q.; et al. General Analysis of Heat Shock Factors in the Cymbidium ensifolium Genome Provided Insights into Their Evolution and Special Roles with Response to Temperature. Int. J. Mol. Sci. 2024, 25, 1002. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as Designed by Its Users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.-H.; Hu, S. Evolview v2: An Online Visualization and Management Tool for Customized and Annotated Phylogenetic Trees. Nucleic Acids Res 2016, 44, W236–W241. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Q.; Zhang, M.-M.; He, X.; Zhao, X.; Wang, L.; Lan, S.; Liu, Z.-J. Genome-Wide Identification and Expression Analysis of the GRAS Gene Family and Their Responses to Heat Stress in Cymbidium goeringii. Int. J. Mol. Sci. 2024, 25, 6363. [Google Scholar] [CrossRef]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van Den Berg, C.; Schuiteman, A. An Updated Classification of Orchidaceae: Updated Classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Demianski, A.J.; Chung, K.M.; Kunkel, B.N. Analysis of Arabidopsis JAZ Gene Expression during Pseudomonas syringae Pathogenesis. Mol. Plant Pathol. 2012, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, X.; Wang, P.; Sun, Y.; Yue, C.; Ye, N. Genome-Wide and Expression Pattern Analysis of JAZ Family Involved in Stress Responses and Postharvest Processing Treatments in Camellia Sinensis. Sci. Rep. 2020, 10, 2792. [Google Scholar] [CrossRef]
- Tao, J.; Jia, H.; Wu, M.; Zhong, W.; Jia, D.; Wang, Z.; Huang, C. Genome-Wide Identification and Characterization of the TIFY Gene Family in Kiwifruit. BMC Genom. 2022, 23, 179. [Google Scholar] [CrossRef]
- Ye, H.; Du, H.; Tang, N.; Li, X.; Xiong, L. Identification and Expression Profiling Analysis of TIFY Family Genes Involved in Stress and Phytohormone Responses in Rice. Plant Mol. Biol. 2009, 71, 291–305. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Wang, H.; Li, J.; Guo, C.; Gao, H.; Zheng, Y.; Fan, C.; Wang, X. Genome-Wide Identification and Analysis of the Apple (Malus × Domestica Borkh.) TIFY Gene Family. Tree Genet. Genomes 2015, 11, 808. [Google Scholar] [CrossRef]
- Wang, H.; Leng, X.; Xu, X.; Li, C. Comprehensive Analysis of the TIFY Gene Family and Its Expression Profiles under Phytohormone Treatment and Abiotic Stresses in Roots of Populus trichocarpa. Forests 2020, 11, 315. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Q.; Li, M.; Zhai, J.; Wu, S.; Ahmad, S.; Lan, S.; Peng, D.; Liu, Z.-J. Genome-Wide Identification and Expression Pattern Analysis of TIFY Family Genes Reveal Their Potential Roles in Phalaenopsis Aphrodite Flower Opening. Int. J. Mol. Sci. 2024, 25, 5422. [Google Scholar] [CrossRef]
- Chini, A.; Ben-Romdhane, W.; Hassairi, A.; Aboul-Soud, M.A.M. Identification of TIFY/JAZ Family Genes in Solanum lycopersicum and Their Regulation in Response to Abiotic Stresses. PLoS ONE 2017, 12, e0177381. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xia, X.-C.; Han, L.-H.; Ni, P.; Yan, J.-Q.; Tao, M.; Huang, G.-Q.; Li, X.-B. Genome-Wide Identification and Characterization of JAZ Gene Family in Upland Cotton (Gossypium hirsutum). Sci. Rep. 2017, 7, 2788. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Yu, H.; Pan, H.; Qiu, K.; Xie, Q.; Chen, H.; Fu, S.; Zhang, J.; Zhou, H. Genome-Wide Analysis of the Gene Structure, Expression and Protein Interactions of the Peach (Prunus persica) TIFY Gene Family. Front. Plant Sci. 2022, 13, 792802. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar Pérez, A.; Nagels Durand, A.; Vanden Bossche, R.; De Clercq, R.; Persiau, G.; Van Wees, S.C.M.; Pieterse, C.M.J.; Gevaert, K.; De Jaeger, G.; Goossens, A.; et al. The Non-JAZ TIFY Protein TIFY8 from Arabidopsis Thaliana Is a Transcriptional Repressor. PLoS ONE 2014, 9, e84891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pan, X.; Yu, Y.; Zhu, Y.; Kong, F.; Sun, X.; Wang, F. Overexpression of a TIFY Family Gene, GsJAZ2, Exhibits Enhanced Tolerance to Alkaline Stress in Soybean. Mol. Breed. 2020, 40, 33. [Google Scholar] [CrossRef]
- Lyons, E.; Pedersen, B.; Kane, J.; Alam, M.; Ming, R.; Tang, H.; Wang, X.; Bowers, J.; Paterson, A.; Lisch, D.; et al. Finding and Comparing Syntenic Regions among Arabidopsis and the Outgroups Papaya, Poplar, and Grape: CoGe with Rosids. Plant Physiol. 2008, 148, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Heidari, P.; Faraji, S.; Ahmadizadeh, M.; Ahmar, S.; Mora-Poblete, F. New Insights Into Structure and Function of TIFY Genes in Zea mays and Solanum lycopersicum: A Genome-Wide Comprehensive Analysis. Front. Genet. 2021, 12, 657970. [Google Scholar] [CrossRef]
- Lv, G.; Han, R.; Shi, J.; Chen, K.; Liu, G.; Yu, Q.; Yang, C.; Jiang, J. Genome-Wide Identification of the TIFY Family Reveals JAZ Subfamily Function in Response to Hormone Treatment in Betula platyphylla. BMC Plant Biol. 2023, 23, 143. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, F.; Yang, G.; Liu, X.; Peng, S. Identification of TIFY Gene Family in Walnut and Analysis of Its Expression under Abiotic Stresses. BMC Genom. 2022, 23, 190. [Google Scholar] [CrossRef]
- Chung, H.S.; Howe, G.A. A Critical Role for the TIFY Motif in Repression of Jasmonate Signaling by a Stabilized Splice Variant of the JASMONATE ZIM-Domain Protein JAZ10 in Arabidopsis. Plant Cell 2009, 21, 131–145. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-M.; He, X.; Huang, Y.; Zheng, Q.; Zhao, X.; Wang, L.; Liu, Z.-J.; Lan, S. Genome-Wide Analysis of the TIFY Gene Family in Three Cymbidium Species and Its Response to Heat Stress in Cymbidium goeringii. Horticulturae 2024, 10, 796. https://doi.org/10.3390/horticulturae10080796
Zhang M-M, He X, Huang Y, Zheng Q, Zhao X, Wang L, Liu Z-J, Lan S. Genome-Wide Analysis of the TIFY Gene Family in Three Cymbidium Species and Its Response to Heat Stress in Cymbidium goeringii. Horticulturae. 2024; 10(8):796. https://doi.org/10.3390/horticulturae10080796
Chicago/Turabian StyleZhang, Meng-Meng, Xin He, Ye Huang, Qinyao Zheng, Xuewei Zhao, Linying Wang, Zhong-Jian Liu, and Siren Lan. 2024. "Genome-Wide Analysis of the TIFY Gene Family in Three Cymbidium Species and Its Response to Heat Stress in Cymbidium goeringii" Horticulturae 10, no. 8: 796. https://doi.org/10.3390/horticulturae10080796
APA StyleZhang, M.-M., He, X., Huang, Y., Zheng, Q., Zhao, X., Wang, L., Liu, Z.-J., & Lan, S. (2024). Genome-Wide Analysis of the TIFY Gene Family in Three Cymbidium Species and Its Response to Heat Stress in Cymbidium goeringii. Horticulturae, 10(8), 796. https://doi.org/10.3390/horticulturae10080796






