Abstract
The spotted wing drosophila, Drosophila suzukii (Matsumura), is currently distributed in the main soft-skinned fruits production areas in China and 59 other countries, presenting a significant threat to importing nations. Optimal phytosanitary treatments, including fumigation, irradiation, and cold treatment, have been developed to prevent the international movement of this invasive fly. To determine the most cold-tolerant stage and facilitate the development of the technical schedules requested by the Technical Panel on Phytosanitary Treatment (TPPT), cold treatments of D. suzukii immature stages in ‘Red Globe’ grapes were conducted. Dose–mortality data at 0 °C and 2 °C from repeated trials were subjected to analysis of covariance, linear regression, and probit analysis. Results identified 3 d old pupae as the most cold-tolerant stage, followed by 1 d old pupae, 4 d old larvae, and 6 h old eggs with similar tolerance. The 2 d old larvae were the most sensitive stage. In subsequent confirmatory tests, 3 d old pupae were subjected to cold treatment at 0 °C for 9 and 10 days, and at 2 °C for 10 and 12 days, based on the probit estimation of the probit-9 value. No adult emergence occurred in the confirmatory tests except for one deformed adult from a 2 °C 10 d treatment. Therefore, the recommended treatment schedule requires fruit temperatures below 0.00 °C (or 1.62 °C) for no less than 10 (or 12) continuous days, with treatment efficacy not less than 99.9960% (or 99.9955%) at a 95% confidence level, respectively. These schedules are intended for submission to TPPT for the development of phytosanitary treatment standards.
1. Introduction
The spotted wing drosophila, Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae), is a highly adaptive insect and poses a global threat that affects more than 60 types of berries and soft-skinned stone fruits [1,2]. This fly generates both direct and indirect economic impacts through yield losses, shorter shelf life of infested fruit, and increased production costs [3]. D. suzukii is unique in its ability to lay eggs on healthy, ripening fruits using its hard ovipositor, which directly leads to economic losses due to larval consumption of the fruit pulp from within. This primary damage is further exacerbated by secondary decay caused by ensuing fungal or bacterial infections around the feeding area [4]. Ever since it was introduced simultaneously into California, Spain, and Italy in 2008, this Asian pest has been responsible for massive crop destruction of up to 100%. Due to global trade and the initial lack of regulation over the spread of any Drosophila, the fly has spread rapidly throughout the temperate regions of Europe, North America, and South America [2]. It is currently distributed in about 60 countries/regions [2,5]. Certain countries have imposed restrictions on the trade of fresh fruit, and postharvest phytosanitary treatments (PT) are therefore required before shipment of the infested commodity [6].
Currently, cold treatment is a widely studied and commonly used PT measure against fruit flies and moths in international trade of fruits and vegetables [6,7,8]. For spotted wing drosophila, Kanzawa (1939) reported that exposure to 1.67 °C for 96 h kills all eggs and larvae [9]. Aly et al. (2017) found that 1.1 °C for 72 h neutralizes them, extending development and reducing eggs and larvae in blueberries and raspberries [10]. Feeding larvae are more cold-resistant than wandering larvae, with some surviving 72 h at 0 °C, though none last beyond 120 h [11]. Kim et al. (2018) found that pupae are more cold-tolerant than eggs and larvae, and all the immature stages have been eradicated after 6 days at 1 °C, or 8 days at 1.5 °C and 2 °C [12]. In a previous study [13], however, mature eggs were identified as the most cold-tolerant immature stage based on single dose (exposure time) testing. Therefore, determining the most cold-tolerant stage(s) is critical for setting up PT schedules.
Other measures for PT of D. suzukii have also been developed, including fumigation with methyl bromide, phosphine, and ethyl formate (EF) [3,6,14], as well as irradiation treatment at a minimum absorbed dose of 80 Gy [15,16]. In recent years, modified atmosphere (MA), essential oils, and combined treatments (cold + MA, EF + MA) have shown potential for the disinfestation of immature stages [17,18,19,20,21]. Unfortunately, none or very few of these methods are used for PT of fruits.
Drosophila suzukii exhibits a broad host range, including grapes, stone fruits, and berries [3,5]. The ‘Red Globe’ grape variety, known for its high-quality yield, superior taste, and significant export potential, plays a major role in the export market [22]. Therefore, developing phytosanitary cold-treatment schedules is essential to support the growth of China’s grape exports [5,13]. Additionally, since the topic of cold treatment for D. suzukii on grapes, based on the results of Wang et al. (2020) [13], was proposed to the secretariat of International Plant Protection Convention (IPPC) in 2021 and subsequently added to the list of topics in May 2022. The Technical Panel on Phytosanitary Treatments (TPPT) needs to determine which is the most cold-tolerant stage of the pest [23,24]. Confirmatory tests should be conducted accordingly if the egg is not the most tolerant stage [25,26].
Thus, the study aims to conduct dose–response only, or confirmatory cold-treatment tests together, to establish treatment schedules for D. suzukii [25,26]. The primary dose–response test involves exploring the cold tolerance variances across the different immature life stages infesting ‘Red Globe’ grapes, and determining the most cold-tolerant stage. Confirmatory tests may be necessary to validate the minimum exposure time required to achieve mortality of the most cold-tolerant stage, thereby demonstrating quarantine and biosecurity effectiveness [25,26]. This will also contribute to setting up the IPPC PT standard to facilitate the international movement of infested fruits.
2. Materials and Methods
2.1. Insect Rearing
The progeny of D. suzukii used in this investigation was originally collected from infested mulberry and cherry fruits in orchards in Yantai City, Shandong Province, China, during the spring and summer seasons. It was reared in the rearing room at the Chinese Academy of Inspection and Quarantine (CAIQ) in Beijing, China, and replaced with a wild population within a year. The adults were kept in cages (30 × 40 × 40 cm) with pure water, a dry powder mixture (sucrose, brewer’s yeast, casein in a ratio of approximately 9:3:1), and grapes for 3–5 days. A larval diet, modified from an artificial diet for Bactrocera papayae [27], was also used to maintain the population. The rearing room conditions were controlled at 26 ± 1 °C, 50–70% relative humidity, with a photoperiod of 14:10 (L:D) hours.
2.2. Insect Preparation
2.2.1. Egg Collection
Pesticide-free table grapes (cultivar ‘Red Globe’), purchased from a local market near the laboratory for collecting D. suzukii eggs and rearing larvae, were stored in the refrigerator at about 0 °C. Before egg collection, grapes (40 ± 5 g for each) of similar hardness, color, and size were washed with clean water, dried, warmed to room temperature, and a longitudinal crack was made with a scalpel [12,13]. The grapes were then placed in the rearing cage for egg-laying by the flies (2000–3000 adults per cage). Two hours later, the infested grapes were removed and kept in the rearing room for hatching, which began after five to six hours. Four to six-hour-old eggs (6 h old eggs) were then used for the treatment.
2.2.2. Larvae Rearing
The 2 h infested grapes were placed on a layer of artificial diet (to absorb grape juice) in a plastic cup for larval development. Since most of the egg hatched within 24 h [13], the 2 d and 4 d old larvae correspond to eggs that have developed for 3 and 5 days, respectively.
2.2.3. Pupae Development
The late-developed larvae (maturated) leave the fruits and pupated in the artificial diet or stay in the fruit for pupation. The pupae were checked daily and kept in a plastic box covered with 3–4 layers of moist papers to maintain high moisture content. In this investigation, the 1 d and 3 d old pupae were subjected to cold treatment, as adult emergence of D. suzukii begins from 4 d old pupae [13].
2.3. Dose–Response Tests
2.3.1. Cold-Treatment Facilities
A refrigerated warehouse (5.0 × 3.1 × 2.5 m) in CAIQ was utilized for the dose–response and large-scale confirmatory cold-treatment tests. Temperature recording was conducted using a temperature recorder (1586A SUPER-DAQ Precision Temperature Scanner, Fluke Co., Everett, WA, USA) and probes (Pt100, Chong Qing Well Co., Chongqing, China) in monitoring temperature changes every 5 min. The uncertainty of the temperature recording system is less than 0.01 °C.
2.3.2. Experimental Design
To identify the cold tolerance of D. suzukii immature stage, 6 h old eggs, 2 d and 4 d old larvae, and 1 d and 3 d old pupae were subjected to cold treatments at 0 °C and 2 °C with a serial of exposure time ranging from 8 h to 14 days in the dose–response tests. For each developmental stage, 3 to 4 grapes containing ≥120 individuals were placed into a plastic cup (Ф 6 cm, h 5.5 cm) and treated as 1 replicate, with each exposure time having 4 replicates [25,26]. All the dose–response tests at 0 °C and 2 °C were repeated twice (labeled as Test-1 and Test-2, respectively).
2.3.3. Cold-Treatment Tests
The grapes, kept in plastic cups and infested with eggs and larvae, or non-infested but mixed with D. suzukii pupae, were subjected to cold treatment in a refrigerated warehouse at 0 °C and 2 °C, respectively. To maintain a stable temperature, all plastic cups were placed in foam boxes, which were then placed inside a transparent plastic container (100 × 60 × 60 cm). Treatment was deemed to have started once all the fruit centers reached the target temperature. As the exposure time elapsed, the treated samples and plastic cups were taken out of the warehouse and transferred to the insect-rearing room for further development. Evaluation criteria for treatment efficacy included mortality of the treated stages. The duration for mortality evaluation after treatment was 4 days for eggs, 4 days for larvae, and 7–10 days for pupae. Mortality was determined by the absence of egg or pupal shell openings, lack of movement in larvae upon needle puncture, or a change in insect color to black.
2.4. Confirmatory Tests
After comparing the dose–mortality data with analysis of covariance (ANCOVA), linear regression, and probit analysis, the 3 d old pupa was determined to be the most cold-tolerant stage. Subsequently, these pupae were reared from grapes, placed together with fresh grapes into plastic boxes (11.0 × 11.0 × 5.5 cm), and subjected to large-scale confirmatory tests at 0 °C and 2 °C, respectively. Each treatment was repeated 6 times to ensure the mortality of more than 30,000 pupae, meeting quarantine security requirements [28]. Following cold treatment, the pupae were then incubated or reared in the rearing room maintained at 26 ± 1 °C for 7 to 10 days, and survivorship (development into adult) was assessed.
2.5. Statistical Analysis
To make comparisons of cold tolerance between life stages, all the dose–response data on percentage mortality of D. suzukii were firstly adjusted for control mortality using Abbott’s formula [29], then arcsine transformed, and subjected to ANCOVA by using the Tukey model [30,31]. Finally, linear regression was used to estimate the minimum time leading to 100% mortality. Data used in the regression and analysis included any exposure time causing mortality between 0% and 100%, as well as the least exposure time causing 100% mortality. Data Processing System (DPS) software (Version 20.05) was used for the analysis [30].
Probit analysis using the computer program PoloPlus 2.0 [32] was performed to analyze the relationship between exposure time and mortality in each stage of D. suzukii treated at 0 °C or 2 °C. It estimated the minimum exposure time for achieving 50%, 90%, 99%, and 99.9968% (probit-9) mortality at a 95% confidence level (CL), along with their 95% confidence interval (CI) for tolerance comparison among stages. The significant difference in tolerance was determined if the 95% CIs did not overlap [31,33]. The estimating value, including the 95% CI of probit-9, can be used as the target exposure time for conducting the confirmatory test [8,12,13,15,25,28].
For the large-scale confirmatory tests, the mortality proportion (1 − Pu) related to the treated number of D. suzukii pupae with zero survivors was calculated by the Equation (1):
where Pu is the acceptable level of survivorship, C is the CL, and n is the number of insects treated with no survivor [34]. Furthermore, the number (n) treated in confirmatory tests should be adjusted based on the control survivorship for calculating the current PT efficacy [28,35,36].
1 − Pu = (1 − C)1/n
3. Results
3.1. ANCOVA on Dose–Response Data at 0 °C
In the dose–response test at 0 °C, the mortality of D. suzukii increased with the increasing exposure time in all the tests (Figure 1). For the treated 6 h old egg, 2 d and 4 d old larvae, and 1 d and 3 d old pupae, the minimum exposure times causing 100% mortality were 96, 60, 96, 144, and 144 h, respectively, in Test-1, and 96, 48, 96, 120, and 168 h, respectively, in Test-2. The difference between the two trials may be attributed to variations in treatment temperature (0.15 (0.02–0.51) °C vs. 0.1 (−0.17–0.27) °C), sample conditions, and experimental errors. Regarding the effect of different developmental stages on tolerance to cold treatment, the order of tolerance is as follows: 3 d old pupa > 1 d old pupa > 4 d old larva ≥ 6 h old egg > 2 d old larva (Figure 1).
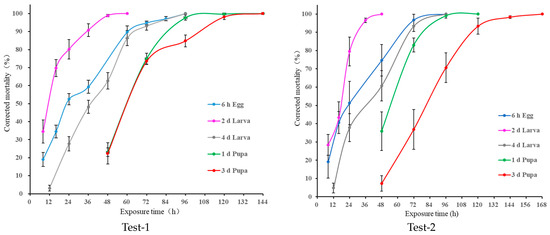
Figure 1.
The exposure time–mortality (mean ± SD, adjusted with Abbott’s formula) of immature stages of Drosophila suzukii treated under 0 °C cold-treatment conditions. Left: Test-1; Right: Test-2.
Additionally, the ANCOVA results in Test-1 and Test-2 also showed that the mortality was significantly affected by the stage (F = 37.15, df = 4, 114, p < 0.0001; F = 19.27, df = 4, 94, p < 0.0001), exposure time (F = 2184.16, df = 1, 114, p < 0.0001; F = 1479.87, df = 1, 94, p < 0.0001), and the interaction stage × exposure time (F = 14.88, df = 4, 114, p < 0.0001; F = 30.67, df = 4, 94, p < 0.0001). The significant cold tolerance for D. suzukii is as follows: 2 d old larva < 6 h old egg ≤ 4 d old larva < 1 d old pupa < 3 d old pupa. In addition, the estimated exposure time for causing 100% mortality (Table 1) also supports this conclusion.

Table 1.
Linear regression to estimate the minimum time causing 100% mortality of Drosophila suzukii treated at 0 °C.
3.2. ANCOVA on Dose–Response Data at 2 °C
When the immature stages of D. suzukii were subjected to cold treatment at 2 °C, the mortality also increased with increasing exposure time in all the tests (Figure 2). However, the minimum exposure time causing 100% mortality was longer than that for treatment at 0 °C, since the temperature had increased to 1.61 (1.44–1.85) °C and 1.85 (1.48–2.26) °C. The estimated minimum exposure times leading to 100% mortality for treatment of the 6 h old egg, 2 d and 4 d old larva, and 1d and 3 d old pupa were 120, 72, 120, 192, and 192 h, respectively, in Test-1, and 144, 96, 120, 168, and 192 h, respectively, in Test-2. For the effect of developmental stages, the tolerance is as cold treatment at 0 °C except for 4 d old larvae ≥6 h old eggs.
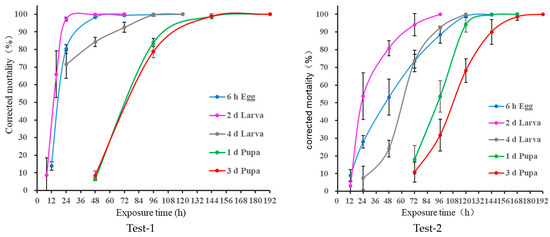
Figure 2.
The exposure time–mortality (mean ± SD, adjusted with Abbott’s formula) of immature stages of Drosophila suzukii treated under 2 °C cold-treatment conditions. Left: Test-1; Right: Test-2.
Similarly, the ANCOVA results in Test-1 and Test-2 also showed that the mortality was significantly affected by the stage (F = 6.32, df = 4, 74, p = 0.0002; F = 42.42, df = 4, 98, p < 0.0001), exposure time (F = 229.74, df = 1, 74, p < 0.0001; F = 1758.63, df = 1, 98, p < 0.0001), and the interaction stage × exposure time (F = 8.71, df = 4, 74, p <0.0001; F = 17.53, df = 4, 98, p < 0.0001). Therefore, the cold tolerance for D. suzukii is the same as that under 0 °C, except for the 6 h old egg and 4 d old larva, which present similar tolerance. The estimates on causing 100% mortality (Table 2) also support this result.

Table 2.
Linear regression to estimate the minimum time leading to 100% mortality of Drosophila suzukii treated at 2 °C.
3.3. Probit Analysis on Dose–Response Data
To compare the cold tolerance of D. suzukii stages, results derived from probit analysis using the probit model are listed in Table 3 and Table 4, where the exposure time was not converted. These results include the estimated exposure times for LT50, LT90, and LT99 and their corresponding CI at 95% CL, as well as slope information. For the different stages treated at 0 °C, only a few of the 95% CI for LT50, LT90, and LT99 overlapped, indicating significant tolerance difference. The 2 d old larva, obtaining the smallest value, was the most sensitive stage, while the 3 d old pupa, with the largest value, was the most tolerant stage. The 1 d old pupa was significantly more sensitive than the 3 d old pupa, and the 4 d old larva and 6 h old egg had similar tolerance to cold treatment. Therefore, the probit analysis yielded the same tolerance sequence as the ANCOVA and linear regression results.

Table 3.
Probit estimation of minimum lethal time (LT) for Drosophila suzukii treated at 0 °C in grapes.

Table 4.
Probit estimation of minimum lethal time (LT) for Drosophila suzukii treated at 2 °C in grapes.
For the cold treatments under 2 °C, the estimated exposure times for LT50, LT90, and LT99 are notably longer than those for treatments at 0 °C. However, the cold tolerance among the stages is similar at 2 °C. Therefore, the 2 d old larva was defined as the most susceptible stage, while the 3 d old pupa was deemed the most resistant stage. This result aligns with the analyses from ANCOVA and linear regression.
Furthermore, the probit-9 values for the 3 d old pupa were analyzed using the probit model, both with and without logarithmic transformation of exposure time, as shown in Table 5. When the exposure time is logarithm transformed, the value is larger than that derived from the non-transformed data, which closely matches the value for 100% mortality in the dose–response tests (Figure 1 and Figure 2) and estimations from linear regression (Table 1 and Table 2). Therefore, the value derived from the probit model with logarithmic conversion was utilized as the target exposure time for conducting the subsequent large-scale confirmatory tests.

Table 5.
Probit analysis on 3 d old pupae of Drosophila suzukii treated under 0 °C and 2 °C.
3.4. Confirmatory Tests
3.4.1. Confirmatory Tests at 0 °C
The confirmatory test at 0 °C initially selected 9 days as the target exposure time, since at least 7 days were needed to achieve 100% mortality in the dose–response test, and the lower interval for probit-9 estimation ranged between 8.85 and 9.18 days (Table 5). Moreover, confirmatory tests typically commence from the lower CI of probit-9 estimation [37,38]. As a result, no D. suzukii adult emergence was observed from a total of 31,987 3 d old pupae treated at temperatures ranging from −0.06 to 0.23 °C, whereas the control group showed successful development, with adult emergence rates ranging from 95.61% to 96.69% (Table 6).

Table 6.
The confirmatory cold treatments of Drosophila suzukii 3 d old pupa on grape at 0 °C.
A target exposure time of 10 days was used in the second batch of confirmatory tests conducted in May 2024, since one previous confirmatory test at 2 °C failed to achieve the desired efficacy using a 10-day exposure, based on the lower CI for probit-9. As a result, no adult emergence was observed from a total of 44,211 treated 3 d old pupae, while the mortality for the control group was less than 4.19% (Table 6).
Therefore, a total of 76,198 pupae underwent cold treatment in the confirmatory test. The treatment efficacy, calculated using formula (1) and adjusted number of insects based on the control group, was determined to be 99.9960%. Then, the technical schedule for phytosanitary cold treatment of D. suzukii is as follows: maintaining fruit temperature below 0.00 °C for no less than 10 continuous days. There is a 95% CL that treatment according to this schedule will eliminate not less than 99.9960% of immature stages of spotted wing drosophila [25,26].
3.4.2. Confirmatory Tests at 2 °C
The confirmatory tests at 2 °C were initially conducted with a target exposure time of 10 days. However, no adult emergence was observed in two tests, with only 1 adult emerging out of 10,851 treated pupae (Table 7). This result indicates that the lower CI estimation is sometimes too short to achieve the expected goals, as the minimum time causing 100% mortality is 8 days in the dose–response test (Figure 2). Consequently, the target duration for the confirmatory test was extended to 12 days, which is slightly higher than the means of the probit-9 estimation (Table 5 and Table 7).

Table 7.
The confirmatory cold treatments of Drosophila suzukii 3 d old pupa on grape at 2 °C.
As a result, no adult emergence was observed from a total of 46,980 treated 3 d old pupae in the second batch of confirmatory tests, whereas the mortality in the control group was less than 4.57%. Therefore, a total of 69,496 pupae underwent cold treatment and mortality assessment in the confirmatory test. The treatment efficacy, calculated using formula (1) and adjusted based on the number treated in the control group, was determined to be 99.9955% at a 95% CL.
In summary, the technical schedule for phytosanitary cold treatment of D. suzukii, based on the confirmatory tests at 2 °C, is as follows: maintaining fruit temperature below 1.62 °C for no less than 12 continuous days, achieving a treatment efficacy of no less than 99.9955% at a 95% CL for disinfestation of spotted wing drosophila [25,26].
4. Discussion
Research on phytosanitary treatment requires conducting dose–response tests to compare tolerances and determine the most tolerant stage(s), followed by large-scale confirmatory tests on the most tolerant stage(s) to validate the estimated dose and determine treatment efficacy [25,26,39]. Phytosanitary cold treatments, such as those for D. suzukii, should adhere to international standards and guidelines, which recommend setting test temperatures in a gradient with at least five doses in the dose–response test [35,40,41,42]. Standard measures were then utilized in this cold-treatment study to examine the tolerance of all immature stages at 0 °C and 2 °C. The same variety of ‘Red Globe’ grapes used by Wang et al. (2020) in cold-treatment studies for D. suzukii [13] were also utilized to avoid varietal influence. Based on ANCOVA, linear regression, and probit analysis [28,42], the results showed significant tolerance differences between stages, with the 3 d old pupa being the most tolerant stage, followed by the 1 d old pupa, 4 d old larva, and 6 h old egg. The 2 d old larva was the most susceptible stage. These findings differ from Wang et al. (2020) [13] but are consistent with those of Kim et al. (2018) [12].
Kim et al. (2018) [12] conducted a dose–response test on spotted wing drosophila reared on an artificial diet at three temperatures (1 °C, 1.5 °C, and 2 °C) with nine exposure times to develop a phytosanitary cold-treatment schedule. Through probit analysis of the dose–response data, their findings suggested that at 2 d to 3 d old, pupae are at their most tolerant stage and eggs are their most sensitive, which matches our results. However, unlike this study, they did not differentiate larval ages; we found that 2 d old larvae were the most susceptible (Table 1, Table 2, Table 3 and Table 4), possibly due to age variation.
In contrast, Wang et al. (2020) [13] treated the eggs, first to third instar larvae, young, and late pupae with a single dose of 12 h at 0 °C or 24 h at 2 °C. Their results, analyzed using one-way ANOVA on mortality, showed that late-aged eggs were the most tolerant stage, with no significant differences found among larvae and pupae. This finding contradicts the results of Kim et al. (2018) [12] and our study. The discrepancy might be due to inconsistent insect stage (all the eggs were collected in 24 h), insufficient insect numbers per replicate, response variability, and the single dose approach representing only the initial threshold for the dose–response. To accurately assess pest tolerance, dose–response studies with more than five doses are crucial, as emphasized by international research protocols from IPPC (2003) [43], NAPPO (2011) [35], and TPPT (2019) [25]. These tests help determine the most cold-tolerant life stage and identify the temperature–time combination that provides a specified efficacy level, as required by TPPT (2019) [25] and PMRG (2019) [26], and are therefore recommended in phytosanitary research [35,42,44,45].
Probit analysis has been widely used to analyze dose–response data to estimate the lethal dose (fumigant concentration, temperature exposure time, radiation dose, etc.), compare tolerances, and provide reference doses for confirmatory tests [28,34,37]. The dose is normally logarithmically transformed, and this transformation appears to provide a good fit for data in this study (Table 5) and other cold-treatment studies, such as those for six species of the genus Bactrocera and Zeugodacus [42,46] and Z. tau [43]. However, better estimations have been obtained from non-transformed radiation doses. This is evident in studies on the phytosanitary irradiation treatment of Z. tau [45], B. dorsalis [47], Pseudococcus baliteus [38], P. jackbeardsleyi [48], and Paracoccus marginatus [49]. Additionally, the estimation for probit-9 based on non-transformed doses has been validated in large-scale confirmatory tests.
However, estimation using logarithmically transformed doses is more suitable for this cold treatment [32,37,45]. Only one deformed adult emerged after a 10-day treatment at 2 °C (Table 7), which corresponds to the lower 95% CI for probit-9 (Table 5) [37]. This suggests that a higher dose or the mean of probit-9 is recommended for confirmatory testing. A minimum mean temperature of 0.00 °C (or 1.62 °C) in a confirmatory test was set as the temperature limit in the schedules for PT of D. suzukii in grapes [25,26]. Therefore, the cold-treatment schedules are also suitable for postharvest storage of ‘Red Globe’ table grapes. In China, the optimal storage temperature for ‘Red Globe’ grapes is −1 to 0 °C with a relative humidity of 90% to 95% for over a month [50,51]. Two other research studies on the quality evaluation of this variety also show that a 12-day cold treatment at 0 °C and 1 °C, or a 14-day treatment at 0 °C and 2 °C, does not significantly damage quality [13,52].
Globalization, characterized by increased trade and human mobility, along with environmental changes, significantly facilitates the introduction and establishment of D. suzukii, a highly invasive species, in new regions beyond its native habitats [3,5,6]. Cold-treatment schedules, achieving a treatment efficacy of at least 99.9960% or 99.9955% at a 95% CL, can meet phytosanitary requirements and offer an organic and flexible method for treating this fly in international trade [24,28]. Furthermore, this study underscores the use of cold-treatment technology to preserve fruit quality during storage and transportation. The cold treatment can easily integrate with cold-chain transportation systems and other PT measures for fresh fruits and vegetables [19,20,28]. Therefore, the establishment and adoption of IPPC phytosanitary standards are crucial to curb the spread of D. suzukii and facilitate safe international trade [24,25].
In addition, future research should explore wider temperature ranges, for example 3 °C and 5 °C, to better understand temperature thresholds for D. suzukii mortality. Additionally, comparing different grape varieties could further clarify their impact on treatment efficacy and fruit quality maintenance. Addressing these gaps will enhance PT standards and ensure effective pest control strategies in diverse agricultural settings.
5. Conclusions
In conclusion, this study has comprehensively evaluated phytosanitary cold-treatment methods for managing D. suzukii in ‘Red Globe’ grapes, addressing the significant threat this invasive species poses to global fruit production. Through rigorous dose–response tests and confirmatory tests at 0 °C and 2 °C, we determined the 3 d old pupae to be the most cold-tolerant stage, followed by 1 d old pupae, 4 d old larvae, and 6 h old eggs, with 2 d old larvae exhibiting the highest susceptibility. Confirmatory tests validated the efficacy of maintaining fruit temperatures below 0.00 °C (or 1.62 °C) for 10 (or 12) continuous days, achieving treatment efficacies not less than 99.9960% (or 99.9955%) at a 95% CL. These results support the development of an IPPC PT standard, crucial for international trade compliance and the maintenance of fruit quality.
Future research should continue to refine these treatment protocols and explore additional factors influencing treatment efficacy under varied environmental conditions, ensuring robust protection against invasive pests in global movement of agricultural and food products.
Author Contributions
Conceptualization, G.-P.Z., Z.-H.L., B.L., Y.F., and W.-N.G.; methodology, G.-P.Z., Z.-H.L., B.L., Y.F., Q.-Y.Z., and T.-B.M.; formal analysis, T.-B.M., Q.-Y.Z., G.-P.Z., and W.-N.G.; investigation, T.-B.M., Q.-Y.Z., and G.-P.Z.; resources, B.L., Y.F., and W.-N.G.; data curation, T.-B.M., Q.-Y.Z., and G.-P.Z.; validation, Y.F., and W.-N.G.; writing—original draft preparation, G.-P.Z., T.-B.M., B.L., Z.-H.L., Q.-Y.Z., and W.-N.G.; writing—review and editing, G.-P.Z., Z.-H.L., B.L., Q.-Y.Z., and W.-N.G.; supervision, G.-P.Z., and Z.-H.L.; project administration, G.-P.Z.; funding acquisition, G.-P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chinese National Key R & D Program (2021YFF0601901) and the National Natural Science Foundation of China (no. 62272060).
Data Availability Statement
All data presented in this study are available in the article.
Acknowledgments
We would like to thank Yi-Fan Zhai and his research teams (the Institute of Plant Protection, Shandong Academy of Agricultural Sciences, China) for insect sourcing, and the anonymous reviewers for their valuable suggestions and modifications to the draft of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adrion, J.R.; Kousathanas, A.; Pascual, M.; Burrack, H.J.; Haddad, N.M.; Bergland, A.O.; Machado, H.; Sackton, T.B.; Schlenke, T.A.; Watada, M.; et al. Drosophila suzukii: The Genetic Footprint of a Recent, Worldwide Invasion. Mol. Biol. Evol. 2014, 31, 3148–3163. [Google Scholar] [CrossRef] [PubMed]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Èntomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef] [PubMed]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- CABI Compendium. Drosophila suzukii (Spotted Wing Drosophila) Datasheet (Updated on 2 April 2024). 2024. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.109283 (accessed on 6 May 2024).
- Walse, S.S.; Cha, D.H.; Lee, B.H.; Follett, P.A. Postharvest quarantine treatments for Drosophila suzukii in fresh fruit. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020; pp. 255–267. [Google Scholar]
- Li, Z.; Jiang, F.; Ma, X.; Fang, Y.; Sun, Z.; Qin, Y.; Wang, Q. Review on prevention and control techniques of Tephritidae invasion. Plant Quar. 2013, 27, 1–10. (In Chinese) [Google Scholar]
- Fang, Y.; Kang, F.; Zhan, G.; Ma, C.; Li, Y.; Wang, L.; Wei, Y.; Gao, X.; Li, Z.; Wang, Y. The Effects of a Cold Disinfestation on Bactrocera dorsalis Survival and Navel Orange Quality. Insects 2019, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T. Studies on Drosophila suzukii Matsumura (Abstract). Rev. Appl. Entomol. 1939, 29, 622. [Google Scholar]
- Aly, M.F.K.; Kraus, D.A.; Burrack, H.J. Effects of Postharvest Cold Storage on the Development and Survival of Immature Drosophila suzukii (Diptera: Drosophilidae) in Artificial Diet and Fruit. J. Econ. Èntomol. 2017, 110, 87–93. [Google Scholar] [CrossRef]
- Jakobs, R.; Ahmadi, B.; Houben, S.; Gariepy, T.D.; Sinclair, B.J. Cold tolerance of third-instar Drosophila suzukii larvae. J. Insect Physiol. 2017, 96, 45–52. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.S.; Jeong, J.S.; Choi, D.S.; Park, J.; Kim, I. Phytosanitary cold treatment of spotted-wing drosophila, Drosophila suzukii (Diptera: Drosophilidae) in ‘Campbell Early’ grape. J. Econ. Entomol. 2018, 111, 1638–1643. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, G.; Ren, L.; Sun, S.; Dang, H.; Zhai, Y.; Yin, H.; Li, Z.; Liu, B. Cold disinfestation for ‘Red Globe’ grape (Rhamnales: Vitaceae) infested with Drosophila suzukii (Diptera: Drosophilidae). J. Insect Sci. 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Walse, S.S.; Jimenez, L.R.; Hall, W.A.; Tebbets, J.S.; Obenland, D.M. Optimizing postharvest methyl bromide treatments to control spotted wing drosophila, Drosophila suzukii, in sweet cherries from Western USA. J. Asia-Pac. Èntomol. 2016, 19, 223–232. [Google Scholar] [CrossRef]
- Follett, P.A.; Swedman, A.; Price, D.K. Postharvest Irradiation Treatment for Quarantine Control of Drosophila suzukii (Diptera: Drosophilidae) in Fresh Commodities. J. Econ. Èntomol. 2014, 107, 964–969. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Park, C.G. X-ray radiation and developmental inhibition of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Int. J. Radiat. Biol. 2016, 92, 849–854. [Google Scholar] [CrossRef]
- Follett, P.A.; Swedman, A.; Mackey, B. Effect of Low-Oxygen Conditions Created by Modified Atmosphere Packaging on Radiation Tolerance in Drosophila suzukii (Diptera: Drosophilidae) in Sweet Cherries. J. Econ. Èntomol. 2018, 111, 141–145. [Google Scholar] [CrossRef]
- Yang, X.B.; Liu, Y.B. Nitric oxide fumigation for control of spotted wing drosophila (Diptera: Drosophilidae) in strawberries. J. Econ. Entomol. 2018, 111, 1180–1184. [Google Scholar] [CrossRef]
- Mostafa, M.; Ibn Amor, A.; Admane, N.; Anfora, G.; Bubici, G.; Verrastro, V.; Scarano, L.; El Moujabber, M.; Baser, N. Reduction of Post-Harvest Injuries Caused by Drosophila suzukii in Some Cultivars of Sweet Cherries Using a High Carbon Dioxide Level and Cold Storage. Insects 2021, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-C.; Kim, H.-K.; Koo, H.-N.; Kim, B.-S.; Yang, J.-O.; Kim, G.-H. Synergistic Effect of Cold Treatment Combined with Ethyl Formate Fumigation against Drosophila suzukii (Diptera: Drosophilidae). Insects 2022, 13, 664. [Google Scholar] [CrossRef]
- Bošković, D.; Vuković, S.; Lazić, S.; Baser, N.; Čulum, D.; Tekić, D.; Žunić, A.; Šušnjar, A.; Šunjka, D. Insecticidal Activity of Selected Essential Oils against Drosophila suzukii (Diptera: Drosophilidae). Plants 2023, 12, 3727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, P. Research progress of postharvest preservation technology on ‘Red Globe’ grape. North. Hortic. 2016, 10, 181–184. (In Chinese) [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Report of the 2022 September Meeting of the Technical Panel on Phytosanitary Treatments, 12–18 September 2022; FAO: Roma, Italy, 2022; pp. 1–23. [Google Scholar]
- IPPC (International Plant Protection Convention). List of Topics for IPPC Standards. (Updated on 15 February 2024) 2024. Available online: https://www.ippc.int/en/core-activities/standards-setting/list-topics-ippc-standards/list (accessed on 6 May 2024).
- Liu, B.; Li, B.; Zhan, G.; Zha, T.; Wang, Y.; Ma, C. Forced hot-air treatment against Bactrocera papayae (Diptera: Tephritidae) in papaya. Appl. Èntomol. Zool. 2017, 52, 531–541. [Google Scholar] [CrossRef]
- TPPT (Technical Panel on Phytosanitary Treatments). Treatment Research Guidelines; Secretariat IPPC: Rome, Italy, 2019. [Google Scholar]
- PMRG (Phytosanitary Measures Research Group). Guidelines for the Development of Cold Disinfestation Treatments for Fruit Fly Host Commodities; Secretariat of IPPC: Rome, Italy, 2019. [Google Scholar]
- Follett, P.A.; Neven, L.G. Current Trends in Quarantine Entomology. Annu. Rev. Èntomol. 2006, 59, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Èntomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- DPS (Data Processing System), Version 13.5. User’s Guide. Hangzhou RuiFeng Information Technology Co., Ltd.: Hangzhou, China, 2010.
- Zhan, G.; Zhao, J.; Ma, F.; Liu, B.; Zhong, Y.; Song, Z.; Zhao, Q.; Chen, N.; Ma, C. Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres. Insects 2020, 11, 526. [Google Scholar] [CrossRef]
- LeOra Software, Version 2.0. User’s Guide: PoloPlus Probit and Logit Analysis. LeOra Software: Berkeley, CA, USA, 2002.
- Wheeler, M.W.; Park, R.M.; Bailer, A.J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef]
- Couey, H.M.; Chew, V. Confidence Limits and Sample Size in Quarantine Research. J. Econ. Èntomol. 1986, 79, 887–890. [Google Scholar] [CrossRef]
- NAPPO (North American Plant Protection Organization). NAPPO Regional Standards for Phytosanitary Measures (RSPM) 34: Development of Phytosanitary Treatment Protocols for Regulated Arthropod Pests of Fresh Fruits or Vegetables; NAPPO: Ottawa, ON, Canada, 2011; p. 14. [Google Scholar]
- IPPC (International Plant Protection Convention). IPPC Procedure Manual for Standard Setting; Secretariat of IPPC: Rome, Italy, 2012. [Google Scholar]
- West, M.; Hallman, G.J. Estimation of dose requirements for extreme levels of efficacy. In Proceedings of the 25th Conference on Applied Statistics in Agriculture, Manhattan, KS, USA, 28–30 April 2013. [Google Scholar]
- Zhao, Q.Y.; Ma, F.H.; Deng, W.; Li, Z.H.; Song, Z.J.; Ma, C.; Ren, Y.L.; Du, X.; Zhan, G.P. Phytosanitary treatment of the aerial root mealybug, Pseudococcus baliteus (Hemiptera: Pseudococcidae). J. Econ. Entomol. 2023, 116, 1567–1574. [Google Scholar] [CrossRef]
- IPPC. ISPM #28: Phytosanitary Treatments for Regulated Pests; Secretariat of IPPC: Rome, Italy, 2007. [Google Scholar]
- Hallman, G.J.; Mangan, R.L. Concerns with temperature quarantine treatment research. In Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reduction, San Diego, CA, USA, 3–5 November 1997; Obenauf, G.L., Ed.; Office of Methyl Bromide Alternatives Outreach: Fresno, CA, USA, 1997; pp. 79-1–79-4. [Google Scholar]
- Wang, Y.; Wu, H.; Li, X.; Zhan, G. The progress and application for research protocol on phytosanitary treatment of arthropods. Plant Quar. 2016, 30, 1–5. (In Chinese) [Google Scholar]
- Myers, S.W.; Cancio-Martinez, E.; Hallman, G.J.; Fontenot, E.A.; Vreysen, M.J. Relative Tolerance of Six Bactrocera (Diptera: Tephritidae) Species to Phytosanitary Cold Treatment. J. Econ. Èntomol. 2016, 109, 2341–2347. [Google Scholar] [CrossRef]
- Dias, V.S.; Hallman, G.J.; Araújo, A.S.; Lima, I.V.G.; Galvão-Silva, F.L.; Caravantes, L.A.; Rivera, M.N.G.; Aguilar, J.S. High cold tolerance and differential population response of third instars from the Zeugodacus tau complex to phytosanitary cold treatment in navel oranges. Postharvest Biol. Tec. 2023, 203, 112392. [Google Scholar] [CrossRef]
- IPPC (International Plant Protection Convention). ISPM #18: Requirements for the Use of Irradiation as a Phytosanitary Measure; Food and Agricultural Organization: Rome, Italy, 2003; (Revised in 2023). [Google Scholar]
- Zhan, G.; Ren, L.; Shao, Y.; Wang, Q.; Yu, D.; Wang, Y.; Li, T. Gamma irradiation as a phytosanitary treatment of Bactrocera tau (Diptera: Tephritidae) in pumpkin fruits. J. Econ. Entomol. 2015, 108, 88–94. [Google Scholar]
- Doorenweerd, C.; Leblanc, L.; Norrbom, A.L.; Jose, M.S.; Rubinoff, D. A global checklist of the 932 fruit fly species in the tribe Dacini (Diptera, Tephritidae). ZooKeys 2018, 730, 19–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, J.; Wu, M.; Jiao, X.; Wang, Z.; Liang, F.; Zhan, G. Gamma radiation as a phytosanitary treatment against larvae and pupae of Bactrocera dorsalis (Diptera: Tephritidae) in guava fruits. Food Control 2017, 72, 360–366. [Google Scholar] [CrossRef]
- Zhan, G.; Shao, Y.; Yu, Q.; Xu, L.; Liu, B.; Wang, Y.; Wang, Q. Phytosanitary irradiation of Jack Beardsley mealybug (Hemiptera: Pseudococcidae) females on rambutan (Sapindales: Sapindaceae) fruits. Fla. Entomol. 2016, 99 Pt 2, 114–120. [Google Scholar]
- Song, Z.-J.; Zhao, Q.-Y.; Ma, C.; Chen, R.-R.; Ma, T.-B.; Li, Z.-H.; Zhan, G.-P. Quarantine disinfestation of papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) using Gamma and X-rays irradiation. Insects 2023, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, N. Study on the Storage and Preservation of ‘Red Globe’ Grapes in Xinjiang. Agric. Tech. 2015, 35, 112–159. [Google Scholar]
- Wu, S. Different effects of pre-cooling methods on “Red Globe” grape. China Fruit Veg. 2015, 8, 1–3. (In Chinese) [Google Scholar]
- Wuernisha, K.; Che, F.B.; Zhang, T.; Li, P.; Hu, B.W. Effect of different temperature on quality and physiological index of postharvest ‘Red Globe’ grape during storage. Xinjiang Agric. Sci. 2010, 47, 82–86. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).