1. Introduction
South Africa is ranked as the 10th largest fresh citrus producer globally, with soft citrus accounting for 27% of the production area, of which ‘Nadorcott’ (
Citrus reticulata Blanco) is the most widely planted mandarin. South Africa exports 69% of its production (2,133,585 tons) [
1]. In comparison, 25% is used for processing, and the remaining volume of fruit is consumed locally, resulting in South Africa being the second largest citrus exporter [
1].
In addition to preharvest growing conditions, the storage life and quality of citrus fruit are affected by the handling and environmental factors that the fruit is exposed to during the postharvest chain [
2]. During postharvest handling, the fruit continues with its essential metabolic processes, as it is active during fruit development. Of all these various physiological and biochemical changes that occur within the fruit, respiration is considered a critical driver, as it affects the postharvest potential of the fruit, either directly or indirectly, through changes in the biochemical composition of the fruit [
3]. Blanke and Bower [
4] have stated that developing citrus fruit has the highest number of stomata (ca. 200,000) in comparison to other fruit types, and consequently, a high respiration rate is evident. The respiration rate of citrus fruit decreases with development towards maturity, with a relatively low rate in mature fruit [
5,
6], which finally results in associated changes in the fruit’s biochemical and physical properties after harvest [
7].
Physical changes are ascribed to moisture loss, which causes fruit softening and the shrivelling of the peel and consequently accelerates fruit deterioration [
8]. The biochemical changes involved, in most instances, are losses in organic acids and sugars, which are used as substrates in the respiration process, providing energy to the fruit to maintain cellular functions [
9,
10], and consequently affecting the taste [
11]. Cold storage and shipping conditions are important to ensure that the fruit maintains its good quality, with no rind physiological disorders and defects present, along with good internal and nutritional quality on reaching the consumer within the targeted export markets [
2]. High relative humidity (RH) (85–95%) and low temperature conditions are critical to controlling the fruit deterioration rate and thereby ensuring that the qualities of the fruit are maintained for longer by preventing excessive moisture loss [
8,
12]. Furthermore, when fruit is subjected to cold storage (i.e., 2 to 8 °C), the rate of biochemical changes is reduced and in addition, the development of decay, i.e., blue and green mold caused by
Penicillium spp. is retarded or prevented, which in turn prolongs the fruit’s storage life [
9,
13].
There are, however, phytosanitary protocols that need to be met to allow the shipment of citrus fruit from South Africa to certain countries [
14]. Currently, soft citrus are shipped between −0.6 °C (chilling) and 4 °C (non-chilling temperature), with the temperature and cold-storage duration (days) being dependent on the export market [
15,
16].
During the cold chain, the fruit experiences certain gradual physiological changes. ‘Washington Navel’ (
C. sinensis (L.) Osbeck) oranges, which were stored at 4 °C for an extended storage time of five months, showed an improvement in the rind colour, along with an increase in the total soluble solids (TSS), sugars and weight loss percentage, while the citric acid content decreased [
17]. ‘Or’ and ‘Odem’ mandarins stored at 2, 5 and 8 °C showed no increase in TSS, but the acids decreased, leading to an increase in the sugar-to-acid ratio during storage [
13]. In addition, these changes may differ between cultivars or varieties. The physiological changes of citrus fruit stored at 15 °C and 95% RH have shown contrasting changes in the °Brix, citric acid and sugars (sucrose, glucose and fructose) content amongst different citrus varieties, ‘Hamlin’ orange, ‘Marsh’ grapefruit (
C. paradisi Macf.) and ‘Robinson’ tangerine (
C. tangerina) [
18]. Furthermore, studies by Matsumoto et al. [
19] and Carmona et al. [
20] reported that the carotenoid content increased more rapidly during storage at moderate to high temperatures (12 to 20 °C) as compared to lower temperatures (2 to 5 °C) for both ‘Satsuma’ mandarin (
C. unshiu Marc.) and ‘Navelina’ orange. Matsumoto et al. [
19] proposed that citrus fruit carotenoid biosynthesis is temperature sensitive. In addition to this, Van Wyk et al. [
21] found that the rind colouration was enhanced (more orange) and the carotenoid content increased in ‘Palmer Navel’ sweet oranges shipped at 4.5 °C as opposed to −0.6 °C. The same effect was seen in a study by Tietel et al. [
13], reporting that “mandarin fruit stored at 8 °C developed a better rind colour than fruit stored at 2 and 5 °C”.
Continuous exposure to low temperatures (0 to 10 °C), however, may result in chilling injury (CI) in citrus fruit, which is considered a physiological disorder [
22,
23,
24,
25] that is characterized by a localized discolouration of the rind, resulting in depressions in the fruit surface [
26]. CI incidence is determined by various factors, i.e., cultivar, species, season and maturity index [
27].
Rind disorders, such as rind staining or rind breakdown (RB), generally referred to as ‘postharvest non-chilling peel pitting’ and may develop at non-chilling temperatures, have been the subject of revision [
2,
25,
28]. These disorders are generally characterized by depressions in the flavedo, which becomes dry and turns brown and black [
12]. Thes postharvest physiological rind disorders affect the fruit’s external appearance, thereby reducing marketability. Cronje et al. [
29] proposed that citrus fruit sensitivity to RB is largely determined during preharvest conditions, but with symptom development occurring only after a signal is triggered during postharvest handling.
Studies have reported that canopy positions, which are known to receive different light levels [
30] have a marked influence on postharvest physiological disorders, i.e., CI in ‘Marsh’ grapefruit [
31], and rind breakdown in ‘Nules Clementine’ mandarin [
29]. Furthermore, it has been reported that the light levels influence the carbohydrate content (glucose, sucrose and fructose) in the rind, with generally higher carbohydrate levels in the rind of outside canopy ‘Nules Clementine’ fruit [
30], even though in one season, no differences were found. A study on ‘Marsh’ grapefruit, showed no difference between canopy positions [
32]. In addition, fruit covering studies where light was excluded from the fruit also reported a lower carbohydrate content in the flavedo of both ‘Miyagawa wase’ [
33] and ‘Nules Clementine’ mandarins [
34] and a reduction in carotenoid and ascorbic acid content in oranges and mandarin fruits [
35,
36].
Production of citrus fruit under a shade net is a relatively recent orchard management technique used to protect fruit against rind blemishes such as sunburn through light interception [
37,
38] and wind damage [
39]. Shade nets, however, may impact the external and internal quality parameters of citrus fruit both positively and negatively. Syvertsen et al. [
40] reported that rind colour of ‘Spring’ navel oranges was enhanced by shade net, while Jifon and Syvertsen [
41] reported a delay in rind colour expression in ‘Ruby Red’ grapefruit and ‘Hamlin’ orange fruit. Mira-Garcia et al. [
42] found no difference in rind colour for ‘Bearss’ lime (
C. latifolia Tan). In terms of internal quality, shade nets enhanced the sugar/acid ratio of the juice in ‘Orri’ mandarin [
39], with no effect in ‘Spring Navel’ oranges [
40] but with a reduction in ‘Ruby Red’ grapefruits and ‘Hamlin’ sweet oranges [
41]. Cohen et al. [
43] reported that sugars and acids in ‘Marsh’ grapefruit were not influenced by shade netting, while in ‘Hamlin’ oranges, the °Brix was reduced in the presence of shade nets [
41].
The postharvest potential of citrus fruit is determined by the rind and its ability to resist microbial attack and physiological disorder development, in addition to retarding senescence [
3]. In addition, Cronje et al. [
29,
30] proposed that a well-developed rind, with higher carbohydrate and carotenoid content and good rind colour before postharvest storage will result in a good physiological condition of the rind during storage. Van Wyk et al. [
21] proposed that the initial rind colour is important in determining the final quality after cold storage. Based on this, it can also be postulated that a fruit of good internal quality at harvest will have a better storage behaviour, since some changes are evident during cold storage. However, to date, there is no available literature on the effect of preharvest shade net on the postharvest storage potential of citrus fruit at various long-term cold-storage temperatures.
This study aimed to determine the effect of 20% white shade netting on the external and internal quality parameters of ‘Nadorcott’ mandarin fruit during long-term cold storage at −0.6 and 4 °C. Aspects such as changes in the rind and pulp colour, internal quality parameters (soluble solid content (SSC), citric acid percentage and the SSC-to-acid ratio), carotenoid concentration and fruit weight loss over the storage duration at these temperatures were evaluated in addition to the incidence of physiological disorders such as chilling injury and staining.
2. Materials and Methods
2.1. Plant Material and Site Location
Fruit was obtained from ‘Nadorcott’ mandarin (C. reticulata Blanco) trees, grafted on ‘Carrizo citrange’ (Poncirus trifoliata × C. sinensis) rootstock within a commercial orchard in Citrusdal (−32.542140, 19.011877), Western Cape Province, South Africa. Trees were planted in 2012 in a north-to-south row orientation, with a tree spacing of 5.5 m between and 2.5 m within rows. The orchard management followed standard citriculture practices for both treatments, including irrigation and nutrition, as well as pest and disease management. Fruit was harvested over two consecutive seasons, on 7 July 2016 (first season) and 10 July 2017 (second season).
2.2. Experimental Design and Treatments
The experimental layout was a randomized complete block design (RCBD), where each experimental block was divided into two plots of 75 m × 25 m, with the treatments being randomly allocated to the plots within a block and replicated four times per treatment (n = 4). The treatments consisted of either the shade netting or the control (no netting). The shade plots were covered with permanent 20% white shade netting (Plusnet, 13 Bussing Road, Aureus, Randfontein, 1759, Gauteng, South Africa) after flowering in Sept. 2015 of the first season. The shade net structure was not fully enclosed on the sides (halfway down). To compensate for a border effect, trees in the first 10 m at either side of the rows and the first two rows from the sides of each plot were excluded from data collection.
Three adjacent trees per block were selected, uniform in size, health and crop load, which served as sampling units per replicate for each treatment.
2.3. Fruit Sampling and Measurements
Fruit was harvested from a central position within the canopy, at approximately 1–1.5 m from the ground level and 0–20 cm from the outside part into the canopy, on the eastern side of the tree. The fruits were all uniform in colour and free of rind blemishes, with a fruit diameter between 55–74 mm. A total of 280 fruits per block replicates were sampled in the first season, and 140 fruits per block replicate were sampled in the second season.
In the first season, 40 fruits per block replicate (n = 4) of each treatment were randomly allocated to be stored at either 4 °C or −0.6 °C for 14, 27 or 34 d, respectively. The remaining 40 fruits of each replicate were subjected to immediate quality analysis on day 0 (at harvest), with no exposure to cold storage. The same process was repeated during the second season; however, the fruit number was reduced to 20 fruit per replicate due to the low variation observed in the first season.
To increase potential susceptibility to rind pitting and staining, fruits were dehydrated at 25–30 °C for 3 d, in both seasons, before cold storage. After that, the fruits were rehydrated for 1 d at room temperature by placing wet paper towels in each berry tray and covering it with plastic, to achieve approximately 100% RH. The fruit did not receive any of the postharvest treatments that are commercial practices for citrus fruit, i.e., the application of wax or thiabendazole prior to cold storage.
During the second season, the weight loss of the fruit over the cold-storage period was calculated using the following equation:
Following cold storage at the respective storage regimes, the fruits were subjected to a 7 d shelf-life period at 20–22 °C, whereafter the fruits were evaluated for internal and external quality parameters.
2.4. External and Internal Quality Analysis
External quality assessment included the determination of rind colour on the most vivid coloured side of the fruit by means of a chroma meter (CR-400, Konica Minolta Sensing, Inc., Osaka, Japan), immediately prior to the removal of the flavedo in preparation for rind pigment analyses. The rind and pulp colour were expressed as the Hunter a/b ratio, obtained by dividing the Hunter a value by the Hunter b value.
The flavedo was removed with a lemon zester from the fruit sample for each treatment replicate and pooled accordingly before being frozen in liquid nitrogen. Thereafter, it was freeze-dried (Christ Beta 1-8 LD, Christ, Osterode am Harz, Germany) for 2 d before milled to a fine powder with an analytical grinder (Yellow line, A10, IKA-Werke, Staufen, Germany) and stored at −80 °C until rind pigment analysis (see
Section 2.6).
To measure internal quality parameters, following flavedo removal, the fruit was cut in half along the longitudinal plane (from stem to calyx end) for the measurement of pulp colour with a chroma meter (CR-400, Konica Minolta Sensing, Inc., Osaka, Japan) on one pulp segment, whereafter extraction of juice using a citrus juicer (8-SA10, Sunkist®, Chicago, IL, USA) was performed.
The juice was strained through a muslin cloth to remove the pulp particles, whereafter the soluble solids content (SSC), measured as °Brix, was determined with a digital refractometer (PR-32 Palette, ATAGO CO, Tokyo, Japan). From the extracted juice, a 50 mL sample was used to determine the citric acid content, with a potentiometric titrator (888 Titrando, Metrohm AG, Herisua, Switzerland), using Tiamo™ software (Version 2.5). Due to citric acid being the major component of organic acids within Citrus, the percentage citric acid was also used in calculating the sugar-to-acid ratio (°Brix/citric acid percentage).
2.5. Postharvest Rind Disorders
Fruit was rated for detected incidences of staining following the respective storage days and shelf-life period, based on a visual rating system, where 0 = no incidence and 3 = severe (
Figure 1). The staining incidence was calculated as a staining index to provide an estimate of severity and a staining percentage, by using the equations below:
2.6. Rind Pigment Analysis
The chlorophyll and carotenoid content in the flavedo were determined spectrophotometrically. Two extractions per replicate were done in the first season and increased to three in the second season.
Extractions were performed in diminished light conditions to limit direct light exposure. A sample of 0.050–0.055 g freeze-dried and milled flavedo was weighed into a Kimax tube before adding 2 mL absolute ethanol (99.9% v/v) [Merck (Pty) Ltd., Modderfontein, Gauteng, South Africa] and vortexed until the solution was well dispersed. The tubes were placed in a shaker (KS 500, IKA-Werke GmbH&Co. KG, Staufen, Germany) for 30 min at a speed of 265 rpm, whereafter the tube was centrifuged (5810 R, Eppendorf AG, Hamburg, Germany) for 5 min at 4000 rpm and 4 °C. The alcoholic supernatant was transferred to a savant vial that was closed to prevent evaporation. The ethanol extraction procedure was repeated, with the alcoholic phases subsequently pooled. Thereafter, 2 mL n-hexane (≥97.0% v/v) [Sigma-Aldrich, Co., St. Louis, MO, USA] containing butylated hydroxytoluene (BHT) (100 mg·L−1) [Sigma-Aldrich Co., St. Louis, MO, USA] was added to the pellet. The tube was vortexed and then shaken at 265 rpm for 15 min.
The tube was then centrifuged, and the extraction solution was transferred to the Savant tube containing the ethanol fraction, which was closed to prevent evaporation. The hexane extraction was repeated twice following the first extraction.
The extraction was dried under vacuum using a speedvac concentrator (SC210A, Thermo Fisher Scientific, Asheville, NC, USA), set at low-to-medium temperature. The dried extracts were reconstituted with 10 mL pure acetone (100% v/v) [Merck (Pty) Ltd., Modderfontein, Gauteng, South Africa]. The acetone volume was, however, increased up to 16 mL, as rind colour development progressed.
The pigment-containing solution was then transferred to microtubes and centrifuged (5417 R, Eppendorf AG, Hamburg, Germany) for 7 min, 10,000 rpm, and 4 °C before being transferred to a UV-specific cuvette. The absorbance was measured with a spectrophotometer (Cary 60 UV-Vis, Agilent, Santa Clara, CA, USA) at 470, 644 and 662 nm wavelengths, respectively, with 100% acetone as the blank solution. The concentrations of chlorophyll a (C
a), chlorophyll b (C
b) and carotenoids (C
x+c), expressed as µg∙mL
−1, were determined by substituting the absorbance values into equations based on Lichtenthaler [
44] for 100% acetone (
v/
v).
Concentration values were converted to express data as µg∙g
−1 dry weight (DW) for chlorophyll a (C
Ca), chlorophyll b (C
Cb) and carotenoids (C
car) using the equations below, where vf represents the final volume of acetone in L and the unit for flavedo mass in g:
2.7. Statistical Analysis
Statistical analyses were conducted using Statistica 13’s VEPAC module (TIBICO Software Inc., Santa Clara, CA, USA, (version 13), 2017). The mixed model was used to account for repeated measures that were performed on the same blocks over time. Treatment (control and shade-net fruit) and storage duration (days) were entered as fixed effects, and block nested in treatment as a random effect. For cases where there were technical reps within a block (e.g., fruit coming from the same block were individually measured i.e., rind colour), blockstorage duration nested in treatment was further added as a random effect. Fisher’s Least Significant Difference (LSD) tests were used for post hoc testing.
Normal probability plots were inspected to check the normality of the data, and for cases where there were prominent deviations from normality, Box-Cox transformations were performed. The staining index and percentage were analyzed by a one-way analysis of variance (ANOVA).
4. Discussion
The production and delivery of high-quality fruit in the market remain key factors in ensuring competitiveness in the fresh fruit industry, driving producers to incorporate new technology, such as shade netting, to realize these goals. The impact of shade netting on fruit development and its effect on the postharvest potential of citrus fruit are largely unknown. The results from the current study on the impact of 20% white shade netting on ‘Nadorcott’ mandarin fruit when subjected to extended periods of postharvest cold storage reported no negative effects on internal or external fruit quality.
Moisture loss in citrus fruit during postharvest handling and storage is mainly ascribed to water loss through the rind, causing fruit softening and the shrivelling of the rind. Water-based waxing as a postharvest treatment is generally applied to reduce water loss in citrus fruit during storage [
8,
13,
45]. In this study, fruit was deliberately not treated with waxes to avoid interferences of the treatment in the evaluation of the possible impact of preharvest cultivation conditions, such as exposure to shade netting versus open cultivation (no shade netting), on moisture loss of fruit during postharvest. Our results showed that preharvest shading did not affect fruit weight loss during long-term cold storage, irrespective of whether the storage temperatures were at 4 or −0.6 °C.
The results from our study concurred with those of Lee et al. [
38], where preharvest shading did not influence the weight loss of ‘Ponkan’ mandarin. However, in their study, the fruit was stored at 13.5 or 25 °C.
The results from our study concur with both those of Cohen et al. [
26] in ‘Marsh’ grapefruit and ‘Villa franca’ lemon (
C. limon L. Burm.f.), as well as with those of Çandir et al. [
17] in ‘Washington’ Navel, who reported that weight loss increased during cold storage. However, the weight loss reported by Cohen et al. [
26] and Çandir et al. [
17] was not as pronounced as that recorded in our study. This discrepancy may be mostly attributed to the fact that the fruit in the current study was only evaluated after an additional 7 d shelf-life period at 20 to 22 °C, which exacerbated the extent of weight loss. Such an extended shelf period is known to influence weight loss, as reported by Cohen et al. [
26] in grapefruit and lemon fruit after being transferred from cold storage to a shelf life of three days at 20 °C. However, it is important to note that none of these aspects resulted in any difference in fruit weight loss between the shade-net fruit and control treatments, suggesting that the netting conditions assessed did not produce a negative impact in the ability the epidermis of the peel to control water exchange during subsequent postharvest cold storage.
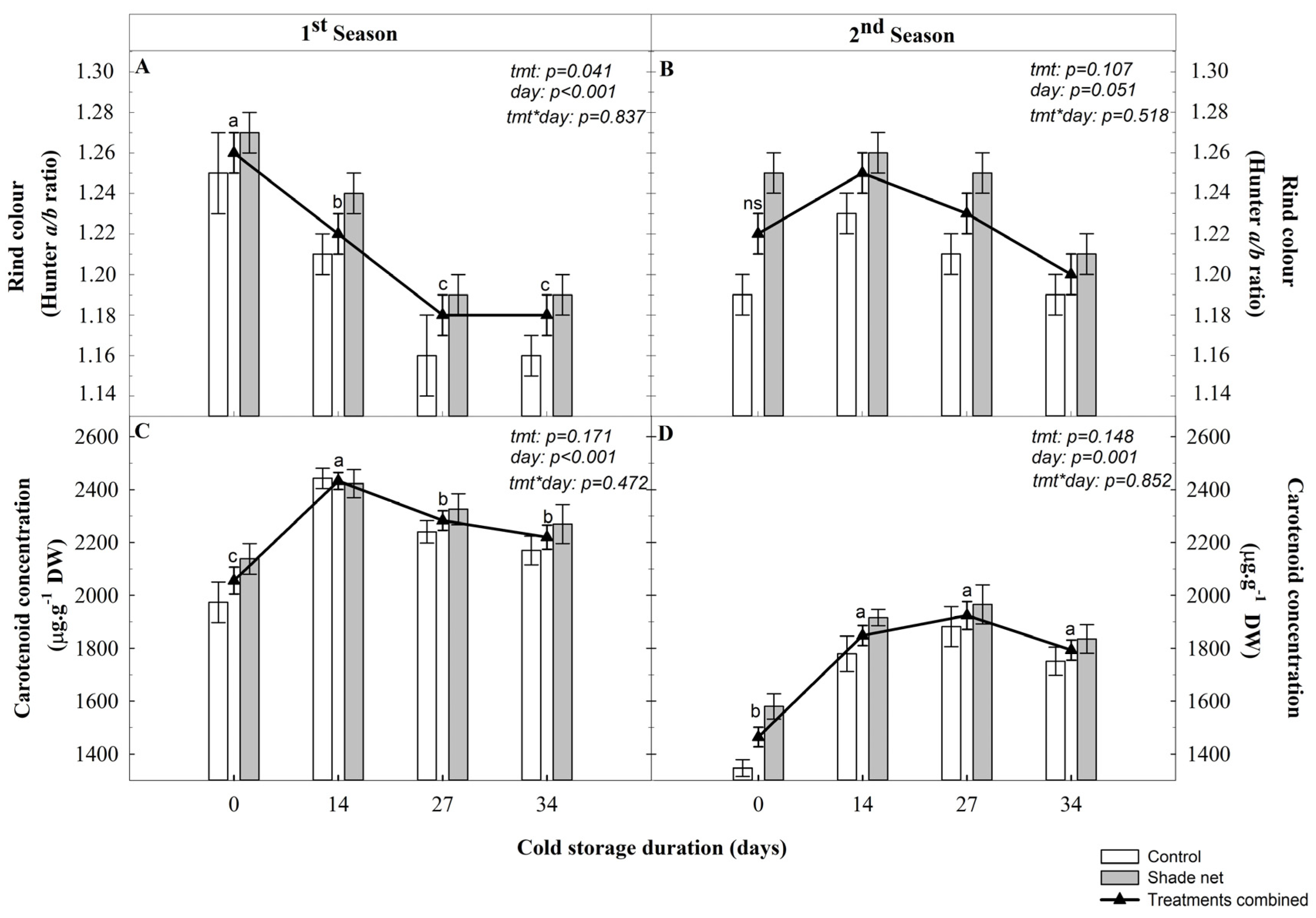
Rind colour largely determines consumers’ acceptance. In
Citrus, carotenoids are responsible for the characteristic orange rind colouration [
46,
47]. In the current study, rind colouration, quantified as the Hunter
a/
b ratio, was not affected by the shade netting treatment at harvest or during cold storage except in the first season for fruit stored at −0.6 °C. However, no visual difference could be detected (personal observation). Seasonal variation in colour development was seen, as illustrated by the different Hunter
a/
b ratios of the fruit stored at 4 °C. In the first season, the
a/
b ratio decreased after 14 d of cold storage, whereas in the second season, an increase in the
a/
b ratio values occurred. In addition, the starting point at harvest was lower in the second season compared to the first. The fruit stored at −0.6 °C showed a decrease in the Hunter
a/
b ratio during the first season, with no cold storage effect evident in the second season. However, there was a trend for fruit stored for 34 d to have a lower ratio in general. Although numerical differences existed in the
a/
b ratio, such changes did not produce visible differences in the external colour (probably due to the high
a/
b values) and, therefore, had little or no commercial importance.
These results on fruit colouration are consistent with those on carotenoid content, since shade netting did not affect the carotenoid content throughout fruit development, as determined at harvest or during cold storage at 4 or −0.6 °C, regardless of the season. However, the storage duration significantly affected the carotenoid content of both treatments, resulting in a general increase in carotenoid content. The difference in rind colouration development between the two seasons might be explained by a delay in rind carotenoid synthesis observed in the second season, resulting in less coloured fruit. Therefore, full colouration could only be achieved during cold storage. Carmona et al. [
20] reported that when ‘Navelina’ oranges were harvested at the breaker stage (−0.11 Hunter
a/
b ratio, indicating that they were not fully coloured) and stored at 2 °C, there was a slight increase in the rind colour, as opposed to the full coloured fruit, which showed no change in colour. Furthermore, the total carotenoid content was not always directly related to colour, at least as determined by the Hunter
a/
b ratio, as differences in the orange and yellow carotenoid ratio impacted the actual flavedo colouration [
20,
48]. It is interesting to note that although the Hunter
a/
b ratio (colour) decreased in the first season during storage at both temperatures, the carotenoid content increased, suggesting changes in the different carotenoid species which did not directly contribute to the rind colour, or to the
a/
b Hunter value.
Cold storage is known to influence rind colouration. Tietel et al. [
13] reported ‘Or’ and ‘Odem’ mandarin fruit exposed to 2 and 5 °C developed a paler rind colour than fruit stored at 8 °C. In contrast, Carmona et al. [
20] found no change in the rind colour and carotenoid content of full coloured ‘Navelina’ orange fruit stored at 2 °C compared to fruit kept at 12 °C. In our study, the fruit from the first season stored at both 4 or −0.6 °C showed a reduction in rind colour, which was in agreement with the findings in a previous study by Van Wyk et al. [
21] on ‘Palmer Navel’ oranges, but contradicting with those reported by Cronje et al. [
29] for ‘Nules Clementine’, where an improvement in rind colouration was observed during storage. The increase in the carotenoid content for the fruit in cold storage recorded in our study followed similar trends to those reported for ‘Nules Clementine’, stored at both −0.5 and 7.5 °C [
29], and for ‘Satsuma’ mandarins stored at 5 °C [
19]. This apparent discrepancy may be explained by an equilibrium between carotenoid synthesis and catabolism, which favours the accumulation of carotenoids with little to no impact in external colouration. In this context, citrus fruit carotenoid biosynthesis is considered temperature-sensitive [
19].
Moreover, different levels of specific carotenoids are known to be present and differentially accumulated in the various commercial citrus varieties. The interaction of these factors may result in different storage behaviour of the fruit, explaining the contradicting reports obtained from studies on different cultivars [
49]. This concurs with Rodrigo et al. [
50], who proposed that the regulatory process of pigmentation in
Citrus fruit differs amongst species.
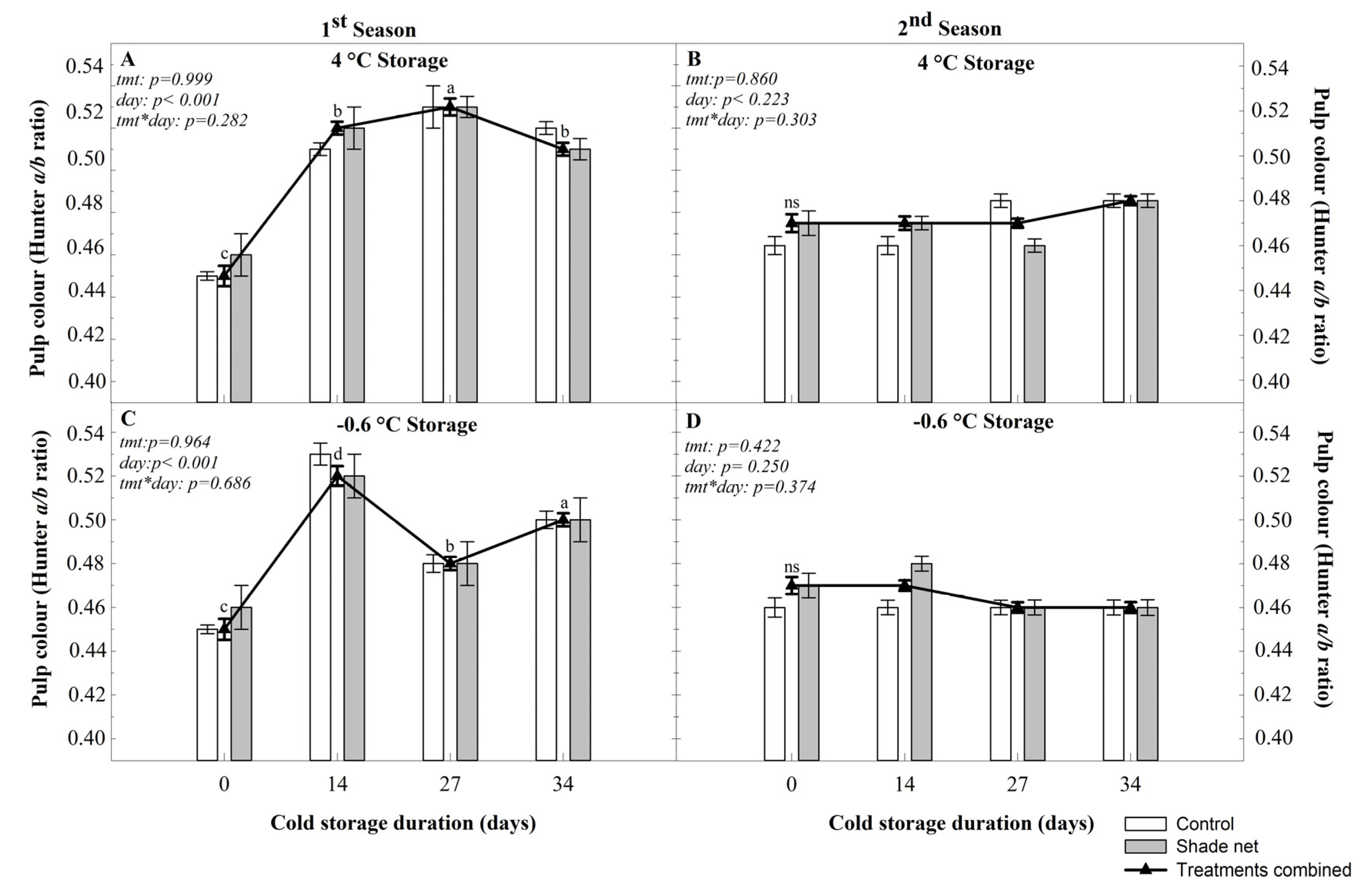
With regards to pulp colouration, no shade net effect was seen, and both subsequent cold-storage temperatures had similar trends, with only an increase in pulp colouration seen during the first season. Due to the lack of comparable literature, no conclusions can be drawn about the reason for the seasonal difference.
The lack of postharvest physiological disorders, along with rind colour, is responsible for determining the appearance and, consequently, the value of the fruit. Rind staining of ‘Nadorcott’ mandarin (
Figure 1) occurred in fruit stored at both cold storage temperatures. Yet, there was no difference when the fruit from the shade netting and the control treatment were compared. The highest staining incidence occurred in the first season, indicating the impact of seasonal macroclimatic factors on fruit rind sensitivity during fruit development [
7,
29]. The absence of any negative impact from the shade netting on postharvest disorders in ‘Nadorcott’ mandarin indicates that the physiological and biochemical changes occurring under the shade conditions assessed did not have a detrimental effect on the postharvest performance of the peel, which is a key result with great commercial significance.
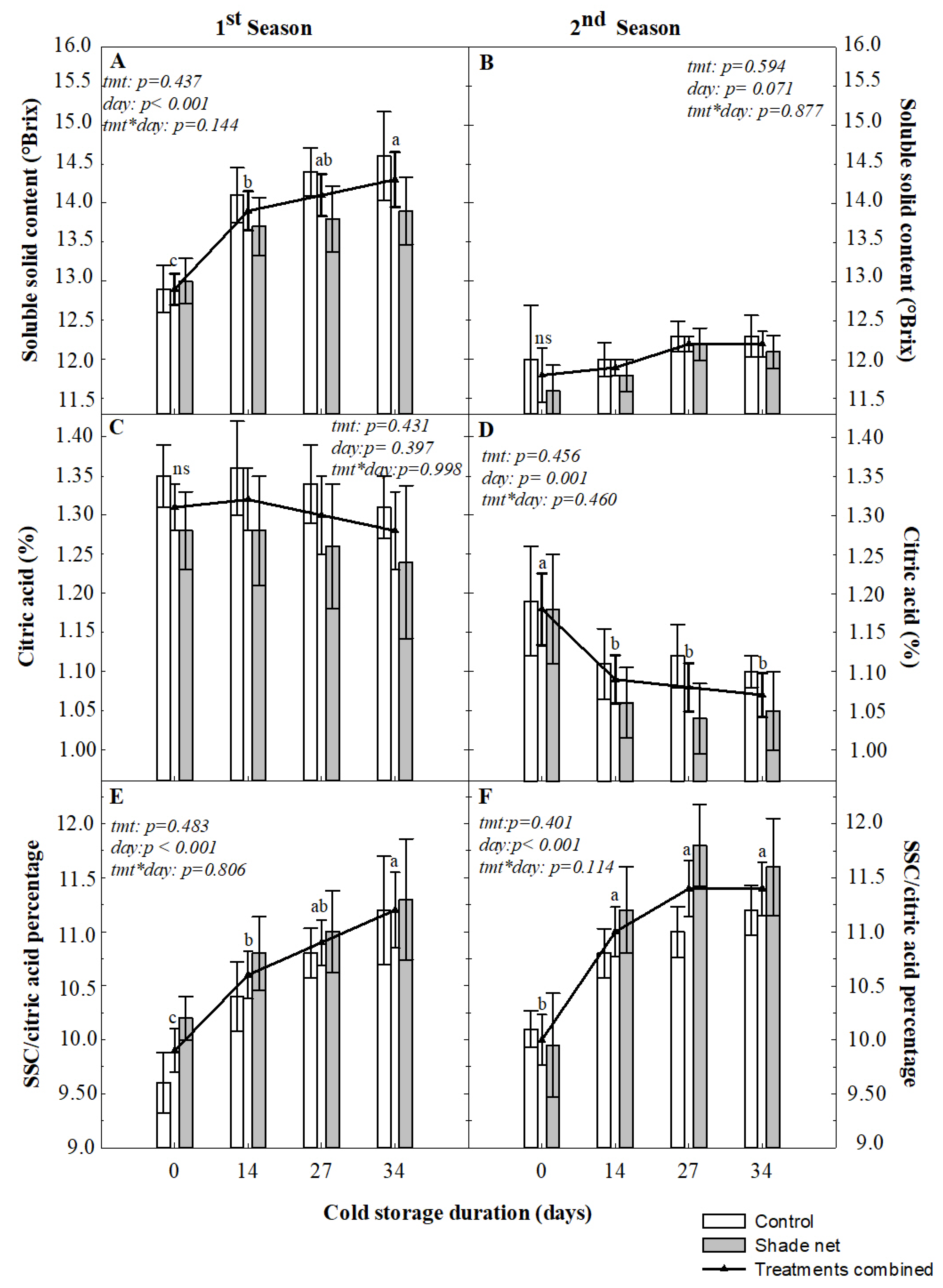
The taste and flavour of mandarin fruit, which is determined by the pulp SSC, acid content and the sugar/acid ratio, are important in shaping consumers’ perception of quality [
13]. Preharvest shade netting did not influence the SSC, citric acid content or their ratio at harvest and during storage at either 4 or −0.6 °C. Similar findings were reported for ‘Ponkan’ mandarin, with late shading application not influencing the SSC or titratable acidity [
38]. Due to the increase in SSC and decrease in acid, the SSC/citric acid ratio increased during both seasons when the fruit were subjected to extended periods of cold storage. There is limited research on how mandarin fruit responds to cold storage at chilling temperatures < 0 °C. The observed increase in the ratio reported in our study, due to a decrease in acid content, could be beneficial for producing mandarins with more acceptable quality for export markets, especially after shipment at often mandatory ultra-low temperature regimes. Mature citrus fruit generally has a relatively low respiration rate [
6], which declines during storage at low temperatures, as compared with fruit not exposed to cold storage [
29]. In addition, acids tend to decrease much faster than sugars under storage [
51].
During postharvest storage there are, however, changes that occur in the individual sugars of the juice (glucose, fructose and sucrose), which directly affect the Brix content, as reported by Echeverria and Ismail [
18]. The increase in SSC during the first season can be ascribed to the known changes in these individual sugars. Even though some changes in the internal quality parameter did occur during cold storage, they were still well within the range set for export standards for late mandarins, where a minimum of 12 °Brix, 0.85% acid and a sugar/acid ratio of 10 are allowed [
52]. However, a sub-minimum value for SSC at 11.8 was recorded on some storage days during the second season, at both storage temperatures, a value that is still close to the acceptable standards.
As with rind colouration and the reported disorder incidences, the values measured to quantify internal quality also differed between seasons, indicating the likely impact of macroclimatic effects prevalent between seasons. However, these preharvest conditions were not altered by the shade netting to such an extent as to result in any difference in internal fruit quality. In addition, it was concluded by various researchers that the unique genetics of each mandarin variety play a significant role in how the fruit responds to low temperatures during storage [
13,
18,
53]. Overall, our results from the current study under climatic conditions characteristics of Citrusdal, where the trial site was located, demonstrated that the 20% shade netting conditions used for ‘Nadorcott’ mandarin did not have a detrimental effect on external and internal fruit quality. These conditions can be suitable agronomical growing conditions for avoiding winds, excessive irradiation and other adverse situations.