An Overview of N2O Emissions from Cropping Systems and Current Strategies to Improve Nitrogen Use Efficiency
Abstract
1. Introduction
2. Soil Nitrous Oxide Emissions from Arable Soils: Microbial Processes and Key Controlling Factors
2.1. Nitrous Oxide Production Pathways
- (a)
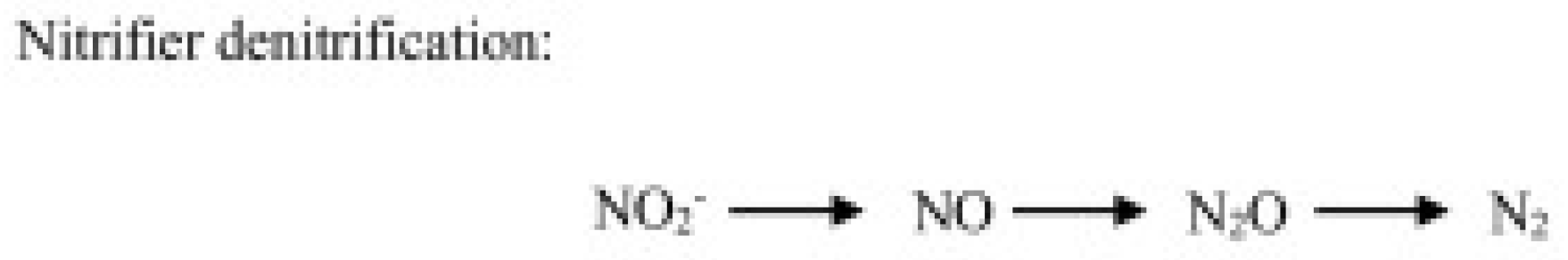
- Nitrification-related pathways, including ammonia (hydroxylamine) oxidation and nitrite oxidation—respectively operated by chemoautotrophic ammonia-oxidizing bacteria (AOB) and archaea (AOA), and nitrite-oxidizing bacteria (NOB)—and the nitrifier denitrification consisting in the reduction in nitrite by ammonia-oxidizing bacteria (Figure 1).
- (b)
- Heterotrophic nitrification, operated by heterotrophic bacteria and fungi, consists of ammonia or organic compound oxidation (Figure 2).
- (c)
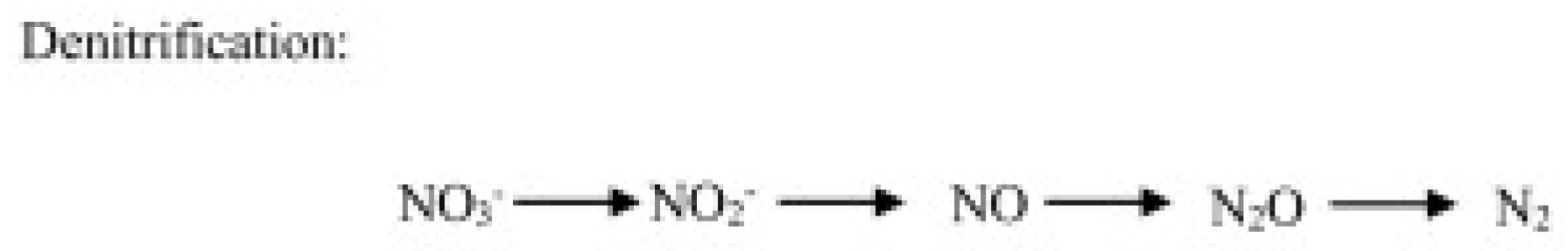
- Heterotrophic denitrification, operated by bacteria and fungi, consists of a microbial respiratory process that reduces oxidized mineral forms of N to gases, including N2O (Figure 3).
2.2. Drivers of Nitrous Oxide Production and Soil Emissions
2.2.1. O2 Levels
2.2.2. Water Content
2.2.3. pH
2.2.4. Temperature
2.2.5. Nitrogen and Carbon Availability
3. Strategies for Improving Nitrogen Use Efficiency by Crops and Mitigating N2O Emissions in Agro-Ecosystems
3.1. Nitrogen Use Efficiency (NUE)
- -
- Breeding N-efficient genotypes. In the past, several research has been carried out in order to select physiological, biochemical, and molecular traits affecting nitrogen uptake by plants through roots. In particular, studies have addressed the spatial root arrangement in the soil evaluating the root depth, lateral root expansion, and root length densities, together with a variety of components, such as roots and root segments [63]. Studies have also been carried out in order to understand the molecular basis of the transport systems involved in the absorption of NO3− from the soil and its systemic fluxes within plants, as well as studies that have been addressed to identify the Ammonium Transporters (AMTs) and the gene family of the Amino acid Transport Family (ATF) [63]. All that knowledge has made it possible to start crop breeding programs with high nitrogen absorption through the introgression of the improved trait into the gene pool of the new genotype [63].
- -
- Adopting appropriate agronomic practices, with particular reference to, e.g., tillage, crop rotation, green manuring, intercropping, grafting, the use of biostimulants, and, obviously, organic/mineral fertilization. This latter aspect (mineral fertilization) is understandably receiving a lot of attention, for example, through the 4R Nutrient Stewardship [67,68], which provides a framework for applying the right nutrient source at the right rate, at the right time, and at the right place. Recently, the use of biostimulants to improve NUE by crops has received more and more attention, with encouraging results. Navarro-León et al. [69] demonstrated that L-α-amino-acid-based biostimulants enhance plant productivity through improved photosynthesis and increased the assimilation of essential nutrients, such as nitrogen (N). Cozzolino et al. [70] found that legume-derived protein hydrolysates and the extract of brown seaweed Ecklonia maxima applied on the leaves improved the yield and quality of tomato grown in the field. The use of lignite-derived humic substances applied to the soil has also proven effective in improving NUE through an improvement in nitrogen uptake efficiency (NUpE) and nitrogen utilization efficiency (NUtE) by tomato crops [71]. Among biostimulants, microbial biostimulants also are currently used in agriculture to improve the efficiency of the use of fertilizers and, in particular, N fertilizers. Bacteria and other microorganisms, through their metabolic processes, exude or secrete a wide range of biochemical compounds into the soil that allow plants to efficiently use nutrients [72].
- Determination of the N demand from the crop.
- Determination of the right N fertilizer rates to meet crop demand, considering the interactions between crop uptake, soil supply, environmental risks, and field operation logistics.
- Correct application techniques, favoring localized over broadcast applications.
- Selection of the proper nutrient source to ensure the best match between nutrient availability and crop requirements.
3.2. Slow- and Controlled-Release Fertilizers (SRFs and CRFs)
3.3. Nitrification Inhibitors (NIs)
3.4. Fertigation
4. Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge Univ Press: Cambridge, UK, 2007. [Google Scholar]
- Tubiello, F.N.; Salvatore, M.; Cóndor Golec, R.D.; Ferrara, A.; Rossi, S.; Biancalani, R.; Federici, S.; Jacobs, H.; Flammini, A. Agriculture, Forestry and Other Land Use Emissions by Sources and Removals by Sinks; ESS Working Paper No. 2; FAO: Rome, Italy, 2014. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, T.K.; Kongshaug, G. Energy Consumption and Greenhouse Gas Emissions in Fertiliser Production; Proceedings No. 509; The International Fertiliser Society: New York, NY, USA, 2004. [Google Scholar]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilizers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef] [PubMed]
- Mumford, M.T.; Rowlings, D.W.; Scheer, C.; De Rosa, D.; Grace, P.R. Effect of irrigation scheduling on nitrous oxide emissions in intensively managed pastures. Agric. Ecosyst. Environ. 2019, 272, 126–134. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, Y.; Mu, Y.; Liu, J.; He, K. Effect of N fertilizer types on N2O and NO emissions under drip fertigation from an agricultural field in the North China Plain. Sci. Total Environ. 2019, 715, 136903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Bruggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Global Chang. Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Chang, C.; Larney, V.; Travis, G.R. Greenhouse gas emissions during cattle feedlot manure composting. J. Environ. Qual. 2002, 31, 376–386. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors that influence nitrous oxide emissions from agricultural soils as well as their representation in simulation models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Drastig, K.; Meyer-Aurich, A.; Ellmer, F.; Baumecker, M. Irrigation, soil organic carbon and N2O emissions. A review. Agron. Sustain. Dev. 2013, 33, 733–749. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, W.; Du, Y.; Sun, J.; Cui, B.; Zhang, E.; Wang, Y.; Siddique, K.H.M. Effect of aerated drip irrigation and nitrogen doses on N2O emissions, microbial activity, and yield of tomato and muskmelon under greenhouse conditions. Agric. Water Manag. 2022, 283, 108321. [Google Scholar] [CrossRef]
- Smith, H.P.J.; Reinsch, T.; Swanepoel, P.A.; Kluß, C.; Taube, F. Grazing under irrigation affects N2O-emissions substantially in south Africa. Atmosphere 2020, 11, 925. [Google Scholar] [CrossRef]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Conant, R.T.; Berdanier, A.B.; Grace, P.R. Patterns and trends in nitrogen use and nitrogen recovery efficiency in world agriculture. Global Biogeochem. Cycles 2023, 27, 558–566. [Google Scholar] [CrossRef]
- Basile, A.P.; Bonfante, A.; De Mascelis, R.; Terribile, F.; Brenna, S.; Acutis, M. Nitrate leaching under maize cropping systems in Po Valley (Italy). Agric. Ecosyst. Environ. 2012, 147, 57–65. [Google Scholar]
- Bancheri, M.; Coppola, A.; Colombi, A.; Basile, A. The extended transfer function model for the simulation of pesticides transport along the unsaturated zone. In Proceedings of the 3rd ISMC Conference—Advances in Modeling Soil Systems, Online, 18–22 May 2021. [Google Scholar]
- Hafner, S.D.; Pacholski, A.; Bittman, S.; Burchill, W.; Bussink, W.; Chatigny, M.; Carozzi, M.; Gnemont, S.; Häni, C.; Hansen, M.; et al. The ALFAM2 database on ammonia emission from field-applied manure: Description and illustrative analysis. Agric. For. Meteorol. 2018, 258, 66–79. [Google Scholar] [CrossRef]
- Abalos, D.; Recous, S.; Butterbach-Bahl, K.; De Notaris, C.; Rittl, T.F.; Topp, C.F.E.; Petersen, S.O.; Hansen, S.; Bleken, M.A.; Rees, R.M.; et al. A review and meta-analysis of mitigation measures for nitrous oxide emissions from crop residues. Sci. Tot. Environ. 2022, 828, 154388. [Google Scholar] [CrossRef] [PubMed]
- Basso, B.; Liu, L.; Ritchie, J.T. A comprehensive review of the CERES-wheat, -maize and-rice models’ performances. Adv. Agron. 2022, 136, 27–132. [Google Scholar]
- Martinez-Feria, R.A.; Castellano, M.J.; Dietzel, R.N.; Helmers, M.J.; Liebman, M.; Huber, I.; Archontoulis, S.V. Linking crop- and soil-based approaches to evaluate system nitrogen-use efficiency and tradeoffs. Agric. Ecosyst. Environ. 2018, 256, 131–143. [Google Scholar] [CrossRef]
- Kushwaha, C.P.; Tripathi, S.K.; Singh, K.P. Variations in soil microbial biomass and N availability due to residue and tillage management in a dryland rice agro-ecosystem. Soil Till. Res. 2000, 56, 153–166. [Google Scholar] [CrossRef]
- Huang, L.; Chakrabarti, S.; Cooper, J. Ammonia-oxidizing archaea are integral to nitrogen cycling in a highly fertile agricultural soil. ISME Commun. 2021, 1, 19. [Google Scholar] [CrossRef]
- Hu, T.; Wei, J.; Du, L. The effect of biochar on nitrogen availability and bacterial community in farmland. Ann. Microbiol. 2023, 73, 4. [Google Scholar] [CrossRef]
- Poly, F.; Wertz, S.; Brothier, E.; Degrange, V. First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol. Ecol. 2008, 63, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lücker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.J. Heterotrophic nitrification—An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.J.; Watmough, N.J. Inorganic nitrogen metabolism in bacteria. Curr. Opin. Chem. Biol. 1999, 3, 207–219. [Google Scholar] [CrossRef]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.W.; Wakagi, T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R Soc. Lond B Biol. Sci. 2012, 5, 1186–1194. [Google Scholar] [CrossRef]
- Groffman, P.M. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol. Appl. 2006, 6, 2091–2122. [Google Scholar] [CrossRef]
- Wolf, B. Grazing-induced reduction of natural nitrous oxide release from continental steppe. Nature 2010, 464, 881–884. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr. Opin. Environ. Sustain. 2011, 3, 389–395. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobe, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Orellana, L.H.; Chee-Sanford, J.C.; Sanford, R.A.; Löffler, F.E.; Konstantinidis, K.T. Year-round shotgun metagenomes reveal stable microbial communities in agricultural soils and novel ammonia oxidizers responding to fertilization. Appl. Environ. Microbiol. 2017, 84, e01646-17. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D. High-throughput sequencing and metagenomics: Moving forward in the culture-independent analysis of food microbial ecology. Appl. Environ. Microbiol. 2013, 79, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.-G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total. Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Conrad, R. Influence of O2 availability on no and N2O release by nitrification and denitrification in soils. Global Chang. Biol. 1998, 4, 387–396. [Google Scholar] [CrossRef]
- Song, X.; Wei, H.; Rees, R.M.; Ju, X. Soil oxygen depletion and corresponding nitrous oxide production at hot moments in an agricultural soil. Environ Pollut. 2022, 292, 118345. [Google Scholar] [CrossRef] [PubMed]
- Saxton, K.E.; Rawls, W.; Romberger, J.S.; Papendick, R.I. Estimating generalized soil-water characteristics from texture. Soil Sci. Soc. Am. J. 1986, 50, 1031–1036. [Google Scholar] [CrossRef]
- Carbonell-Bojollo, R.M.; Veroz-González, Ó.; GonzálezSánchez, E.J.; Ordóñez-Fernández, R.; Moreno-García, M.; RepulloRuibérriz de Torres, M.A. Soil management, irrigation and fertilisation strategies for N2O emissions mitigation in mediterranean agricultural systems. Agronomy 2022, 12, 1349. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, Y.; Zhang, J.; Liu, Q.; Han, J.; Zhang, L. Effects of water and nitrogen management on N2O emissions and NH3 volatilization from a vineyard in North China. Agric. Water Manag. 2022, 266, 107601. [Google Scholar] [CrossRef]
- Lu, L.; Han, W.; Zhang, J.; Wu, Y.C.; Wang, B.; Lin, X. Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J. 2012, 6, 1978–1984. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhong, W.; Cai, Z. Organic nitrogen stimulates the heterotrophic nitrification rate in an acidic forest soil. Soil Biol. Biochem. 2015, 80, 293–295. [Google Scholar] [CrossRef]
- Brenzinger, K.; Dörsch, P.; Braker, G. pH-driven shifts in overall and transcriptionally active denitrifiers control gaseous product stoichiometry in growth experiments with extracted bacteria from soil. Front. Microbiol. 2015, 6, 961. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mørkved, P.T.; Frostegård, A.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef]
- Shaaban, M.; Peng, Q.; Lin, S.; Wu, Y.; Zhao, J.; Hu, R. Nitrous oxide emission from two acidic soils as affected by dolomite application. Soil Res. 2014, 52, 841–848. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.; Wu, L.; Hu, R.; Younas, A.; Nunez-Delgado, A.; Xu, P.; Sun, Z.; Lin, S.; Xu, X.; et al. The effects of pH change through liming on soil N2O emissions. Processes 2020, 8, 702. [Google Scholar] [CrossRef]
- Lai, T.V.; Denton, M.V. N2O and N2 emissions from denitrification respond differently to temperature and nitrogen supply. J. Soils Sed. 2018, 18, 1548–1557. [Google Scholar] [CrossRef]
- Cui, P.; Chen, Z.; Fan, F.; Yin, C.; Song, A.; Li, T.; Zhang, H.; Liang, Y. Soil texture is an easily overlooked factor affecting the temperature sensitivity of N2O emissions. Sci. Total Environ. 2023, 862, 160648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, L.; Wang, C.; Wan, Y.; Yang, R.; Mou, J.; Liu, J.; Wu, Y.; Tang, S.; Zhu, T.; et al. Carbon and nitrogen fractions control soil N2O emissions and related functional genes under land-use change in the tropics. Environ. Pollut. 2023, 335, 122370. [Google Scholar] [CrossRef]
- Akiyama, H.; Tsuruta, H.; Watanabe, T. N2O and NO Emissions from Soils after the Application of Different Chemical Fertilizers; Chemosphere: Global Change Science:: Oxford, UK, 2000; Volume 2, pp. 313–320. [Google Scholar]
- Schlüter, S.; Henjes, S.; Zawallich, J.; Bergaust, L.; Horn, M.; Ippisch, O.; Vogel, H.-J.; Dörsch, P. Denitrification in soil aggregate analogues-effect of aggregate size and oxygen Diffusion. Front. Environ. Sci. 2018, 6, 17. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Coutinho, J.; Trindade, H.; Jensen, L.S. Chemical properties of agro-waste compost affect greenhouse gas emission from soils through changed C and N mineralisation. Biol. Fertil. Soils. 2021, 57, 781–792. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, J.; Yang, W.; Cai, Z. Effects of organic material amendment and water content on NO, N2O, and N2 emissions in a nitrate-rich vegetable soil. Biol. Fertil. Soils. 2012, 49, 53–163. [Google Scholar] [CrossRef]
- Lu, C.; Bowman, D.; Rufty, T.; Shi, W. Reactive nitrogen in turfgrass systems: Relations to soil physical, chemical, and biological properties. J. Environ. Qual. 2015, 44, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B.; Martínez-Gaitan, C.; Gallardo, M.; Giménez, C.; Fernández, M.D. Identification of irrigation and N management practices that contribute to nitrate leaching loss from an intensive vegetable production system by use of a comprehensive survey. Agri Water Manag. 2017, 89, 261–274. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Lammerts van Bueren, E.T.; Struik, P.C. Diverse concepts of breeding for nitrogen use efficiency. A review. Agron. Sustain. Dev. 2017, 37, 50. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Kubo, K.; Burn, S.I.G.; Draycott, A. Apparent recovery of fertilizer N by vegetable crops. Soil Sci. Plant Nutr. 1989, 35, 367–381. [Google Scholar] [CrossRef]
- Ferrante, A.; Nocito, F.F.; Morgutti, S.; Sacchi, G.A. Plant breeding for improving nutrient uptake and utilization efficiency. In Advances in Research on Fertilization Management of Vegetable Crops; Advances in Fertilization Management of Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 221–246. [Google Scholar]
- Tremblay, N.; Scharpf, H.C.; Weier, U.; Laurence, H.; Owen, J. Nitrogen Management in Field Vegetables: A Guide to efficient fertilization. Horticultural Research and Development Centre, Canada. 2001. Available online: https://publications.gc.ca/collections/Collection/A42-92-2001E.pdf (accessed on 27 April 2023).
- Benincasa, P.; Guiducci, M.; Tei, F. The nitrogen use efficiency: Meaning and sources of variation—Case studies on three vegetable crops in Central Italy. HortTechnology 2011, 21, 266–273. [Google Scholar] [CrossRef]
- Tei, F.; De Neve, S.; de Haan, J.; Kristensen, H.L. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 240, 106316. [Google Scholar] [CrossRef]
- Reetz, H.F., Jr.; Heffer, P.; Bruulsema, T.W. Chapter 4: 4R nutrient stewardship: A global framework for sustainable fertilizer management. In Managing Water and Fertilizer for Sustainable Agricultural Intensification; Drechseler, P., Heffer, P., Magen, H., Mikkelsen, R., Wichelns, D., Eds.; International Fertilizer Industry Association (IFA), International Water Management Institute (IWMI), International Plant Nutrition Institute (IPNI), and International Potash Institute (IPI): Paris, France, 2015; pp. 65–86. [Google Scholar]
- Bruulsema, T. Managing nutrients to mitigate soil pollution. Environ. Pollut. 2018, 243, 1602–1605. [Google Scholar] [CrossRef]
- Navarro-León, E.; López-Moreno, F.J.; Borda, E.; Marín, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Effect of L-amino acid-based biostimulants on nitrogen use efficiency (NUE) in lettuce plants. J. Sci. Food Agric. 2022, 102, 7098–7106. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar application of plant-based biostimulants improve yield and upgrade qualitative characteristics of processing tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Qin, K.; Dong, X.; Leskovar, D.I. Improving tomato nitrogen use efficiency with lignite-derived humic substances. Sci. Hortic. 2023, 321, 112243. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.S. Enhanced nitrogen fertilizer technologies support the ‘4R’ concept to optimize crop production and minimize environmental losses. Soil Res. 2017, 55, 463–472. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Tot. Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Haque, K.M.S.; Khan, M.Z.H. A review on application of controlled released fertilizers influencing the sustainable agricultural production: A Cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- Morgan, K.T.; Cushman, K.E.; Sato, S. Release Mechanisms for slow- and controlled-release fertilizers and strategies for their use in vegetable production. HortTechnology 2009, 19, 10–12. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, J.; Liang, T.; Tan, H. Response of bacterial compositions to the use of slow-release fertilizers with long-acting agents and synergists. Appl. Soil Ecol. 2023, 182, 104699. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Coulter, J.A.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Gan, Y. Slow-release fertilizer improves the growth, quality, and nutrient utilization of wintering chinese chives (Allium tuberosum Rottler ex Spreng.). Agronomy 2020, 10, 381. [Google Scholar] [CrossRef]
- Simonne, E.H.; Hutchinson, C.M. Controlled-release fertilizers for vegetable production in the era of best management practices: Teaching New Tricks to an Old Dog. HortTechnology 2005, 15, 36–46. [Google Scholar] [CrossRef]
- Van Eerd, L.L. Nitrogen dynamics and yields of fresh bean and sweet corn with different cover crops and planting dates. Nutr. Cycl. Agroecosyst. 2018, 111, 33–46. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, B.; Zhou, Y.; Zhao, M.; Chen, Y.; Zhang, J.; Wang, J.; Liang, L. Effects of long-term controlled-release urea on soil greenhouse gas emissions in an open-field lettuce system. Plants 2024, 13, 1071. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Caballero-Molada, M.; Atares, S.; García, C.; Vicente, O. New eco-friendly polymeric-coated urea fertilizers enhanced crop yield in wheat. Agronomy 2020, 10, 438. [Google Scholar] [CrossRef]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous overview on the controlled release fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef]
- Shaviv, A. Advances in controlled release of fertilizers. Adv. Agron. 2000, 71, 1–49. [Google Scholar]
- Pasda, G.; Hähndel, R.; Zerulla, W. Effect of fertilizers with the new nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biol. Fertil. Soils 2001, 34, 85–97. [Google Scholar] [CrossRef]
- Gilsanz, C.; Báez, D.; Misselbrook, T.H.; Dhanoa, M.S.; Cárdena, L.M. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP. Agric. Ecosyst. Environ. 2016, 216, 1–8. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, L.; Hu, S.; Compton, J.A.; Greaver, T.; Li, Q. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Global Chang. Biol. 2015, 21, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, I.; Lamsfus, C.; Aparicio-Tejo, P.; Muro, J. The influence of 3, 4-di- methylpyrazole phosphate and dicyandiamide on reducing nitrate accumulation in spinach under Mediterranean conditions. J. Agric. Sci. 2006, 144, 555–562. [Google Scholar] [CrossRef]
- Guardia, G.; Marsden, K.; Vallejo, A.; Jones, D.L.; Chadwick, D.R. Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci. Total Environ. 2018, 624, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.; Opoku, A.; De Neve, S.; Boeckx, P.; Can Cleemput, O.; Hofman, G. Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues. Biol. Fertil. Soils 2006, 43, 62–68. [Google Scholar] [CrossRef]
- Drury, C.F.; Yang, X.; Reynolds, W.D.; Calder, W.; Oloya, T.O.; Woodley, A.L. Combining urease and nitrification inhibitors with incorporation reduces ammonia and nitrous oxide emissions and increases corn yields. J. Environ. Qual. 2017, 46, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Corrochano-Monsalve, M.; Bozal-Leorri, A.; Sánchez, C.; González-Murua, C.; Estavillo, J.-M. Joint application of urease and nitrification inhibitors to diminish gaseous nitrogen losses under different tillage systems. J. Clean. Prod. 2017, 289, 125701. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.D.B. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, P.; Li, B. Effects of fertigation scheme on N uptake and N use efficiency in cotton. Plant Soil 2007, 290, 115–126. [Google Scholar] [CrossRef]
- Lv, H.; Lin, S.; Wang, Y.; Lian, X.; Zhao, Y.; Li, Y.; Du, J.; Wang, Z.; Wang, J.; Butterbach-Bahl, K. Drip fertigation significantly reduces nitrogen leaching in solar greenhouse vegetable production system. Environ. Pollut. 2019, 245, 694–701. [Google Scholar] [CrossRef]
- Lamm, F.R.; Schlegel, A.J.; Clark, G.A. Development of a best management practice for nitrogen fertigation of corn using SDI. Appl. Eng. Agric. 2004, 20, 211–220. [Google Scholar] [CrossRef]
- Bhat, R.; Sujatha, S. Soil fertility and nutrient uptake by arecanut (Areca catechu L.) as affected by level and frequency of fertigation in a laterite soil. Agric. Water Manag. 2009, 96, 445–456. [Google Scholar] [CrossRef]
- Silber, A.; Xu, G.; Levkovitch, I.; Soriano, S.; Bilu, A.; Wallach, R. High fertigation frequency: The effects on uptake of nutrients, water and plant growth. Plant Soil 2003, 253, 467–477. [Google Scholar] [CrossRef]
- Wolff, M.W.; Hopmans, J.W.; Stockert, C.M.; Burger, M.; Sanden, B.L.; Smart, D.R. Effects of drip fertigation frequency and N-source on soil N2O production in almonds. Agric. Ecosyst. Environ. 2017, 238, 67–77. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Souza, T.R.; Zambrosi, F.C.B.; Boaretto, R.M.; Mattos, D., Jr. Nitrogen fertilizer forms affect the nitrogen use efficiency in fertigated citrus groves. J. Plant Nutrit. Soil Sci. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Bozal-Leorri, A.; Subbarao, G.V.; Kishii, M.; Urmeneta, L.; Kommerell, V.; Karwat, H.; Braun, H.J.; Aparicio-Tejo, P.M.; Ortiz-Monasterio, I.; González-Murua, C.; et al. Biological nitrification inhibitor-trait enhances nitrogen uptake by suppressing nitrifier activity and improves ammonium assimilation in two elite wheat varieties. Front. Plant Sci. 2022, 13, 1034219. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ishikawa, T.; Ito, O.; Nakahara, K.; Wang, H.Y.; Berry, W.L. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant Soil 2006, 288, 101–112. [Google Scholar] [CrossRef]
- Lan, T.; Li, M.; He, X.; Deng, O.; Zhou, W.; Luo, L. Effects of synthetic nitrification inhibitor (3,4-dimethylpyrazole phosphate; DMPP) and biological nitrification inhibitor (methyl 3-(4-hydroxyphenyl) propionate; MHPP) on the gross N nitrification rate and ammonia oxidizers in two contrasting soils. Biol. Fertil. Soils 2022, 58, 333–344. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Searchinger, T.D. A “more ammonium solution” to mitigate nitrogen pollution and boost crop yields. Proc. Natl. Acad. Sci. USA 2021, 118, 2107576118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manco, A.; Giaccone, M.; Zenone, T.; Onofri, A.; Tei, F.; Farneselli, M.; Gabbrielli, M.; Allegrezza, M.; Perego, A.; Magliulo, V.; et al. An Overview of N2O Emissions from Cropping Systems and Current Strategies to Improve Nitrogen Use Efficiency. Horticulturae 2024, 10, 754. https://doi.org/10.3390/horticulturae10070754
Manco A, Giaccone M, Zenone T, Onofri A, Tei F, Farneselli M, Gabbrielli M, Allegrezza M, Perego A, Magliulo V, et al. An Overview of N2O Emissions from Cropping Systems and Current Strategies to Improve Nitrogen Use Efficiency. Horticulturae. 2024; 10(7):754. https://doi.org/10.3390/horticulturae10070754
Chicago/Turabian StyleManco, Antonio, Matteo Giaccone, Terenzio Zenone, Andrea Onofri, Francesco Tei, Michela Farneselli, Mara Gabbrielli, Marina Allegrezza, Alessia Perego, Vincenzo Magliulo, and et al. 2024. "An Overview of N2O Emissions from Cropping Systems and Current Strategies to Improve Nitrogen Use Efficiency" Horticulturae 10, no. 7: 754. https://doi.org/10.3390/horticulturae10070754
APA StyleManco, A., Giaccone, M., Zenone, T., Onofri, A., Tei, F., Farneselli, M., Gabbrielli, M., Allegrezza, M., Perego, A., Magliulo, V., & Vitale, L. (2024). An Overview of N2O Emissions from Cropping Systems and Current Strategies to Improve Nitrogen Use Efficiency. Horticulturae, 10(7), 754. https://doi.org/10.3390/horticulturae10070754









