Cytokinin Oxidase (CKX) Family Members in Potato (Solanum tuberosum): Genome-Wide Identification and Expression Patterns at Seedling Stage under Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of CKX Members in Potato
2.2. Analysis of the Characteristics of CKX Genes in Potato
2.3. Plant Materials and Conditions
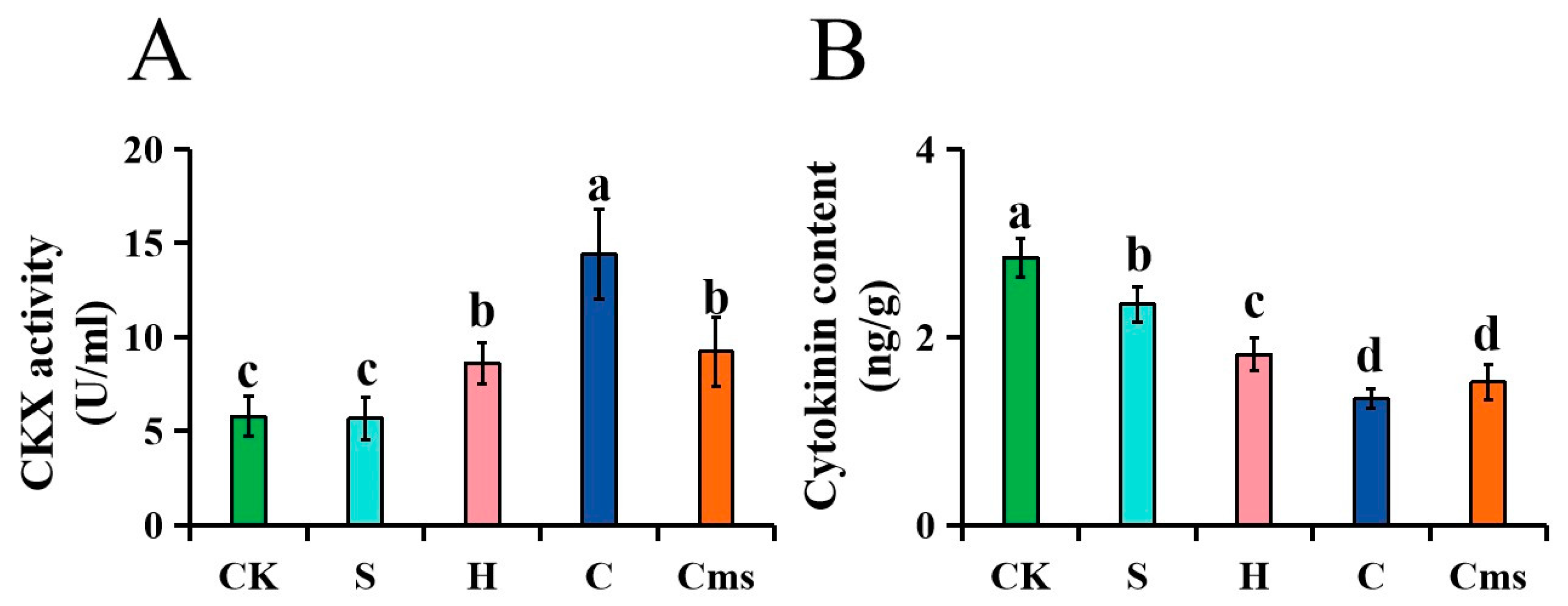
2.4. Measurement of the Cytokinin Content
2.5. The CKX Members’ Expression Levels
2.6. Data Analysis
3. Results
3.1. Identification of CKX Family Members in the Potato Genome
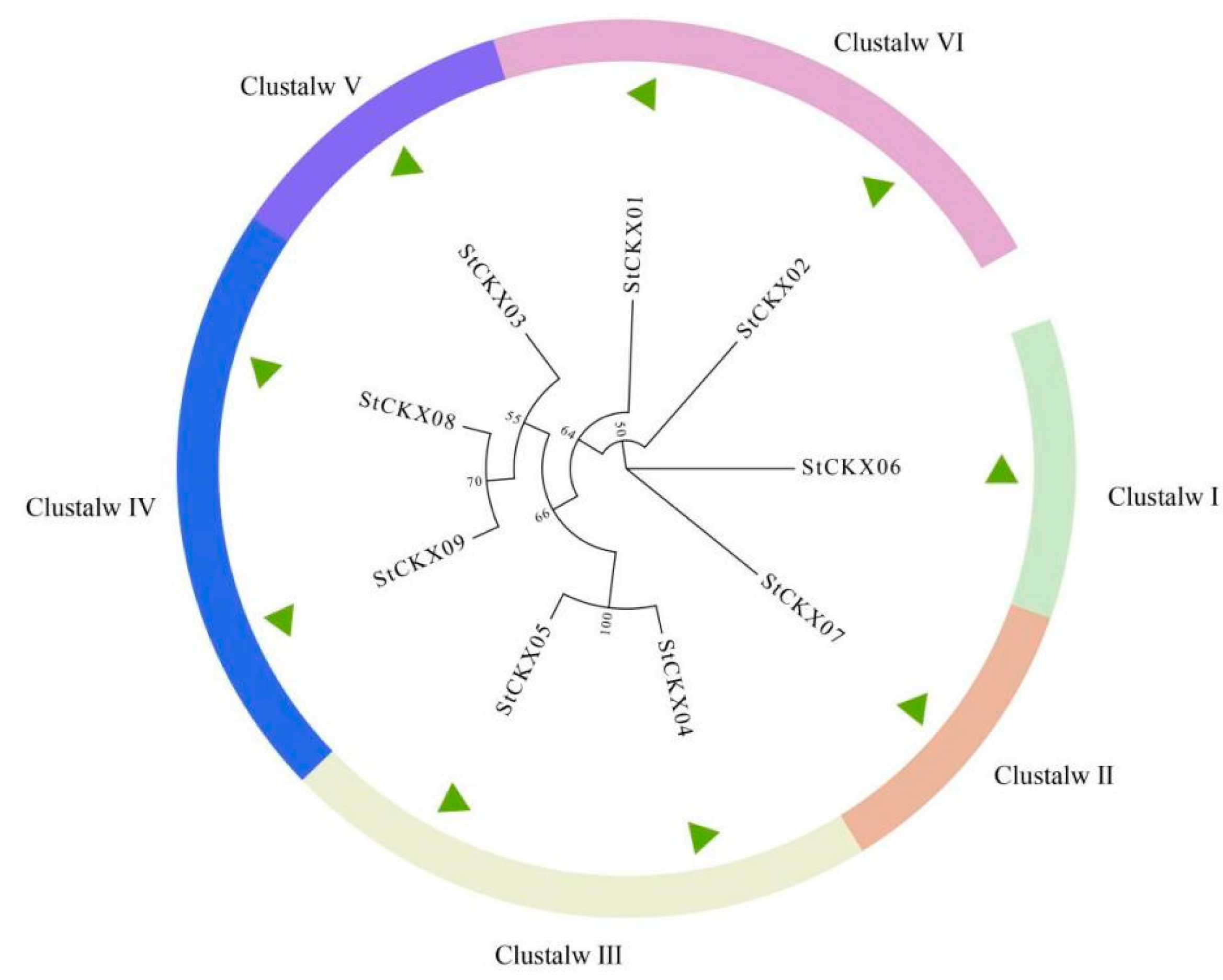
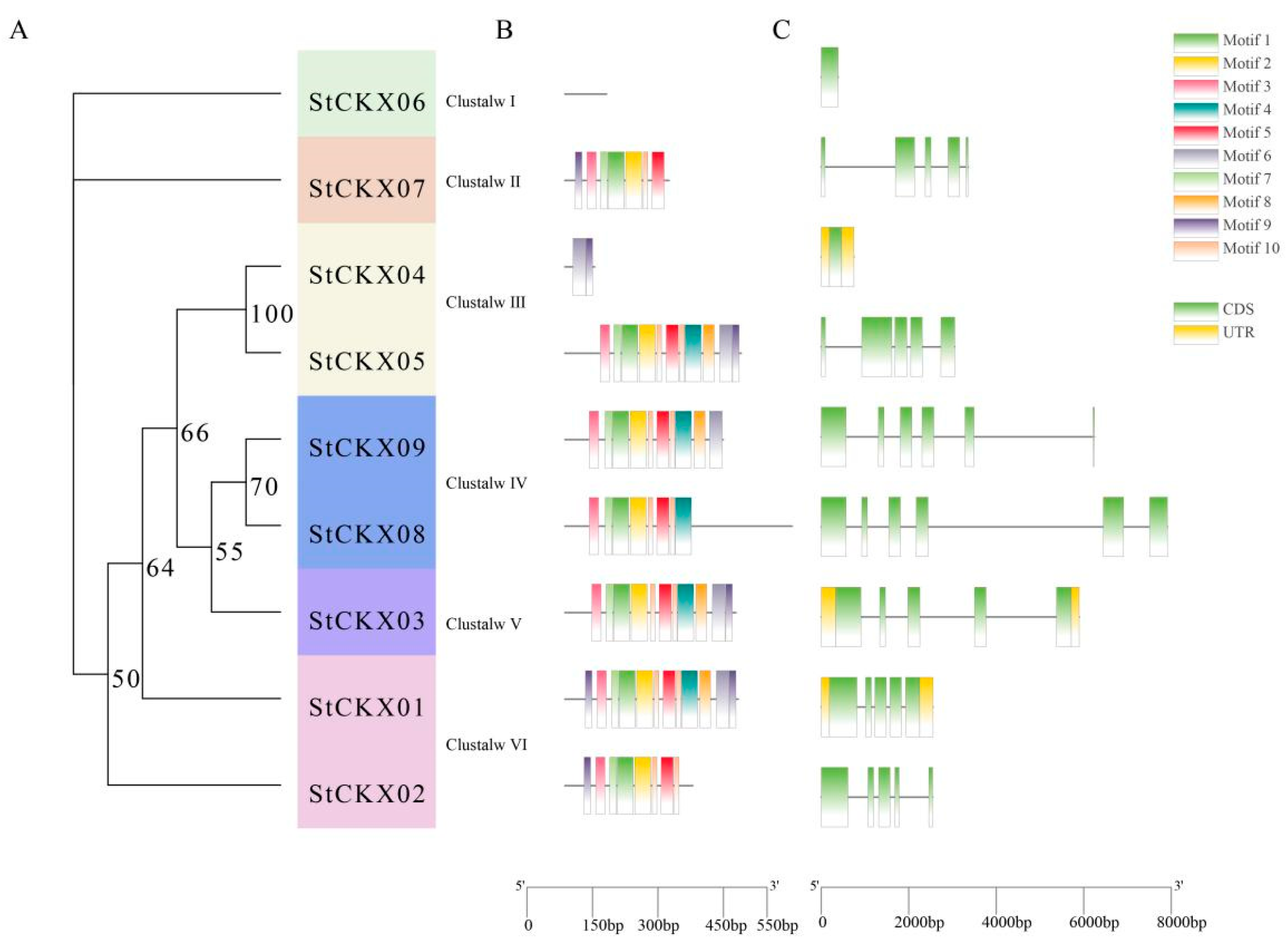
3.2. Evolutionary Analysis of StCKXs
3.3. Comparative Motif and Gene Structure Analysis of StCKXs
3.4. Comprehensive Analysis of the CKXs
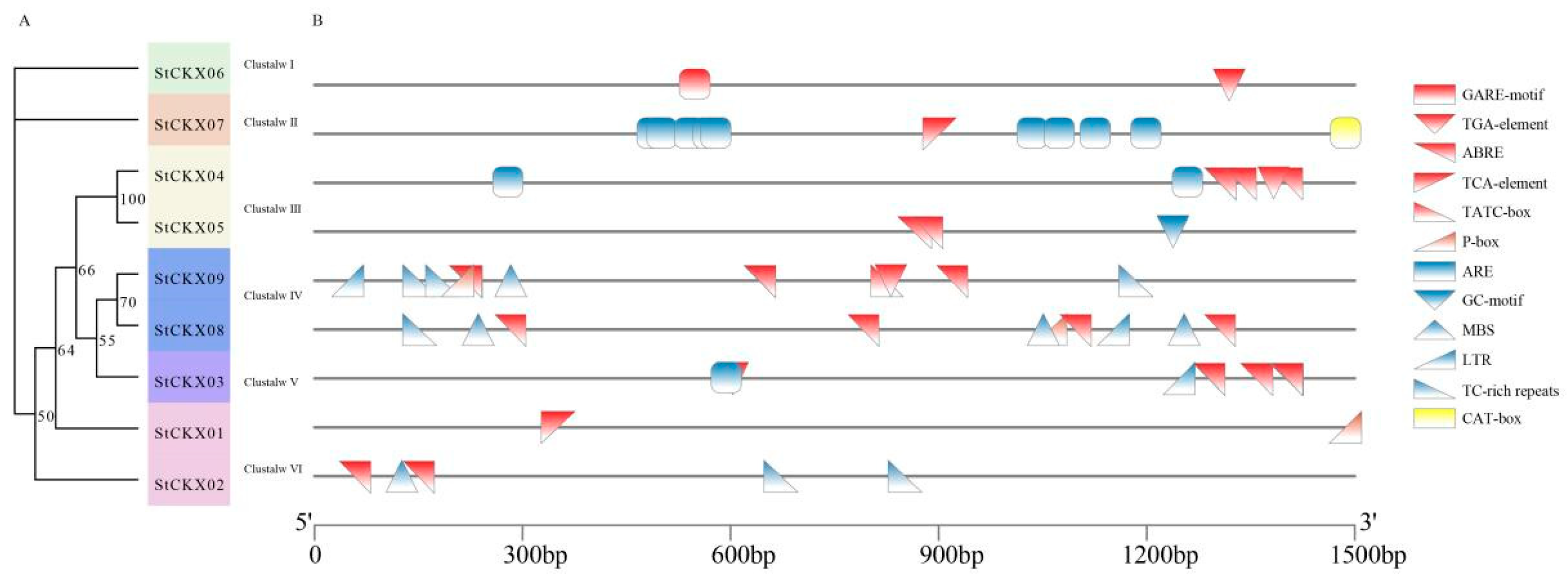
3.5. Analysis of the cis-Acting Elements of StCKXs
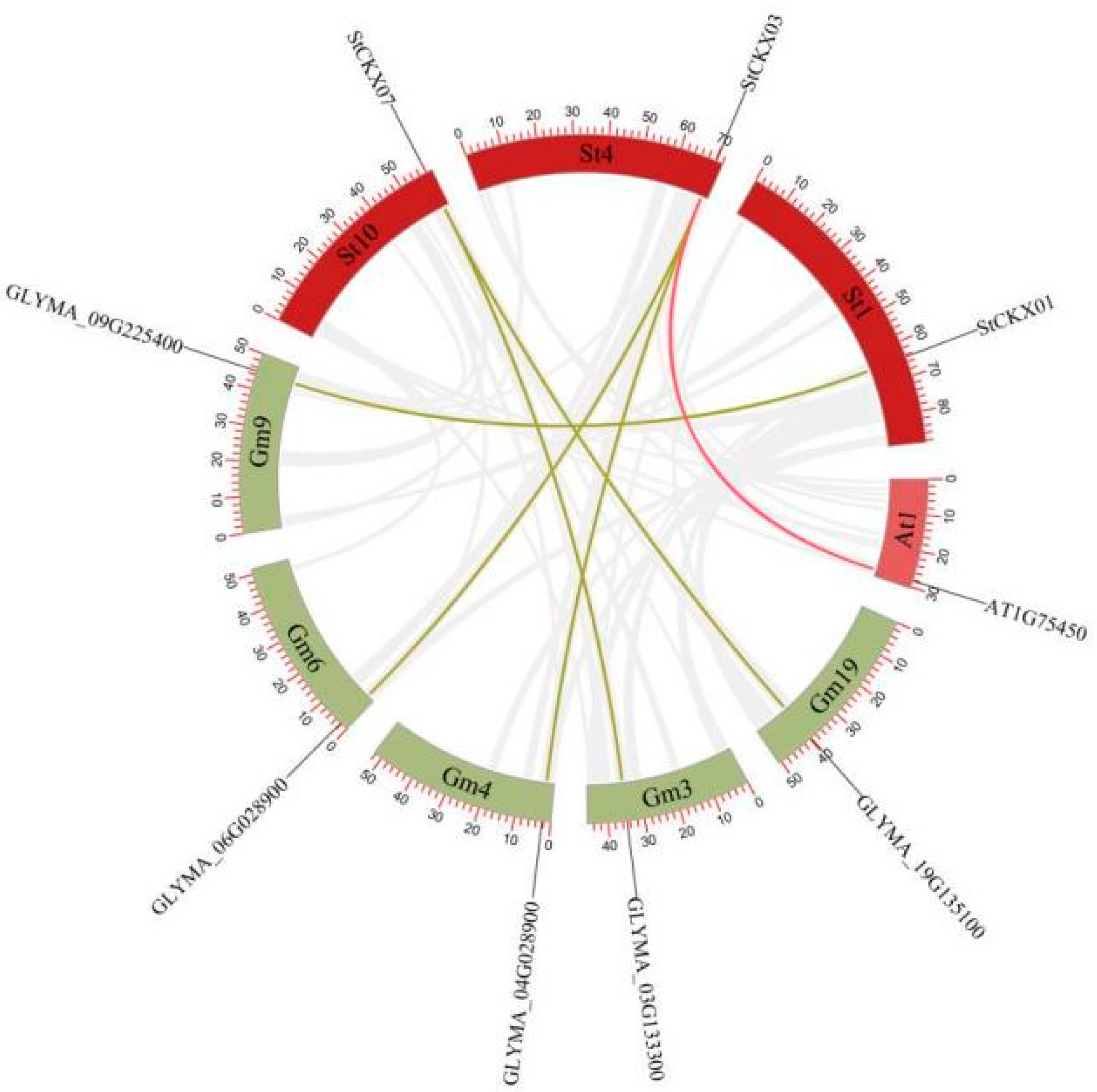
3.6. Collinearity Analysis of StCKXs
3.7. Gene Ontology Enrichment Analysis of StCKXs
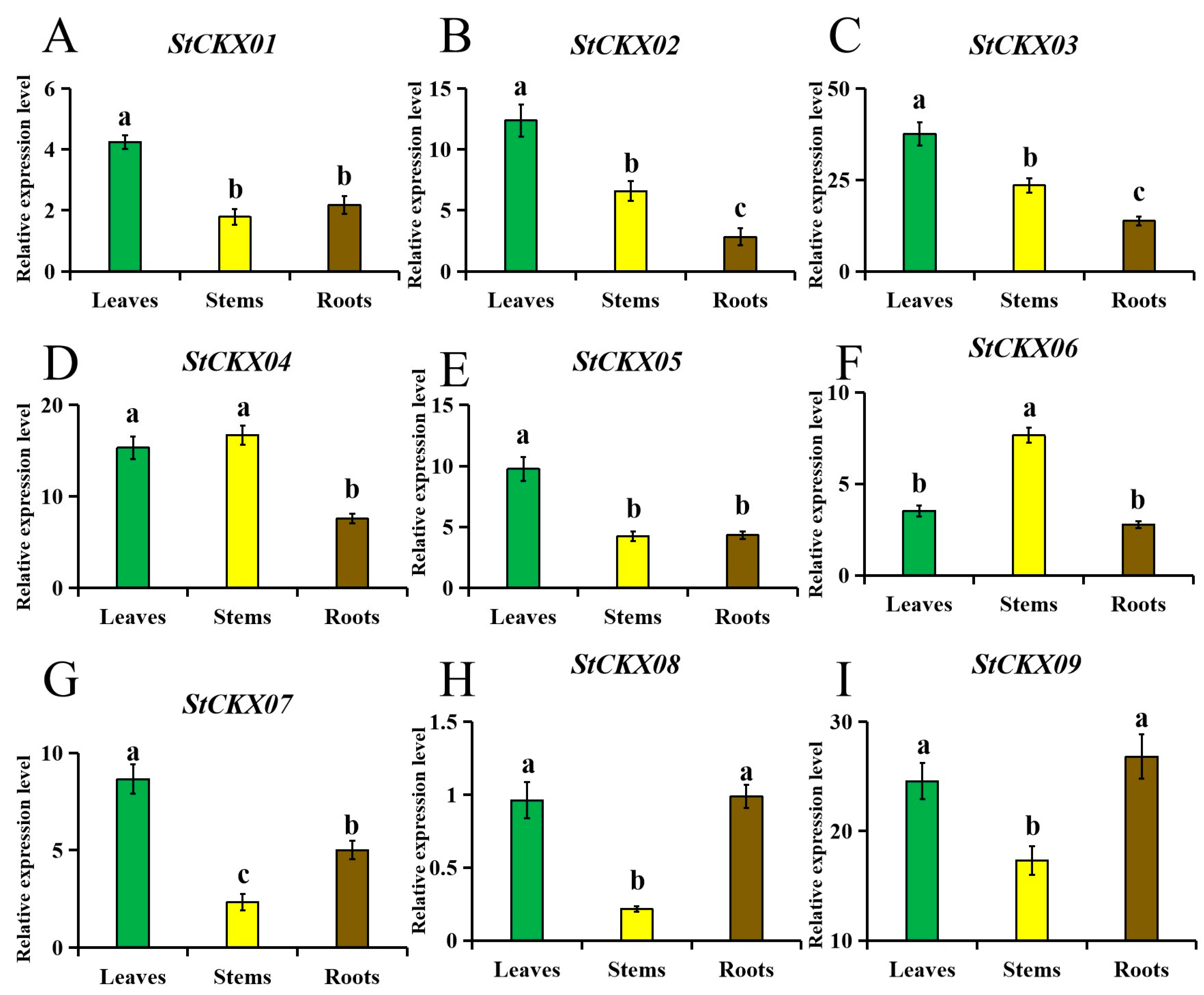
3.8. Tissue-Specific Expression Patterns of StCKXs
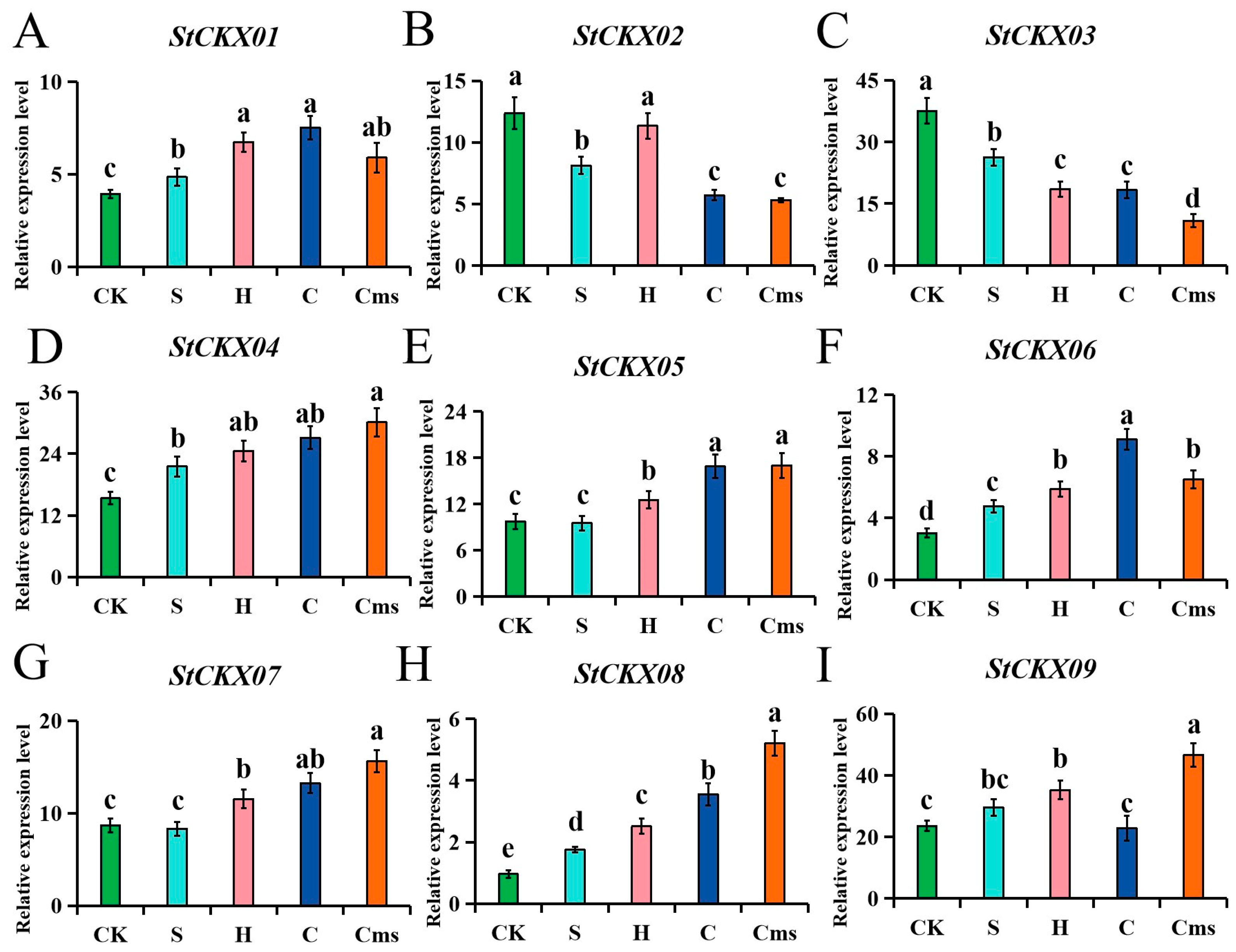
3.9. Expression Patterns of StCKXs Exposed to Various Stress Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wong, C.; Alabadí, D.; Blázquez, M.A. Spatial Regulation of Plant Hormone Action. J. Exp. Bot. 2023, 74, 6089–6103. [Google Scholar] [CrossRef] [PubMed]
- Wybouw, B.; De Rybel, B. Cytokinin—A Developing Story. Trends Plant Sci. 2019, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinin Signaling in Plant Development. Dev. Biol. 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular Mechanism of Cytokinin-Activated Cell Division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Rashotte, A.M. The Evolution of Cytokinin Signaling and Its Role in Development before Angiosperms. Semin. Cell Dev. Biol. 2021, 109, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef] [PubMed]
- Hrtyan, M.; Šliková, E.; Hejátko, J.; Růžička, K. RNA Processing in Auxin and Cytokinin Pathways. J. Exp. Bot. 2015, 66, 4897–4912. [Google Scholar] [CrossRef] [PubMed]
- Drenichev, M.S.; Oslovsky, V.E.; Tararov, V.I.; Mikhailov, S.N. Synthesis of N6 -Substituted Adenosines as Cytokinin Nucleosides. Curr. Protoc. Nucleic Acid Chem. 2018, 72, 14–15. [Google Scholar] [CrossRef]
- Liu, C.-F.; Yang, N.; Teng, R.-M.; Li, J.-W.; Chen, Y.; Hu, Z.-H.; Li, T.; Zhuang, J. Exogenous Methyl Jasmonate and Cytokinin Antagonistically Regulate Lignin Biosynthesis by Mediating CsHCT Expression in Camellia Sinensis. Protoplasma 2023, 260, 869–884. [Google Scholar] [CrossRef]
- Perilli, S.; Moubayidin, L.; Sabatini, S. The Molecular Basis of Cytokinin Function. Curr. Opin. Plant Biol. 2010, 13, 21–26. [Google Scholar] [CrossRef]
- Hoyerová, K.; Hošek, P. New Insights into the Metabolism and Role of Cytokinin N-Glucosides in Plants. Front. Plant Sci. 2020, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.J.; Rashotte, A.M. Cytokinin Inhibition of Leaf Senescence. Plant Signal. Behav. 2013, 8, e24737. [Google Scholar] [CrossRef] [PubMed]
- Lomin, S.N.; Myakushina, Y.A.; Kolachevskaya, O.O.; Getman, I.A.; Savelieva, E.M.; Arkhipov, D.V.; Deigraf, S.V.; Romanov, G.A. Global View on the Cytokinin Regulatory System in Potato. Front. Plant Sci. 2020, 11, 613624. [Google Scholar] [CrossRef] [PubMed]
- Blume, R.; Yemets, A.; Korkhovyi, V.; Radchuk, V.; Rakhmetov, D.; Blume, Y. Genome-Wide Identification and Analysis of the Cytokinin Oxidase/Dehydrogenase (Ckx) Gene Family in Finger Millet (Eleusine coracana). Front. Genet. 2022, 13, 963789. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Weber, H.; Niemann, M.C.E.; Theisl, L.; Leonte, G.; Novák, O.; Werner, T. Arabidopsis HIPP Proteins Regulate Endoplasmic Reticulum-Associated Degradation of CKX Proteins and Cytokinin Responses. Mol. Plant 2021, 14, 1918–1934. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. Evolution of the Cytokinin Dehydrogenase (CKX) Domain. J. Mol. Evol. 2021, 89, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, H.; Jiskrová, E.; Vojta, P.; Mrízová, K.; Kokáš, F.; Čudejková, M.M.; Bergougnoux, V.; Plíhal, O.; Klimešová, J.; Novák, O. Transgenic Barley Overexpressing a Cytokinin Dehydrogenase Gene Shows Greater Tolerance to Drought Stress. N. Biotechnol. 2016, 33, 692–705. [Google Scholar] [CrossRef]
- Bartrina, I.; Otto, E.; Strnad, M.; Werner, T.; Schmülling, T. Cytokinin Regulates the Activity of Reproductive Meristems, Flower Organ Size, Ovule Formation, and Thus Seed Yield in Arabidopsis thaliana. Plant Cell 2011, 23, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, Y.; Chen, B.; Wang, W.; Sun, H.; Sun, H.; Li, J.; Zhao, Q. OsCKX2 Regulates Phosphate Deficiency Tolerance by Modulating Cytokinin in Rice. Plant Sci. 2022, 319, 111257. [Google Scholar] [CrossRef]
- Gasparis, S.; Przyborowski, M.; Kała, M.; Nadolska-Orczyk, A. Knockout of the HvCKX1 or HvCKX3 Gene in Barley (Hordeum vulgare L.) by RNA-Guided Cas9 Nuclease Affects the Regulation of Cytokinin Metabolism and Root Morphology. Cells 2019, 8, 782. [Google Scholar] [CrossRef]
- Prerostova, S.; Černý, M.; Dobrev, P.I.; Motyka, V.; Hluskova, L.; Zupkova, B.; Gaudinova, A.; Knirsch, V.; Janda, T.; Brzobohatý, B. Light Regulates the Cytokinin-Dependent Cold Stress Responses in Arabidopsis. Front. Plant Sci. 2021, 11, 608711. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; An, J.; You, C. Molecular Cloning and Tolerance Identification of Apple Cytokinin Oxidase Gene MdCKX7.2. Acta Hortic. Sin. 2019, 46, 409. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, L.; Hao, Z.; Wu, W.; Xu, L.; Yang, Y.; Zhang, J.; Lu, Y.; Shi, J.; Chen, J. Genome-Wide Identification and Abiotic-Stress-Responsive Expression of CKX Gene Family in Liriodendron chinense. Plants 2023, 12, 2157. [Google Scholar] [CrossRef]
- Li, T.; Luo, K.; Wang, C.; Wu, L.; Pan, J.; Wang, M.; Liu, J.; Li, Y.; Yao, J.; Chen, W.; et al. GhCKX14 Responding to Drought Stress by Modulating Antioxi-Dative Enzyme Activity in Gossypium hirsutum Compared to CKX Family Genes. BMC Plant Biol. 2023, 23, 409. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic-Momcilov, M.; Krstic-Milosevic, D.; Trifunovic, S.; Ciric, A.; Glamoclija, J.; Jevremovic, S.; Subotic, A. Antimicrobial Activity, Antioxidant Potential and Total Phenolic Content of Transgenic Atckx1 Centaury (Centaurium erythraea Rafn.) Plants Grown In Vitro. Environ. Eng. Manag. J. 2019, 18, 2063–2072. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Kuznetsova, E.S.; Dolgikh, V.A.; Sobolev, A.V.; Presniakov, I.A.; Olenev, A.V.; Rahaman, B.; Saha-Dasgupta, T.; Zakharov, K.V.; Zvereva, E.A. Crystal Structure, Physical Properties, and Electronic and Magnetic Structure of the Spin S = 5/2 Zigzag Chain Compound Bi2Fe(SeO3)2OCl3. Inorg. Chem. 2014, 53, 5830–5838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Xu, J.; Zhao, W.; Yang, Y.; Wang, L.; Wang, Q.; Yin, Z.; Sun, H.; Mao, Y. Genome-Wide Identification of Cytokinin Oxidase Family Members in Common Bean (Phaseolus vulgaris): Identification, Phylogeny, Expansion, and Expression Pattern Analyses at the Sprout Stage under Abiotic Stress. Sci. Hortic. 2023, 315, 111974. [Google Scholar] [CrossRef]
- Ma, J.; Xie, L.; Zhao, Q.; Sun, Y.; Zhang, D. Cyclanilide Induces Lateral Bud Outgrowth by Modulating Cytokinin Biosynthesis and Signalling Pathways in Apple Identified via Transcriptome Analysis. Int. J. Mol. Sci. 2022, 23, 581. [Google Scholar] [CrossRef] [PubMed]
- Schmülling, T.; Werner, T.; Riefler, M.; Krupková, E.; Bartrina, Y.; Manns, I. Structure and Function of Cytokinin Oxidase/Dehydrogenase Genes of Maize, Rice, Arabidopsis and Other Species. J. Plant Res. 2003, 116, 241–252. [Google Scholar] [CrossRef]
- Macková, H.; Hronková, M.; Dobrá, J.; Turečková, V.; Novák, O.; Lubovská, Z.; Motyka, V.; Haisel, D.; Hájek, T.; Prášil, I.T. Enhanced Drought and Heat Stress Tolerance of Tobacco Plants with Ectopically Enhanced Cytokinin Oxidase/Dehydrogenase Gene Expression. J. Exp. Bot. 2013, 64, 2805–2815. [Google Scholar] [CrossRef]
- Jain, P.; Singh, A.; Iquebal, M.A.; Jaiswal, S.; Kumar, S.; Kumar, D.; Rai, A. Genome-Wide Analysis and Evolutionary Perspective of the Cytokinin Dehydrogenase Gene Family in Wheat (Triticum aestivum L.). Front. Genet. 2022, 13, 931659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Xin, Q. Genome-Wide Analysis and Identification of Cytokinin Oxidase/Dehydrogenase (CKX) Gene Family in Foxtail Millet (Setaria italica). Crop J. 2014, 2, 244–254. [Google Scholar] [CrossRef]
- Suttle, J.C.; Huckle, L.L.; Lu, S.; Knauber, D.C. Potato Tuber Cytokinin Oxidase/Dehydrogenase Genes: Biochemical Properties, Activity, and Expression during Tuber Dormancy Progression. J. Plant Physiol. 2014, 171, 448–457. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and Human Health. Crit. Rev. Food Sci. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Li, F.; Deng, H.; Wang, Y.; Li, X.; Chen, X.; Liu, L.; Zhang, H. Potato Growth, Photosynthesis, Yield, and Quality Response to Regulated Deficit Drip Irrigation under Film Mulching in a Cold and Arid Environment. Sci. Rep. 2021, 11, 15888. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, C.; Mostaguir, K.; Appel, R.D.; Lisacek, F. The World-2DPAGE Constellation to Promote and Publish Gel-Based Proteomics Data through the ExPASy Server. J. Proteom. 2008, 71, 245–248. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for In Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Paterson, A.H. MCScanX-Transposed: Detecting Transposed Gene Duplications Based on Multiple Colinearity Scans. Bioinformatics 2013, 29, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Geng, J.; Du, Y.; Zhao, Q.; Zhang, W.; Fang, Q.; Yin, Z.; Li, J.; Yuan, X.; Fan, Y. Heat Shock Transcription Factor (Hsf) Gene Family in Common Bean (Phaseolus vulgaris): Genome-Wide Identification, Phylogeny, Evolutionary Expansion and Expression Analyses at the Sprout Stage under Abiotic Stress. BMC Plant Biol. 2022, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Sagcan, H.; Turgut Kara, N. Detection of Potato Ring Rot Pathogen Clavibacter michiganensis subsp. Sepedonicus by Loop-Mediated Isothermal Amplification (LAMP) Assay. Sci. Rep. 2019, 9, 20393. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Reid, R.; Freese, D.; Li, C.; Watkins, J.; Shi, H.; Zhang, H.; Loraine, A.; Song, B.-H. Salt Tolerance Response Revealed by RNA-Seq in a Diploid Halophytic Wild Relative of Sweet Potato. Sci. Rep. 2017, 7, 9624. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Duricki, D.A.; Soleman, S.; Moon, L.D.F. Analysis of Longitudinal Data from Animals with Missing Values Using SPSS. Nat. Protoc. 2016, 11, 1112–1129. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Song, J.; Jameson, P.E. Cytokinin Dehydrogenase: A Genetic Target for Yield Improvement in Wheat. Plant Biotechnol. J. 2020, 18, 614–630. [Google Scholar] [CrossRef]
- Mameaux, S.; Cockram, J.; Thiel, T.; Steuernagel, B.; Stein, N.; Taudien, S.; Jack, P.; Werner, P.; Gray, J.C.; Greenland, A.J. Molecular, Phylogenetic and Comparative Genomic Analysis of the Cytokinin Oxidase/Dehydrogenase Gene Family in the Poaceae. Plant Biotechnol. J. 2012, 10, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, W.; Gasparis, S.; Boczkowska, M.; Rajchel, I.K.; Kała, M.; Orczyk, W.; Nadolska-Orczyk, A. Expression Patterns of HvCKX Genes Indicate Their Role in Growth and Reproductive Development of Barley. PLoS ONE 2014, 9, e115729. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Kambhampati, S.; Kisiala, A.; Seegobin, M.; Emery, R.J.N. The Soybean (Glycine max L.) Cytokinin Oxidase/Dehydrogenase Multigene Family; Identification of Natural Variations for Altered Cytokinin Content and Seed Yield. Plant Direct 2021, 5, e00308. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Yang, W.; Shan, Q.; Sajjad, M.; Zhang, A. Genome-Wide Identification and Expression Analysis of New Cytokinin Metabolic Genes in Bread Wheat (Triticum aestivum L.). PeerJ 2019, 7, e6300. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, J.; Lin, S.; Liu, D.; Gu, T.; Wu, H.; Trigiano, R.N.; McAvoy, R.; Huang, J.; Li, Y. Evolution and Roles of Cytokinin Genes in Angiosperms 2: Do Ancient CKXs Play Housekeeping Roles While Non-Ancient CKXs Play Regulatory Roles. Hortic. Res. 2020, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Zhu, H.; Ji, W.; Hou, Y.; Meng, Y.; Wen, J.; Mysore, K.S.; Li, X.; Lin, H. Genome-Wide Identification and Characterization of Cytokinin Oxidase/Dehydrogenase Family Genes in Medicago truncatula. J. Plant Physiol. 2021, 256, 153308. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Ma, J.-Q.; Zhang, L.-Y.; Yang, B.; Tang, X.-Y.; Huang, L.; Zhou, X.-T.; Lu, K.; Li, J.-N. Genome-Wide Identification and Expression Profiling of Cytokinin Oxidase/Dehydrogenase (CKX) Genes Reveal Likely Roles in Pod Development and Stress Responses in Oilseed Rape (Brassica napus L.). Genes 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, Y.; Zhang, M.; Liu, Y.; Kong, L.; Zou, M.; Lu, G.; Cao, J.; Yu, X. Identification, Expression, and Comparative Genomic Analysis of the IPT and CKX Gene Families in Chinese Cabbage (Brassica rapa ssp. Pekinensis). BMC Genom. 2013, 14, 594. [Google Scholar] [CrossRef]
- Tan, M.; Li, G.; Qi, S.; Liu, X.; Chen, X.; Ma, J.; Zhang, D.; Han, M. Identification and Expression Analysis of the IPT and CKX Gene Families during Axillary Bud Outgrowth in Apple (Malus domestica Borkh.). Gene 2018, 651, 106–117. [Google Scholar] [CrossRef]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an Inflorescence Meristem-specific Cytokinin Oxidase—OsCKX2 in Rice Reduces Yield Penalty under Salinity Stress Condition. Plant Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Z.; Gu, Y.; Li, W.; Wang, W.; Yuan, X.; Zhang, Y.; Yuan, M.; Du, J.; Zhao, Q. Genome-Wide Identification of the Soybean Cytokinin Oxidase/Dehydrogenase Gene Family and Its Diverse Roles in Response to Multiple Abiotic Stress. Front. Plant Sci. 2023, 14, 1163219. [Google Scholar] [CrossRef] [PubMed]
- Farber, M.; Attia, Z.; Weiss, D. Cytokinin Activity Increases Stomatal Density and Transpiration Rate in Tomato. J. Exp. Bot. 2016, 67, 6351–6362. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, B.; Zhang, J.; Chang, L. Cytokinin Oxidase Gene CKX5 Is Modulated in the Immunity of Arabidopsis to Botrytis cinerea. PLoS ONE 2024, 19, e0298260. [Google Scholar] [CrossRef] [PubMed]
- Hyoung, S.; Cho, S.H.; Chung, J.H.; So, W.M.; Cui, M.H.; Shin, J.S. Cytokinin Oxidase PpCKX1 Plays Regulatory Roles in Development and Enhances Dehydration and Salt Tolerance in Physcomitrella patens. Plant Cell Rep. 2020, 39, 419–430. [Google Scholar] [CrossRef]


| Gene Name | chr | Location | CDS Length | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index |
|---|---|---|---|---|---|---|---|
| StCKX01 | 1 | 64568552-64571106 | 387 | 14,886.85 | 5.33 | 36.62 | 89.06 |
| StCKX02 | 4 | 11108702-11111248 | 963 | 35,494.13 | 5.36 | 36.03 | 84.72 |
| StCKX03 | 4 | 70221729-70227626 | 276 | 11,095.66 | 8.82 | 50.1 | 60.99 |
| StCKX04 | 8 | 33888474-33891981 | 1629 | 60,572.18 | 6.14 | 34.95 | 93.45 |
| StCKX05 | 8 | 33930753-33933803 | 1464 | 55,122.1 | 6.47 | 42.83 | 91.01 |
| StCKX06 | 10 | 52575434-52575820 | 2100 | 79,314.95 | 8.62 | 41.4 | 91.29 |
| StCKX07 | 10 | 57972972-57976328 | 1182 | 43,988.23 | 6.42 | 33.64 | 95.24 |
| StCKX08 | 12 | 3611311-3619224 | 1581 | 59,107.35 | 6.1 | 36.02 | 90.44 |
| StCKX09 | 12 | 3650404-3656647 | 1248 | 45,612.36 | 7.12 | 32.86 | 97.01 |
| GO_Accession | Description | Term_Type | Q-Value |
|---|---|---|---|
| GO:0008762 | UDP-N-acetylmuramate dehydrogenase activity | molecular_function | 0.00 |
| GO:0009308 | Amine metabolic process | biological_process | 0.00 |
| GO:0009690 | Cytokinin metabolic process | biological_process | 0.00 |
| GO:0010817 | Regulation of hormone levels | biological_process | 0.00 |
| GO:0016614 | Oxidoreductase activity | molecular_function | 0.00 |
| GO:0019139 | Cytokinin dehydrogenase activity | molecular_function | 0.00 |
| GO:0034754 | Cellular hormone metabolic process | biological_process | 0.00 |
| GO:0042445 | Hormone metabolic process | biological_process | 0.00 |
| GO:0048037 | Cofactor binding | molecular_function | 0.01 |
| GO:0050660 | Flavin adenine dinucleotide binding | molecular_function | 0.01 |
| GO:0050662 | Coenzyme binding | molecular_function | 0.01 |
| GO:0065008 | Regulation of biological quality | biological_process | 0.01 |
| GO:0055114 | Oxidation-reduction process | biological_process | 0.02 |
| GO:1901564 | Organonitrogen compound metabolic process | biological_process | 0.02 |
| GO:0016491 | Oxidoreductase activity | molecular_function | 0.02 |
| GO:0065007 | Biological regulation | biological_process | 0.03 |
| GO:0043168 | Anion binding | molecular_function | 0.04 |
| GO:0000166 | Nucleotide binding | molecular_function | 0.04 |
| GO:1901265 | Nucleoside phosphate binding | molecular_function | 0.04 |
| GO:0036094 | Small molecule binding | molecular_function | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liu, S.; Wang, S.; Xu, F.; Liu, Z.; Jia, B. Cytokinin Oxidase (CKX) Family Members in Potato (Solanum tuberosum): Genome-Wide Identification and Expression Patterns at Seedling Stage under Stress. Horticulturae 2024, 10, 737. https://doi.org/10.3390/horticulturae10070737
Zhang W, Liu S, Wang S, Xu F, Liu Z, Jia B. Cytokinin Oxidase (CKX) Family Members in Potato (Solanum tuberosum): Genome-Wide Identification and Expression Patterns at Seedling Stage under Stress. Horticulturae. 2024; 10(7):737. https://doi.org/10.3390/horticulturae10070737
Chicago/Turabian StyleZhang, Wei, Shangwu Liu, Shaopeng Wang, Feifei Xu, Zhenyu Liu, and Bei Jia. 2024. "Cytokinin Oxidase (CKX) Family Members in Potato (Solanum tuberosum): Genome-Wide Identification and Expression Patterns at Seedling Stage under Stress" Horticulturae 10, no. 7: 737. https://doi.org/10.3390/horticulturae10070737
APA StyleZhang, W., Liu, S., Wang, S., Xu, F., Liu, Z., & Jia, B. (2024). Cytokinin Oxidase (CKX) Family Members in Potato (Solanum tuberosum): Genome-Wide Identification and Expression Patterns at Seedling Stage under Stress. Horticulturae, 10(7), 737. https://doi.org/10.3390/horticulturae10070737






