Abstract
The export of trimmed coconuts necessitates controlling microbial growth and browning, often achieved through the use of sodium metabisulfite (SMS). However, SMS can elicit allergic reactions in operators. To address this concern, ultrafine bubble (UFBs) technology was applied to reduce the SMS concentration. Trimmed coconuts were dipped in either a 1.5% SMS solution or a combination of 1.5% SMS with UFBs (1.5% SMS-UFBs) and compared to coconuts dipped or not dipped in a 3% SMS solution. All treated coconuts were then wrapped with polyvinyl chloride (PVC) film and stored at 2–4 °C for 2 months, followed by transfer to storage at 8–10 °C for an additional 14 days. The results indicated that halving the SMS concentration, with or without UFB application, effectively controlled microbial growth and browning, comparable to using 3% SMS. No contamination of E. coli or Salmonella spp. was detected. The mesocarp whiteness, browning index, polyphenol oxidase (PPO) activity, and total phenolic content of coconuts treated with 1.5% SMS or 1.5% SMS-UFBs did not differ significantly from those dipped in 3% SMS solution (p > 0.05). Similarly, the quality of coconut water and coconut meat in SMS or SMS-UFB treatments did not show significant differences. In dry seasons, using 1.5% SMS with or without UFBs yielded comparable results to those obtained using 3% SMS. However, in wet seasons, the synergistic effects of UFBs on reducing microbial growth incidence were observed, similar to the impact achieved with 3% SMS, whereas 1.5% SMS alone did not inhibit microbial growth. Overall, UFB technology shows promise in reducing SMS concentration by 50% for trimmed young coconuts throughout the year.
1. Introduction
The aromatic coconut (Cocos nucifera L.) ranks second among tropical fruit exports from Thailand, with a recorded export value of approximately 15 billion Baht in 2023 [1]. To meet export demands, green coconut fruits are often trimmed into diamond or cylindrical shapes, a practice popularized for export purposes [2,3]. Trimming alters the coconut’s appearance, rendering it akin to a “semi-fresh-cut” product, as the edible portion remains enclosed within the nut, distinguishing it from conventional fresh-cut fruits and vegetables. Browning is a primary concern with trimmed coconut, and in addition to browning, microbial growth poses another challenge. Therefore, sodium metabisulfite (SMS) has been utilized to inhibit the browning process in trimmed coconut.
Currently, the use of sulfite agents is the most efficient and cheapest chemical approach for controlling browning. Sulfite agents such as SMS, potassium metabisulfite, and sulfur dioxide, alongside chlorine, have become prevalent in efforts to prolong the shelf-life of trimmed coconut. In Thailand, a common practice involves immersing trimmed coconut in a 1–3% SMS solution for 2–5 min, followed by wrapping in plastic film to inhibit enzymatic browning [4]. Mohpraman and Siriphanich (2012) [2] recommended an SMS concentration of ≤5% for ≤5 min of dipping. It is noted that higher SMS concentrations and prolonged dipping times facilitate SMS penetration into the nut’s interior through air channels in the husk and via the soft eye. Commercially, a concentration of 3–5% SMS and a dipping duration of 3–5 min are typical and safe. However, concerns have arisen regarding allergenic effects associated with these substances [5,6]. SMS can induce asthma and skin irritation in some consumers [7]. In addition, SMS is used in marine shrimp harvesting to prevent the occurrence of black spots. Shrimps are soaked in an SMS solution in ice, which is disposed of in sewages that run into marine canals, creating an environmental hazard [8]. From this evidence, the waste solution of SMS after use might affect the environment if discharged into the environment without treatment.
In many countries, the use of sulfites on fresh fruits and vegetables to maintain color and prevent microbial growth has been banned [7,9], except for table grapes, lychees, and blueberries [10,11]. In Thailand, the Ministry of Public Health has expressed concerns about the use of sulfites in foods and has established regulations for food additives. According to the Ministry of Public Health announcement (Issue 389) in 2018, the maximum residue limit for sulfites in fresh-cut vegetables is 50 mg/kg (ppm) [12]. In the case of trimmed young coconuts, the use of SMS is allowed in Thailand and many other countries because SMS does not come into direct contact with the edible part. Previous reports indicate that SMS residue is only found in the coconut mesocarp (husk), and there is a potential risk of SMS penetrating through the soft eye if used in high concentrations or for prolonged soaking [2]. Concerns regarding its adverse health effects and environmental impact necessitate the exploration of alternative preservation methods or the reduction in SMS concentration. Previously, other chemicals, such as those generally recognized as safe (GRAS), citric acid, sodium chloride (NaCl), peroxyacetic acid, or a combination thereof, were considered to replace SMS for controlling browning and microbial growth [3,13]. However, these chemicals were unable to completely replace SMS for prolonging storage life for 8 weeks plus 14 days on the shelf.
In recent years, ultrafine bubble (UFBs) technology has emerged as a promising solution for various applications, including food preservation and microbial control [14]. UFBs, also known as nanobubbles (NBs), are gas bubbles smaller than 1 µm and are invisible to the human eye [15]. UFBs can be generated in a liquid by adjusting gas pressure, ultrasonic intensity, or stirring intensity. Standard techniques for preparing UFBs include mechanical stirring, gas dissolution release, pressure changes, and cavitation. Additionally, UFBs can be produced using microfluidic systems and nanoporous membranes [16]. Characterized by their minute size and high stability, UFBs offer unique properties that enhance their efficacy in reducing oxidative processes and microbial growth [14,17,18]. Additionally, it has been reported that the collapse of nanobubbles generates reactive oxygen species (ROS), including hydroxyl radicals, singlet oxygen, and superoxide [19,20]. These properties have made UFBs a valuable technology for water treatment and disinfection [14,21]. The advantages of UFBs extend to many fields, including agriculture [22,23]. Previous research using UFBs in agriculture has reported that water containing UFBs with oxygen has a greater impact on plant growth. This includes improving seed germination [19,24] and enhancing the growth of cucumber [22] and tomato plants [25]. The potential of UFB technology presents an opportunity to address the challenges associated with browning and microbial growth in trimmed young aromatic coconuts while minimizing reliance on SMS. Therefore, UFB technology might be useful for reducing the concentration of SMS needed for effective preservation.
This research investigates the feasibility of reducing SMS concentration in the preservation process of trimmed young aromatic coconut through the integration of UFB technology. By exploring the synergistic effects of UFBs on inhibiting enzymatic browning and microbial growth, including maintaining the fruit qualities, this study aims to provide insights into sustainable and efficient preservation strategies for the coconut industry.
2. Materials and Methods
2.1. Plant Material and Chemical Treatments
Young aromatic ‘Khon Jeeb’ coconut fruits were harvested 6–7 months after the inflorescence full bloom. Subsequently, the coconuts were transported by pick-up truck to a packing house located in Samut Sakhon Province, Thailand, where they underwent trimming using a sharp knife. Before trimming, the coconut fruits were washed and graded by floating in water. Trimming involved cutting the top and shaping the fruit to form a cylindrical body and flat base, referred to as the ‘cylindrical’ shape. The average weight of the coconuts was approximately 1.2–1.5 kg each [3]. After trimming, the weight of the trimmed coconut was approximately 450–600 g and 14 × 14 × 14 cm in size.
Ultrafine bubble (UFBs) water was generated using a generator at a water flow rate of 10 L/min and an operating pump pressure of 0.5 MPa. The UFB water generated from the cavitation nozzle was recirculated in a UFB water tank. Tap water was generated a UFB water for preparing SMS-UFBs. The size of UFBs was about 0.5509–0.5586 μm and total particle number concentration was approximately 8.7154 × 106 particle/mL after 15 min of generation time.
Sodium metabisulfite (SMS; food grade; the supplier of SMS is Delta Laboratory company) at concentrations of 3% and 1.5% (w/v) was dissolved in tap water, and SMS at a concentration of 1.5% was dissolved in ultrafine bubbles (1.5% SMS-UFBs). Following trimming, the coconuts were immediately immersed in the treatment solutions for 5 min. The fruits were divided into four groups and immersed in 3% SMS (positive control), 1.5% SMS (50% reduction in SMS), and 1.5% SMS-UFBs compared with non-treated fruit (negative control). Subsequently, the fruits were wrapped in an 11-µm thick PVC film and stored at 2–4 °C with 80–85% RH, replicating export conditions for 2 months at the company. They were then transferred to 8–10 °C every month for an additional 14 days to simulate on-shelf conditions. Treated coconut for simulated on-shelf was transported to a postharvest laboratory at the Department of Horticulture, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Kamphaeng Saen campus within 1.5 h by a van.
The fruits were assessed at 0, 7, and 14 days after transfer to 8–10 °C with a batch of 27 fruits and a total of 81 coconut fruits in three repeats. Each treatment batch was evaluated for appearance, incidence of diseased fruit, mesocarp whiteness, browning index, and quality of coconut water and coconut meat. Coconut mesocarp samples were collected and frozen in liquid nitrogen and then stored at −70 °C until use for total phenolic compounds and polyphenol oxidase (PPO) analysis. Each treatment batch consisted of three biological replicates, with one fruit per replication, to assess fruit quality.
All the experiments were repeated three times. The first and second repeat (dry season) were carried out in February–April 2023 and March–May 2023, respectively. The third repeat (wet season) was carried out in July–October 2023. The experiment in three repeats gave the same result in control browning and fruit qualities; therefore, we represent the data of the first experiment. But, the results of microbial growth incidence and microbial analysis were different between the dry and wet seasons. This part was comparing between the dry season (February–April 2023) and wet season (July–October 2023). The average temperature in dry and wet season was about 29.97 °C and 29.70 °C, respectively. The average precipitation was 25.13 mm in the dry season and 267.10 mm in the wet season.
2.2. Incidence of Microbial Contaminated Fruit
The coconut fruits from each treatment exhibiting microbial-contaminated symptoms, such as turning black, blue-green, orange, or pink on the surface of the mesocarp, were counted, and the percentage was calculated.
2.3. Microbiological Analysis
The coconut mesocarp from trimmed coconuts was collected using aseptic techniques, approximately 50–60 g, in autoclaved plastic bags. Total plate count, yeast and mold, fecal coliforms, E. coli, and Salmonella spp. analyses were conducted on the trimmed coconuts from all treatments.
Twenty-five grams of coconut mesocarp were added to an appropriate amount of 0.1% peptone water to achieve a 10−1 dilution. The sample was then homogenized in a stomacher for 2 min. Subsequently, appropriate 1:10 (1 + 9) dilutions in 0.1% peptone water were made. Dilutions of 10−6 should suffice. A spread-plate method was used to analyze the total plate count and yeast and mold, with results reported in colony-forming units (CFU)/g. Fecal coliform contamination was assessed by the MPN-presumptive test, and results were reported in MPN/100 mL by averaging the counts of triplicate sets. Alternatively, E. coli colonies could be distinguished among the coliform colonies on VRBA by adding 100 µg of 4-methyl-umbelliferyl-β-D-glucuronide (MUG) per ml in the VRBA overlay. After incubation, colonies were observed for bluish fluorescence under longwave UV light, following the method outlined in the FDA’s BAM, 8th edition (2001) [26].
For Salmonella spp. contamination assessment, 25 g of coconut mesocarp was added to sterile lactose broth (1:9 dilution). Salmonella analysis was performed using selective differential plate media. After incubation, colonies typical of Salmonella, which are round and medium-sized, with a red color and black center due to the formation of black hydrogen sulfide, were observed. This procedure also followed the method outlined in the FDA’s BAM, 8th edition (2001) [26].
2.4. Mesocarp Whiteness (W) and Browning Index (BI)
The color changes (L*, a*, and b*; CIE color space) of the fruits from all treatment batches were determined by examining two spots beside the coconut fruit in each fruit as the fixed position, using a color meter (Minolta CR-400, Osaka, Japan). The mesocarp whiteness (W) was calculated following Pérez (2016) [27], and the browning index (BI) was calculated following Hunter and Harold (1987) [28] and Palou et al. (1999) [29]. The W and BI were calculated using the following equation:
where X = (a* + 1.75L*)/(5.646L* + a* − 3.012b*).
W = 0.511L* − 2.324a* − 1.100b*
BI = [(X − 0.31)/0.172] × 100
2.5. Total Phenolic Content of Coconut Mesocarp
The total phenolic content (TPC) was measured using the Folin–Ciocalteu reagent method. Two grams of coconut mesocarp were homogenized with 80% ethanol and then filtered using cheesecloth. The crude extract was centrifuged at 12,000× g at 4 °C for 20 min. The supernatant was diluted 50 times with double-ionized water. One milliliter of the sample was mixed with 5 mL of 2 N Folin–Ciocalteu reagent and then added to 4 mL of 7.5% sodium carbonate. The mixture was incubated at 30 °C in a water bath for 30 min. Subsequently, the test tube was cooled in cold water for 5 min, after which the absorbance (Abs) was measured at 760 nm using a spectrophotometer (Thermo Scientific, Genesys 10S, Madision, WI, USA). Gallic acid (Fluka Chemie AG, Buchs, Switzerland) (0–100 mg/L) was used as a standard to produce the calibration curve. The mean of three readings was used, and the total phenolic content was expressed in mg of gallic acid equivalents (GAE)/g fresh weight (FW) [30].
2.6. Polyphenol Oxidase (PPO) Activity of Coconut Mesocarp
One gram of coconut mesocarp was homogenized with 10 mL of 0.1 M phosphate buffer (pH 7.0), to which 0.2 g of polyvinylpyrrolidone (PVP) was added. The homogenate was filtered using cheesecloth and then centrifuged at 17,400× g at 4 °C for 20 min. The supernatants were collected. Two hundred microliters (200 µL) of supernatant were added to 2750 µL of 0.1 M phosphate buffer and 70 µL of 0.1 M catechol and then incubated at room temperature for 3 min. The absorbance was measured at 420 nm using a spectrophotometer (Thermo Scientific, Genesys 10S, Madison, USA). Protein analysis was performed using the Bradford assay. PPO activity was calculated as units/mg protein. The units are defined as the absorbance at 420 nm per minute [31].
The non-treated fruit was terminated for analysis of total phenolic content and enzyme activity of PPO after storage for one month.
2.7. Coconut Water and Coconut Meat Qualities
Coconut water (liquid endosperm) and coconut meat (solid endosperm) were assessed for total soluble solids (TSS), titratable acidity (TA), and turbidity during storage and after transfer to 10 °C.
The TSS of coconut water was measured using a hand refractometer (ATAGO, Tokyo, Japan). Ten milliliters of coconut water were titrated with 0.1 N sodium hydroxide (NaOH) using 1% phenolphthalein as an indicator [32], and results were expressed as malic acid equivalents per mL. The turbidity of coconut water was assessed as the percentage of transmittance (%T) using a spectrophotometer (Thermo Scientific, Genesys 10S, USA) at a wavelength of 610 nm, as described by Campos et al. (1996) [33], with reversed osmosis water used as a blank.
Coconut meat (solid endosperm) was utilized for TSS and TA measurements. Five grams of coconut meat were homogenized with 15 mL of reverse osmosis (RO) water for 30 s and then centrifuged at 13,200× g at 4 °C for 20 min. The supernatant was used for TSS measurement via a hand refractometer, and 5 mL of supernatant was titrated with 0.05 N NaOH using 1% phenolphthalein as an indicator.
The TSS/TA ratio of coconut water and coconut meat was calculated.
2.8. Statistical Analysis
The experiments were repeated thrice (three times). The experiment had a completely randomized design (CRD). The statistical differences in all parameters were analyzed using analysis of variance (ANOVA). Mean separations were determined using Duncan’s new multiple range test (DMRT) with IBM SPSS statistic 26 programs.
3. Results
3.1. Coconut Fruit Appearance and Incidence of Microbial Contaminated Fruit
3.1.1. Coconut Fruit Appearance
All trimmed coconuts dipped in SMS and stored at 2–4 °C for 0 days before storage and for 1–2 months and then transferred to 8–10 °C for 7 days showed no signs of disease, and the coconut mesocarp remained white. In contrast, non-treated fruits exhibited immediate browning of the mesocarp after trimming, with subsequent fungal occurrence. The appearance of fruits dipped in 1.5% SMS and 1.5% SMS-UFBs stored at 2 °C and subjected to simulated on-shelf conditions for 14 days resembled those treated with 3% SMS (positive control) in the dry season (first repeat) (Figure 1). However, in the wet season (third repeat), fruits dipped in 1.5% SMS showed more signs of disease compared to those dipped in 1.5% SMS-UFBs (Figure S1 and Table 1).
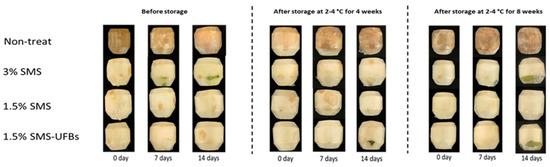
Figure 1.
Trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage), 1 month, and 2 months. After storage, all coconut fruits were transferred to 8–10 °C for 14 days, and photos were taken on days 0, 7, and 14.

Table 1.
Incidence of microbial contaminated fruit of trimmed coconuts dipped in 3% SMS, 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated after storage at 2–4 °C for 2 months.
3.1.2. Incidence of Microbial Contaminated Fruit
In the dry season (first and second repeats), fruits dipped in 3% SMS, 1.5% SMS, and 1.5% SMS-UFBs were not found to be microbial contaminated, while only non-treated fruits exhibited signs of microbial growth. In the wet season (third repeat), fruits dipped in 3% SMS showed no signs of microbial growth, whereas non-treated fruits had the highest incidence of microbial growth. Fruits dipped in 1.5% SMS and 1.5% SMS-UFBs had microbial contaminated incidences of 77.78% and 33.33%, respectively (Table 1).
3.2. Microbial Analysis
All SMS treatments greatly affected the bacterial, yeast, and mold, as well as fecal coliform populations of trimmed young aromatic coconuts in the first repeat of the experiment. The total plate count of coconuts dipped in all SMS treatments was not detected, but it was approximately 1.74 × 106 CFU/g in non-treated fruit. In the wet season of the experiment, only the 3% SMS treatment showed efficiency in controlling bacterial, yeast, and mold growth. Interestingly, the fecal coliform count in the dry season did not differ from that in the wet season (Table 2). E. coli was only found in the non-treated fruit in both season. Salmonella spp. was not found in all treatments of both seasons (Table 3).

Table 2.
Total plate count, yeast and mold, and fecal coliform of trimmed coconuts dipped in 3% SMS, 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated when stored at 2–4 °C for 2 months and then transferred to 8–10 °C for 14 days.

Table 3.
E. coli and Salmonella spp. of trimmed coconuts dipped in 3% SMS, 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated when stored at 2–4 °C for 2 months and then transferred to 8–10 °C for 14 days.
3.3. Mesocarp Whiteness and Browning Index
3.3.1. Whiteness
The mesocarp whiteness of trimmed coconuts before storage (Figure 2A) was approximately 85, gradually increasing over the storage period (Figure 2). Mesocarp whiteness of coconut fruits dipped in 1.5% SMS and 1.5% SMS-UFBs did not differ significantly from those dipped in 3% SMS (Figure 2). In contrast, non-treated fruits exhibited the lowest whiteness, corresponding to a higher browning index, leading to mesocarp browning (Figure 1, Figure 2 and Figure 3). This suggests that reducing SMS concentration to 1.5%, with or without UFBs, demonstrated similar efficacy to 3% SMS in preserving trimmed coconuts.
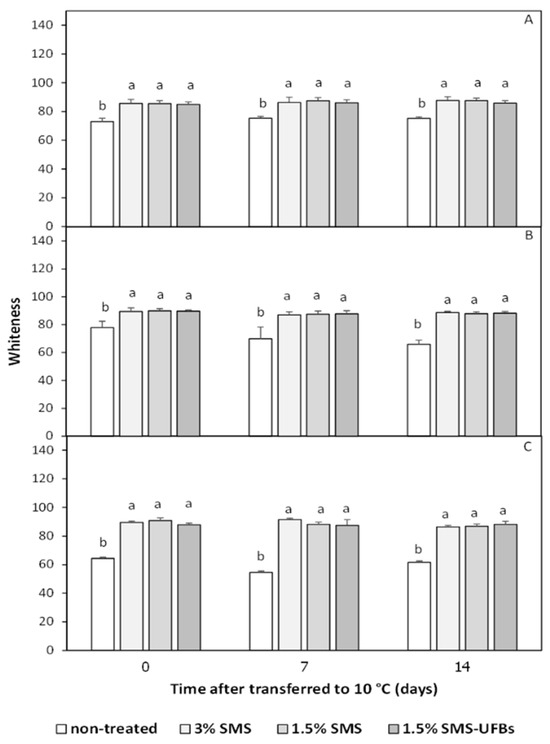
Figure 2.
Mesocarp whiteness of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage) (A), 1 month (B), and 2 months (C). After storage, all coconut fruits were transferred to 8–10 °C for 14 days. Means with the same letter are not significantly different from each other. Error lines represent standard deviation of the mean.
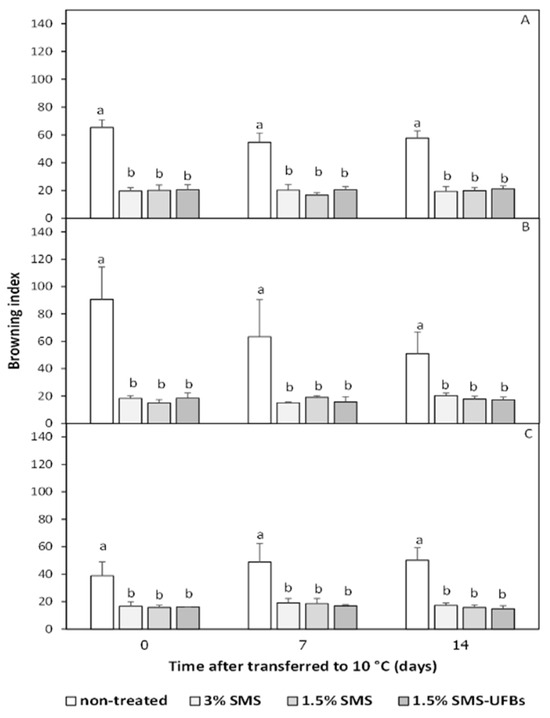
Figure 3.
Mesocarp browning index of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage) (A), 1 month (B), and 2 months (C). After storage, all coconut fruits were transferred to 8–10 °C for 14 days. Means with the same letter are not significantly different from each other. Error lines represent standard deviation of the mean.
3.3.2. Browning Index
The browning index of coconut mesocarp correlated negatively with mesocarp whiteness (Figure 2 and Figure 3). There were no significant differences in the browning index among coconuts treated with SMS, with or without UFBs, when stored at 2–4 °C for 2 months and transferred to 8–10 °C for 14 days. Conversely, non-treated fruits exhibited the highest browning index, which increased after a 2-month storage period. The browning index of all SMS treatments was below 20 (Figure 3).
3.4. Total Phenolic Content in the Mesocarp
The total phenolic content (TPC) in the mesocarp of trimmed coconuts before storage (Figure 4A) was approximately 6000 mgGAE/g FW, gradually increasing over the storage period (Figure 4B,C). TPC in the mesocarp of trimmed coconuts dipped in 1.5% SMS and 1.5% SMS-UFBs did not differ significantly from those dipped in 3% SMS (Figure 4A). In contrast, non-treated fruits exhibited the lowest TPC, corresponding to a higher browning index, leading to mesocarp browning (Figure 1 and Figure 3). After storage for one to two months, the TPC in the mesocarp of trimmed coconuts dipped in 3%SMS remained at a high level as before storage (Figure 4). The results of TPC are related to the activity of PPO (Figure 4 and Figure 5).
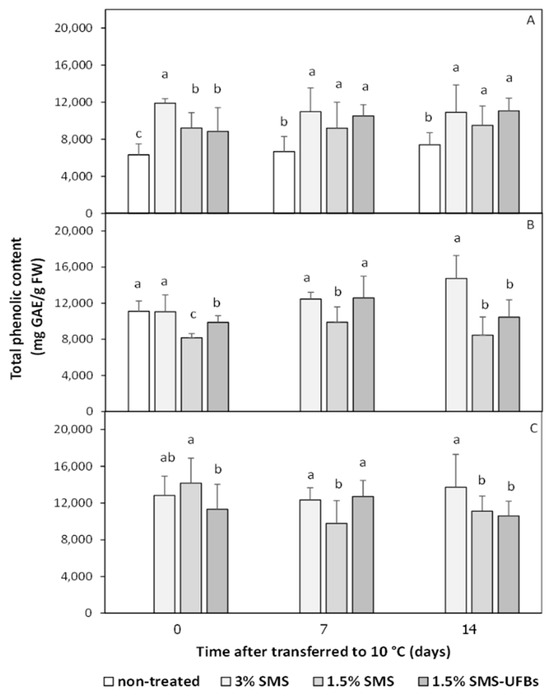
Figure 4.
Total phenolic content in the mesocarp of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage) (A), 1 month (B), and 2 months (C). After storage, all coconut fruits were transferred to 8–10 °C for 14 days. Means with the same letter are not significantly different from each other. Error lines represent standard deviation of the mean.
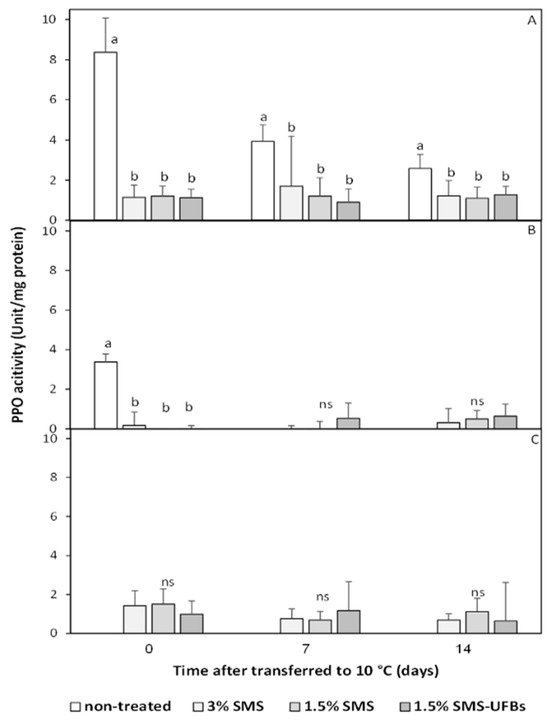
Figure 5.
PPO activity in the mesocarp of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage) (A), 1 month (B), and 2 months (C). After storage, all coconut fruits were transferred to 8–10 °C for 14 days. Means with the same letter are not significantly different from each other. ns: not significantly different. Error lines represent standard deviation of the mean.
3.5. PPO Activity in the Mesocarp
The PPO activity in the mesocarp of non-treated fruit was highest initially (immediately after trimming), and the enzyme activity declined during the 14-day shelf simulation (Figure 5A). In contrast, all trimmed coconuts dipped in SMS or SMS-UFB treatments exhibited low levels of PPO activity, with variations observed among treatments. The PPO activity in the mesocarp of SMS or SMS-UFB treatments was approximately below 2 units/mg protein (Figure 5). Interestingly, the level of PPO activity was lowest after storage for one month (Figure 5B), and then it recovered to levels observed before storage (Figure 5A,C).
3.6. Coconut Water and Coconut Meat Qualities
3.6.1. Coconut Water Qualities
The TSS of coconut water was not significantly different among the treatment groups before storage and when stored at 2–4 °C for 1–2 months and after being transferred to 10 °C for 7 and 14 days. The TSS of coconut water tended to decrease during storage. Initially, the TSS was about 7.5°Brix before storage and then decreased to 6–7°Brix after 2 months of storage (Table 4). The TSS/TA ratio of coconut water in all treatments before storage, when stored at 2–4 °C for one month and after being transferred to 10 °C for 7 and 14 days, was not significantly different. However, the TSS/TA ratio of coconut water from all coconuts stored for 2 months decreased after being transferred to 10 °C, with non-treated fruit exhibiting the lowest TSS/TA ratio (Table 5). Furthermore, the turbidity of coconut water was consistently above 80% transmittance across all treatment groups, indicating clarity (Table 6). However, the turbidity of coconut water from non-treated fruit stored at low temperatures for 2 months and after being transferred to 10 °C for 14 days was below 60% transmittance, indicating that the microorganism on the coconut husk affected the coconut water quality.

Table 4.
Total soluble solids (TSS) of coconut water of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage), 1 month, and 2 months. After storage, all coconut fruits were transferred to 8–10 °C for 14 days. The unit of TSS is °Brix.

Table 5.
The ratio of TSS/TA of coconut water of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage), 1 month, and 2 months. After storage, all coconut fruits were transferred to 8–10 °C for 14 days.

Table 6.
Turbidity or transmittance of coconut water of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage), 1 month, and 2 months. After storage, all coconut fruits were transferred to 8–10 °C for 14 days.
3.6.2. Coconut Meat Qualities
The TSS of coconut meat was around 8–10°Brix both before and after storage at 2–4 °C (Table 7). Compared to coconut water (Table 4 and Table 7), the TSS of coconut meat was higher and did not significantly differ among treatments after being transferred to 10 °C for 14 days (Table 7). The TSS/TA ratio of coconut meat in all treatments before storage, when stored at 2–4 °C for one month and after being transferred to 10 °C for 14 days, did not show significant differences. However, the TSS/TA ratio of coconut meat tended to decrease during storage (Table 8).

Table 7.
Total soluble solids (TSS) of coconut meat of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage), 1 month, and 2 months. After storage, all coconut fruits were transferred to 8–10 °C for 14 days. The unit of TSS is °Brix.

Table 8.
The ratio of TSS/TA of coconut meat of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS, and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage), 1 month, and 2 months. After storage, all coconut fruits were transferred to 8–10 °C for 14 days.
4. Discussion
The experiment was conducted in three repeats. In the first and second repeats (dry season), there was no difference in disease incidence between the 1.5% SMS and 1.5% SMS-UFB treatments. However, in contrast, the third repeat (wet season) revealed that trimmed coconuts dipped in 1.5% SMS had a higher disease incidence, while no disease incidence was observed in fruits dipped in 3% SMS (Table 2). These data suggest that during the wet season, the use of a low concentration of SMS may require the addition of UFBs for controlling microbial growth. Moreover, we found that, in the wet season, the total plate count was more than in the dry season. This is the point of UFBs that have helped control microbial growth because the concentration of hydroxyl radicals (OH⦁) in UFBs generated at 15 min is approximately 3.011 × 10−13 M, with 1.5% SMS-UFB solution containing OH⦁ radicals. These data, accompanied by the decontamination of roselle seed by H2O2 + micro–nano bubbles (MBs), contained the OH⦁ radicals more than those of H2O2 + UV-C [34]. OH⦁ radicals have a potential aid in the removal of attached microorganisms on the surface by enhancing agitation force to facilitate their removal [35].
Interestingly, the total plate count, yeast and mold, and fecal coliform counts in trimmed coconuts dipped in 1.5% SMS and 1.5% SMS-UFBs were not significantly different from those in non-treated fruits and were higher than those dipped in 3% SMS. In this research, the mesocarp samples from each treatment were randomly sampled without using swab techniques. The incidence of microbial growth was observed by the naked eye, and the symptom was revealed on the surface of the trimmed coconut. Further research needs to evaluate microbial growth on the surface by swab technique. All SMS treatments did not detect E. coli, which was only found in contaminated non-treated fruits. The use of 1.5% SMS with and without UFBs is efficient for controlling E. coli as 3% SMS. UFBs’ ability to decrease in size would create a wide surface area for both SMS and the OH⦁ radicals to have better contact with E. coli [14,17,18]. Additionally, Salmonella spp. contamination was not observed in this experiment, indicating good worker hygiene in the coconut packing house and good hygiene practices.
The whiteness and browning index of trimmed coconuts dipped in a 1.5% SMS solution, with and without UFBs, did not differ from those dipped in a 3% SMS solution. Previous research has indicated that browning in coconuts can be controlled by dipping them in an aqueous 1–3% SMS solution for 2–5 min [4,36]. Specifically, the whiteness and browning index of trimmed coconuts dipped in SMS were observed to be over 80 and below 20, respectively. In comparison, trimmed coconuts treated with a salt/acid combination with ozone-ultrafine bubbles (O3UFBs) or under controlled atmospheric conditions (CA) exhibited whiteness values of 25–30 and browning index values over 20 [3,37]. These findings suggest that using a low concentration of SMS results in a whiter and brighter appearance compared to treatments involving salt/acid. The data indicate that a low concentration of SMS is more effective in inhibiting PPO activity.
The prevention of browning using salt/acid works through a different mechanism to inhibit the PPO enzyme. Citric acid acts as a phenolase Cu-chelating agent, and its inhibition of polyphenol oxidase (PPO) is due to this chelating action [38,39]. For NaCl, Ayala-Zavala and Gonzalez-Aguilar (2010) [40] reported that chloride was a weak inhibitor. In contrast, SMS, a reducing agent, reacts with o-quinone to form diphenols, which helps prevent the polymerization of o-quinone into colored pigments [41]. SMS is highly efficient in inhibiting PPO activity and preventing browning. Unal (2007) [42] reported that 0.01 or 0.05 mM of SMS was the most effective in inhibiting PPO, while high concentrations of citric acid (20 mM) and sodium chloride (100 mM) were not as effective.
PPO activity and total phenolic content were correlated with whiteness and browning index. Non-treated fruit exhibited immediate browning after trimming, with a sharp decrease in total phenolic content compared to SMS-treated fruits, while PPO activity reached its highest level. However, PPO activity and total phenolic content did not differ significantly among the SMS treatments. These findings suggest that reducing the SMS concentration, with or without UFBs, had similar results. Therefore, the use of SMS with UFBs did not improve the efficiency of PPO inhibition. Instead, the use of UFBs primarily contributed to controlling microbial growth.
Moreover, after 2 months of storage at 2–4 °C, the trimmed coconuts were found to have a whiter mesocarp than at the beginning of storage. The optimum temperature for PPO activity varies between 20 and 30 °C when using catechol and 4-methyl-catechol as substrates [42,43]. Low temperatures also inhibit PPO activity. Thus, the combination of SMS and low temperature was more effective at inhibiting PPO activity, as indicated by the low level of PPO activity after a month of storage.
The TSS, TSS/TA ratio, and turbidity of coconut water among the four treatments were not significantly different during the 2-month storage period. The TSS of coconut water was around 6.5–7.5°Brix and slightly changed during storage, concurring with Luengwilai et al. (2014) [44]; Jirapong et al. (2018) [45]; and Treesuwan et al. (2022) [37]. However, the TSS and TSS/TA ratio of coconut water from all treatments after storage and then on-shelf simulation trend to decline with on-shelf times (Table 4 and Table 5). The TSS and TSS/TA ratio in coconut water decreased during the on-shelf period due to the consumption of respiratory substrates. Especially, the TSS/TA ratio in coconut water of non-treated fruit storage 2 months sharply decreased (Table 5), while TA in coconut water of their fruit rapidly decreased during on-shelf simulation for 7 and 14 days (Figure S2).
Previously, Luckanatinvong and Sornkeaw (2011) [46] reported that trimmed coconuts dipped in 0.9% SMS for 3–5 min and blanched by hot steaming at 60, 80, and 100 °C and then wrapped with PVC film, maintained fruit quality and inhibited mesocarp browning and microbial growth for one month of storage. However, after two months of storage, it could not control microbial growth. Therefore, until now, no method can completely replace SMS for preventing browning and microbial growth in trimmed young coconuts for 2-month storage plus on shelf simulation. Furthermore, the sulfite residue in the edible portion was tested using the Test Kit for Sulfite (Bleaching Agent) in Food, which was produced by the Government Pharmaceutical Organization (GPO) and provided by the Department of Medical Sciences, Ministry of Public Health, Thailand. The testing revealed that all SMS treatments, with or without UFBs, did not detect any sulfite residue in coconut water and coconut meat. Therefore, reducing the SMS concentration through UFB technology ensures that the edible portion of the coconut fruit is safe for consumption.
5. Conclusions
Halving the SMS concentration, with or without UFB application, effectively controlled microorganisms and browning, comparable to the effects of using 3% SMS. No contamination of E. coli or Salmonella spp. was detected. Mesocarp whiteness, browning index, PPO activity, and total phenolic content in the mesocarp of coconuts treated with 1.5% SMS or 1.5% SMS-UFBs did not differ significantly from those dipped in 3% SMS solution. Similarly, the quality of coconut water and coconut meat in SMS or SMS-UFB treatments did not show significant differences. In dry seasons, the use of 1.5% SMS with or without UFBs yielded comparable results to those obtained using 3% SMS. However, in wet seasons, the synergistic effects of UFBs on reducing the incidence of microbial growth were observed, similar to the effects achieved with 3% SMS, whereas 1.5% SMS alone did not inhibit the incidence of microbial growth. Overall, UFB technology shows promise in reducing SMS concentration by 50% for trimmed young coconuts throughout the year. The findings presented herein contribute to the understanding of UFB technology’s potential in coconut preservation and offer practical solutions for enhancing the shelf life of trimmed coconut for sustainable export in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10070719/s1, Figure S1. Trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 2 months or 8 weeks. After storage, all coconut fruits were transferred to 8–10 °C for 14 days and photos were taken on days 0 and 14. Figure S2. Titratable acidity (TA) of coconut water of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage) (A), 1 month (B) and 2 months (C). After storage, all coconut fruits were transferred to 8–10 °C for 14 days. Error lines represent standard deviation of the mean. Means with the same letter are not significantly different from each other. ns: not significantly different. Figure S3. Titratable acidity (TA) of coconut meat of trimmed coconuts dipped in 3% SMS (positive control), 1.5% SMS and 1.5% SMS-UFBs compared with non-treated (negative control) when stored at 2–4 °C for 0 days (before storage) (A), 1 month (B) and 2 months (C). After storage, all coconut fruits were transferred to 8–10 °C for 14 days. Error lines represent standard deviation of the mean. Means with the same letter are not significantly different from each other. ns: not significantly different.
Author Contributions
Conceptualization, W.I. and S.J.; methodology, W.I., S.J. and S.P.; software S.P.; validation, W.I. and S.P.; formal analysis, S.P. and W.I.; investigation, S.P.; resources, W.I. and S.J.; data curation, S.P. and W.I.; writing—original draft preparation, W.I. and S.P.; writing—review and editing, W.I. and S.J.; visualization, W.I.; supervision, W.I. and S.J.; project administration, W.I.; funding acquisition, W.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Kasetsart University Research and Development Institute (KURDI), grant number: FF(KU) 10.65.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank K Fresh Co., Ltd., Samut Sakhon, Thailand, for providing us with trimmed aromatic coconuts for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Office of Agricultural Economics. Statistics of Thai Agricultural Products Trade with Foreign Countries; Agricultural Information Center, Office of Agricultural Economics, Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2024.
- Mohpraman, K.; Siriphanich, J. Safe use of sodium metabisulfite in young coconuts. Postharvest Biol. Technol. 2012, 65, 76–78. [Google Scholar] [CrossRef]
- Pathomaim, S.; Jarussophon, S.; Arikit, S.; Imsabai, W. Ozone-Ultrafine Bubbles for Reducing Concentration of Citric Acid and Sodium Chloride for Trimmed Young Coconut Preservation. Horticulturae 2023, 9, 284. [Google Scholar] [CrossRef]
- Tongdee, S.C.; Suwannagul, A.; Neamprem, S. Postharvest handling of tender coconut. ASEAN Food J. 1991, 6, 74–75. [Google Scholar]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- Abadias, M.; Alegre, I.; Usall, J.; Torres, R.; Viñas, I. Evaluation of alternative sanitizers to chlorine disinfection for reducing foodborne pathogens in fresh-cut apple. Postharvest Biol. Technol. 2011, 59, 289–297. [Google Scholar] [CrossRef]
- Lien, K.-W.; Hsieh, D.P.; Huang, H.-Y.; Wu, C.-H.; Ni, S.-P.; Ling, M.-P. Food safety risk assessment for estimating dietary intake of sulfites in the Taiwanese population. Toxicol. Rep. 2016, 3, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.M.d.C.M.M.; Cavalcante, A.A.M.; Dantas, A.F.; Pereira, D.L.A.; Rocha, F.C.C.; de Oliveira, F.M.; Da Silva, J. Environmental mutagenicity and toxicity caused by sodium metabisulfite in sea shrimp harvesting in Piauí, Brazil. Chemosphere 2011, 82, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.; Taylor, T. Chemical preservatives and natural antimicrobial compounds. Food Microbiol. Fundam. Front. 2007, 3, 713–745. [Google Scholar]
- European Commission. Commission Regulation (EU) on 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Natskoulis, P.I.; Lappa, I.K.; Panagou, E.Z. Evaluating the efficacy of turbimetric measurements as a rapid screening technique to assess fungal susceptibility to antimicrobial compounds as exemplified by the use of sodium metabisulfite. Food Res. Int. 2018, 106, 1037–1041. [Google Scholar] [CrossRef]
- The Ministry of Public Health Announcement (Issues 389). About Food Additives. 2018. Available online: https://www.ratchakitcha.soc.go.th/DATA/PDF/2561/E/178/1.PDF (accessed on 14 June 2024). (In Thai)
- Nguyen, D.T.N.; Tongkhao, K.; Tongchitpakdee, S. Application of citric acid, sodium chloride and peroxyacetic acid as alternative chemical treatment for organic trimmed aromatic coconut. Chiang Mai Univ. J. Nat. Sci. 2019, 18, 444–460. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice. PLoS ONE 2013, 8, e65339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, T. Preparation Method and Application of Nanobubbles: A Review. Coatings 2023, 13, 1510. [Google Scholar] [CrossRef]
- Ljunggren, S.; Eriksson, J.C. The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction. Colloids Surfaces A Physicochem. Eng. Asp. 1997, 129–130, 151–155. [Google Scholar] [CrossRef]
- Takahashi, M. Base and technological application of micro-bubble and nano-bubble. Mater. Integr. 2009, 22, 2–19. [Google Scholar]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS produced by nanobubbles and their positive and negative effects on vegetable seed germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Maeda, M.; Nishiuchi, Y.; Nagahara, J.; Hata, T.; Zhuowei, Z.; Yoshida, Y.; Watanabe, S.; Ohmori, M. ESR measurement of hydroxyl radicals in micro-nanobubble water. Chem. Lett. 2014, 43, 1907–1908. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Apul, O.G.; Schneider, O.; Garcia-Segura, S.; Westerhoff, P. Nanobubble technologies offer opportunities to improve water treatment. Acc. Chem. Res. 2019, 52, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kitano, M.; Eguchi, H. Water uptake and growth of cucumber plants (Cucumis sativus L.) under control of dissolved O2 concentration in hydroponics. Acta Hortic. 1996, 440, 199–204. [Google Scholar] [CrossRef]
- Minamikawa, K.; Takahashi, M.; Makino, T.; Tago, K.; Hayatsu, M. Irrigation with oxygen-nanobubble water can reduce methane emission and arsenic dissolution in a flooded rice paddy. Environ. Res. Lett. 2015, 10, 084012. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and Its Effect on Seed Germination. ACS Sustain. Chem. Eng. 2015, 4, 1347–1353. [Google Scholar] [CrossRef]
- Wu, Y.; Lyu, T.; Yue, B.; Tonoli, E.; Verderio, E.A.M.; Ma, Y.; Pan, G. Enhancement of Tomato Plant Growth and Productivity in Organic Farming by Agri-Nanotechnology Using Nanobubble Oxygation. J. Agric. Food Chem. 2019, 67, 10823–10831. [Google Scholar] [CrossRef] [PubMed]
- United State Food and Drug Administration. Bacteriological Analytical Manual (BAM). 2001. Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam (accessed on 9 March 2021).
- Pérez, M.d.M.; Ghinea, R.; Rivas, M.J.; Yebra, A.; Ionescu, A.M.; Paravina, R.D.; Herrera, L.J. Development of a customized whiteness index for dentistry based on CIELAB color space. Dent. Mater. 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.S.; Harold, R.W. The Measurement of Appearance; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase Activity and Color of Blanched and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.; van Beek, T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Yang, C.P.; Fujita, S.; Ashrafuzzman, M.D.; Nakamura, N.; Hayashi, N. Purification and characterization of polyphenol oxidase from banana (Musa sapientum L.) pulp. J. Agric. Food Chem. 2000, 48, 2732–2735. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- Campos, C.F.; Souza, P.E.A.; Coelho, J.V.; Gloria, M.B.A. Chemical composition, enzyme activity and effect of enzyme inactivation on flavor quality of green coconut water. J. Food Process. Preserv. 1996, 20, 487–500. [Google Scholar] [CrossRef]
- Phornvillay, S.; Yodsarn, S.; Oonsrithong, J.; Srilaong, V.; Pongprasert, N. A Novel Technique Using Advanced Oxidation Process (UV-C/H2O2) Combined with Micro-Nano Bubbles on Decontamination, Seed Viability, and Enhancing Phytonutrients of Roselle Microgreens. Horticulturae 2022, 8, 53. [Google Scholar] [CrossRef]
- Phaephiphat, A.; Mahakarnchanakul, W. Surface decontamination of Salmonella Typhimurium and Escherichia coli on sweet basil by ozone microbubbles. Cogent Food Agric. 2018, 4, 1558496. [Google Scholar] [CrossRef]
- Paull, R.E.; Ketsa, S. Coconut: Postharvest quality-maintenance guidelines. College of Tropical Agriculture and Human Resources (CTAHR). University of Hawai’i at Manoa, F_N-45. 2015. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/F_N-45.pdf (accessed on 9 March 2021).
- Treesuwan, K.; Jirapakkul, W.; Tongchitpakdee, S.; Chonhenchob, V.; Mahakarnchanakul, W.; Moonmangmee, S.; Tongkhao, K. Effect of controlled atmospheric conditions combined with salt acid immersion on trimmed young coconut qualities during cold storage. Food Packag. Shelf Life 2022, 32, 100857. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, J.; Zauberman, G.; Fuchs, Y. Purification of polyphenol oxidase and the browning control of litchi fruit by glutathione and citric acid. J. Sci. Food Agric. 1999, 79, 950–954. [Google Scholar] [CrossRef]
- Pizzocaro, F.; Torreggiani, D.; Gilardi, G. Inhibition of apple polyphenoloxidase (PPO) by ascorbic acid, citric acid and sodium chloride. J. Food Process. Preserv. 1993, 17, 21–30. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. Use of additives to preserve the quality of fresh-cut fruits and vegetables. In Advances in Fresh-Cut Fruits and Vegetables Processing; Martin-Belloso, O., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 231–254. [Google Scholar]
- Kilic-Akyilmaz, M.; Gulsunoglu, Z. Additives and preservatives. In Handbook of Vegetable Preservation and Processing, 2nd ed.; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 301–318. [Google Scholar]
- Unal, U.M. Properties of polyphenol oxidase from Anamur banana (Musa cavendishii). Food Chem. 2007, 100, 909–913. [Google Scholar] [CrossRef]
- Dogan, M.; Arslan, O.; Dogan, S. Substrate specificity, heatinactivation and inhibition of polyphenol oxidase from different aubergine cultivars. Int. J. Food Sci. Technol. 2002, 37, 415–423. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M.; Pluemjit, O.; Siriphanich, J. Postharvest quality and storage life of ‘Makapuno’ coconut (Cocos nucifera L.). Sci. Hortic. 2014, 175, 105–110. [Google Scholar] [CrossRef]
- Jirapong, C.; Changprasert, S.; Kanlayanarat, S.; Uthairatanakij, A.; Bodhipadma, K.; Noichinda, S.; Wongs-Aree, C. Characterization of the liquid endosperm attributes in young coconut fruit during storage. Int. Food Res. J. 2018, 25, 2650–2656. [Google Scholar]
- Luckanatinvong, V.; Sonkeaw, P.; Siriphanich, J. Quality of blanched aromatic coconut for export. Agric. Sci. J. 2011, 42 (Suppl. 1), 147–150. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).