Abstract
The potential for grapes and wine to be tainted following vineyard exposure to wildfire smoke is well established, with recent studies suggesting hops and apples (and thus beer and cider) can be similarly affected. However, the susceptibility of other crops to ‘smoke taint’ has not yet been investigated. Smoke was applied to a selection of fruits and vegetables, as well as potted lavender plants, and their volatile phenol composition determined by gas chromatography–mass spectrometry to evaluate their susceptibility to contamination by smoke. Volatile phenols were observed in control (unsmoked) capsicum, cherry, lavender, lemon, spinach and tomato samples, typically at ≤18 µg/kg, but 52 µg/kg of guaiacol and 83–416 µg/kg of o- and m-cresol and 4-methylsyringol were detected in tomato and lavender samples, respectively. However, significant increases in volatile phenol concentrations were observed as a consequence of smoke exposure; with the highest volatile phenol levels occurring in smoke-exposed strawberry and lavender samples. Variation in the uptake of volatile phenols by different crops was attributed to differences in their physical properties, i.e., their surface area, texture and/or cuticle composition, while the peel of banana, lemon, and to a lesser extent apple samples, mitigated the permeation of smoke-derived volatile phenols into pulp. Results provide valuable insight into the susceptibility of different crops to smoke contamination.
Keywords:
cresols; fruit; guaiacol; peel; phenol; pulp; smoke; syringol; vegetables; volatile phenols 1. Introduction
For almost two decades, researchers from around the world have been studying the chemical, sensory and physiological impacts of grapevine exposure to wildfire/bushfire smoke [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. The potential for grapes, and therefore wine, to be tainted by smoke has been well established [19] and is attributed to the uptake of smoke-derived volatile compounds, volatile phenols (VPs) in particular, which have been shown to accumulate in grapevine fruit and leaves in glycosylated forms, i.e., as a combination of glucosides, disaccharides and trisaccharides [4,5,15,17,22,23,24,26,36,40]. During fermentation, VP glycosides can be hydrolysed to release their aroma-active aglycones [7,8,17,24], which then impart various smoke-related sensory attributes, e.g., smoky, cold ash, and medicinal aromas and flavours, along with an ashy, drying aftertaste [12,17,20,25,37], colloquially known as ‘smoke taint’. However, post-fermentation, some VP glycosides remain in wine [17,23,24,26,37], and whilst they are seemingly stable at wine pH [21], they can be hydrolysed by salivary enzymes during wine consumption, contributing to the sensory perception of smoke taint [16].
Despite decisive progress towards understanding the consequences of grapevine smoke exposure, smoke taint remains a threat to the long-term economic viability of grape and wine production. As such, global research efforts continue, with a focus on: resolving key knowledge gaps, such as how smoke volatile compounds enter grapes and the identity of other constituents of smoke that might also contribute to smoke taint (e.g., thiophenols [38]); improved sensing and analytical capabilities for monitoring vineyard smoke exposure and quantifying smoke taint in grapes and wine [26,27,28,30,37,39]; and innovative strategies that mitigate either the uptake of smoke volatiles by grapes or their presence in wine [29,31,32]. Achieving these aims will afford the wine industry a suite of options for responding and adapting to the impacts of wildfires made more frequent by climate change. However, to date, there has been little consideration of the potential for smoke to contaminate other horticultural crops, although research has investigated the effects air pollutants on plant physiology, biochemistry and morphology [41,42,43], including the transfer of metals from atmospheric particulate matter into plant tissue (i.e., leaves and fruit) [44,45].
A recent study reported the development and validation of a gas chromatography–mass spectrometry (GC-MS) method for quantification of several VPs (guaiacol, 4-methlylguaiacol, and m- and p-cresols) in control and smoke-exposed hops, and subsequently, in corresponding hop teas and fermentation samples [46]. The results demonstrated that VPs were extracted into hop teas (at up to ~10 µg/L) and transferred into beer (at up to ~5 µg/L), with concentrations dependent on hop dosing rates. Although VP concentrations in beers were below published detection threshold levels (for wine [12,47]), a trained panel concluded that smoke taint was perceptible [46]; however, the authors did not disclose their sensory methodology. Nevertheless, findings suggest the use of smoke-exposed hops during brewing could lead to smoke-tainted beer; presumably, the extent to which beer is tainted depends on some of the same factors that influence the risk of smoke taint in grapes and wine, e.g., the duration of smoke exposure and density of smoke [2,6,30].
From late 2019 into early 2020, a series of bushfires occurred throughout south-eastern Australia, including in New South Wales, where 5.6 million hectares were burned [48]. Significant damage was reported in the apple-producing regions of Batlow and Bilpin, with orchards, machinery and infrastructure (netting, trellising and irrigation systems) being directly or indirectly affected [49]. Whilst devastating, this provided an opportunity to evaluate the potential for smoke to taint apples and cider [50]. Apples were harvested from several orchards that were thought to have been exposed to dense smoke (for up to 30 days) for compositional analysis (i.e., quantification of free and glycosylated VPs, as markers of smoke taint) and for cider production. For comparison, apples were harvested (from the same orchards) in the subsequent year (when there were no fires) and compositional analysis and cider production repeated (to generate control samples). VPs were only detected in cider made from Nicoter apples: the control cider contained 3 µg/L of syringol, whereas the smoke-affected cider comprised 4, 2 and 6 µg/L of guaiacol, 4-methylguaiacol and syringol, respectively [50]. However, the presence of elevated concentrations of VP glycosides in smoke-affected apples and ciders (e.g., 17–39 and 7–15 µg/L of syringol gentiobioside, respectively [50]) provided compositional evidence of smoke taint. Sensory evaluation (by a panel trained to assess smoke taint in wine) confirmed the presence of undesirable smoke characters in ciders made from smoke-affected apples and the study therefore concluded that apples and cider can be tainted when orchards are exposed to smoke.
This study specifically aimed to evaluate the susceptibility of different crops to contamination by smoke: not only fruit and vegetable crops, but also lavender. The study also sought to establish to what extent skin thickness might influence the uptake of smoke-derived VPs, i.e., to determine whether or not the thicker peels of apples, bananas and lemons provide any protection against smoke taint relative to the thinner skins of cherries, grapes, strawberries and tomatoes.
2. Materials and Methods
2.1. Application of Smoke to Different Crops
A selection of fruits and vegetables were purchased from a local produce store (Marketplace, Littlehampton, SA, Australia), while potted lavender plants were purchased from a hardware store (Bunnings, Mount Barker, SA, Australia). Apples (3), bananas (3), capsicum (3), cherries (3 clusters), grape bunches (3), lemons (3), strawberries (3), and tomatoes (3) were suspended (from their stems, in random order) from the middle rack of a wire frame set-up in a purpose-built smoke box [35]. Broccolini florets (3) were suspended from the top rack, while spinach leaves (6) were laid flat on the top rack, and lavender plants (3) were placed on the floor of the smoke box. Barley straw (100 g) was then combusted in a fire box smoker and carried into the smoke box via aluminium exhaust ducting [35], to expose the fruits, vegetables and lavender plants to smoke for 30 min. Immediately after smoke exposure, the peel and pulp of apples, bananas and lemons were separated and the lavender leaves and flowers removed, before all the samples were frozen (−4 °C) in individual ziplock bags (i.e., triplicate samples were stored separately), until needed for compositional analysis (approximately 6 months after smoke exposure); replicate spinach samples each comprised 2 leaves. Control fruit, vegetable and lavender samples (in triplicate) were also frozen, with the peel and pulp of control apples, bananas and lemons also separated.
2.2. Volatile Phenol Analysis
VPs were quantified in the control and smoke-exposed fruit, vegetable and lavender samples using an Agilent 6890N gas chromatograph coupled to a 5973N mass selective detector (Agilent Technologies, Palo Alto, CA, USA), according to stable isotope dilution analysis methods developed and validated for grapes [5]; internal standards (d4-guaiacol, d5-o-cresol, d6-phenol, and d3-syringol) were sourced from CDN Isotopes (Pointe-Claire, QC, Canada).
Cherry, grape, strawberry and tomato samples were homogenized with a T18 Ultra Turrax (IKA, Saufen, Germany), while the remaining samples were snap-frozen in liquid nitrogen and powdered using an A11 basic analytical mill (IKA, Saufen, Germany). Grape and tomato samples were extracted via a previously reported method for grape homogenates [5] with slight modification. Briefly, internal standard solution (100 µL; 5 µg/mL of each of d4-guaiacol, d5-o-cresol, d6-phenol and d3-syringol) was added to grape and tomato homogenate samples (12 g), the resulting sample was mixed thoroughly and centrifuged (3220× g, 5 min), and an aliquot of supernatant (5 mL) was extracted with 2:1 pentane:ethyl acetate (2 mL). For all the remaining samples, aliquots (0.5 g) were placed in ethanol (0.5 mL, overnight with shaking); then, water was added (to give a final mass of 5 g), followed by internal standard (50 µL; 1 µg/mL), and the resulting mixture was extracted with 2:1 pentane:ethyl acetate (2 mL). After extraction with organic solvent, all samples were left to stand for 1 h at ambient temperature, before being centrifuged (2465× g, 3 min), and a portion of the organic layer was transferred to an autosampler vial for instrumental analysis. Instrument operating conditions were as previously reported for grapes [5]. For each of the sample types, one control replicate was spiked with known concentrations of VPs (50 µg/kg for grape and tomato samples; 60 µg/kg for remaining crop samples) and extracted as outlined above. The VPs in grape and tomato samples were quantified using calibration standards prepared in grape homogenate (0–200 µg/kg), while the VPs in other sample matrices were quantified using calibration standards prepared in broccolini (0–200 µg/kg).
Data acquisition and processing were performed using Mass Hunter Workstation software (version B.09.00).
2.3. Statistical Analysis
Volatile phenol data were analysed by multiple unpaired t-tests using GraphPad Prism (version 10.1.0, GraphPad Software, Boston, MA, USA). Principal component analysis was performed using XLSTAT (version 2022, Lumivero, Denver, CO, USA).
3. Results and Discussion
3.1. Sample Preparation and Method Validation
The methodology for the extraction of VPs from grapes, juice and wine have already been well established [5], and this analysis is offered by commercial laboratories around the world. Samples with high water content can be easily homogenised, and adequate volumes can be obtained for liquid–liquid extraction using an organic solvent. In this study, grape and tomato samples were processed according to methodology employed for the analysis of grape homogenate [5]. However, the water content of the remaining sample matrices (apple peel and pulp, banana peel and pulp, broccolini, capsicum, cherry, lavender, lemon peel and pulp, spinach and strawberry) was too low to allow the same processing protocol. Consequently, their preparation was different, and as such, a separate calibration curve (in broccolini) was established for those samples. Broccolini was chosen simply due to its ease of handling, i.e., its low water content yielded a powder upon snap-freezing and grinding. While this study did not set out to develop and fully validate analytical methods for the determination of VPs in different sample matrices, it was nevertheless important to establish quality controls and ensure a level of confidence in the results generated for the different sample types. Each sample matrix was therefore spiked with known concentrations of VPs (50 µg/kg for the grape and tomato samples; 60 µg/kg for all other samples); the percentage recoveries for each analyte were then determined in each matrix (Table 1), taking into account background levels (by subtracting the concentration of any VPs observed in the control samples).

Table 1.
Percentage (%) recoveries for known concentrations of volatile phenols spiked in different crops (50 µg/kg for grape and tomato samples; 60 µg/kg for other crops).
The majority of VPs were adequately recovered in most matrices, i.e., within 20% of their expected values (giving recoveries of 80–120%). Measurement of VPs in lavender and lemon peel was the most problematic due to the large quantity of other volatile compounds that were present in these samples. In some instances, accurate quantification was not possible in these two samples, because interfering (co-eluting) peaks prevented accurate abundances from being obtained for some analytes and/or their corresponding internal standard. Where the area for an internal standard is overestimated, the concentration of an analyte is underestimated, which likely explains the lower percentage recoveries observed for some analytes in the lavender and lemon peel samples (Table 1).
With the exception of the lavender sample, there was good confidence in the results obtained for guaiacol across all sample matrices, with percentage errors for spiked samples being ≤8%. 4-Methylguaiacol concentrations were more likely to be overestimated, but recoveries were all within 30% of their expected values, with the exception of the lavender sample, which was slightly underestimated. The overestimation of 4-methylguaiacol may have been due to background levels in the control samples that were difficult to quantify at low levels (i.e., <10 µg/kg). Recoveries for phenol were good for most sample types (at 84–119%), but phenol could not be quantified in the lavender or lemon peel samples.
Good recoveries were achieved for both o- and m-cresol, although they tended to be slightly overestimated; measurements were within 23% of the expected value, with the exception of lavender (for o-cresol) and lemon peel (for m-cresol). The low recovery of o-cresol in the lavender sample was attributed to the extremely high background levels observed in the control sample (i.e., >400 µg/kg, being well above the calibration range). In the case of lemon peel, adjoining peaks for both the analyte and internal standard (d5-o-cresol) made it difficult to obtain an accurate result for m-cresol. The accuracy of p-cresol quantification was less consistent across the different sample types. It was greatly overestimated for the grape and tomato samples, at approximately double and quadruple the expected values, respectively; the reason for this is unknown. Concentrations of p-cresol in the apple peel and pulp, broccolini, capsicum, cherry, lemon pulp and strawberry samples were within 20% of their expected values (but slightly overestimated with recoveries of 104–119%), while in banana peel and pulp, they were within 30% of their expected values (but underestimated, with recoveries of 72 and 70%, respectively). p-Cresol was not quantifiable in the lavender and lemon peel samples.
Recovery data could only be generated for syringol and 4-methylsyringol in the grape and tomato samples, but recoveries were excellent for these samples, at 99–102%. For the other crop samples, issues were encountered with the calibration curves for syringol and 4-methylsyringol when spiked samples were analysed, preventing quantitation. This result was unusual since the calibration curves for all other VPs generated from the same calibration set were good. Syringol has previously been described as ‘a problematic compound both chromatographically and from a (presumptive) reactivity standpoint’ [37], and issues were similarly attributed to poor chromatography. Fortunately, issues were not encountered after GC column and instrument maintenance.
The results from the spiking experiments indicated that for most analytes and most sample matrices, the methods employed for preparation and extraction were suitable for the adequate quantification of VPs required for this study. However, if the need to routinely measure VPs were to arise in the future, particularly in lavender or lemon peel samples, then additional optimisation and validation of analytical methods for these matrices should be undertaken (but this was beyond the scope of the current study).
3.2. Susceptibility of Different Crops to Smoke Taint
VPs occur naturally in grapes (in free and glycosylated forms), typically at <15 µg/kg, but ‘background’ concentrations vary by cultivar [33,40]. In the current study, none of the VPs measured as markers of smoke taint were detected in the control grapes (cv. Sultana); however, as expected, guaiacol, 4-methylguaiacol, o-, m- and p-cresol, syringol and 4-methylsyringol were detected at elevated concentrations in smoke-exposed grapes, at 87, 2, 60, 10, 22, 18 and 3 µg/kg, respectively (Table 2). With the exception of phenol (which was not detected), volatile phenol concentrations were comparable to levels reported in a previous study involving exposure of Semillon grapes to smoke using the same smoke box and experimental conditions [35]. The inclusion of grapes in the current study was intended to provide a benchmark against which the volatile phenol profiles of other crops could be compared following smoke exposure.

Table 2.
Concentration of volatile phenols (µg/kg) in different crops, with (S) and without (C) exposure to smoke.
VPs were not detected in the peel or pulp of the control apple or banana samples, nor in broccolini, but interestingly, background levels of some VPs were detected in the other control crop samples (Table 2). Guaiacol was detected in the control capsicum, cherry, lemon peel, and tomato samples, at 18, 7, 17 and 52 µg/kg, respectively, while phenol was detected (at 4–7 µg/kg) in lemon pulp, spinach and tomatoes. 4-Methylsyringol, p-cresol and syringol were also detected (at 3–5 µg/kg) in lemon peel and pulp, and in tomato. However, these results were in agreement with previous studies [51,52,53,54]. For example, VPs have been identified in acid and enzyme hydrolysates of strawberry extracts: guaiacol and m-cresol at only 0.5–1.0 µg/kg, but 4-vinylguaiacol and 4-vinylphenol at up to 352 µg/kg and 28 mg/kg, respectively [51]. Guaiacol, phenol, and o- and p-cresol were also amongst the volatile compounds identified in extracts of paprika [52,53], which derives from capsicum. In the case of tomato, guaiacol, methyl salicylate and eugenol are known to be present in fruit in glycosylated forms, and their accumulation as disaccharides vs. trisaccharides determines their release (and sensory impact) when fruit is damaged (e.g., via maceration) [54]; hence, the higher background level of guaiacol observed in tomato homogenate, relative to the other crop samples, is not unexpected. VPs (including guaiacol, phenol and cresols) are also amongst the volatile constituents identified in essential oils extracted or distilled from various plants, including lavender [55].
As is known to occur for grapes, smoke exposure significantly impacted the VP composition of the different crops (Table 2). However, considerable variation was observed in the uptake of VPs, which likely reflects differences in the physical properties of the various crop samples, in particular, their surface area, texture and composition (e.g., the wax present in plant cuticles, which can trap pollutants [56]). Based on VP uptake, strawberries appeared to be the crop most susceptible to smoke contamination, with guaiacol and phenol concentrations each exceeding 1000 µg/kg and 4-methylguaiacol, cresol and syringol concentrations ranging from 101 to 500 µg/kg (Table 2). These levels were more than ten-fold higher than those observed in the smoke-exposed grapes and several fold higher than those observed for smoke-exposed tomatoes. A recent study compared the accumulation of metals from synthetic airborne particles in strawberry and tomato leaves and fruit [45] and reported that on a fresh weight basis, strawberry fruit had the highest accumulation capacity. This was attributed to reduced particle deposition by ‘the perfectly round and very smooth shape of the small cherry tomato compared to the rough and heterogeneous shape of the strawberry fruit’ [45], which provides a plausible explanation for the difference in VP uptake observed for strawberries compared with tomatoes in the current study (Table 2).
The VP content of smoke-exposed capsicum, cherries and spinach were comparable to that of smoke-exposed tomatoes, in that all VPs were significantly elevated relative to their corresponding control samples (with the exception of syringol and 4-methylsyringol, which were not detected in cherries); however, phenol and p-cresol were higher in capsicum and tomatoes, o-cresol was highest in spinach, and m-cresol was higher in capsicum and spinach. Excluding syringol and 4-methylsyringol, the VP content of these samples was also higher than those observed in smoke-affected grapes. In contrast, only 5–7 µg/kg of guaiacol, o- and p-cresol were detected in smoke-exposed broccolini, while 4-methylguaiacol, syringol and 4-methylsyringol were not detected (Table 2); however, phenol and m-cresol levels were considerably elevated, at 225 and 51 µg/kg, respectively. The variation in VP profiles observed amongst the crop samples may reflect relative differences in rates of adsorption for individual VPs, again potentially due to differences in surface area, texture and/or cuticle composition. Nevertheless, the results suggest these crops are susceptible to smoke contamination. Of course, the risk of contamination would still depend on smoke exposure occurring (i) during a given crop’s growing season and (ii) at the altitude at which crops are grown (being ground level for crops such as strawberries). Additionally, crops grown in greenhouses (e.g., tomatoes) would presumably be at significantly less risk of smoke exposure.
Apples, bananas and lemons were included in the current study to enable the extent to which skin thickness might influence the permeation of smoke-derived VPs into fruit pulp to be investigated. Significantly higher levels of guaiacol, phenol and cresols were detected in the peel of smoke-exposed apple samples (being 14–178 µg/kg) compared with the corresponding pulp (being 7–20 µg/kg, Table 2), suggesting the thicker skin of apples (relative to grapes, cherries, strawberries and tomatoes, for example) may have hindered permeation of smoke-derived VPs. Elevated VPs were also found in the peel of smoke-exposed banana and lemon samples, i.e., 7–85 µg/kg of guaiacol, phenol and cresols for banana peel and 12–110 µg/kg of guaiacol, 4-methylguaiacol, m-cresol, syringol and 4-methylsyringol for lemon peel. However, the corresponding banana and lemon pulp samples contained ≤10 µg/kg of p-cresol and/or phenol (Table 2), indicating smoke-derived VPs were almost entirely retained within fruit peel. As such, smoke taint is unlikely to be a concern. Given apples are typically consumed or processed whole (e.g., for juice or cider production), these results suggest there is some risk of perceivable smoke taint, especially where orchards are exposed to smoke for extended periods of time, as was reported for the apple-producing regions of Batlow and Bilpin [49,50]. However, the risk of smoke taint being perceptible in apple juice and cider should be less than that for red wine, given that apple juice is not usually fermented together with peel and pulp (as occurs in red winemaking, thereby facilitating the extraction of smoke taint compounds [7]).
Lavender was included in this study after the owner of a commercial lavender farm sought advice on screening samples for smoke taint following exposure of the owner’s field-grown lavender plants to smoke from a prescribed burn on a neighbouring property. Despite the aforementioned challenges with the quantification of VPs in the lavender samples, it was evident that smoke exposure had a significant impact on VP profiles. Whereas syringol and 4-methylsyringol levels were comparable between the control and smoke-exposed lavender samples and p-cresol could not be quantified (for reasons outlined above), guaiacol, 4-methylguaiacol, phenol, and o- and m-cresol concentrations were substantially elevated due to smoke treatment (Table 2). Indeed, the highest phenol, o- and m-cresol concentrations (nominally 2913, 1365 and 761 µg/kg, respectively) were observed in the smoke-exposed lavender samples, although this might reflect plants being positioned on the floor of the smoke box, adjacent to the exhaust ducting that emitted smoke into the smoke box. Again, these results suggest that, like many of the other crops included in this study, lavender is susceptible to smoke contamination. However, further research is required to establish whether or not the sensory impact of smoke-derived VPs would be perceptible in products (e.g., essential oils) derived from smoke-exposed lavender. The production of lavender essential oil involves distillation [55], and although the phenols associated with smoke taint are described as ‘volatile’, a recent study involving spinning-cone-column distillation of smoke-tainted wine found volatile phenols were not readily transferred into condensate under the low temperature distillation conditions that were employed (i.e., <40 °C, under vacuum) [57]. If the relatively high boiling points and low vapor pressures of VPs resulted in their retention in stillage following distillation, they would have no impact on the sensory properties of essential oils, but further research is needed to confirm this outcome.
Principal component analysis (PCA) of VP data showed separation of the smoke-exposed crop samples according to their VP uptake, illustrating their relative susceptibility to the compositional effects of smoke exposure (Figure 1). The first principal component explained 72.8% of variation, with separation of the samples along the x-axis driven by guaiacol, 4-methylguaiacol, phenol, cresol and 4-methylsyringol concentrations, while the second principal component explained a further 21.5% of variation, with separation along the y-axis reflecting syringol concentrations. The strawberry and lavender samples were positioned on the right side of the PCA biplot, indicating the highest susceptibility to smoke; the capsicum, spinach and tomato samples were also to the right of the y-axis, indicating moderate susceptibility. Notably, these five samples were all positioned to the right of the grape sample, being the sample in which the potential for smoke taint has been well established. The remaining samples were positioned on the left side of the PCA biplot, indicating lower susceptibility to the effects of smoke exposure. This included the apple peel and pulp samples, suggesting apples (and cider) should be at less risk of smoke taint than grapes and wine, in agreement with previous research [50].
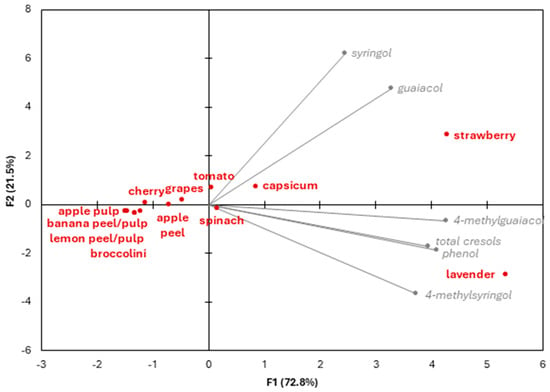
Figure 1.
Principal component analysis biplot of volatile phenol concentrations measured in different crops, following exposure to smoke.
In the current study, only free VPs were measured (and not VP glycosides), since the samples were frozen immediately after smoke exposure, such that no significant glycosylation was expected to have occurred. However, in the event of a ‘real’ scenario involving exposure of a given crop to smoke from a bushfire or prescribed burn, it may be necessary to measure both free and glycosylated VPs to ascertain the extent of any smoke exposure, as routinely occurs for grapes and wine. It is reasonable to expect that exogenous, smoke-derived VPs will accumulate in other crops in glycoconjugate forms, in a manner analogous to that observed for naturally occurring VPs in grapes, apples, strawberries and tomatoes [33,40,49,50,54]. For robust smoke taint diagnostics, it may therefore be necessary for existing analytical methods (developed for grape and wine analysis [5,15,22,23,36,37,40]) to be adapted, optimised and validated for use in other matrices. For now, smoke taint may be a challenge unique to grapes and wine, and to a lesser extent, hops and beer [46], and apples and cider [49,50]. Nevertheless, given that modelling forecasts bushfires will increase in frequency and severity with future climate change, it is likely other crops may be subject to smoke exposure in the future. It is therefore useful to understand the susceptibility of different crops to smoke contamination.
Author Contributions
Conceptualization, R.R. and K.W.; methodology, J.C., R.R. and K.W.; formal analysis, J.C.; resources, K.W.; data curation, K.W.; writing—original draft preparation, J.C. and K.W.; writing—review and editing, R.R.; project administration, K.W.; funding acquisition, K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Research Council (LP210300715), with support from several industry partners.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Yiming Huo, Ysadora Mirabelli-Montan, and Tingting Shi for technical assistance in the field and laboratory.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kennison, K.R.; Wilkinson, K.L.; Williams, H.G.; Smith, J.H.; Gibberd, M.R. Smoke-derived taint in wine: Effect of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. J. Agric. Food Chem. 2007, 55, 10897–10901. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust. J. Grape Wine Res. 2009, 15, 228–237. [Google Scholar] [CrossRef]
- Sheppard, S.I.; Dhesi, M.K.; Eggers, N.J. Effect of pre-veraison and postveraison smoke exposure on guaiacol and 4-methylguaiacol concentration in mature grapes. Am. J. Enol. Vitic. 2009, 60, 98–103. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Dungey, K.A.; Baldock, G.A.; Kennison, K.R.; Wilkinson, K.L. Identification of a β-D-glucopyranoside precursor to guaiacol in grape juice following grapevine exposure to smoke. Anal. Chim. Acta 2010, 660, 143–148. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.A.; Parker, M.; Pardon, K.H.; Black, C.A.; Herderich, M.J.; Jeffery, D.W. Glycosylation of smoke derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar] [CrossRef] [PubMed]
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of smoke application to field-grown Merlot grapevines at key phenological growth stages on wine sensory and chemical properties. Aust. J. Grape Wine Res. 2011, 17, S5–S12. [Google Scholar] [CrossRef]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.A.; Hayasaka, Y.; Fudge, A.L.; Wilkinson, K.L. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- Singh, D.P.; Chong, H.H.; Pitt, K.M.; Cleary, M.; Dokoozlian, N.; Downey, M.O. Guaiacol and 4-methylguaiacol accumulate in wines made from smoke-affected fruit because of hydrolysis of their conjugates. Aust. J. Grape Wine Res. 2011, 17, S13–S21. [Google Scholar] [CrossRef]
- Wilkinson, K.L.; Ristic, R.; Pinchbeck, K.A.; Fudge, A.L.; Singh, D.P.; Pitt, K.M.; Downey, M.O.; Baldock, G.A.; Hayasaka, Y.; Parker, M.; et al. Comparison of methods for the analysis of smoke related phenols and their conjugates in grapes and wine. Aust. J. Grape Wine Res. 2011, 17, S22–S28. [Google Scholar] [CrossRef]
- Fudge, A.L.; Ristic, R.; Wollan, D.; Wilkinson, K.L. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Kelly, D.; Zerihun, A.; Singh, D.P.; Vitzthum von Eckstaedt, C.; Gibberd, M.; Grice, K.; Downey, M. Exposure of grapes to smoke of vegetation with varying lignin composition and accretion of lignin derived putative smoke taint compounds in wine. Food Chem. 2012, 135, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Fudge, A.L.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K.L. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- Bell, T.L.; Stephens, S.L.; Moritz, M.A. Short-term physiological effects of smoke on grapevine leaves. Int. J. Wildland Fire 2013, 22, 933–946. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Parker, M.; Baldock, G.A.; Pardon, K.H.; Black, C.A.; Jeffery, D.W.; Herderich, M.J. Assessing the impact of smoke exposure in grapes: Development and validation of an HPLC-MS/MS method for the quantitative analysis of smoke-derived phenolic glycosides in grapes and wine. J. Agric. Food Chem. 2013, 61, 25–33. [Google Scholar] [CrossRef]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wine. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef]
- Ristic, R.; Fudge, A.L.; Pinchbeck, K.A.; De Bei, R.; Fuentes, S.; Hayasaka, Y.; Tyerman, S.D.; Wilkinson, K.L. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant Physiol. 2016, 28, 67–83. [Google Scholar] [CrossRef]
- Culbert, J.A.; Jiang, W.; Bilogrevic, E.; Likos, D.; Francis, I.L.; Krstic, M.P.; Herderich, M.J. Compositional changes in smoke-affected grape juice as a consequence of activated carbon treatment and the impact on phenolic compounds and smoke flavor in wine. J. Agric. Food Chem. 2021, 69, 10246–10259. [Google Scholar] [CrossRef]
- Krstic, M.P.; Johnson, D.L.; Herderich, M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- De Vries, C.J.; Buica, A.; Brand, J.; Mckay, M. The impact of smoke from vegetation fires on sensory characteristics of Cabernet Sauvignon wines made from affected grapes. S. Afr. J. Enol. Vitic. 2016, 37, 22–31. [Google Scholar] [CrossRef][Green Version]
- Ristic, R.; van der Hulst, L.; Capone, D.L.; Wilkinson, K.L. Impact of bottle aging on smoke-tainted wines from different grape cultivars. J. Agric. Food Chem. 2017, 65, 4146–4152. [Google Scholar] [CrossRef] [PubMed]
- Noestheden, M.; Thiessen, K.; Dennis, E.G.; Zandberg, W.F. Quantitating organoleptic volatile phenols in smoke-exposed Vitis vinifera berries. J. Agric. Food Chem. 2017, 65, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Noestheden, M.; Dennis, E.G.; Romero-Montalvo, E.; DiLabio, G.A.; Zandberg, W.F. Detailed characterization of glycosylated sensory-active volatile phenols in smoke-exposed grapes and wine. Food Chem. 2018, 259, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.; Lerno, L.; Rumbaugh, A.; Girardello, R.; Zweigenbaum, J.; Oberholster, A.; Ebeler, S.E. Changes in smoke-taint volatile-phenol glycosides in wildfire smoke-exposed Cabernet Sauvignon grapes throughout winemaking. Am. J. Enol. Vitic. 2019, 70, 373–381. [Google Scholar] [CrossRef]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Mokwena, L.; Buica, A. Profiling potentially smoke tainted red wines: Volatile phenols and aroma attributes. S. Afr. J. Enol. Vitic. 2019, 40, 1–16. [Google Scholar]
- Szeto, C.; Ristic, R.; Capone, D.; Puglisi, C.; Pagay, V.; Culbert, J.; Jiang, W.; Herderich, M.; Tuke, J.; Wilkinson, K. Uptake and glycosylation of smoke-derived volatile phenols by Cabernet Sauvignon grapes and their subsequent fate during winemaking. Molecules 2020, 25, 3720. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Summerson, V.; Gonzalez Viejo, C.; Tongson, E.; Lipovetzky, N.; Wilkinson, K.L.; Szeto, C.; Unnithan, R.R. Assessment of smoke contamination in grapevine berries and taint in wines due to bushfires using a low-cost e-nose and an artificial intelligence approach. Sensors 2020, 20, 5108. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Alcazar-Magana, A.; Qian, Y.L.; Qian, M.C. Smoke-derived volatile phenol analysis in wine by stir bar sorptive extraction-gas chromatography-mass spectrometry. Molecules 2021, 26, 5613. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli-Montan, Y.A.; Marangon, M.; Graça, A.; Mayr Marangon, C.M.; Wilkinson, K.L. Techniques for mitigating the effects of smoke taint while maintaining quality in wine production: A review. Molecules 2021, 26, 1672. [Google Scholar] [CrossRef]
- Wilkinson, K.; Ristic, R.; McNamara, I.; Loveys, B.; Jiang, W.W.; Krstic, M. Evaluating the potential for smoke from stubble burning to taint grapes and wine. Molecules 2021, 26, 7540. [Google Scholar] [CrossRef]
- Modesti, M.; Szeto, C.; Ristic, R.; Jiang, W.W.; Culbert, J.; Bindon, K.; Catelli, C.; Mencarelli, F.; Tonutti, P.; Wilkinson, K. Potential mitigation of smoke taint in wines by post-harvest ozone treatment of grapes. Molecules 2021, 26, 1798. [Google Scholar] [CrossRef]
- Wilkinson, K.L.; Ristic, R.; Szeto, C.; Capone, D.L.; Yu, L.; Losic, D. Novel use of activated carbon fabric to mitigate smoke taint in grapes and wine. Aust. J. Grape Wine Res. 2022, 28, 500–507. [Google Scholar] [CrossRef]
- Coulter, A.; Baldock, G.; Parker, M.; Hayasaka, Y.; Francis, I.L.; Herderich, M. Concentration of smoke marker compounds in non-smoke-exposed grapes and wine in Australia. Aust. J Grape Wine Res. 2022, 28, 459–474. [Google Scholar] [CrossRef]
- Jiang, W.W.; Bilogrevic, E.; Parker, M.; Francis, I.L.; Leske, P.; Hayasaka, Y.; Barter, S.; Herderich, M. The effect of pre-veraison smoke exposure of grapes on phenolic compounds and smoky flavour in wine. Aust. J. Grape Wine Res. 2022, 2022, 9820204. [Google Scholar] [CrossRef]
- Szeto, C.; Ristic, R.; Wilkinson, K. Thinking inside the box: A novel approach to smoke taint mitigation trials. Molecules 2022, 27, 1667. [Google Scholar] [CrossRef] [PubMed]
- Crews, P.; Dorenbach, P.; Amberchan, G. Appraising California Zinfandel exposure to wildfire smoke using natural product phenolic diglycosides biomarkers. J. Agric. Food Chem. 2022, 70, 11738–11748. [Google Scholar] [CrossRef]
- Favell, J.W.; Wilkinson, K.L.; Zigg, I.; Lyons, S.M.; Ristic, R.; Puglisi, C.J.; Wilkes, E.; Taylor, R.; Kelly, D.; Howell, G.; et al. Correlating sensory assessment of smoke-tainted wines with inter-laboratory study consensus values for volatile phenols. Molecules 2022, 27, 4892. [Google Scholar] [CrossRef]
- Tomasino, E.; Cerrato, D.C.; Aragon, M.; Fryer, J.; Garcia, L.; Ashmore, P.L.; Collins, T.S. A combination of thiophenols and volatile phenols cause the ashy flavor of smoke taint in wine. Food Chem. Adv. 2023, 2, 100256. [Google Scholar] [CrossRef]
- Szeto, C.; Lloyd, N.; Nicolotti, L.; Herderich, M.J.; Wilkinson, K.L. Beyond volatile phenols: An untargeted metabolomic approach to revealing additional markers of smoke taint in grapevines (Vitis vinifera L.) cv. Merlot. J. Agric. Food Chem. 2024, 72, 2018–2033. [Google Scholar] [CrossRef]
- Szeto, C.; Feng, H.; Aui, Q.; Blair, B.; Mayfield, S.; Pan, B.; Wilkinson, K. Exploring variation in grape and wine volatile phenol glycoconjugates to improve evaluation of smoke taint risk. Am. J. Enol. Vitic. 2024, 75, 0750013. [Google Scholar] [CrossRef]
- Farmer, A. Effects of Particulates. In Air Pollution and Plant Life, 2nd ed.; Bell, J.N.B., Treshow, M., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2002; pp. 187–199. [Google Scholar]
- Zalud, P.; Szakova, J.; Sysalova, J.; Tlustos, P. Factors influencing uptake of contaminated particulate matter in leafy vegetables. Cent. Eur. J. Biol. 2012, 7, 519–530. [Google Scholar] [CrossRef]
- Anand, P.; Minab, U.; Kharea, M.; Kumarc, P.; Kota, S.H. Air pollution and plant health response-current status and future directions. Atmos. Pollut. Res. 2022, 13, 101508. [Google Scholar] [CrossRef]
- Jouraeva, V.A.; Johnson, D.L.; Hassett, J.P.; Nowak, D.J. Differences in accumulation of PAHs and metals on the leaves of Tilia×euchlora and Pyrus calleryana. Environ. Pollut. 2002, 120, 331–338. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, I.; Pérez-Vázquez, L.; de Pablos-Pons, F.; Fernández-Espinosa, A.J. Toxic metals from atmospheric particulate matter in food species of tomato (Solanum lycopersicum) and strawberry (Fragaria x ananassa) used in urban gardening. A closed chamber study. Chemosphere 2023, 340, 139921. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Alexander, J. A HS-SPME arrow/GC-MS method for determination of smoke taint-related volatile phenols in Humulus lupulus. J. Am. Soc. Brew. Chem. 2022, 80, 128–135. [Google Scholar] [CrossRef]
- Boidron, J.N.; Chatonnet, P.; Pons, M. Effects of wood on aroma compounds of wine. Conn. Vigne Vin. 1988, 22, 275–294. [Google Scholar]
- Filkov, A.I.; Ngo, T.; Matthews, S.; Telfer, S.; Penman, T.D. Impact of Australia’s catastrophic 2019/20 bushfire season on communities and environment. Retrospective analysis and current trends. J. Saf. Sci. Resil. 2020, 1, 44–56. [Google Scholar] [CrossRef]
- Dodds, K. Bushfires in Apple Orchards: Observations from the 2019–2020 Season (Department of Regional NSW). 2020; p. 36. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/1285392/Bushfires-in-apple-orchards.pdf (accessed on 2 July 2024).
- Dodds, K.A.; Holzapfel, B.P.; Wilkinson, K.L. Assessing the potential for bushfire smoke exposure of apples (Malus domestic Borkh) to affect the composition and sensory characteristics of cider. Acta Hortic. 2024, 1387, 57–66. [Google Scholar] [CrossRef]
- Ubeda, C.; San-Juan, F.; Concejero, B.; Callejón, R.M.; Troncoso, A.M.; Lourdes Morales, M.; Ferreira, V.; Hernández-Orte, P. Glycosidically bound aroma compounds and impact odorants of four strawberry varieties. J. Agric. Food Chem. 2012, 60, 6095–6120. [Google Scholar] [CrossRef]
- Mateo, J.; Aguirrezábal, M.; Domínguez, C.; Zumalacárregui, J.M. Volatile compound in Spanish paprika. J. Food Compos. Anal. 1997, 10, 225–232. [Google Scholar] [CrossRef]
- Martín, A.; Hernández, A.; Aranda, E.; Casquete, R.; Velázquez, R.; Bartolomé, T.; Córdoba, M.G. Impact of volatile composition on the sensorial attributes of dried paprikas. Food Res. Int. 2017, 100, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Tikunov, Y.M.; Molthoff, J.; de Vos, R.C.H.; Beekwilder, J.; van Houwelingen, A.; van der Hooft, J.J.J.; Nijenhuis-de Vries, M.; Labrie, C.W.; Verkerke, W.; van de Geest, H.; et al. Non-Smoky Glycosyltransferase prevents the release of smoky aroma from tomato fruit. Plant Cell 2013, 25, 3067–3078. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadkyay, A.K. Flavors, fragrances, and food additives from cresol derivatives. In Industrial Chemical Cresols and Downstream Derivatives, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 151–167. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B. Phenanthrene sorption by fruit cuticles and potato periderm with different compositional characteristics. J. Agric. Food Chem. 2009, 57, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, C.; Ristic, R.; Saint, J.; Wilkinson, K. Evaluation of spinning cone column distillation as a strategy for remediation of smoke taint in juice and wine. Molecules 2022, 27, 8096. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).