Abstract
Jasminum sambac is an excellent ornamental species that is renowned worldwide for its pure white flowers and strong fragrance. However, its intolerance to low temperatures limits its cultivation range. Methyl jasmonate (MeJA), an essential plant growth regulator, plays a significant role in assisting plants to resist various stresses. Hence, this study was carried out to decipher the capabilities of diverse concentrations of MeJA in helping J. sambac to resist cold stress by measuring different physiological indexes. A normal temperature (15 °C/10 °C) and low temperature (7 °C/2 °C) were applied to J. sambac seedlings, and a one-way analysis of variance followed by a Duncan’s multiple range test was adopted to compare the differences between the indicators under 5 μmol·L−1, 10 μmol·L−1 and 20 μmol·L−1 of MeJA treatments. The results showed that cold stress significantly decreased the contents of soluble sugar and soluble protein, while the application of MeJA at 10 μmol·L−1 and 20 μmol·L−1 resulted in a partial recovery. In addition, cold stress dramatically hindered the accumulation of total chlorophyll in leaves. Exogenous MeJA elevated the total chlorophyll content during the whole sampling period. The hydrogen peroxide and malondialdehyde levels generally increased in response to low temperatures, and they caused adverse effects on J. sambac, whereas this was effectively alleviated through the application of MeJA. MeJA was also able to improve the resistance of J. sambac by boosting the activity of antioxidant enzymes to remove the excess of reactive oxygen species. In conclusion, we highlighted that exogenous MeJA could attenuate the negative consequences of cold stress for J. sambac, and 10 μmol·L−1 of MeJA treatment could be a feasible strategy for enhancing the resistance of J. sambac to low temperatures and promoting its growth.
1. Introduction
Jasminum sambac is an evergreen shrub in the family Oleaceae that is widely cultivated around the world. J. sambac thrives in a warm and humid environment and blooms from May to August. Its flowers are pure white and have a strong fragrance, making it extremely popular [1]. Currently, research is being conducted on J. sambac in various fields. It is used not only for garden landscapes but also for the production of perfumes, herbal teas, and shampoos; hence, it has high economic value [2]. In addition, some reports focus on the chemical composition and pharmacological activities of J. sambac, such as its volatile compounds and secondary metabolites [2,3]. These substances exhibit antioxidant, anti-inflammatory, and sedative properties, and they possess a wide range of applications in cosmetics, pharmaceuticals, and health products [4,5]. Other research aims to understand the growth and flowering mechanisms of this ornamental plant, including its flowering period, flower color regulation, and flowering induction [6,7,8]. Moreover, numerous studies have been conducted on the fragrance components of J. sambac. Studies have shown that benzylacetate, methylbenzoate, (E)-β-ocimene, linalool, and farnesene are the main constituents [9]. However, J. sambac requires strictly environmental conditions, particularly with regard to temperature. Its cultivation range is restricted due to its sensitivity to unfavorable temperatures. It has been recorded that J. sambac is extremely sensitive to low temperatures during its growth and development, showing growth inhibition, frostbite in the shoots and leaves, and a low survival rate [10]. Some studies pointed out that 25–35 °C was the suitable growth temperature for J. sambac. When the temperature was lower than 9.9 °C, most of the leaves fell off, and when the temperature was 3 °C, the leaves suffered from severe frostbite [11]. The experimental results of Cai et al. showed that 4 °C would be harmful to J. sambac growth, which was manifested by the increase in hydrogen peroxide (H2O2) and malondialdehyde (MDA) and the decrease in photosynthesis ability [12]. Therefore, it is crucial to carry out research on cold resistance in J. sambac to uncover its physiological mechanisms for adapting to temperature changes and exploring ways to enhance its cold resistance, which has important scientific significance for its cultivation and the efficient utilization of J. sambac.
The mechanism of the response of plants to low-temperature stress has been widely discussed. It has been reported that Oryza sativa is susceptible to low temperatures and exhibits poor germination and slow seed growth when grown at low temperatures for more than 4 d, sometimes resulting in the death of seedlings [13]. In addition, rice blast spreads on a large scale during periods with low temperatures, causing major losses to rice production [14]. At low temperatures, the content of MDA in Medicago sativa seedlings was found to be low in the early stage, but it was significantly increased in the middle stage; meanwhile, the contents of soluble protein and chlorophyll in seedlings were also sharply decreased due to the adverse environment [15]. Many transcription factors are involved in the regulation of plant cold resistance. For example, the overexpression of CBF/DREB could induce the enhancement of cold resistance of Arabidopsis thaliana, while MYB15 plays a negative regulatory role [16]. Some studies have pointed out that exogenous chitosan oligosaccharide relieved the damage to plants caused by low temperatures by promoting photosynthesis and the carbon process [17]. Thus, plants’ cold resistance can be improved in various ways, among which the utilization of plant growth regulators is common. In recent years, more and more investigations have shown that methyl jasmonate (MeJA) can facilitate the adaptation of plants to low-temperature stress and increase plants’ cold resistance. Prunus persica was found to be susceptible to chilling injury when grown at low temperatures, while the application of MeJA activated the metabolism of α-linolenic acid by improving the degree of fatty acid unsaturation in the cell membrane. In addition, MeJA was found to regulate the signaling pathway of jasmonate acid (JA), collectively contributing to alleviating injury in P. persica [18]. Gul et al. disclosed that MeJA at a concentration of 10 μmol·L−1 could effectively eliminate the negative effects of low temperatures on the growth of Solanum lycopersicum because MeJA reduced the accumulation of H2O2; elevated the activity of superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX); and boosted the photosynthetic attributes [19]. Nonetheless, whether MeJA can improve the cold resistance of J. sambac at low temperatures is unclear.
Based on the above evidence, we hypothesized that a low temperature would lead to a decrease in nutrient content and antioxidant enzyme activity in J. sambac leaves, while exogenous MeJA could effectively eliminate these negative impacts, thus reducing the damage degree of plants. In this study, different concentrations of MeJA were adopted to treat J. sambac seedlings, and the impacts of MeJA on the cold resistance were evaluated by measuring changes in the physiological indexes of the leaves of seedlings on different days after treatment (DAT), in order to screen the optimal MeJA concentration to improve the cold resistance of J. sambac and reveal its physiological mechanism.
2. Materials and Methods
2.1. Overview of Plant Materials and Experimental Conditions
Four-year-old J. sambac seedlings, obtained from the Nanjing Yangzi Jasmine Valley Culture Technology Co., Ltd., (Nanjing, China) were selected as experimental materials. The whole experiment was conducted in the ecological training center of Yangzhou Polytechnic College (119°22′ E, 32°2′ N). The growth of these seedlings was basically identical, and there were no diseases or pests. Our preliminary experiment simulated the effect of artificial cooling on the growth of J. sambac. The data exhibited that when the temperature was maintained at 22–28 °C, J. sambac grew best. When the temperature was between 15 and 22 °C, plants could still grow normally. When the temperature dropped to 7 °C, the leaves of plants wilted and the growth was obviously poor. Hence, low-temperature stress was applied to 27 seedlings for 24 h at 7 °C/2 °C during the day/night in a growth chamber, while 9 seedlings were kept at 15 °C/10 °C during the day/night, which was considered a normal temperature. MeJA (purchased from Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China) was diluted to three concentrations (5 μmol·L−1 MeJA, 10 μmol·L−1 MeJA, and 20 μmol·L−1 MeJA) with distilled water.
2.2. Experimental Design and Sample Collection
Four treatments were designed in this study, including control (CK, normal temperature + distilled water), M1 (low temperature + 5 μmol·L−1 MeJA), M2 (low temperature + 10 μmol·L−1 MeJA), and M3 (low temperature + 20 μmol·L−1 MeJA). Three replicates (each containing three seedlings) were maintained for each treatment. At normal temperatures, approximately 300 mL of MeJA or distilled water was sprayed on the leaves of seedlings in one treatment at the same time for 3 consecutive days, and leaf samples were collected after the third treatment (0 d). After that, samples from M1, M2, and M3 were kept at low temperatures for 5 days, and all samples were taken on the first, third, and fifth days, respectively (Table 1). Samples were immediately frozen in liquid nitrogen for the determination of physiological indicators. In addition to the temperature, the external growth conditions were the same.

Table 1.
Experimental design and sampling of the current study.
2.3. Measurement of Physiological Indices
Soluble sugar content: 0.2 g of J. sambac leaves were precisely weighed and transferred into a 15 mL centrifuge tube. 10 mL of distilled water was introduced into the tube, and the mixture was subjected to extraction in a boiling water bath for 30 min. After removal and cooling, centrifugation was performed, and the supernatant was transferred to a 25 mL volumetric flask. Subsequently, 10 mL of distilled water was added to the residue, and the mixture was further extracted in a boiling water bath for 20 min. After removal and cooling, the entire mixture was transferred to the 25 mL volumetric flask. The centrifuge tube and residue were repeatedly rinsed, and the volume was adjusted to the calibration mark before filtering. 1 mL of the diluted sample was pipetted into a 20 mL test tube, and distilled water was added to increase the volume to 2 mL. Anthrone-ethyl acetate and concentrated sulfuric acid solution were then added in sequence, and the mixture was developed for color. The absorbance at a wavelength of 630 nm was measured by a Beckman DU 800 UV-visible spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA, the same hereafter). The soluble sugar content in the leaves was calculated by referring to the method of Wang [20].
Soluble protein content: 0.3 g of leaves was weighed and placed in a mortar. It was then ground into a uniform paste by adding 2 mL of distilled water. The paste was transferred to a centrifuge tube, and the mortar was rinsed with 6 mL of water. The rinse solution was collected in the same centrifuge tube. The mixture was left at room temperature (20–25 °C) for 2 h to allow for thorough extraction. Subsequently, it was centrifuged at 4000 r·min−1 for 20 min, and the precipitate was discarded. The supernatant was transferred to a 10 mL volumetric flask and filled up to the mark with distilled water, thus producing an extract solution that was ready for testing. 0.5 mL of the sample extract solution was taken, and 5 mL of Coomassie Blue solution was added. After mixing and allowing the mixture to stand for 2 min, it was developed for color. The absorbance at a wavelength of 560 nm was measured by a spectrophotometer. The soluble protein content was calculated by referring to the method of Wang [20].
Total chlorophyll content: Ten leaves were carefully chosen and sliced along the main vein. Subsequently, 0.2 g of these sliced pieces was placed in a triangular flask, accompanied by 25 mL of 96% ethanol; the flask was sealed and protected from light for 24 h. Once the sliced pieces turned white, 2 mL of the supernatant was combined with an additional 4 mL of 96% ethanol, and 96% ethanol was taken as a blank. The mixture was developed for color. The absorbance at wavelength of 665 nm and 649 nm was measured by a spectrophotometer. The chlorophyll content was calculated by referring to the method of Wang [20].
Peroxidase (POD), SOD, and CAT activity: 0.2 g of leaves was pulverized with 5 mL of phosphate buffer (pH 7.8) to produce a homogenized mixture. This involved mixing 0.1 mL of the supernatant with 2.9 mL of the reaction solution, which contained 28 µL of guaiacol and 19 µL of H2O2. The POD activity was then estimated with a spectrophotometer at a wavelength of 470 nm using the guaiacol method [21]. For the determination of the SOD activity, a nitro tetrazolium blue chloride (NBT) photochemical reduction method was employed. Initially, the homogenate was transferred to a centrifuge tube and centrifuged at 10,000 r·min−1 for 20 min. Subsequently, the reaction system was configured using various components, including 1.5 mL of 50 mmol·L−1 phosphate buffer, 0.3 mL of 130 mmol·L−1 methionine, 0.3 mL of 750 µmol·L−1 nitroblue tetrazolium, 0.3 mL of 100 µmol·L−1 ethylene diamine tetraacetic acid, 0.3 mL of 20 µmol·L−1 riboflavin, and 50 µL of either phosphate buffer or supernatant. Finally, the SOD activity was estimated using a spectrophotometer at a wavelength of 560 nm according to the method of Jiang [22]. After preheating to 25 °C, 0.6 mL of 0.1 mol·L−1 H2O2 was added to the test tube to initiate color development, and the absorbance at a wavelength of 405 nm was subsequently measured to calculate the CAT activity [20].
MDA and H2O2 content: To assess the MDA content, the barbituric acid colorimetric method was employed. Initially, the homogenate underwent centrifugation at 3000 r·min−1 for 20 min. Then, 2 mL of the resulting supernatant was combined with 2 mL of 0.6% thiobarbituric acid in a tube and subjected to boiling for 20 min. Ultimately, the absorbance of the supernatant at 450 nm, 532 nm, and 600 nm was measured to calculate the MDA content [23]. 1 mL of the sample was extracted using a pipette, and 5% titanium sulfate and concentrated ammonia were consecutively added. After the formation of a precipitate, the mixture was centrifuged at 3000 r·min−1 for 10 min, and the supernatant was discarded. The precipitate was washed three to five times with acetone, and 5 mL of sulfuric acid was added to develop the color. The absorbance at a wavelength of 415 nm was then measured to calculate the H2O2 content [20].
2.4. Data Collection and Analysis
The experimental data were presented as the mean ± SD for three replicates. Data processing and figure generation were conducted using Excel (Office 2019 Pro Plus, Microsoft Corporation, Redmond, WA, USA). One-way analysis of variance (ANOVA) was evaluated using SPSS 26.0 (IBM, Armonk, NY, USA), followed by a Duncan’s multiple-range test. p-values less than 0.05 were considered statistically significant.
3. Results
3.1. Effects of MeJA on Nutrient and Chlorophyll Contents in J. sambac Leaves
The influence of varying concentrations of MeJA on the soluble sugar content in J. sambac leaves exhibited diverse trends (Figure 1A). At 0 DAT, all three MeJA treatments significantly increased the soluble sugar content, with increases ranging from 39.1% to 44.8%. The soluble sugar content in the leaves of the M2 treatment was the highest (45.8 mg·g−1 FW). One day later, the soluble sugar content in the leaves of all treatments and CK decreased slightly. The soluble sugar content in the CK leaves was 33.1 mg·g−1 FW, which was not significantly different from that in the leaves treated with M3 (35.7 mg·g−1 FW), but it was significantly lower than that in the leaves of the M1 and M2 treatments, which were 37.3 mg·g−1 FW and 42.5 mg·g−1 FW, respectively. After that, the soluble sugar content continued to decline, but there were no significant differences between the content in the CK leaves and that in the leaves of the M1 and M3 treatments. At this time, the soluble sugar content in the leaves of the M2 treatment was 39.6 mg·g−1 FW, which was 1.24, 1.13, and 1.17 times that in the other three, respectively. On the fifth day, the soluble sugar content in the CK and M2 leaves slightly increased, while the soluble sugar content in the M1 and M3 leaves continued to decline. At this time, the soluble sugar content in the M3 leaves was the lowest (30.6 mg·g−1 FW), and it was significantly lower than that in the M2 leaves.
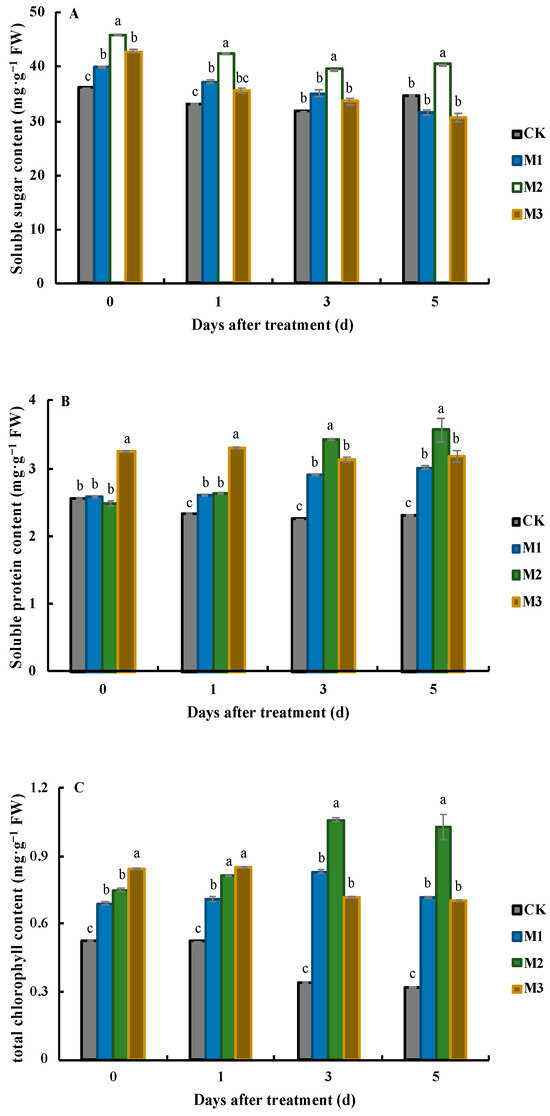
Figure 1.
Effects of MeJA application on soluble sugar (A), soluble protein (B), and total chlorophyll (C) at different days after treatment. Values represent the mean ± SD, and different lowercase letters indicate significant differences at the 0.05 level.
After treatment with different concentrations of MeJA, the soluble protein content in the leaves of J. sambac showed an overall upward trend (Figure 1B). At 0 DAT, the soluble protein content in the leaves of the CK, M1, and M2 treatments was very close, and there were no significant differences between them. All of them had significantly lower soluble protein content than that in the leaves of the M3 treatment (3.25 mg·g−1 FW). At 1 DAT, the soluble protein content in the leaves of the M3 treatment remained the highest, at 3.31 mg·g−1 FW, which was 1.43, 1.26, and 1.25 times that of the CK, M1, and M2, respectively. At 3 DAT, there were significant changes in the soluble protein content in the leaves under the different treatments. The soluble protein content in the leaves treated with M2 significantly increased, while the soluble protein content in the leaves treated with M3 decreased by 34%. There were no significant differences in the soluble protein content between the M3 and M1 treatments, but the content was significantly higher than that in the CK leaves. On the last day of sampling, the trend of the soluble protein content was similar to that in the previous stage. The soluble protein content in the CK leaves was the lowest, at 2.31 mg·g−1 FW, which was only 64% of the soluble protein content in the leaves of the M2 treatment. There were no significant differences in the soluble protein content between the M1 and M3 treatments.
The application of different concentrations of MeJA significantly elevated the total chlorophyll content in the leaves of J. sambac (Figure 1C). In the early stages (0 DAT and 1 DAT), the increase in the total chlorophyll content in the treated leaves was relatively small, and the content was similar among different treatments. However, in the later stages (3 DAT and 5 DAT), the total chlorophyll content in the treated leaves significantly increased. At 0 DAT, the total chlorophyll content in the leaves of the M3 treatment was the highest, reaching 0.84 mg·g−1 FW, which was significantly higher than that in the other three treatments. The total chlorophyll content in the leaves of the M1 and M2 treatments was 0.69 mg·g−1 FW and 0.75 mg·g−1 FW, respectively, with no significant difference between them. After that, the total chlorophyll content in the leaves slightly increased. Except for the CK leaves, whose total chlorophyll content was only 0.52 mg·g−1 FW, the total chlorophyll content in the leaves treated with MeJA at the three concentrations exceeded 0.71 mg·g−1 FW, and M3 remained the treatment with the highest value. After 2 days, the total chlorophyll content in the leaves of the M2 treatment reached the highest level, at 1.06 mg·g−1 FW, which was also the highest content during the whole treatment, and was significantly higher than that in the other treatments. Furthermore, the total chlorophyll content in the CK leaves decreased to 0.34 mg·g−1 FW. At 5 DAT, the total chlorophyll content in the CK leaves and the leaves of the three MeJA treatments slightly decreased compared with that in the previous period. The total chlorophyll content in the leaves of the M1 and M3 was very close, at 0.72 mg·g−1 FW and 0.70 mg·g−1 FW, respectively; both were significantly lower than the total chlorophyll content in the leaves of the M2 (1.03 mg·g−1 FW), but significantly higher than the total chlorophyll content in the CK leaves (0.32 mg·g−1 FW).
3.2. Effects of MeJA on MDA and H2O2 Contents in J. sambac Leaves
After treatment with different concentrations of MeJA, the MDA content in the leaves remained at a high level throughout the experiment (Figure 2A). At 0 DAT, the MDA content in the leaves of the M2 treatment was the lowest, at 62.6 nmol·g−1 FW, which was significantly lower than that in the CK leaves and those of the other two MeJA treatments. At 1 DAT, the MDA content in the leaves of the M3 treatment was the highest (82.1 nmol·g−1 FW) and was significantly higher than that in the other treatments. At this time, the MDA content in the CK leaves was higher than that in the leaves of the M1 and M2 treatments, but the difference between the three was not significant, ranging from 64.5 nmol·g−1 FW to 73.6 nmol·g−1 FW. The trend of the MDA content in the leaves of J. sambac was consistent between the third and fifth days after treatment, with the highest MDA content being found in the CK leaves, followed by the M3 treatment, and the lowest MDA content was found in the M2 treatment. Overall, as the duration of low-temperature stress increased, the MDA content in the leaves of different treatments showed an upward trend.
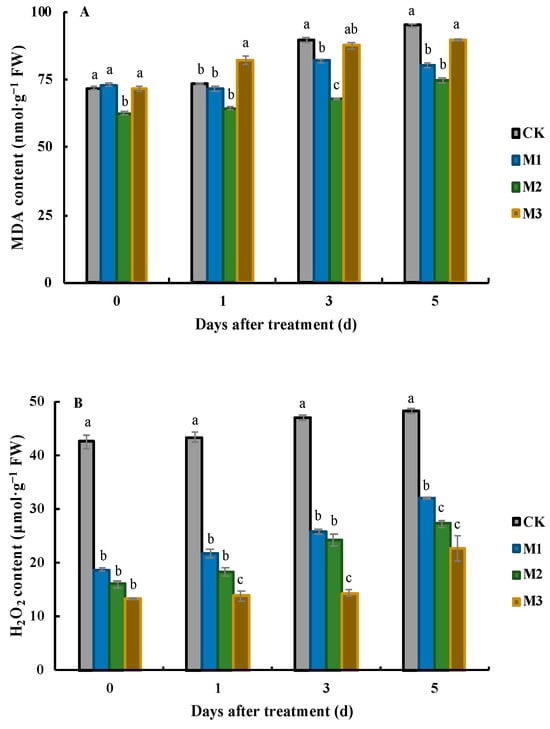
Figure 2.
Effects of MeJA application on MDA (A) and H2O2 (B) on different days after treatment. Values represent the mean ± SD, and different lowercase letters indicate significant differences at the 0.05 level.
The trend of H2O2 content in the leaves of different treatments was similar to that of the MDA content, and the H2O2 content in the CK leaves remained the highest during the whole experiment (Figure 2B). At 0 DAT, the H2O2 content in the CK leaves was 42.6 μmol·g−1 FW, which was significantly higher than that in the leaves treated with the three MeJA treatments: 18.8 μmol·g−1 FW, 16.0 μmol·g−1 FW, and 13.5 μmol·g−1 FW, respectively. After one day, the H2O2 content in the leaves of the four treatments slightly increased in comparison with that at 0 DAT, but the overall trend remained the same. On the third day after treatment, the H2O2 content in the leaves of the M3 treatment (14.4 μmol·g−1 FW) was significantly lower than that of the leaves of the other treatments. At this time, the H2O2 content in the leaves of the M1 and M2 was 1.79 and 1.68 times higher, respectively, than that in the M3 leaves. Two days later, the H2O2 content in the CK leaves increased to 48.2 μmol·g−1 FW, which was significantly higher than that in the leaves of the three MeJA treatments, while the difference in H2O2 content between the M2 and M3 treatments was not significant.
3.3. Effects of MeJA on CAT, SOD, and POD Activities in J. sambac Leaves
It was found that the CAT activity in the leaves of the M2 treatment was the highest at all sampling times (Figure 3A). During the first sampling period, the CAT activity in the leaves of the M2 treatment reached 10.7 U·g−1 FW, which was 1.21, 1.21, and 1.20 times higher than that in the leaves of the CK, M1, and M3, respectively. There were no significant differences in CAT activity between these three treatments. After that, the CAT activity in the leaves of the M2 and M3 treatments decreased, while the CAT activity in the CK and M1 leaves slightly increased. At this time, the CAT activity was the highest in the M2 treatment (9.68 U·g−1 FW) and the lowest in the M3 treatment (7.69 U·g−1 FW). At 3 DAT, the CAT activity in the leaves of the M1 treatment dropped to the lowest level, at 8.95 U·g−1 FW, which was not significantly different from the CAT activity in the leaves of the M3 treatment (9.47 U·g−1 FW), but was significantly lower than that in the leaves of the CK and M2 treatment (10.4 U·g−1 FW and 11.6 U·g−1 FW, respectively). During the last sampling period, the CAT activity in the leaves of the M2 treatment continued to increase and was significantly higher than that in the leaves treated with the other three treatments. At this time, the CAT activity in the CK leaves was only 8.15 U·g−1 FW.
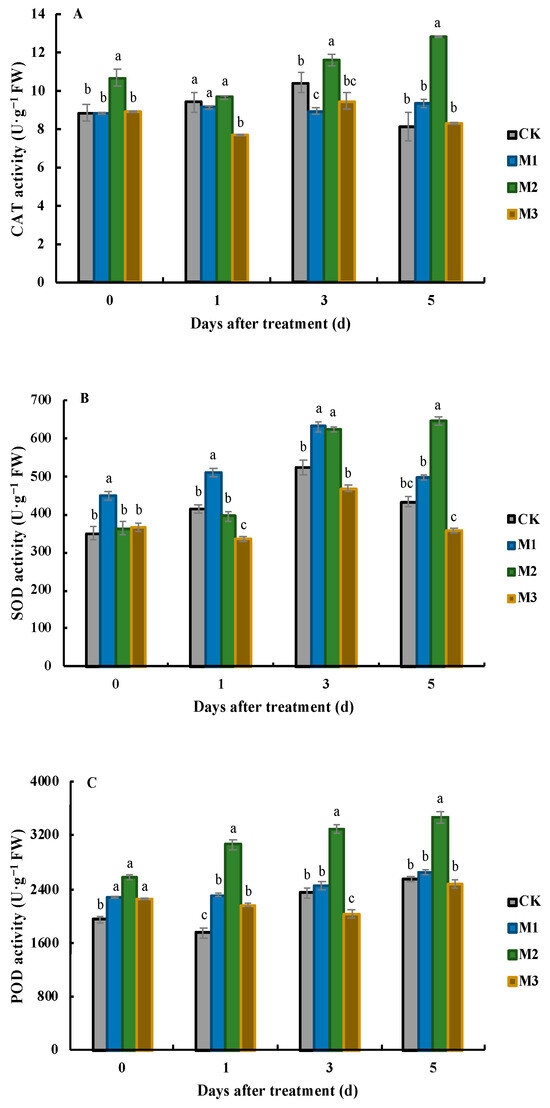
Figure 3.
Effects of MeJA application on CAT (A), SOD (B), and POD (C) on different days after treatment. Values represent the mean ± SD, and different lowercase letters indicate significant differences at the 0.05 level.
The trend in the SOD activity in the leaves under different treatments varied (Figure 3B). The SOD activity in the leaves of the CK and M1 treatment firstly increased and then decreased, while the SOD activity in the leaves of the M2 treatment continuously increased. In contrast, the SOD activity in the leaves of the M3 treatment initially decreased, then increased, and finally decreased again. During the first two sampling periods, the SOD activity in the leaves of the M1 treatment was the highest, reaching 449.5 U·g−1 FW and 510.9 U·g−1 FW, respectively, which was significantly higher than the values of the other treatments. At 0 DAT and 1 DAT, the SOD activity in the leaves of the CK and M3 treatment was the lowest. On the third day, although the SOD activity in the leaves of the M1 treatment remained the highest, the difference of the SOD activity in the leaves of the M2 treatment narrowed, with values of 629.7 U·g−1 FW and 623.3 U·g−1 FW, respectively. Two days later, the SOD activity in the leaves of the M2 treatment reached the highest level (643.4 U·g−1 FW), which was significantly higher than that in the leaves of the other treatments. The SOD activity in the leaves of the M1 treatment was second (496.1 U·g−1 FW), but the difference was not significant when compared with the SOD activity in the CK leaves (432.2 U·g−1 FW). The SOD activity in the leaves of the M3 treatment was only 354.9 U·g−1 FW.
The POD activity in the leaves of each treatment generally displayed an upward trend (Figure 3C). Specifically, the POD activity in the CK leaves was the lowest at 0 DAT (1944.8 U·g−1 FW), and it was significantly lower than that of the other treatments. During this period, the POD activity in the leaves of the M2 treatment was the highest, reaching 2572.6 U·g−1 FW. One day later, the POD activity in the leaves of the M2 treatment further increased its lead over the other treatments, and it was 1.32 times higher than that in the leaves of the M1 treatment and 1.42 times higher than that in the leaves of the M3 treatment. The POD activity in the CK leaves was only 57% of that in the leaves of the M2 treatment. During the next two sampling periods, except for the decrease in POD activity in the leaves of the M3 treatment, the POD activity in the leaves of the other three treatments increased, with the largest increase (9.2%) occurring in the CK leaves.
4. Discussion
As global climate change occurs irregularly, suitable growth environments for plants are constrained. Low temperature is one of the challenges that plants encounter during their growth and development, and it can inflict chilling and freezing injuries on them. Low temperatures negatively affect plant productivity and determine the geographic distribution of plants. Plant leaves turn yellow and wilt under chilling injury, while plants in tropical and subtropical regions may even die. Freezing injury may cause leaf tissue to soften or die due to the loss of pressure [24]. The length and width of Triticum aestivum grains decreased with the increasing severity and duration of cold stress, which was possibly due to the effects of low temperatures on enzyme activity and a decrease in grain-filling rates, which in turn reduced grain dry matter accumulation and, ultimately, decreased grain size [25,26]. Most varieties of Vitis vinifera are sensitive to low temperatures and are vulnerable to freezing injuries during winter, sometimes leading to plant death. Research has indicated that exposure to sub-zero temperatures could cause damage to grape cell membranes and the leakage of intracellular electrolytes, and this damage increases with decreasing temperatures or prolonged durations [27].
J. sambac, an evergreen shrub belonging to the genus Jasminum, is an ideal ornamental plant due to its pure white flowers and strong fragrance. Additionally, it possesses high medicinal value and is used in the production of tea, perfumes, cosmetics, and more, making it widely cultivated in many countries. In China, the cultivation of J. sambac dates back over 2000 years [8], and today, large-scale plantations can still be found in Guangxi, Fujian, and other provinces. Given its significant economic and ecological value, extensive research has been conducted on it, primarily focusing on the structural characteristics of its petals, the photosynthetic capacity of its leaves, and its floral fragrance [9,28,29]. However, J. sambac is a plant that prefers warmth and is not tolerant to cold, exhibiting poor growth performance at low temperatures, which hinders its cultivation and promotion. Therefore, enhancing the cold tolerance of J. sambac is a crucial aspect of efficient cultivation techniques. MeJA plays an important role in regulating signal transduction in plant defense genes and has been proven to improve cold tolerance in Malus × domestica, Olea europaea, Citrus limon, and other plants [30,31,32]. In this study, different concentrations of MeJA were applied to J. sambac leaves, and the effect of MeJA on cold resistance was evaluated by measuring the trends of changes in physiological indexes in the leaves at different time points under low-temperature stress.
Soluble sugar serves as a crucial substance for osmotic adjustment in plants, and an increase in its content can prevent water loss from plant cells, maintain the stability of cell membrane structures, and participate in regulating stress resistance [33]. In this experiment, the application of various concentrations of MeJA significantly boosted the soluble sugar content in leaves during the initial phase of low-temperature stress (0–3 d). Among these treatments, the M2 concentration exhibited the most profound increase. However, in the later stage of stress (5 d), only the M2 treatment resulted in an increased soluble sugar content, while the others exhibited a decrease. A possible reason for the rise in soluble sugar content induced by the MeJA treatment was the activation of a series of genes related to soluble sugar synthesis that encoded enzymes with increased activity, leading to an increase in the soluble sugar content [34]. The exogenous addition of MeJA enhanced the cold tolerance of J. sambac by increasing the soluble sugar content through the entry of MeJA into plant leaves via the stomata, the hydrolysis into JA by esterases, and the realization of long-distance signal transduction, inducing defense responses in plants [35]. Regarding the varied effects of the M2, M1, and M3 treatments on the soluble sugar content on the fifth day, it was possible that the M2 treatment represented a suitable inductive concentration that could maintain or elevate the activity of enzymes related to sugar metabolism for a longer period. In our study, MeJA generally improved the content of soluble protein in leaves, with the M3 treatment having an apparent effect in the early stage and the M2 treatment having an effect in the later stage. Soluble protein is also an essential substance for osmotic adjustment in plants, and its accumulation in the cytoplasm can prevent protoplast dehydration and enhance plant cold tolerance [36,37]. Nonetheless, in a study conducted by Lu et al., the soluble protein content in Cocos nucifera leaves first increased and then decreased at low temperatures [38], which was different from our results. This indicated that although soluble protein in C. nucifera leaves actively regulated the damage to cell membranes caused by low temperatures, this regulatory effect was weakened with prolonged low-temperature stress. In addition, their experimental results were obtained without artificial intervention, resulting in a decreasing trend of the soluble protein content. In contrast, we exogenously applied MeJA to J. sambac leaves, and accordingly, the soluble protein content in the leaves remained at a relatively high level, suggesting that MeJA promoted the accumulation of soluble protein, thereby enhancing the cold tolerance of J. sambac. Similar experimental results were also obtained in O. europaea [31].
It has been reported that low temperature adversely affects photosynthesis in plants, reducing their efficiency in utilizing light energy, damaging the chloroplast structures in leaf cells, inducing chlorophyll degradation, and impacting photosynthetic productivity, thereby hindering plant growth and development. Chlorophyll, an essential pigment in plants, reflects the strength of photosynthetic capacity and ensures the normal functioning of photosynthesis. Research has shown that under low-temperature stress, the accumulation of chlorophyll content in plant leaves is significantly inhibited [39]. Our experiment reached a similar conclusion. Specifically, the total chlorophyll content in J. sambac leaves without the MeJA treatment continuously decreased, indicating that low temperatures disrupted the balance between chlorophyll synthesis and degradation, leading to a reduction in the activity of enzymes related to chlorophyll synthesis, hindering chlorophyll synthesis, or increasing the rate of chlorophyll decomposition and damage to chloroplast structures [40]. But after the exogenous application of MeJA, the negative impact of low-temperature stress was effectively mitigated and the chlorophyll content was significantly increased, particularly in the leaves of the M2 treatment, where the chlorophyll content increased the most. This manifested that MeJA was able to enhance the protective function of plant leaves, eliminate toxic substances produced by low-temperature stress in a timely manner, protect chloroplasts from damage, promote pigment synthesis, maintain a high level of chlorophyll content in plants, and sustain photosynthetic capacity. Fan et al. pointed out that 1 μmol·L−1 MeJA also increased the chlorophyll content in Vigna sinensis at low temperatures, protecting the photosynthetic system [41]. Nevertheless, some studies have implied that the effect of MeJA on chlorophyll content is dose-dependent, and high concentrations of MeJA may reduce the chlorophyll content, weaken plant photosynthetic rates, and induce stomatal closure [42].
H2O2 is a kind of reactive oxygen species (ROS), and when its accumulation exceeds a certain limit, it can lead to oxidative stress, membrane peroxidation, and cell death in plants, while MDA is a byproduct of lipid peroxidation; the content of both is often used to measure the degree of plant exposure to low-temperature stress [43]. The ability of plants to adapt to stressful environments depends on their capacity to eliminate the ROS. Plants adapt to low-temperature stress by regulating their enzymatic reaction system to maintain a balanced ROS metabolism, thereby protecting plant cells from or reducing damage. When the dynamic balance of the ROS in plants is disrupted, their protective and defensive mechanisms change accordingly. They decompose the ROS through enzymatic and non-enzymatic antioxidant systems; the enzymatic defense system mainly consists of SOD, CAT, and POD. SOD is a scavenger of peroxide anions, which can disproportionate anions into H2O2 and oxygen. At the same time, POD and CAT catalyze the conversion of H2O2 into oxygen and water [44,45], thereby alleviating the damage caused by excessive ROS accumulation to cell membranes and assisting plants in resisting low-temperature stress. In the current experiment, we observed a continuous increase in the H2O2 content during the whole stress period, and the content in the CK leaves was significantly higher than that in the three treatment groups. However, the MDA content displayed a significant increase from the initial to the middle and late stages of stress, and the overall MDA content in the CK leaves was higher than that in the treatment groups. Compared with the CK leaves, the M2 leaves demonstrated a remarkably decreased MDA content in all sampling periods. These findings indicate that low temperature caused considerable adverse effects on J. sambac seedlings. To cope with these effects, the activity of SOD, POD, and CAT in MeJA-treated leaves increased to varying degrees, demonstrating that they effectively scavenged the ROS generated by low temperatures. Overall, the M2 treatment achieved the best results. The increase in SOD activity was slightly higher than the increase in POD and CAT activity, which may be attributed to the lower decomposition capacity of POD and CAT for H2O2 produced by SOD [38]. The research by Repkina [46] and Gul [19] revealed that MeJA also increased the activity of SOD, POD, and CAT in the leaves of T. aestivum and S. lycopersicum. They concluded that MeJA induced the expression of genes encoding these enzymes. The aforementioned evidence validated the experimental finding that MeJA application bolstered the enzymatic antioxidant system in J. sambac, effectively scavenging the excessive ROS, thereby mitigating the adverse effects of low temperatures on plants [47].
5. Conclusions
Our study revealed that the external application of MeJA could alleviate the detrimental impacts of cold stress on the growth and development of J. sambac, thereby enhancing its resilience to cold conditions. MeJA at a concentration of 10 μmol·L−1 had the best effect. This was mainly achieved by increasing the content of nutrients (soluble sugar and soluble protein) and chlorophyll as well as the activity of antioxidant enzymes (CAT, SOD, and POD) in leaves. Therefore, the rational application of MeJA is essential for evaluating and improving the cold resistance of J. sambac.
Author Contributions
Conceptualization, C.C. and K.Y.; methodology, H.C.; software, C.C.; formal analysis, C.C. and H.C.; investigation, C.C. and H.C.; writing—original draft preparation, C.C.; writing—review and editing, K.Y.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yangzhou ‘Lvyangjinfeng’ Excellent Doctoral Project (YZLYJFJH2022YXBS019) and the Science and Technology Vice President Project of Jiangsu Province (FZ20230140).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
We sincerely thank General Manager Wang Jihua, from Nanjing Yangzi Jasmine Valley Culture Technology Co., Ltd. for providing experimental materials for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, Y.; Liu, Z.Y.; Lyu, M.L.; Yuan, Y.; Wu, B.H. Characterization of JsWOX1 and JsWOX4 during callus and root induction in the shrub species Jasminum sambac. Plants 2019, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Supanivatin, P.; Siriwattanayotin, S.; Thipayarat, A.; Ekkaphan, P.; Wongwiwat, J. Effect of overfilled solvent and storage time of subcritical extraction of Jasminum sambac on yield, antioxidant activity, antimicrobial activity and tentative volatile compounds. Plants 2023, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Zhang, H.L.; Wan, C.; He, X.; Huang, J.F.; Lyu, M.L.; Yuan, Y.; Wu, B.H. Characterization of two BAHD acetyltransferases highly expressed in the flowers of Jasminum sambac (L.) Aiton. Plants 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Pharmacological and therapeutic effects of Jasminum sambac—A review. Indo Am. J. Pharm. Sci. 2018, 5, 1766–1778. [Google Scholar]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wang, W.; Huang, J.F.; Wang, Y.T.; Hu, L.; Yuan, Y.; Lyu, M.L.; Wu, B.H. Role of gibberellin and its three GID1 receptors in Jasminum sambac stem elongation and flowering. Planta 2022, 255, 17. [Google Scholar] [CrossRef]
- Dhanasekaran, D. Influence of growth regulating chemicals on growth and flowering in Jasmine (Jasminum sambac Ait.). J. Hortic. Sci. 2018, 13, 221–226. [Google Scholar] [CrossRef]
- Deng, Y.M.; Jia, X.P.; Liang, L.J.; Gu, C.S.; Sun, X.B. Morphological anatomy, sporogenesis and gametogenesis in flowering process of jasmine (Jasminum sambac Aiton). Sci. Hortic. 2016, 198, 257–266. [Google Scholar] [CrossRef]
- Bera, P.; Mukherjee, C.; Mitra, A. Enzymatic production and emission of floral scent volatiles in Jasminum sambac. Plant Sci. 2016, 256, 25. [Google Scholar] [CrossRef]
- Deng, Y.M.; Qi, X.Y. Research progress on effects of external factors on growth of jasmine. Jiangsu Agric. Sci. 2019, 47, 62–65. (In Chinese) [Google Scholar]
- Niu, S.H. Discussion on the cultivation and management technology of Jasminum sambac. Hortic. Seed 2023, 43, 37–41. (In Chinese) [Google Scholar]
- Cai, H.; He, M.Y.; Ma, K.; Huang, Y.G.; Wang, Y. Salicylic acid alleviates cold-induced photosynthesis inhibition and oxidative stress in Jasminum sambac. Turk. J. Biol. 2015, 39, 241–247. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.R.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Najeeb, S.; Mahender, A.; Anandan, A.; Hussain, W.; Li, Z.; Ali, J. Genetics and breeding of low-temperature stress tolerance in rice. In Rice Improvement: Physiological, Molecular Breeding and Genetic Perspectives; Ali, J., Wani, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 221–280. [Google Scholar]
- Bao, G.Z.; Tang, W.Y.; An, Q.R.; Liu, Y.X.; Tian, J.Q.; Zhao, N.; Zhu, S.N. Physiological effects of the combined stresses of freezing-thawing, acid precipitation and deicing salt on alfalfa seedlings. BMC Plant Biol. 2020, 20, 204. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Zhang, Q.Q.; Ou, L.N.; Ji, D.Z.; Liu, T.; Lan, R.M.; Li, X.Y.; Jin, L.H. Response to the cold stress signaling of the tea plant (Camellia sinensis) elicited by chitosan oligosaccharide. Agronomy 2020, 10, 915. [Google Scholar] [CrossRef]
- Huan, C.; Yang, X.H.; Wang, L.F.; Kebbeh, M.; Wang, Y.X.; Dai, B.G.; Shen, S.L.; Zheng, X.L.; Zhou, H.J. Methyl jasmonate treatment regulates α-linolenic acid metabolism and jasmonate acid signaling pathway to improve chilling tolerance in both stony hard and melting flesh peaches. Postharvest Biol. Technol. 2022, 190, 111960. [Google Scholar] [CrossRef]
- Gul, N.; Masoodi, K.Z.; Ramazan, S.; Mir, J.I.; Aslam, S. Study on the impact of exogenously applied methyl jasmonate concentrations on Solanum lycopersicum under low temperature stress. BMC Plant Biol. 2023, 23, 437. [Google Scholar] [CrossRef]
- Wang, X.Q. Principles and Techniques of Plant Physiological and Biochemical Tests; Higher Education Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Ranieri, A.; Castagna, A.; Baldan, B.; Soldatini, G.F. Iron deficiency differently affects peroxidase isoforms in sunflower. J. Exp. Bot. 2001, 52, 25–35. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, J.H. Water stress -induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulated the activities of antioxidant enzymes in the maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Ni, M.; Yu, F.Y. A study on petal morphological and physiological characteristics of Styrax japonicus during the flowering period. Agronomy 2021, 11, 1498. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J. Cold stress signaling networks in Arabidopsis. J. Plant Biol. 2013, 56, 69–76. [Google Scholar] [CrossRef]
- Liu, L.L.; Song, H.; Shi, K.J.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.X.; Zhu, Y. Response of wheat grain quality to low temperature during jointing and booting stages-on the importance of considering canopy temperature. Agric. For. Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhao, Y.; Li, L.Y.; Xu, X.; Yang, L.; Luo, Z.; Wang, B.B.; Ma, S.Y.; Fan, Y.H.; Huang, Z.L. The effects of short-term exposure to low temperatures during the booting stage on starch synthesis and yields in wheat grain. Front. Plant Sci. 2021, 12, 684784. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.H.; Yao, M.H.; Yao, F.; Wang, Z.L.; Wang, H.; Li, H. Transcriptomics reveals the effect of short-term freezing on the signal transduction and metabolism of grapevine. Int. J. Mol. Sci. 2023, 24, 3884. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.M.; Li, C.C.; Shao, Q.S.; Ye, X.Q.; She, J.M. Differential response of double petal and multi petal jasmine to shading: I. Photosynthetic characteristics and chloroplast ultrastructure. Plant Physiol. Bioch. 2012, 55, 93–102. [Google Scholar] [CrossRef]
- Wang, P.J.; Fang, J.P.; Lin, H.Z.; Yang, W.W.; Yu, J.X.; Hong, Y.P.; Jiang, M.; Gu, M.; Chen, Q.; Zheng, Y.; et al. Genomes of single- and double-petal jasmines (Jasminum sambac) provide insights into their divergence time and structural variations. Plant Biotechnol. J. 2022, 20, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Xu, H.F.; Liu, W.J.; Wang, N.; Qu, C.Z.; Jiang, S.H.; Fang, H.C.; Zhang, Z.Y.; Chen, X.S. Methyl jasmonate enhances apple’ cold tolerance through the JAZ-MYC2 pathway. Plant Cell 2019, 136, 75–84. [Google Scholar] [CrossRef]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Enhancement of freezing tolerance of olive leaves by foliar application of methyl jasmonate and 24-epibrassinolide through changes in some metabolites and antioxidant activity. Sci. Hortic. 2021, 284, 110127. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Chen, C.; Lai, J.Y.; Chen, H.; Yu, F.Y. Zinc oxide nanoparticles enhanced growth of tea trees via modulating antioxidant activity and secondary metabolites. Horticulturae 2023, 9, 631. [Google Scholar] [CrossRef]
- Wang, K.T.; Lei, C.Y.; Tan, M.L.; Wang, J.S.; Li, C.H.; Zou, Y.Y. Increased soluble sugar accumulation in postharvest peaches in response to different defense priming elicitors. Hortic. Environ. Biotechnol. 2023, 64, 115–131. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ren, Y.; Yang, D.Y.; Liu, H.D.; Zhang, Y.S.; Wang, X.J.; Bai, F.J.; Cheng, S.R. Foliar methyl jasmonate (MeJA) application increased 2-acetyl-1-Pyrroline (2-AP) content and modulated antioxidant attributes and yield formation in fragrant rice. J. Plant Physiol. 2023, 282, 153946. [Google Scholar] [CrossRef] [PubMed]
- George, I.S.; Pascovici, D.; Mirzaei, M.; Haynes, P.A. Quantitative proteomic analysis of cabernet sauvignon grape cells exposed to thermal stresses reveals alterations in sugar and phenylpropanoid metabolism. Proteomics 2015, 15, 3048–3060. [Google Scholar] [CrossRef]
- Zhu, A.D.; Li, W.Y.; Ye, J.L.; Sun, X.H.; Ding, Y.D.; Cheng, Y.J.; Deng, X.X. Microarray expression profiling of postharvest ponkan mandarin (Citrus reticulata) fruit under cold storage reveals regulatory gene candidates and implications on soluble sugars metabolism. J. Int. Plant Biol. 2011, 53, 358–374. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Yang, W.B.; Dong, Z.G.; Tang, L.X.; Liu, Y.Y.; Xie, S.Y.; Yang, Y.D. Integrated transcriptomic and metabolomics analyses reveal molecular responses to cold stress in coconut (Cocos nucifera L.) seedlings. Int. J. Mol. Sci. 2023, 24, 14563. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.W.; Wang, R.J.; Gong, J.J.; Zhu, T.Q.; Long, S.; Guo, H.; Liu, T.Y.; Yang, P.Z.; Xu, Y.F. Combined cold and drought stress-induced response of photosynthesis and osmotic adjustment in Elymus nutans Griseb. Agronomy 2023, 13, 2368. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Han, Q.H.; Ding, C.B.; Huang, Y.; Liao, J.Q.; Chen, T.; Feng, S.L.; Zhou, L.J.; Zhang, Z.W.; Chen, Y.E.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef]
- Fan, L.L.; Wang, Q.; Lv, J.W.; Gao, L.P.; Zuo, J.H.; Shi, J.Y. Amelioration of postharvest chilling injury in cowpea (Vigna sinensis) by methyl jasmonate (MeJA) treatments. Sci. Hortic. 2016, 203, 95–101. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Hamayun, M.; Kang, S.M.; Lee, I.J. Foliar application of methyl jasmonate induced physio-hormonal changes in Pisum sativum under diverse temperature regimes. Plant Physiol. Biochem. 2015, 96, 406–416. [Google Scholar] [CrossRef]
- Elatafi, E.; Elshahat, A.; Yu, X.; Li, S.N.; Lu, S.W.; Dong, T.Y.; Fang, J.G. Effects of different storage temperatures and methyl jasmonate on grape quality and antioxidant activity. Horticulturae 2023, 9, 1282. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; El-Kersh, M.A.; Al-Omair, M.A. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS ONE 2015, 10, e0144509. [Google Scholar] [CrossRef] [PubMed]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Repkina, N.; Ignatenko, A.; Holoptseva, E.; Miszalskl, Z.; Kaszycki, P.; Talanova, V. Exogenous methyl jasmonate improves cold tolerance with parallel induction of two cold-regulated (COR) genes expression in Triticum aestivum L. Plants 2021, 10, 1421. [Google Scholar] [CrossRef]
- Nyanasaigran, L.; Ramasamy, S.; Gautam, A.; Guleria, P.; Kumar, V.; Yaacob, J.S. Methyl jasmonate elicitation improves the growth performance and biosynthesis of antioxidant metabolites in Portulaca oleracea through ROS modulation. Ind. Crop. Prod. 2024, 216, 118709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).