Overexpression of the CpCOR413PM1 Gene from Wintersweet (Chimonanthus praecox) Enhances Cold and Drought Tolerance in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning of CpCOR413PM1 and Bioinformatic Analysis
2.2. RNA Extraction and qRT-PCR Analysis
2.3. Protein Expression, Purification, and Concentration
2.4. Resistance Analysis of E. coli
2.5. Plant Material and Growth Conditions
2.6. Vector Construction and Plant Transformation
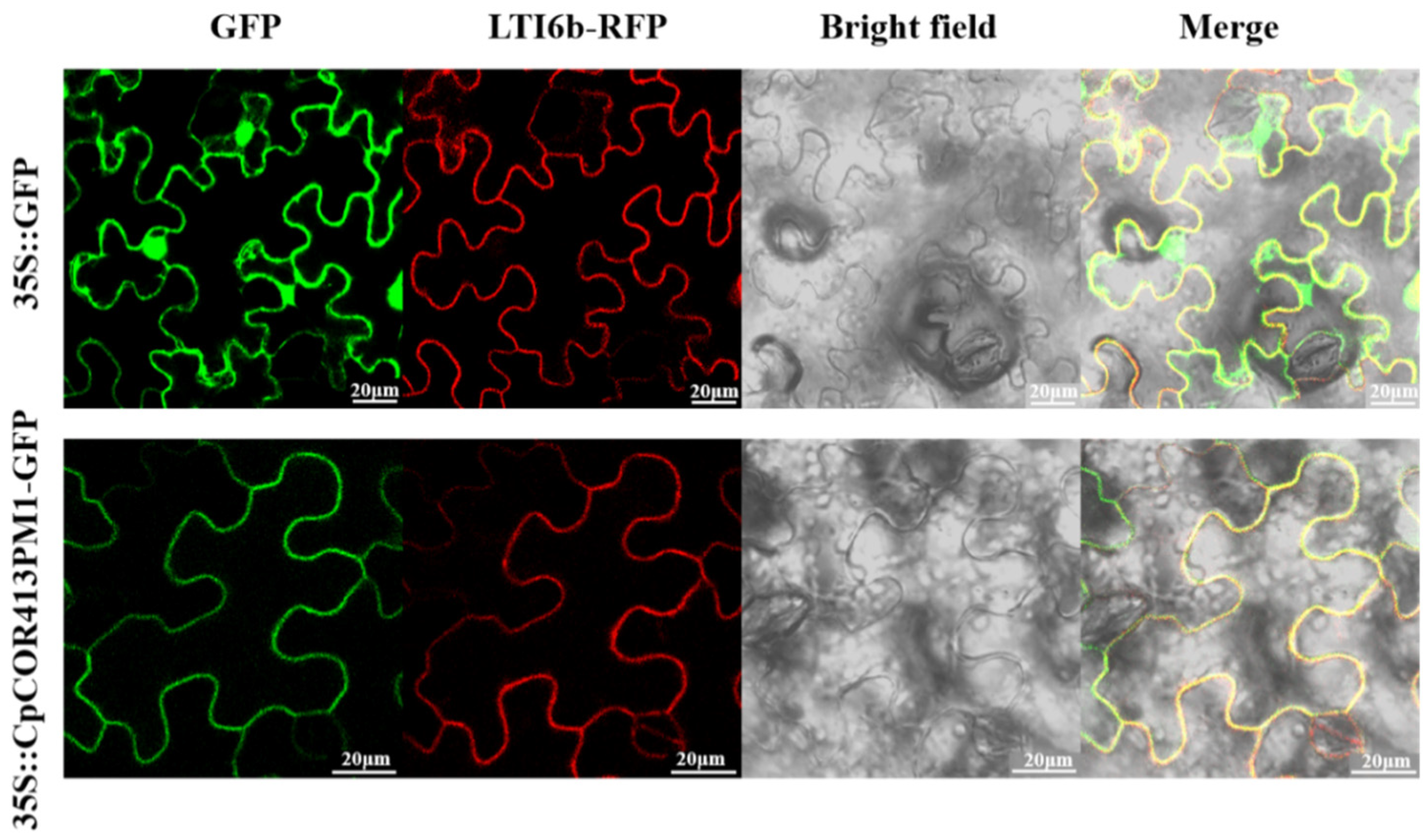
2.7. Subcellular Localization of CpCOR413PM1
2.8. Stress Experimental Conditions
2.9. Measurement of Physiological Indicators
2.10. Cloning and Analysis of the Upstream Sequence of CpCOR413PM1
2.11. Statistical Analysis
3. Results
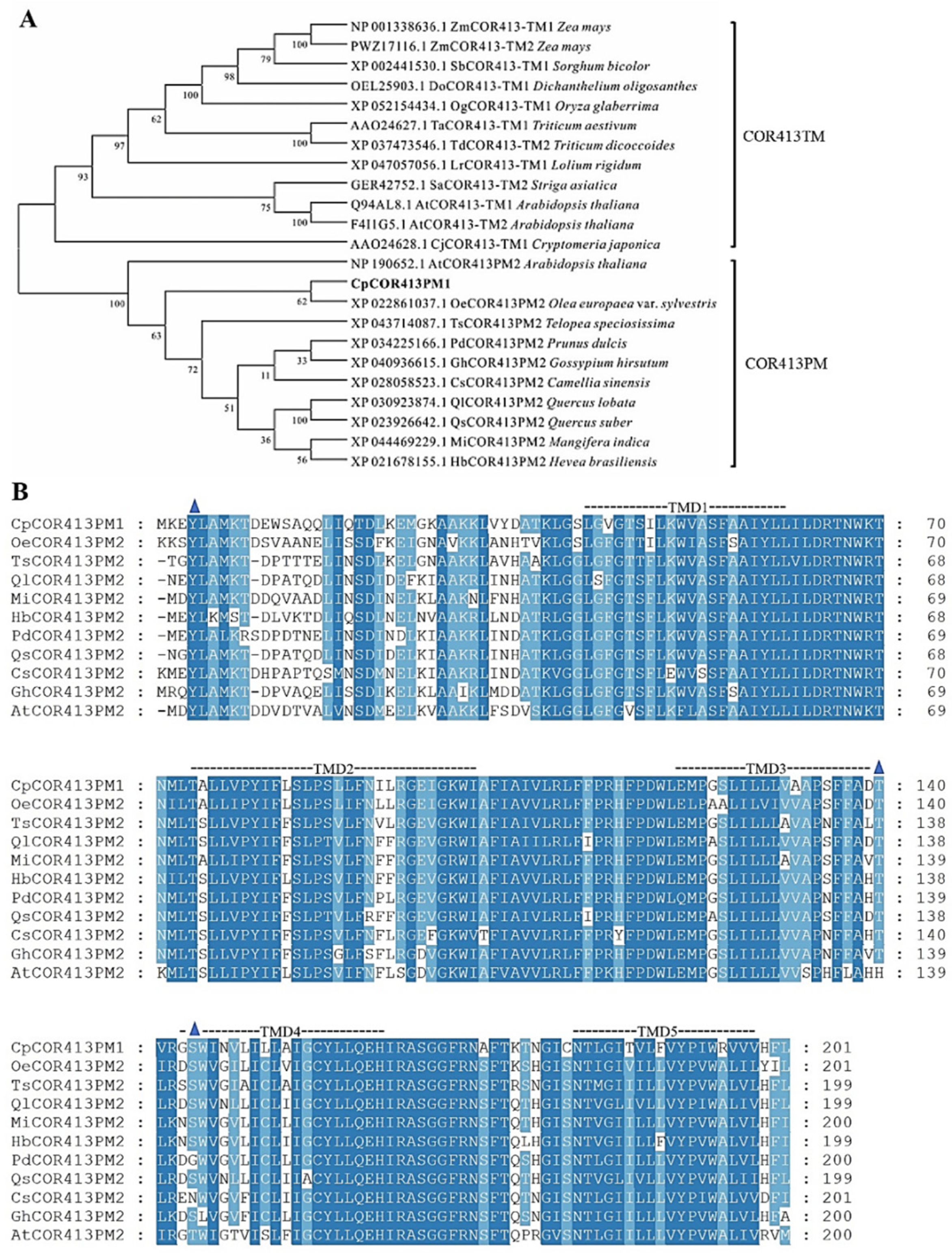
3.1. Cloning and Sequence Analysis of CpCOR413PM1
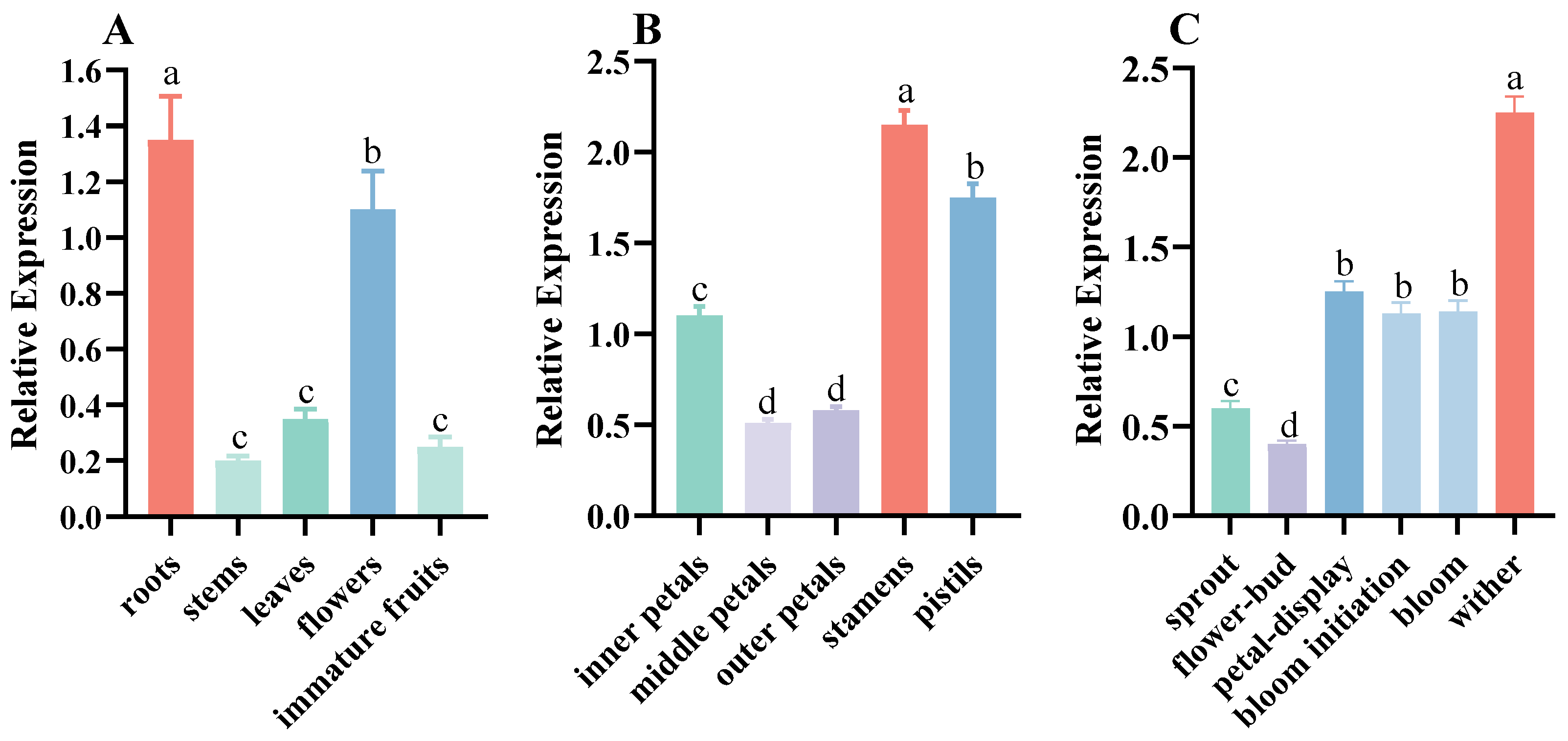
3.2. Tissue-Specific Expression of CpCOR413PM1
3.3. Resistance of E. coli Overexpressing the CpCOR413PM1 Gene
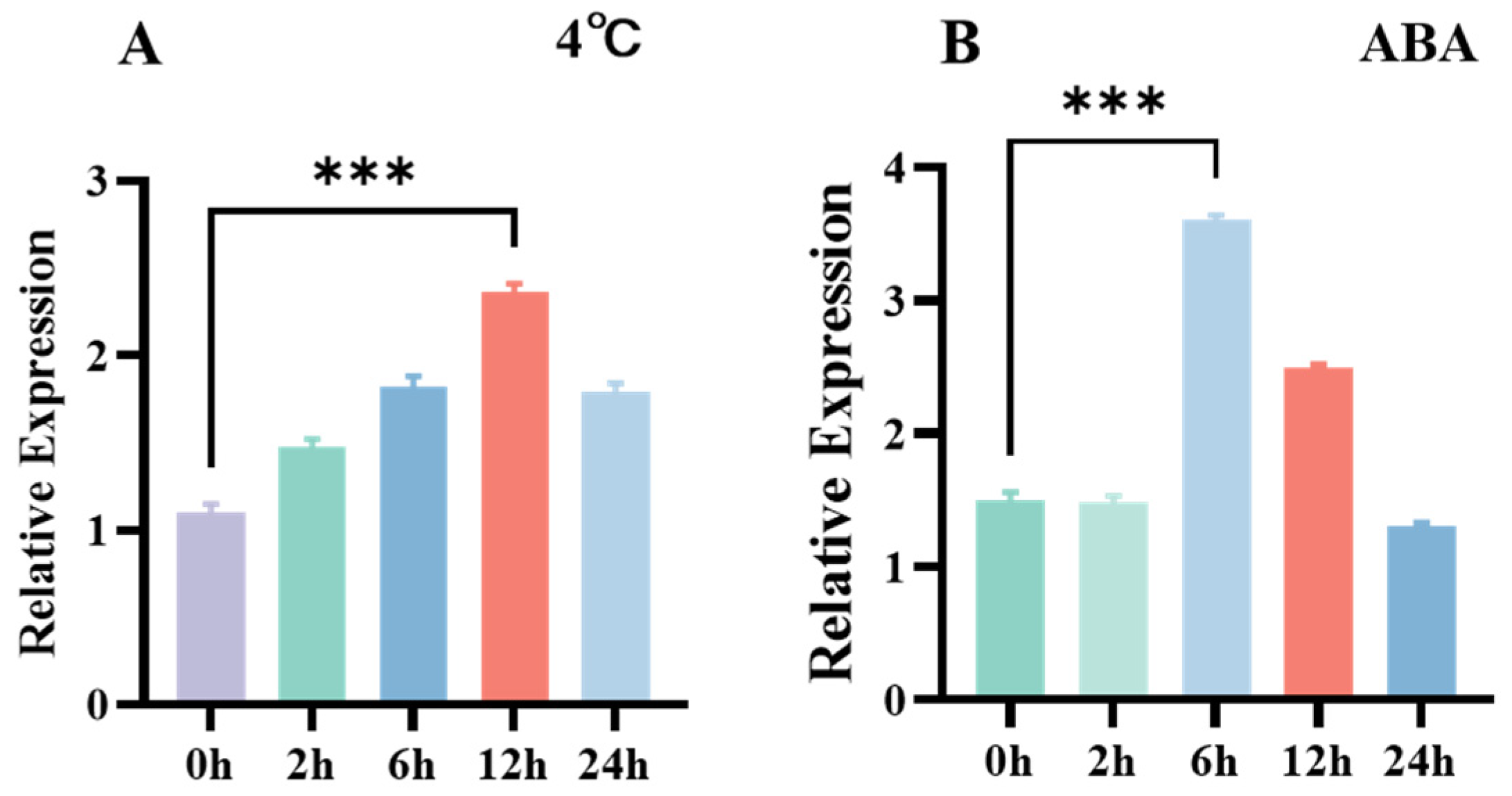
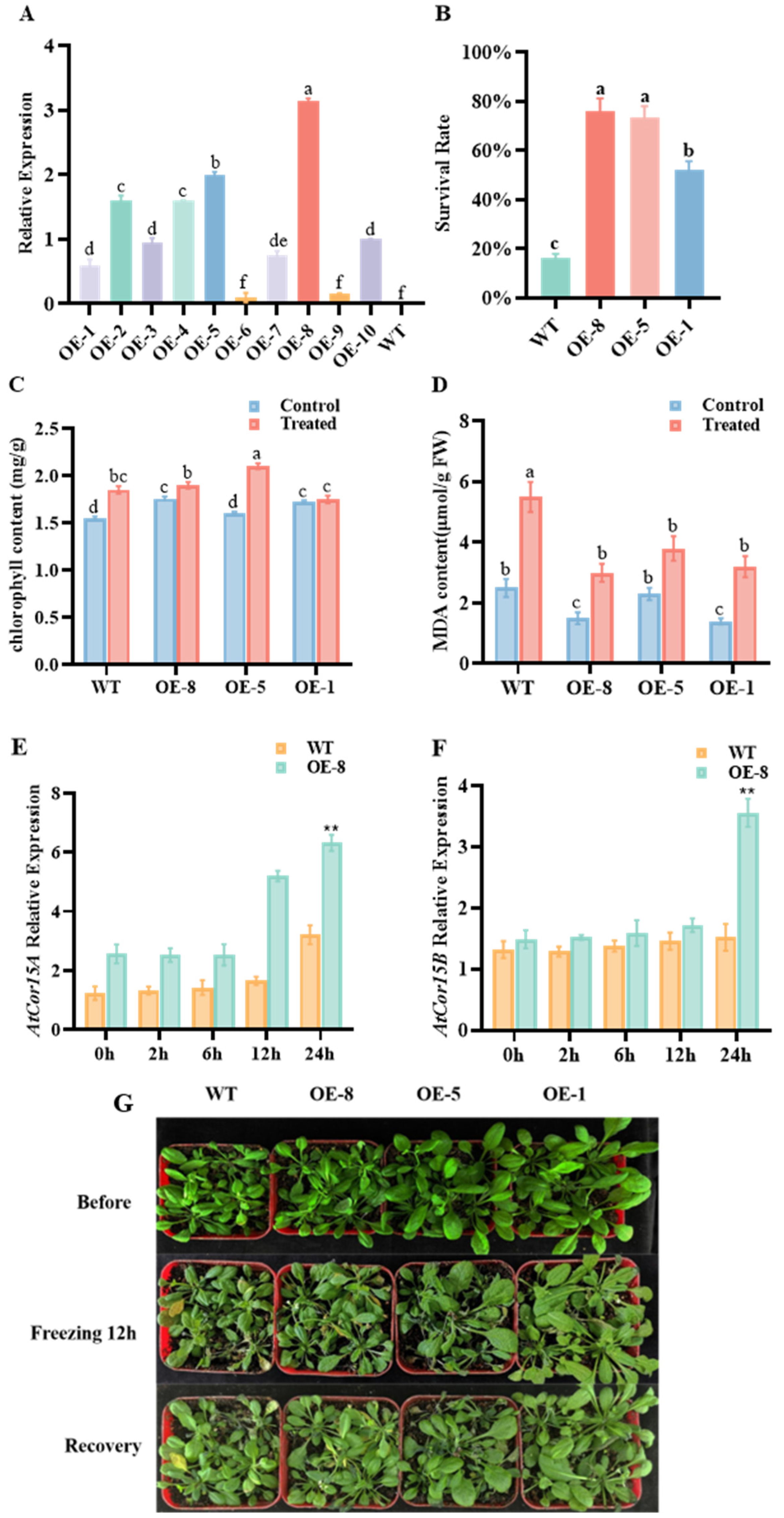
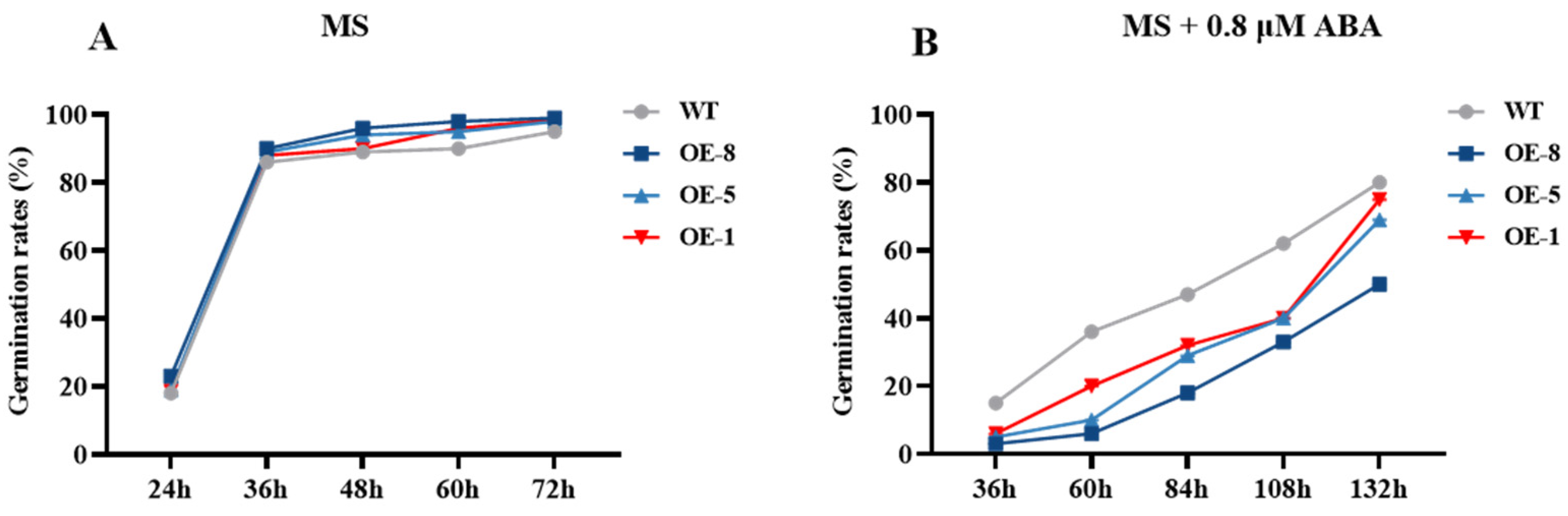
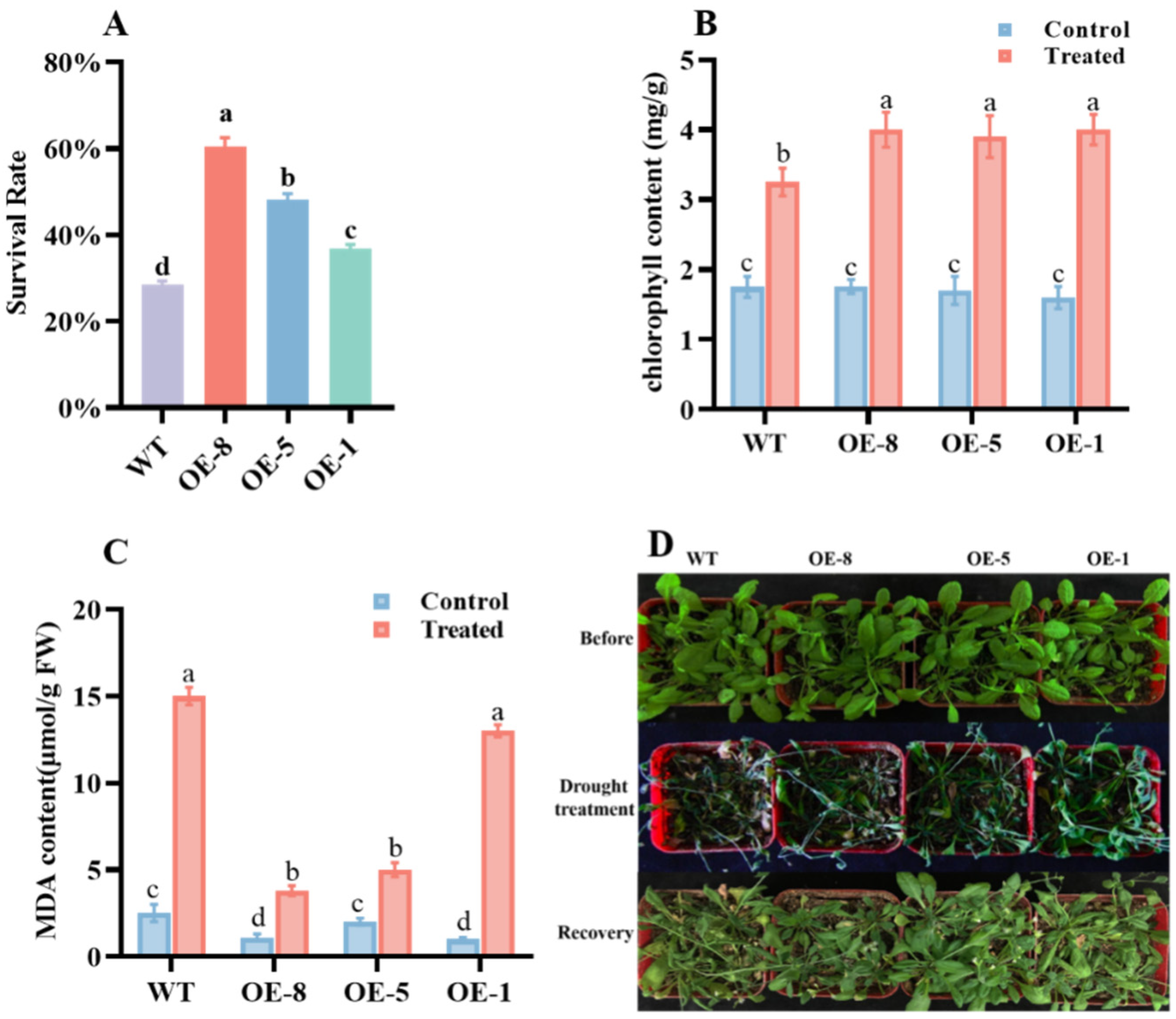
3.4. Effects of CpCOR413PM1 Overexpression on Stress Tolerance in Arabidopsis
3.5. Functional Characterization of the CpCOR413PM1 Promoter
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Okawa, K.; Nakayama, K.; Kakizaki, T.; Yamashita, T.; Inaba, T. Identification and characterization of Cor413im proteins as novel components of the chloroplast inner envelope. Plant Cell Environ. 2008, 31, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Breton, G.; Danyluk, J.; Charron, J.B.; Sarhan, F. Expression profiling and bioinformatic analyses of a novel stress-regulated multispanning transmembrane protein family from cereals and Arabidopsis. Plant Physiol. 2003, 132, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Garwe, D.; Thomson, J.A.; Mundree, S.G. XVSAP1 from Xerophyta viscosa improves osmotic-, salinity- and high-temperature-stress tolerance in Arabidopsis. Biotechnol. J. 2006, 1, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhong, H.; Ren, F.; Guo, Q.Q.; Hu, X.P.; Li, X.B. A novel cold-regulated gene, COR25, of Brassica napus is involved in plant response and tolerance to cold stress. Plant Cell Rep. 2011, 30, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, G.; Zhao, W.; Yang, M.; Ma, N.; Kong, F.; Dong, X.; Meng, Q. SlCOR413IM1: A novel cold-regulation gene from tomato, enhances drought stress tolerance in tobacco. Plant Physiol. 2017, 216, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, J.F.; Chang, Q.S.; Gu, C.S.; Song, A.P.; Chen, S.M.; Dong, B.; Chen, F.D. Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Mol. Biol. Rep. 2014, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Machuka, J.; Bashiardes, S.; Ruben, E.; Spooner, K.; Cuming, A.; Knight, C.; Cove, D. Sequence analysis of expressed sequence tags from an ABA-treated cDNA library identifies stress response genes in the moss Physcomitrella patens. Plant Cell Physiol. 1999, 40, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Cuming, A.C.; Cho, S.H.; Kamisugi, Y.; Graham, H.; Quatrano, R.S. Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol. 2007, 176, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Liu, E.; Li, H.; Li, Y.; Feng, S.; Gong, S.; Wang, J. PsCor413pm2, a Plasma Membrane-Localized, Cold-Regulated Protein from Phlox subulata, Confers Low Temperature Tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2579. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Liu, J.; Lv, Y.; Yu, C.; Li, H.; Zhao, T.; Liu, B. The OsABF1 transcription factor improves drought tolerance by activating the transcription of COR413-TM1 in rice. J. Exp. Bot. 2017, 68, 4695–4707. [Google Scholar] [CrossRef]
- Sui, S.Z.; Luo, J.H.; Ma, J.; Zhu, Q.L.; Lei, X.H.; Li, M.Y. Generation and Analysis of Expressed Sequence Tags from (Wintersweet) Flowers for Discovering Stress-Responsive and Floral Development-Related Genes. Comp. Funct. Genom. 2012, 2012, 134596. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Huang, R.W.; Ma, J.; Sui, S.Z.; Guo, Y.L.; Liu, D.F.; Li, Z.N.; Lin, Y.C.; Li, M.Y. Two C3H Type Zinc Finger Protein Genes and from Affect Stamen Development in. Genes 2017, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Schleifenbaum, F.; Elgass, K.; Sackrow, M.; Caesar, K.; Berendzen, K.; Meixner, A.J.; Harter, K. Fluorescence Intensity Decay Shape Analysis Microscopy (FIDSAM) for Quantitative and Sensitive Live-Cell Imaging: A Novel Technique for Fluorescence Microscopy of Endogenously Expressed Fusion-Proteins. Mol. Plant 2010, 3, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Zhou, L.; Lang, D.Y.; Zhang, W.J.; Wang, J.H.; Gao, X.J.; Wu, X.L.; Fu, X.Y.; Zhang, X.H. Physiological Mechanisms of Salt and Drought Induced Stress Effects on Root Biomass and Secondary Metabolites in Stellaria dichotoma. Int. J. Agric. Biol. 2019, 22, 1285–1292. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Michiels, A.; Van den Ende, W.; Tucker, M.; Van Riet, L.; Van Laere, A. Extraction of high-quality genomic DNA from latex-containing plants. Anal. Biochem. 2003, 315, 85–89. [Google Scholar] [CrossRef]
- Lv, Q.D.; Qiu, J.; Liu, J.; Li, Z.; Zhang, W.T.; Wang, Q.; Fang, J.; Pan, J.J.; Chen, Z.D.; Cheng, W.L.; et al. The Chimonanthus salicifolius genome provides insight into magnoliid evolution and flavonoid biosynthesis. Plant J. 2020, 103, 1910–1923. [Google Scholar] [CrossRef]
- Danyluk, J.; Perron, A.; Houde, M.; Limin, A.; Fowler, B.; Benhamou, N.; Sarhan, F. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell 1998, 10, 623–638. [Google Scholar] [CrossRef]
- Wang, B.G.; Zhang, Q.; Wang, L.G.; Duan, K.; Pan, A.H.; Tang, X.M.; Sui, S.Z.; Li, M.Y. The AGL6-like Gene CpAGL6, a Potential Regulator of Floral Time and Organ Identity in Wintersweet (Chimonanthus praecox). J. Plant Growth Regul. 2011, 30, 343–352. [Google Scholar] [CrossRef]
- Dure, L.; Greenway, S.C.; Galau, G.A. Developmental Biochemistry of Cottonseed Embryogenesis and Germination—Changing Messenger Ribonucleic-Acid Populations as Shown by In vitro and In vivo Protein-Synthesis. Biochemistry 1981, 20, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Goddard, N.J.; Dunn, M.A.; Zhang, L.; White, A.J.; Jack, P.L.; Hughes, M.A. Molecular Analysis and Spatial Expression Pattern of a Low-Temperature-Specific Barley Gene, Blt101. Plant Mol. Biol. 1993, 23, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L.; Niemi, K.J.; Brambl, R. Altered Gene-Expression during Cold-Acclimation of Spinach. Proc. Natl. Acad. Sci. USA 1985, 82, 3673–3677. [Google Scholar] [CrossRef] [PubMed]
- Steponkus, P.L.; Uemura, M.; Joseph, R.A.; Gilmour, S.J.; Thomashow, M.F. Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1998, 95, 14570–14575. [Google Scholar] [CrossRef]
- Jin, Y.N.; Zhai, S.S.; Wang, W.J.; Ding, X.H.; Guo, Z.F.; Bai, L.P.; Wang, S. Identification of genes from the ICE-CBF-COR pathway under cold stress in Aegilops-Triticum composite group and the evolution analysis with those from Triticeae. Physiol. Mol. Biol. Plants 2018, 24, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Peng, D.; Bai, L.P.; Ma, H.; Chen, L.J.; Zhao, M.H.; Xu, Z.J.; Guo, Z.F. Molecular switch for cold acclimation—Anatomy of the cold-inducible promoter in plants. Biochemistry 2013, 78, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Du, D.L.; An, Y.; Yang, W.R.; Wang, J.; Cheng, T.R.; Zhang, Q.X. Overexpression of Prunus mume Dehydrin Genes in Tobacco Enhances Tolerance to Cold and Drought. Front. Plant Sci. 2017, 8, 151. [Google Scholar] [CrossRef]
- Ma, X.C.; Chen, C.; Yang, M.M.; Dong, X.C.; Lv, W.; Meng, Q.W. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol. Biochem. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.Z. PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2005, 331, 325–332. [Google Scholar]
- Zhou, A.M.; Sun, H.W.; Feng, S.; Zhou, M.; Gong, S.F.; Wang, J.G.; Zhang, S.Z. A novel cold-regulated gene from Phlox subulata, PsCor413im1, enhances low temperature tolerance in Arabidopsis. Biochem. Biophys. Res. Commun. 2018, 495, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wang, X.W.; Ban, Q.Y.; Zhu, X.X.; Jiang, C.J.; Wei, C.L.; Bennetzen, J.L. Comparative transcriptomic analysis reveals gene expression associated with cold adaptation in the tea plant Camellia sinensis. BMC Genom. 2019, 20, 624. [Google Scholar] [CrossRef] [PubMed]
- Bhyan, S.B.; Minami, A.; Kaneko, Y.; Suzuki, S.; Arakawa, K.; Sakata, Y.; Takezawa, D. Cold acclimation in the moss Physcomitrella patens involves abscisic acid-dependent signaling. J. Plant Physiol. 2012, 169, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.T.; Zhang, J.K.; Zhang, Q.; Li, X.L.; Li, M.J.; Yang, Y.Z.; Zhou, J.; Wei, Q.P.; Zhou, B.B. Exogenous application of acetic acid enhances drought tolerance by influencing the MAPK signaling pathway induced by ABA and JA in apple plants. Tree Physiol. 2022, 42, 1827–1840. [Google Scholar] [CrossRef]
- Yue, Z.Y.; Liu, H.; Ma, F.W. The Malus carotenoid cleavage dioxygenase 7 is involved in stress response and regulated by basic pentacysteine 1. Sci. Hortic. 2015, 192, 264–270. [Google Scholar] [CrossRef]


| No. | Regulatory Element | Quantities | Sequence | Function of Site |
|---|---|---|---|---|
| 1 | ABRE | 7 | ACGTG | Cis-acting elements involved in abscisic acid response |
| 2 | ARE | 3 | AAACCA | Cis-acting regulatory element essential for anaerobic induction |
| 3 | AuxRR-core | 1 | GGTCCAT | Cis-acting regulatory element involved in auxin responsiveness |
| 4 | ERE | 4 | ATTTCAAA | Ethylene-responsive element |
| 5 | CGTCA motif | 3 | CGTCA | Methyl jasmonate-responsive element |
| 6 | TCA element | 1 | CCATCTTTTT | Salicylate-responsive element |
| 7 | TGACG motif | 3 | TGACG | Methyl jasmonate-responsive element |
| 8 | MBS | 1 | CAACTG | Responds to drought stress |
| 9 | Box 4 | 4 | ATTAAT | Part of a conserved DNA |
| 10 | AE Box | 1 | AGAAACAA | Module or cis-acting |
| 11 | I-Box | 1 | GCATACCAAT | Regulatory element |
| 12 | G-Box | 4 | CACGTT/CACGTG | Involved in light |
| 13 | G-box | 3 | GCCACGTGGA | Responsiveness |
| 14 | CAT-box | 1 | GCCACT | Cis-acting elements involved in the regulation of phloem tissue expression |
| 15 | Circadian | 1 | CAAAGATATC | Cis-acting elements involved in the regulation of circadian rhythms |
| 16 | MYB | 3 | TAACTG/CAACTG/CAACCA | Response to drought and salinity stress tolerance |
| 17 | MYC | 4 | CATGTG/CAATTG | Response to drought and abscisic acid signals |
| 18 | STRE | 1 | AGGGG | Stress-responsive elements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Lin, Y.; Wei, G.; Hu, X.; Zheng, Y.; Ma, J. Overexpression of the CpCOR413PM1 Gene from Wintersweet (Chimonanthus praecox) Enhances Cold and Drought Tolerance in Arabidopsis. Horticulturae 2024, 10, 599. https://doi.org/10.3390/horticulturae10060599
Deng Y, Lin Y, Wei G, Hu X, Zheng Y, Ma J. Overexpression of the CpCOR413PM1 Gene from Wintersweet (Chimonanthus praecox) Enhances Cold and Drought Tolerance in Arabidopsis. Horticulturae. 2024; 10(6):599. https://doi.org/10.3390/horticulturae10060599
Chicago/Turabian StyleDeng, Yeyuan, Yi Lin, Guo Wei, Xiaoqian Hu, Yanghui Zheng, and Jing Ma. 2024. "Overexpression of the CpCOR413PM1 Gene from Wintersweet (Chimonanthus praecox) Enhances Cold and Drought Tolerance in Arabidopsis" Horticulturae 10, no. 6: 599. https://doi.org/10.3390/horticulturae10060599
APA StyleDeng, Y., Lin, Y., Wei, G., Hu, X., Zheng, Y., & Ma, J. (2024). Overexpression of the CpCOR413PM1 Gene from Wintersweet (Chimonanthus praecox) Enhances Cold and Drought Tolerance in Arabidopsis. Horticulturae, 10(6), 599. https://doi.org/10.3390/horticulturae10060599







