Evaluating the Effect of Weed Placement on the Growth of Container-Grown Plants and Herbicide Application around Container Drain Holes and Root Pruning Containers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Effect of Three Different Weed Placement Scenarios on the Growth of Container-Grown Pentas (Pentas lanceolata) and Golden Dewdrop (Duranta erecta)
2.2. Experiment 2: Effect of Two Different Weed Placements on the Growth of Container-Grown Pentas (Pentas lanceolata)
2.3. Experiment 3: Evaluate the Safety of Herbicide Application on Root Pruning Containers Using Common Ornamental Plants
2.4. Statistical Analysis
3. Results and Discussion
3.1. Experiment 1: Effect of Three Different Weed Placement Scenarios on the Growth of Container-Grown Ornamental Plants
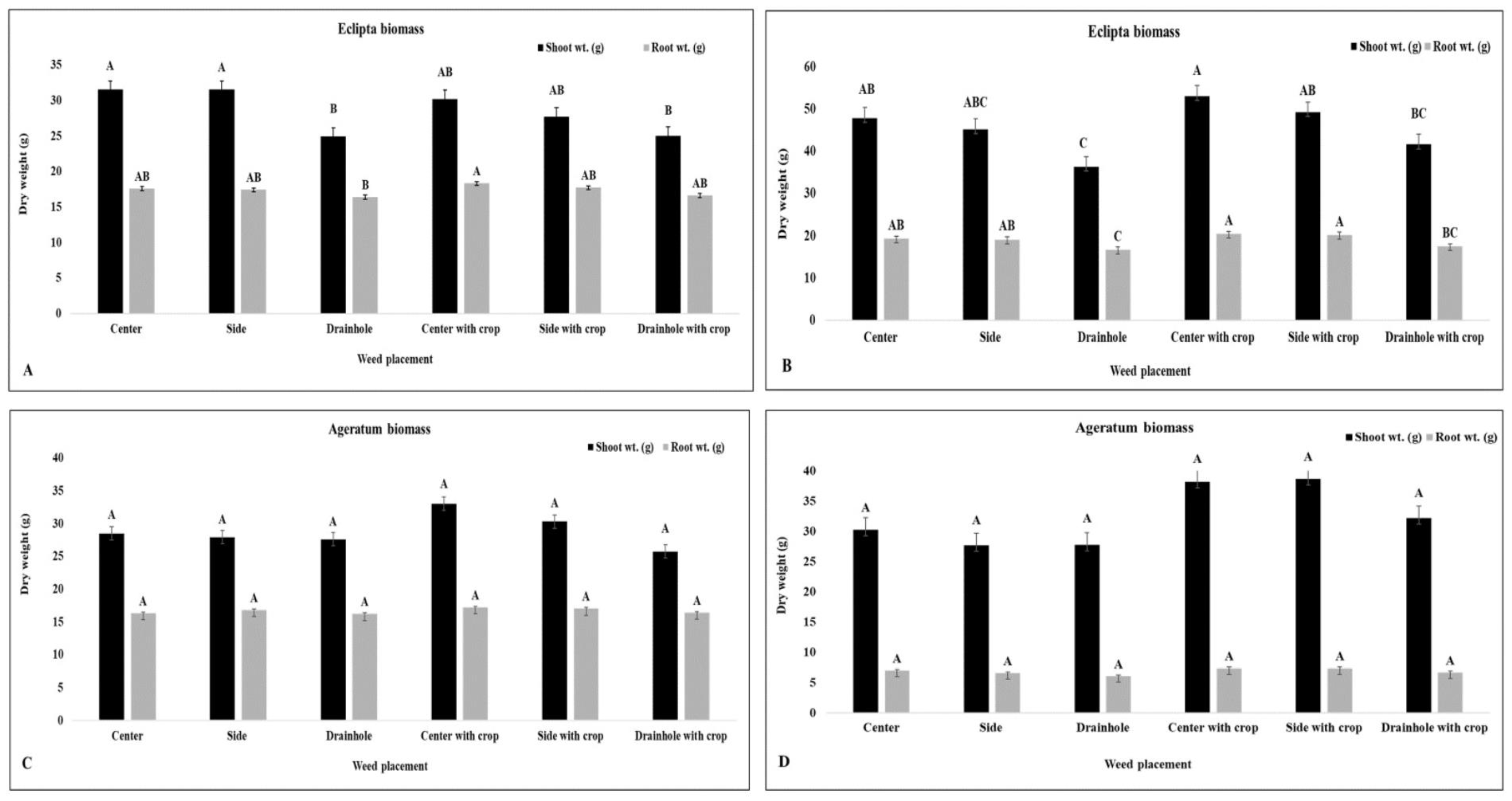
3.1.1. Effect of Eclipta Placement on Container-Grown Golden Dewdrop and Pentas
3.1.2. Effect of Ageratum Placement on Container-Grown Golden Dewdrop and Pentas
3.2. Experiment 2: Effect of Two Different Weed Placements on the Growth of Container-Grown Pentas (Pentas lanceolata)
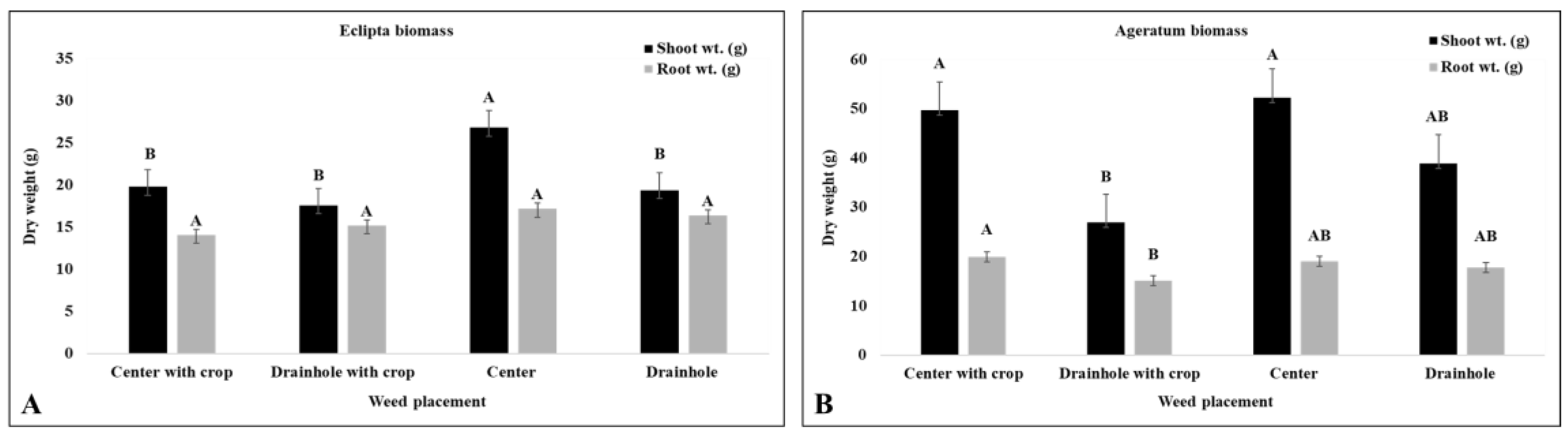
3.2.1. Competitive Effect of Ageratum and Eclipta Placement on Container-Grown Pentas
3.2.2. The Competitive Effect of Container-Grown Pentas on Eclipta and Ageratum
3.3. Experiment 3: Evaluate the Safety of Herbicide Application on Root Pruning Containers Using Common Ornamental Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- U.S. Department of Agriculture, National Agricultural Statistics Service 2019. 2019 Census of Horticulture Specialties 5th June. 2023. Available online: http://www.agcensus.usda.gov/Publications/2019 (accessed on 5 March 2024).
- Ingram, D.L.; Hall, C.R.; Knight, J. Carbon footprint and variable costs of production components for a container-grown evergreen shrub using life cycle assessment: An east coast US model. HortScience 2016, 51, 989–994. [Google Scholar] [CrossRef]
- Ingram, D.L.; Hall, C.R.; Knight, J. Comparison of three production scenarios for Buxus microphylla var. japonica ‘Green Beauty’marketed in a No. 3 container on the west coast using life cycle assessment. HortScience 2017, 52, 357–365. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Duval, J.R. The Effect of Container Size. Horttechnology 1998, 8, 495–498. [Google Scholar] [CrossRef]
- Berchielli-Robertson, D.L.; Gilliam, C.H.; Fare, D.C. Competitive effects of weeds on the growth of container-grown plants. HortScience 1990, 25, 77–79. [Google Scholar] [CrossRef]
- Fretz, T.A. Weed Competition in Container Grown Japanese Holly. HortScience 1972, 7, 485–486. [Google Scholar] [CrossRef]
- Walker, K.L.; Williams, D.J. Grass Interference in Container-Grown Bailey’s Redosier Dogwood (Cornus× baileyi). Weed Sci. 1988, 36, 621–624. [Google Scholar] [CrossRef]
- Khamare, Y.; Marble, S.C.; Pearson, B.J.; Chen, J.; Devkota, P. Effect of Weed Competition on Growth of Container Grown Ornamentals Plants in Four Different Container Sizes. Horticulturae 2023, 9, 317. [Google Scholar] [CrossRef]
- Bethke, J.A.; Cloyd, R.A. Pesticide use in ornamental production: What are the benefits? Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.V.; Gilliam, C.H.; Altland, J.E.; Wehtje, G.R.; Sibley, J.L. Diuron: Postemergence Oxalis control in container-grown plants. J. Environ. Hortic. 2004, 22, 45–49. [Google Scholar] [CrossRef]
- Adams, D. Chemical weed control in containerized hardy ornamental nursery stock. Prof. Hortic. 1990, 4, 70–75. [Google Scholar]
- Marble, S.C.; Pickens, J. Weed Control for Ornamentals Inside Greenhouses and Other Enclosed Structures: ENH1267/EP528, 11/2015. EDIS. 2016, 5-5. Available online: https://edis.ifas.ufl.edu/publication/EP528 (accessed on 20 April 2024).
- Norcini, J.G.; Bolques, A.; Stamps, R.H. Container Nursery Weed Control: Sanitation Practices to Prevent Weed Seed Contamination; ENH1050; Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2010. [Google Scholar]
- Neal, J. Greenhouse Weed Control. 2015, North Carolina Cooperative Extension Resources Horticulture Information Leaflet. Available online: http://content.ces.ncsu.edu/greenhouse-weed-control (accessed on 15 April 2024).
- Altland, J.E.; Gilliam, C.H.; Wehtje, G. Weed control in field nurseries. HortTechnology 2003, 13, 9–14. [Google Scholar] [CrossRef]
- Neal, J.C. Biology and Management of Nursery Weeds©. Comb. Proc. Int. Plant Propagators’ Soc. 2003, 53, 120. Available online: http://admin.ipps.org/uploads/53_20.pdf (accessed on 1 March 2024).
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds. Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Salisbury, E.J. The biology of garden weeds. Part 2. J. R. Hortic. Soc. 1962, 87, 458–470. [Google Scholar]
- Stewart, C.J.; Marble, S.C.; Pearson, B.J.; Wilson, P.C. Impact of container nursery production practices on weed growth and herbicide performance. HortScience 2017, 52, 1593–1600. [Google Scholar] [CrossRef]
- Gilliam, C.H.; Foster, W.J.; Adrain, J.L.; Shumack, R.L. A survey of weed control costs and strategies in container production nurseries. J. Environ. Hortic. 1990, 8, 133–135. [Google Scholar] [CrossRef]
- Mortensen, D.A.; Coble, H.D. The influence of soil water content on common cocklebur (Xanthium strumarium) interference in soybeans (Glycine max). Weed Sci. 1989, 37, 76–83. [Google Scholar] [CrossRef]
- von Wettberg, E.J.; Weiner, J. Effects of distance to crop rows and to conspecific neighbours on the size of Brassica napus and Veronica persica weeds. Basic Appl. Ecol. 2004, 5, 35–41. [Google Scholar] [CrossRef][Green Version]
- Henry, W.T.; Bauman, T.T. Interference between soybeans (Glycine max) and common cocklebur (Xanthium strumarium) under Indiana field conditions. Weed Sci. 1989, 37, 753–760. [Google Scholar] [CrossRef]
- Balisky, A.C.; Salonius, P.; Walli, C.; Brinkman, D. Seedling roots and forest floor: Misplaced and neglected aspects of British Columbia’s reforestation effort? For. Chron. 1995, 71, 59–65. [Google Scholar] [CrossRef]
- Gilman, E.F.; Harchick, C.; Paz, M. Effect of container type on root form and growth of red maple. J. Environ. Hortic. 2010, 28, 1–7. [Google Scholar]
- Marler, T.E.; Willis, D. Chemical or air root-pruning containers improve carambola, longan, and mango seedling root morphology and initial root growth after transplanting. J. Environ. Hortic. 1996, 14, 47–49. [Google Scholar] [CrossRef]
- Van Sambeek, J.W.; Godsey, L.D.; Walter, W.D.; Garrett, H.E.; Dwyer, J.P. Field performance of Quercus bicolor established as repeatedly air-root-pruned container and bareroot planting stock. Open J. For. 2016, 6, 163. [Google Scholar] [CrossRef]
- Khamare, Y.; Marble, S.C.; Chandler, A. Fertilizer placement effects on eclipta (Eclipta prostrata) growth and competition with container-grown ornamentals. Weed Sci. 2020, 68, 496–502. [Google Scholar] [CrossRef]
- Dhawan, R.S. Germination potential and growth behaviour of Eclipta alba. Indian J. Weed Sci. 2007, 39, 116–119. [Google Scholar]
- Case, L.T.; Mathers, H.M.; Senesac, A.F. A review of weed control practices in container nurseries. HortTechnology 2005, 15, 535–545. [Google Scholar] [CrossRef]
- Saha, D.; Marble, S.C.; Stewart, C.; Chandler, A. Preemergence and postemergence control of artilleryweed (Pilea microphylla) in container nurseries and landscapes. Weed Technol. 2017, 31, 574–581. [Google Scholar] [CrossRef]
- Alister, C.; Kogan, M.; Pino, I. Differential phytotoxicity of glyphosate in maize seedlings following applications to roots or shoot. Weed Res. 2005, 45, 27–32. [Google Scholar] [CrossRef]
- Penn, D.J.; Lynch, J.M. Toxicity of glyphosate applied to roots of barley seedlings. New Phytologist. 1982, 90, 51–55. [Google Scholar] [CrossRef]
- Petersen, I.L.; Hansen HC, B.; Ravn, H.W.; Sørensen, J.C.; Sørensen, H. Metabolic effects in rapeseed (Brassica napus L.) seedlings after root exposure to glyphosate. Pestic. Biochem. Physiol. 2007, 89, 220–229. [Google Scholar] [CrossRef]
- Pline, W.A.; Wilcut, J.W.; Edmisten, K.L.; Wells, R. Physiological and morphological response of glyphosate-resistant and non-glyphosate-resistant cotton seedlings to root-absorbed glyphosate. Pestic. Biochem. Physiol. 2002, 73, 48–58. [Google Scholar] [CrossRef]


| Common Name | Trade Name | Rate (v/v) a | Manufacturer |
|---|---|---|---|
| Glyphosate | Roundup Pro® | 2% | Bayer Environmental Science, Research Triangle Park, NC, USA |
| Diquat b | Reward® | 0.58% | Syngenta Crop Protection, LLC; Greensboro, NC, USA |
| Pelargonic acid | Scythe® | 5% | Gowan Company, Yuma, AZ, USA |
| Glufosinate | Finale® | 3.12% | BASF Corporation, Research Triangle Park, NC, USA |
| Treatments c | Golden Dewdrop | Pentas | ||||||
|---|---|---|---|---|---|---|---|---|
| Growth Index a | Biomass b | Growth Index | Biomass | |||||
| Eclipta Placement | 8 WAP | 12 WAP | Shoot wt. (g) | Root wt. (g) | 8 WAP | 12 WAP | Shoot wt. (g) | Root wt. (g) |
| Center | 15.1 a d | 17.4 a | 18.0 b | 16.5 a | 28.4 ab | 31.8 b | 25.4 b | 17.2 b |
| Side | 16.8 a | 21.8 a | 18.7 ab | 17.2 a | 26.1 b | 32.1 b | 26.1 b | 17.6 b |
| Drain hole | 19.5 a | 22.5 a | 19.5 ab | 17.6 a | 35.9 a | 44.5 a | 38.6 a | 19.0 a |
| Control | 18.4 a | 18.7 a | 20.3 a | 17.3 a | 36.1 a | 44.6 a | 41.2 a | 19.7 a |
| Ageratum placement | ||||||||
| Center | 16.0 ab | 19.6 ab | 18.0 a | 16.3 a | 18.6 b | 21.8 c | 14.4 a | 5.5 a |
| Side | 14.4 b | 16.4 b | 18.0 a | 16.8 a | 23.8 b | 24.8 bc | 17.7 a | 4.7 a |
| Drain hole | 21.6 a | 23.2 a | 20.6 a | 17.3 a | 27.7 ab | 35.0 ab | 19.9 a | 6.4 a |
| Control | 18.7 ab | 24.4 a | 20.2 a | 17.2 a | 34.9 a | 44.6 a | 22.2 a | 5.4 a |
| Treatments c | Pentas | |||
|---|---|---|---|---|
| Growth Index a | Biomass b | |||
| 6 WAP | 10 WAP | Shoot wt. (g) | Root wt. (g) | |
| Ageratum Placement | ||||
| Center | 25.8 b d | 31.7 b | 24.9 b | 17.6 b |
| Drain hole | 29.8 ab | 39.2 a | 36.4 a | 18.3 a |
| Control | 33.9 a | 44.9 a | 39.8 a | 18.5 a |
| Eclipta placement | ||||
| Center | 31.8 a | 44.1 a | 39.5 b | 17.9 b |
| Drain hole | 33.1 a | 47.2 a | 45.0 ab | 18.6 ab |
| Control | 35.7 a | 47.8 a | 48.6 a | 18.8 a |
| Herbicide b | Growth Index a | |||
|---|---|---|---|---|
| Loropetulam | Podocarpus | Japanese Holly | Drift Roses | |
| Glyphosate (RoundUp Pro) | 49.4 b e | 46.3 a | 41.8 a | 26.2 a |
| Diquat (Reward) c | 51.5 ab | 42.2 a | 41.9 a | 26.3 a |
| Pelargonic acid (Scythe) | 53.4 ab | 42.4 a | 44.2 a | 25.1 a |
| Glufosinate (Finale) | 53.6 ab | 44.9 a | 42.4 a | 25.7 a |
| Check d | 55.5 a | 43.7 a | 43.5 a | 26.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamare, Y.; Marble, S.C. Evaluating the Effect of Weed Placement on the Growth of Container-Grown Plants and Herbicide Application around Container Drain Holes and Root Pruning Containers. Horticulturae 2024, 10, 661. https://doi.org/10.3390/horticulturae10070661
Khamare Y, Marble SC. Evaluating the Effect of Weed Placement on the Growth of Container-Grown Plants and Herbicide Application around Container Drain Holes and Root Pruning Containers. Horticulturae. 2024; 10(7):661. https://doi.org/10.3390/horticulturae10070661
Chicago/Turabian StyleKhamare, Yuvraj, and Stephen C. Marble. 2024. "Evaluating the Effect of Weed Placement on the Growth of Container-Grown Plants and Herbicide Application around Container Drain Holes and Root Pruning Containers" Horticulturae 10, no. 7: 661. https://doi.org/10.3390/horticulturae10070661
APA StyleKhamare, Y., & Marble, S. C. (2024). Evaluating the Effect of Weed Placement on the Growth of Container-Grown Plants and Herbicide Application around Container Drain Holes and Root Pruning Containers. Horticulturae, 10(7), 661. https://doi.org/10.3390/horticulturae10070661






