Resilient Response to Combined Heat and Drought Stress Conditions of a Tomato Germplasm Collection, Including Natural and Ethyl Methanesulfonate-Induced Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Nursery Screening Trials
2.3. Thermography Analysis
2.4. Combined Drought and Heat Stress Greenhouse Trials
2.5. TILLING and EcoTILLING Analysis for Mutation Detection
3. Results
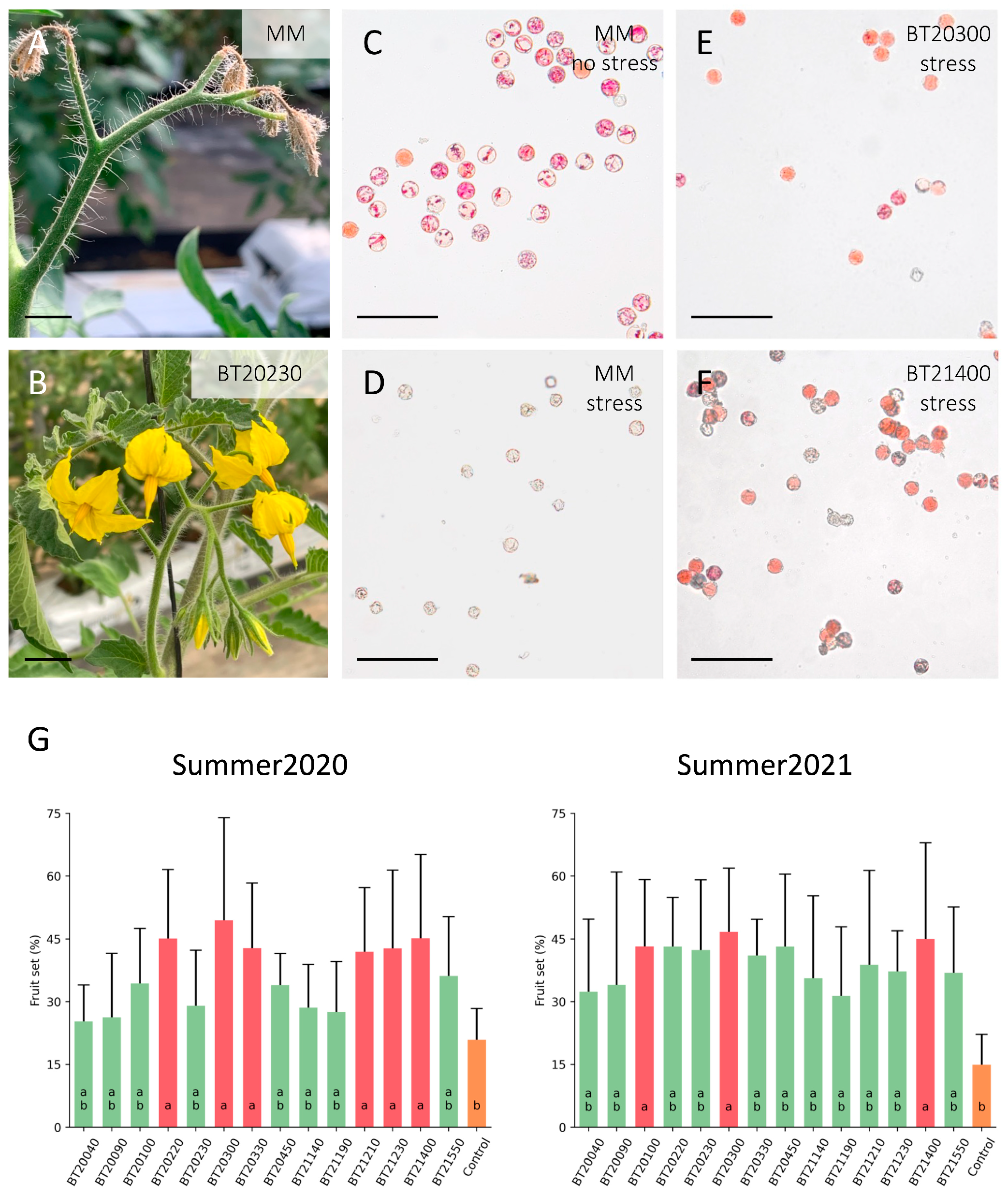
3.1. Screening for Combined Heat and Drought Stress Tolerance in Nursery Conditions
3.2. Stress Tolerance of Selected Germplasm under Climatic Chamber Conditions
3.3. Characterization of Stress Tolerance Response under Greenhouse Conditions
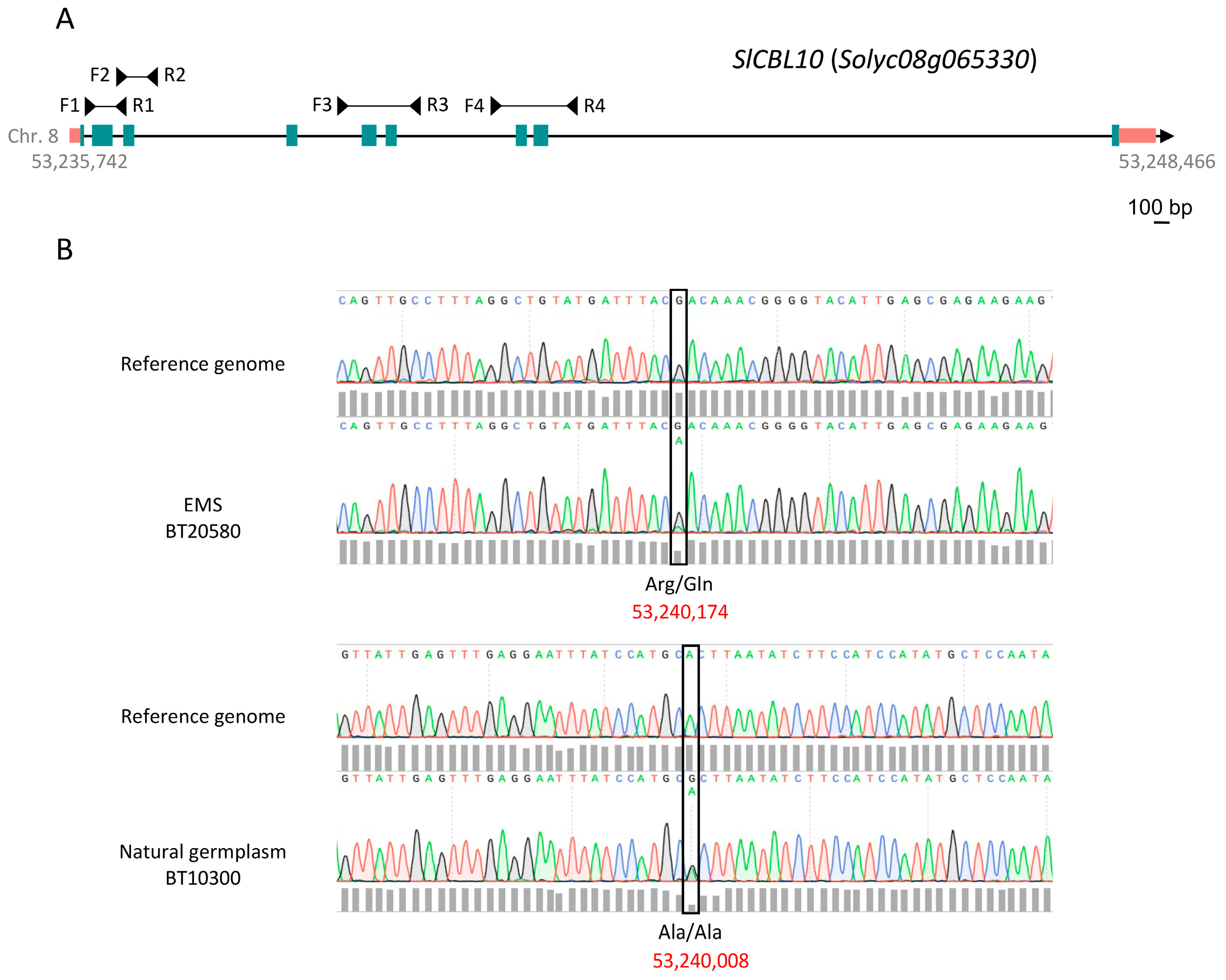
3.4. TILLING and Eco-TILLING Analysis for Mutant Variant Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stocker, T.F. Climate change. The closing door of climate targets. Science 2013, 339, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global Synthesis of Drought Effects on Maize and Wheat Production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Alsamir, M.; Ahmad Nabil Arief, V.; Mahmood, T.; Trethowan, R. Phenotypic diversity and marker-trait association studies under heat stress in tomato (Solanum lycopersicum L.). Aust. J. Crop Sci. 2019, 13, 578–587. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Macian, F.; Ordovás, J.M. Advances in Understanding the Molecular Basis of the Mediterranean Diet Effect. Annu. Rev. Food Sci. 2018, 9, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Capel, C.; Yuste-Lisbona, F.J.; López-Casado, G.; Angosto, T.; Heredia, A.; Cuartero, J.; Fernández-Muñoz, R.; Lozano, R.; Capel, J. QTL mapping of fruit mineral contents provides new chances for molecular breeding of tomato nutritional traits. Theor. Appl. Genet. 2017, 130, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, M.J.; Nájera, I.; Baixauli, C.; Gil, D.; Montoro, T.; Soriano, V.; Olivieri, F.; Rigano, M.M.; Ganeva, D.; Grozeva-Tileva, S.; et al. Identification of tomato accessions as source of new genes for improving heat tolerance: From controlled experiments to field. BMC Plant Biol. 2021, 21, 345. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Alsamir, M.; Ahmad, N.M.; Keitel, C.; Mahmood, T.; Trethowan, R. Identification of High-Temperature Tolerant and Agronomically Viable Tomato (S. lycopersicum) Genotypes from a Diverse Germplasm Collection. Adv. Crop Sci. Tech. 2017, 5, 299. [Google Scholar] [CrossRef]
- Sato, S.; Kamiyama, M.; Iwata, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Srividhya, S. Impact of drought on flowering, yield and quality parameters in diverse genotypes of tomato (Solanum lycopersicum L.). Adv. Hort. Sci. 2016, 30, 3–11. [Google Scholar]
- Tripodi, P.; Soler, S.; Campanelli, G.; Díez, M.J.; Esposito, S.; Sestili, S.; Figàs, M.R.; Leteo, F.; Casanova, C.; Platani, C.; et al. Genome wide association mapping for agronomic, fruit quality, and root architectural traits in tomato under organic farming conditions. BMC Plant Biol. 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, N.; Rieu, I.; Vriezen, W.H. Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod. 2016, 29, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.H. Back into the wild: Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Capel, C.; Nieto-Canseco, R.; Ortiz-Atienza, A.; Bretones, S.; López-Fábregas, J.D.; Quevedo-Colmena, A.S.; Lebrón, R.; Barragán-Lozano, T.; Villalobos-Ramírez, V.; et al. A Tomato EMS-Mutagenized Population Provides New Valuable Resources for Gene Discovery and Breeding of Developmental Traits. Plants 2022, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Micol-Ponce, R.; García-Alcázar, M.; Lebrón, R.; Capel, C.; Pineda, B.; García-Sogo, B.; Alché, J.D.; Ortiz-Atienza, A.; Bretones, S.; Yuste-Lisbona, F.J.; et al. Tomato POLLEN DEFICIENT 2 encodes a G-type lectin receptor kinase required for viable pollen grain formation. J. Exp. Bot. 2023, 74, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Pineda, B.; Ortíz-Atienza, A.; Plasencia, F.A.; Drevensek, S.; García-Sogo, B.; Yuste-Lisbona, F.J.; Barrero-Gil, J.; Atarés, A.; Flores, F.B.; et al. The SlCBL10 Calcineurin B-Like Protein Ensures Plant Growth under Salt Stress by Regulating Na+ and Ca2+ Homeostasis. Plant Physiol. 2018, 176, 1676–1693. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, F.A.; Estrada, Y.; Flores, F.B.; Ortiz-Atienza, A.; Lozano, R.; Egea, I. The Ca2+ Sensor Calcineurin B-Like Protein 10 in Plants: Emerging New Crucial Roles for Plant Abiotic Stress Tolerance. Front. Plant Sci. 2021, 11, 599944. [Google Scholar] [CrossRef] [PubMed]
- Estrada, Y.; Plasencia, F.; Ortiz-Atienza, A.; Faura, C.; Flores, F.B.; Lozano, R.; Egea, I. A novel function of the tomato CALCINEURIN-B LIKE 10 gene as a root-located negative regulator of salt stress. Plant Cell Environ. 2023, 46, 3433–3444. [Google Scholar] [CrossRef] [PubMed]
- Riahi, K.; Schaeffer, R.; Arango, J.; Calvin, K.; Guivarch, C.; Hasegawa, T.; Jiang, K.; Kriegler, E.; Matthews, R.; Peters, G.P.; et al. Mitigation pathways compatible with long-term goals. In IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Pham, D.; Hoshikawa, K.; Fujita, S.; Fukumoto, S.; Hirai, T.; Shinozaki, Y.; Ezura, H. A tomato heat-tolerant mutant shows improved pollen fertility and fruit-setting under long-term ambient high temperature. Environ. Exp. Bot. 2020, 178, 104150. [Google Scholar] [CrossRef]
- Xu, J.; Wolters-Arts, M.; Mariani, C.; Huber, H.; Rieu, I. Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef]
- Müller, F.; Xu, J.; Kristensen, L.; Wolters-Arts, M.; de Groot, P.F.; Jansma, S.Y.; Mariani, C.; Park, S.; Rieu, I. High-Temperature-Induced Defects in Tomato (Solanum lycopersicum) Anther and Pollen Development Are Associated with Reduced Expression of B-Class Floral Patterning Genes. PLoS ONE 2016, 11, e0167614. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Min, L.; Ma, Y.; Zeeshan, M.; Jin, S.; Zhang, X. High-temperature stress in crops: Male sterility, yield loss and potential remedy approaches. Plant Biotechnol. J. 2023, 21, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Pécrix, Y.; Rallo, G.; Folzer, H.; Cigna, M.; Gudin, S.; Le Bris, M. Polyploidization mechanisms: Temperature environment can induce diploid gamete formation in Rosa sp. J. Exp. Bot. 2011, 62, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-Dwamena, C.; Sodedji, F.A.K.; Asante, I.K.; Danquah, E.Y. Accelerating Breeding for Heat Tolerance in Tomato (Solanum lycopersicum L.): An Integrated Approach. Agronomy 2019, 9, 720. [Google Scholar] [CrossRef]
- Gonzalo, M.J.; Li, Y.C.; Chen, K.Y.; Gil, D.; Montoro, T.; Nájera, I.; Baixauli, C.; Granell, A.; Monforte, A.J. Genetic Control of Reproductive Traits in Tomatoes Under High Temperature. Front. Plant Sci. 2020, 24, 326. [Google Scholar] [CrossRef] [PubMed]
- Bineau, E.; Diouf, I.; Carretero, Y.; Duboscq, R.; Bitton, F.; Djari, A.; Zouine, M.; Causse, M. Genetic diversity of tomato response to heat stress at the QTL and transcriptome levels. Plant J. 2021, 107, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.; El-Shershaby, A.; Carriero, F.; Summerer, S.; Petrozza, A.; Iannacone, R.; Schleiff, E.; Fragkostefanakis, S. Identification and Characterization of a Thermotolerant TILLING Allele of Heat Shock Binding Protein 1 in Tomato. Genes 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, R.; Micol-Ponce, R.; Ozuna, C.V.; Castañeda, L.; Capel, C.; Fernández-Lozano, A.; Ortiz-Atienza, A.; Bretones, S.; Pérez-Jiménez, J.M.; Quevedo-Colmena, A.S.; et al. Resilient Response to Combined Heat and Drought Stress Conditions of a Tomato Germplasm Collection, Including Natural and Ethyl Methanesulfonate-Induced Variants. Horticulturae 2024, 10, 552. https://doi.org/10.3390/horticulturae10060552
Fonseca R, Micol-Ponce R, Ozuna CV, Castañeda L, Capel C, Fernández-Lozano A, Ortiz-Atienza A, Bretones S, Pérez-Jiménez JM, Quevedo-Colmena AS, et al. Resilient Response to Combined Heat and Drought Stress Conditions of a Tomato Germplasm Collection, Including Natural and Ethyl Methanesulfonate-Induced Variants. Horticulturae. 2024; 10(6):552. https://doi.org/10.3390/horticulturae10060552
Chicago/Turabian StyleFonseca, Rocío, Rosa Micol-Ponce, Carmen V. Ozuna, Laura Castañeda, Carmen Capel, Antonia Fernández-Lozano, Ana Ortiz-Atienza, Sandra Bretones, José M. Pérez-Jiménez, Abraham S. Quevedo-Colmena, and et al. 2024. "Resilient Response to Combined Heat and Drought Stress Conditions of a Tomato Germplasm Collection, Including Natural and Ethyl Methanesulfonate-Induced Variants" Horticulturae 10, no. 6: 552. https://doi.org/10.3390/horticulturae10060552
APA StyleFonseca, R., Micol-Ponce, R., Ozuna, C. V., Castañeda, L., Capel, C., Fernández-Lozano, A., Ortiz-Atienza, A., Bretones, S., Pérez-Jiménez, J. M., Quevedo-Colmena, A. S., López-Fábregas, J. D., Barragán-Lozano, T., Lebrón, R., Faura, C., Capel, J., Angosto, T., Egea, I., Yuste-Lisbona, F. J., & Lozano, R. (2024). Resilient Response to Combined Heat and Drought Stress Conditions of a Tomato Germplasm Collection, Including Natural and Ethyl Methanesulfonate-Induced Variants. Horticulturae, 10(6), 552. https://doi.org/10.3390/horticulturae10060552





