Effect of Different Fertigation Scheduling Methods on the Yields and Photosynthetic Parameters of Drip-Fertigated Chinese Chive (Allium tuberosum) Grown in a Horticultural Greenhouse
Abstract
1. Introduction
2. Materials and Methods
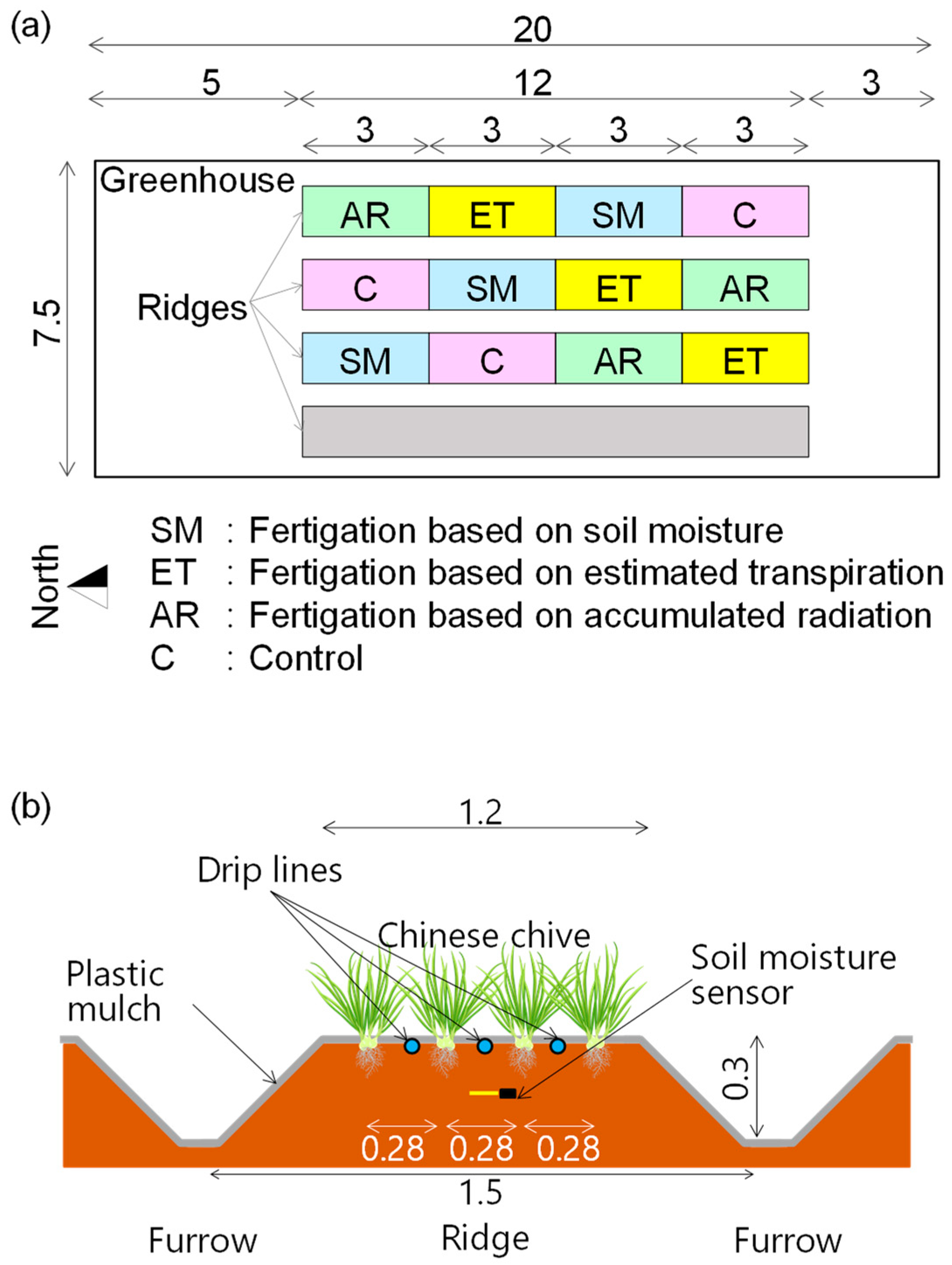
2.1. Plant Material and Experimental Greenhouse
2.2. Fertigation Treatments
2.2.1. Control
2.2.2. Fertigation Based on Accumulated Radiation (AR)
2.2.3. Fertigation Based on Estimated Evapotranspiration (ET)
2.2.4. Fertigation Based on Soil Moisture (SM)
2.3. Measurement of Leaf Photosynthetic Parameters
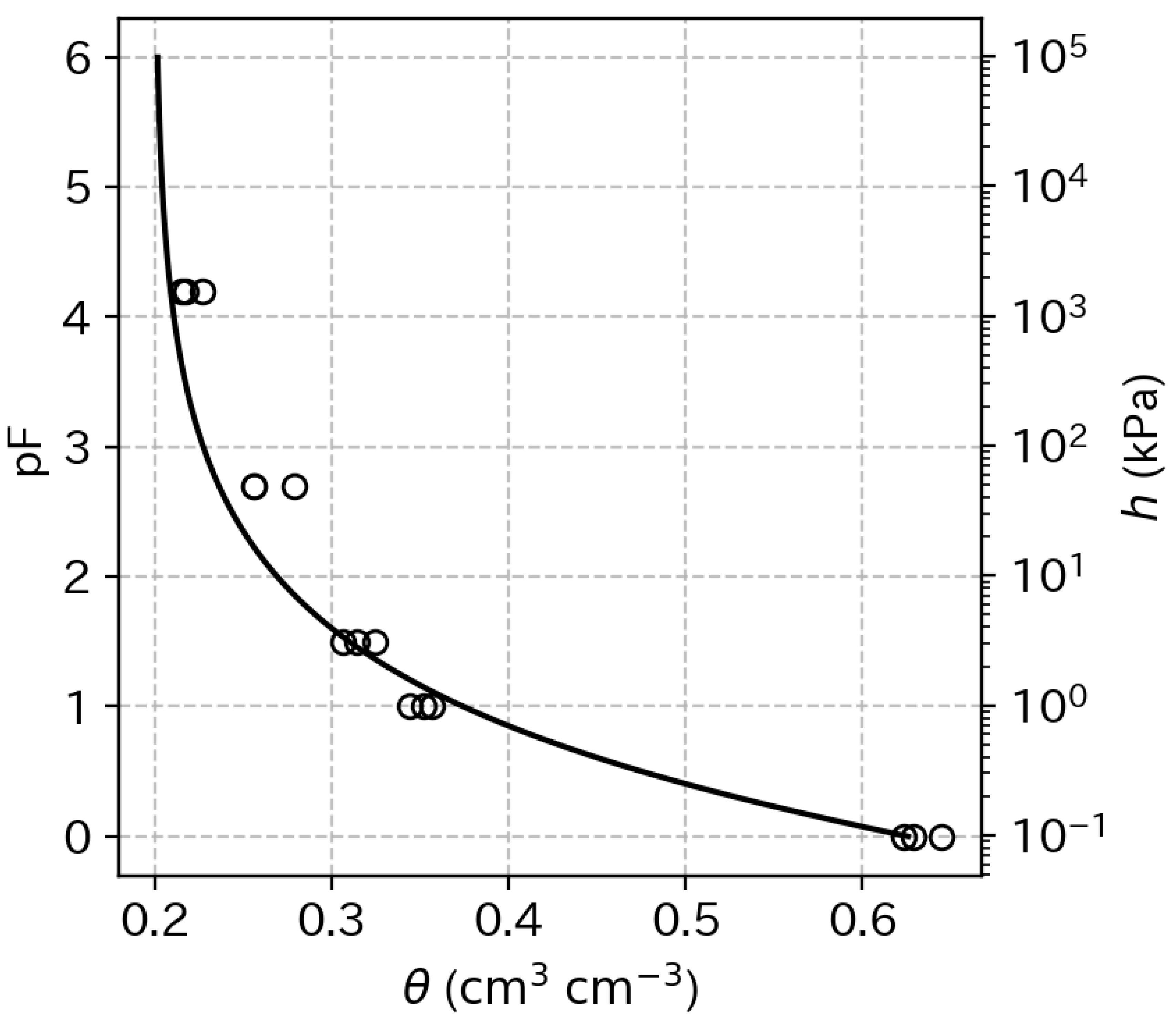
2.4. Measurement of Soil Water Retention Characteristics
2.5. Statistical Analysis
3. Results
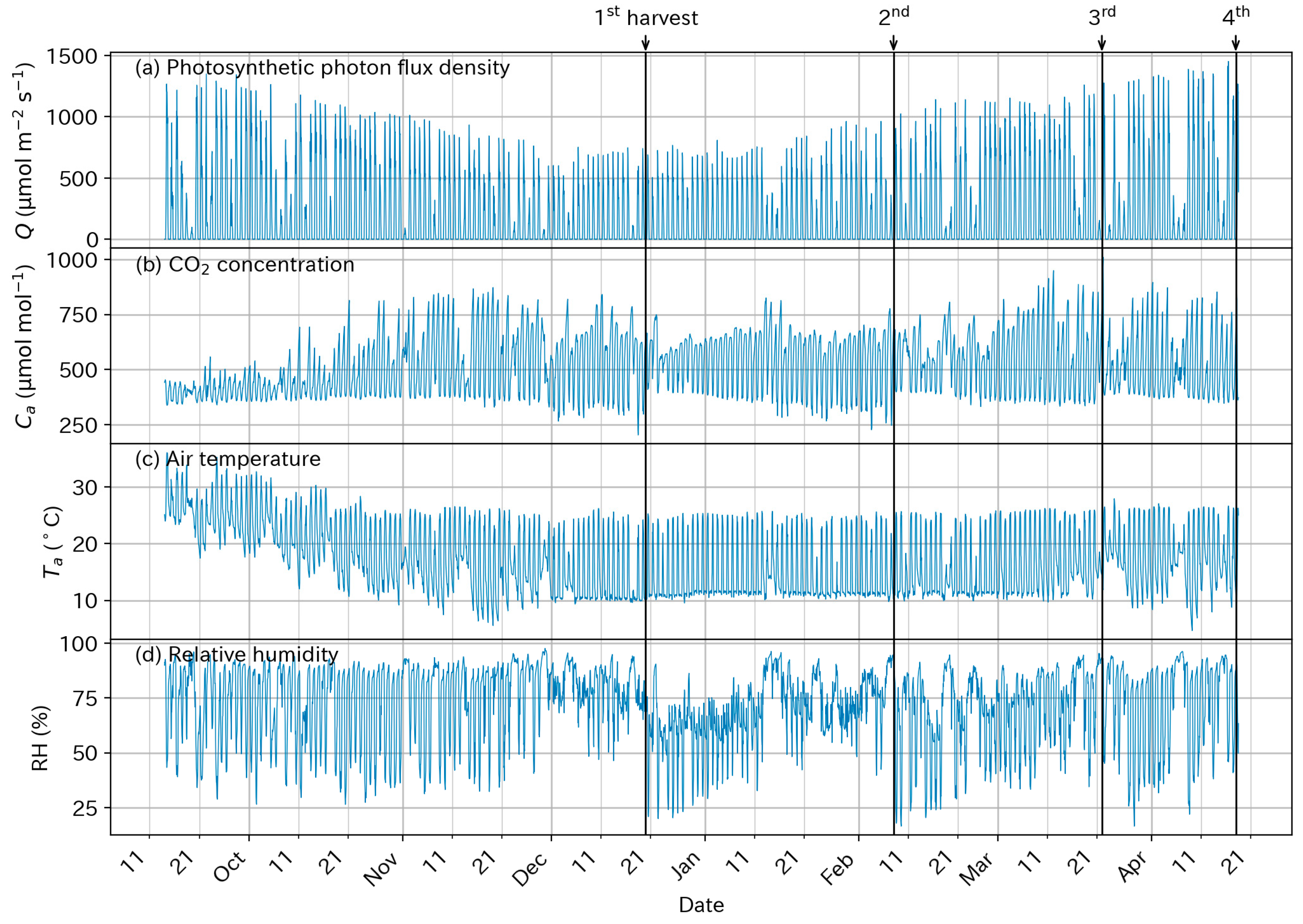
3.1. Above-Ground Environmental Conditions
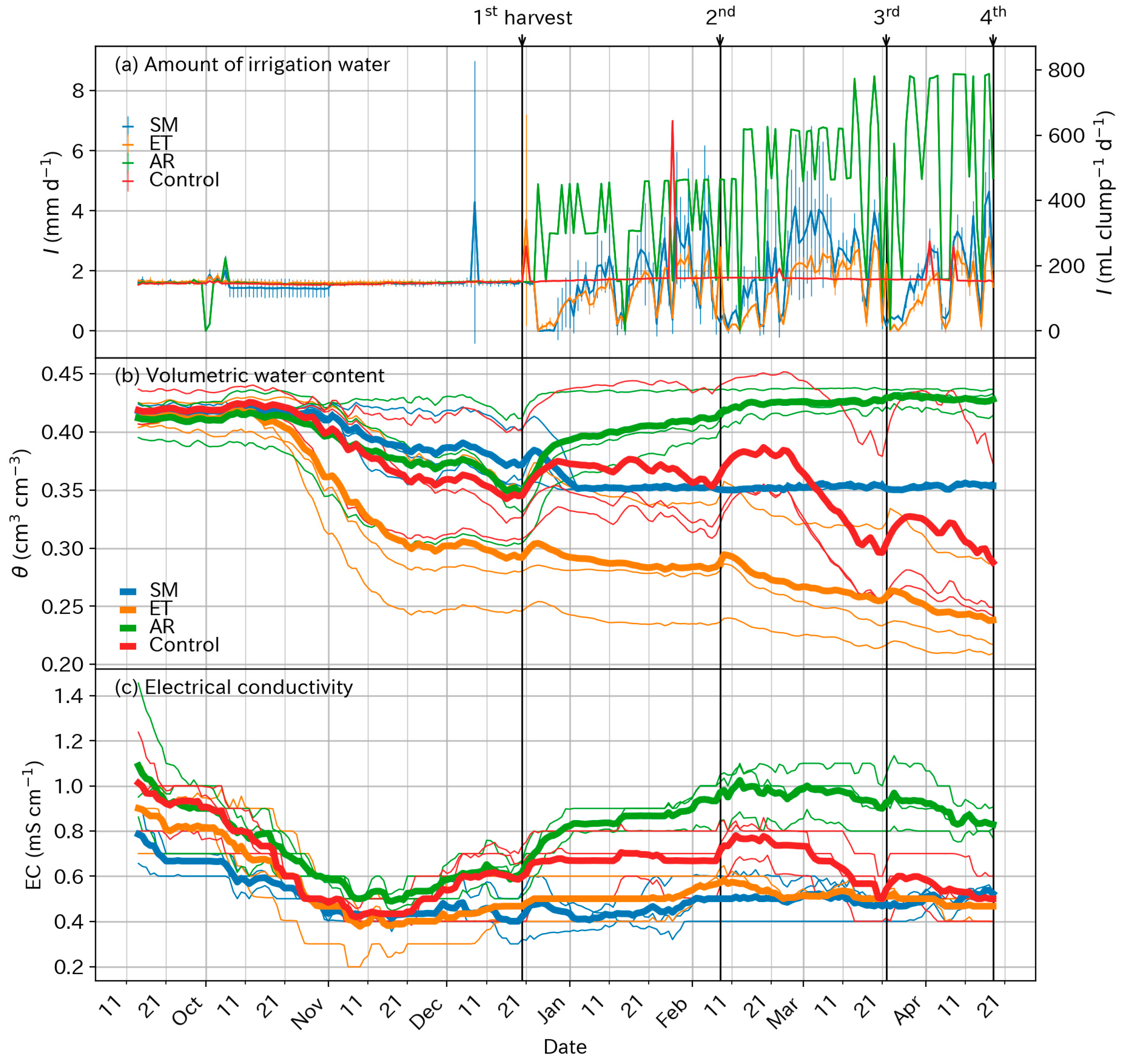
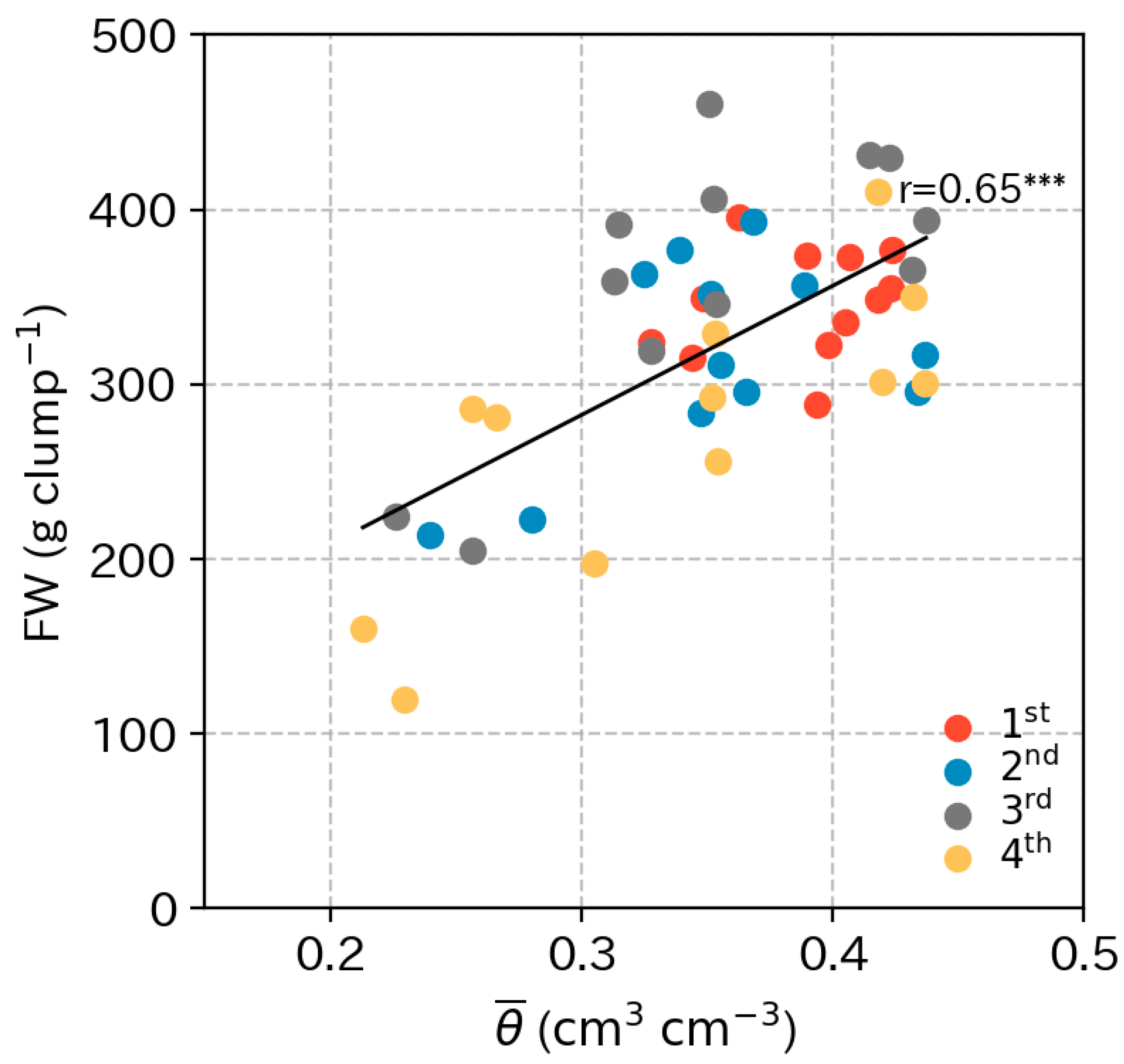
3.2. Supplied Water and Soil Conditions
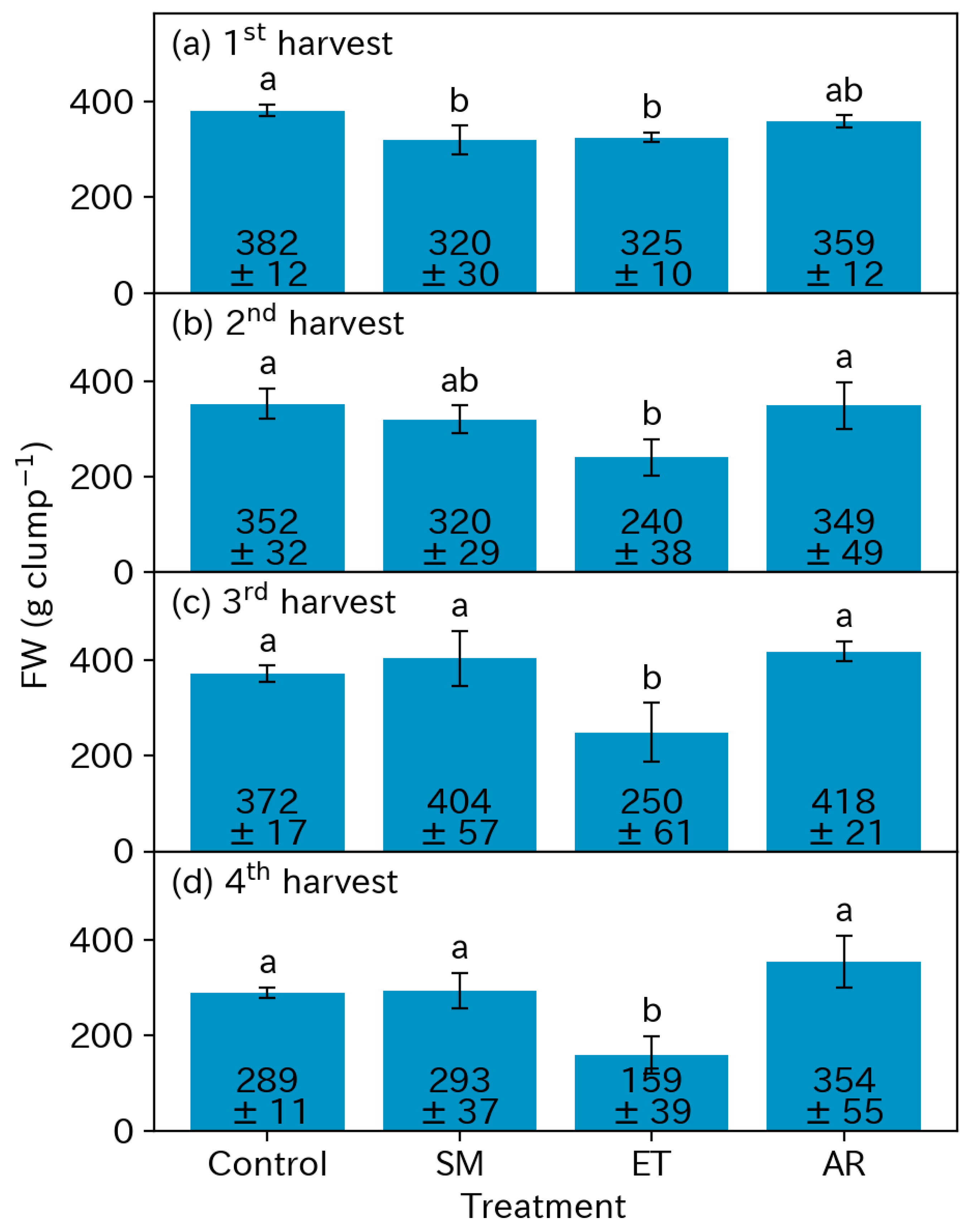
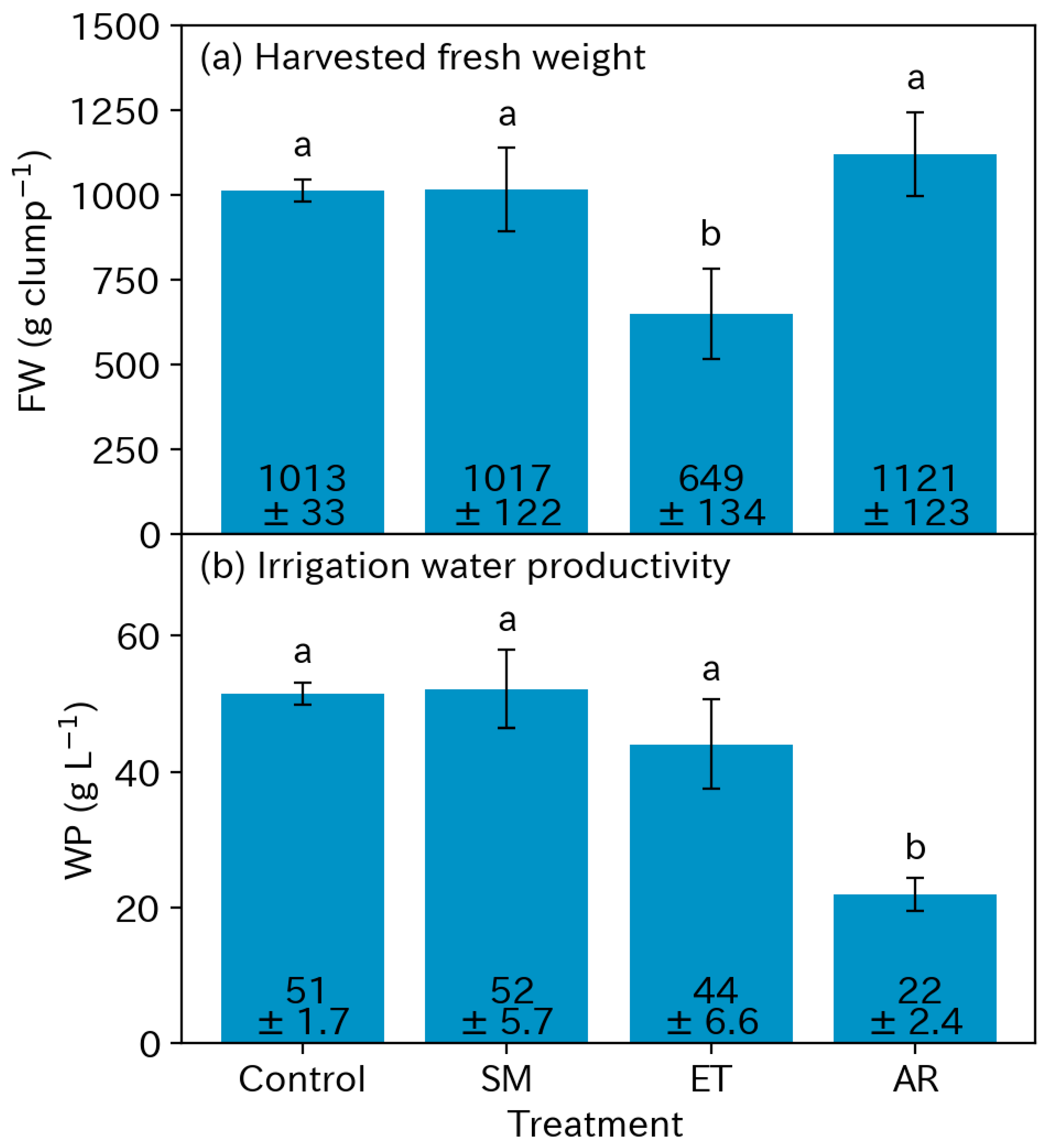
3.3. Yields
3.4. Water Productivity
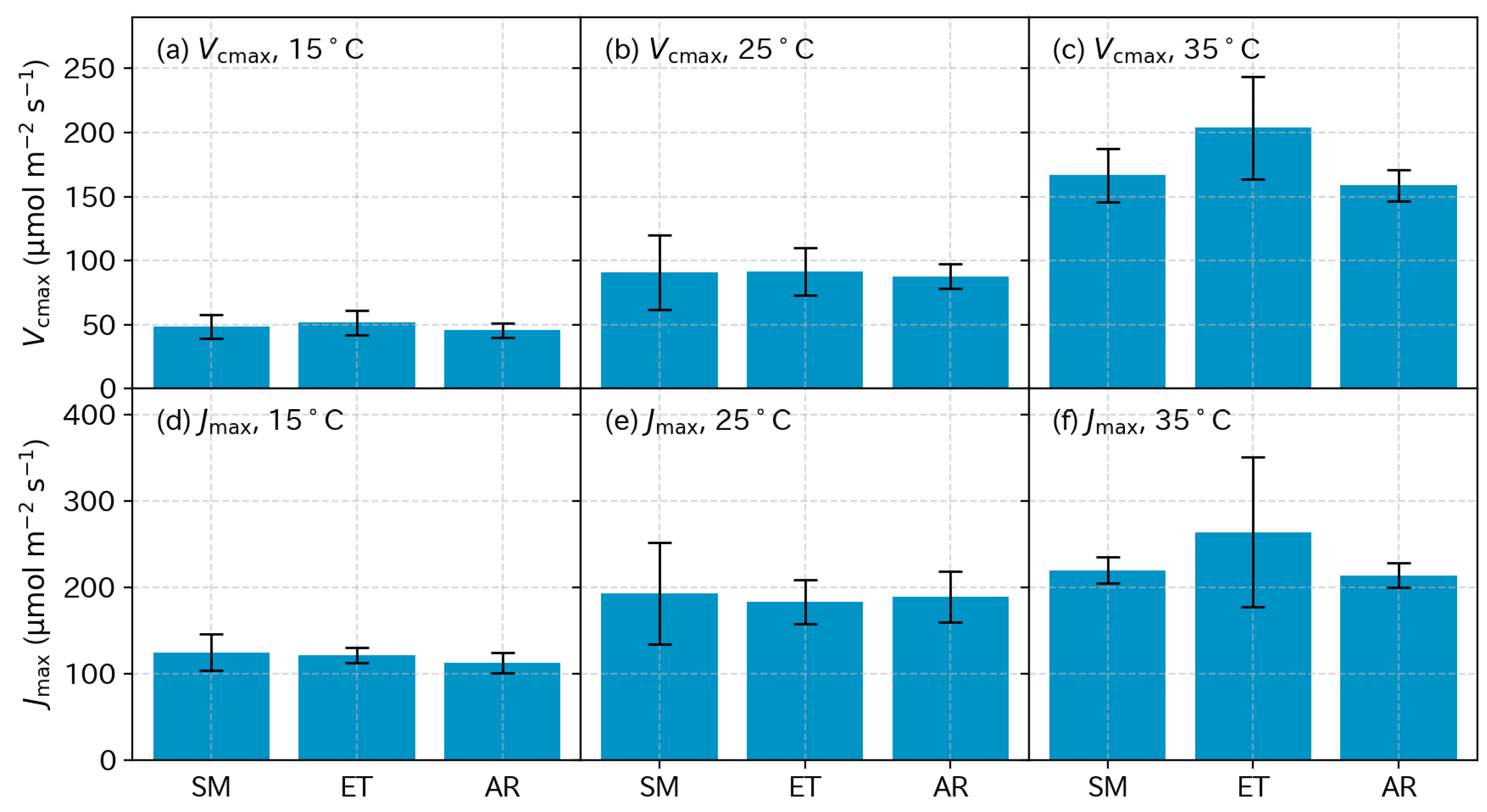
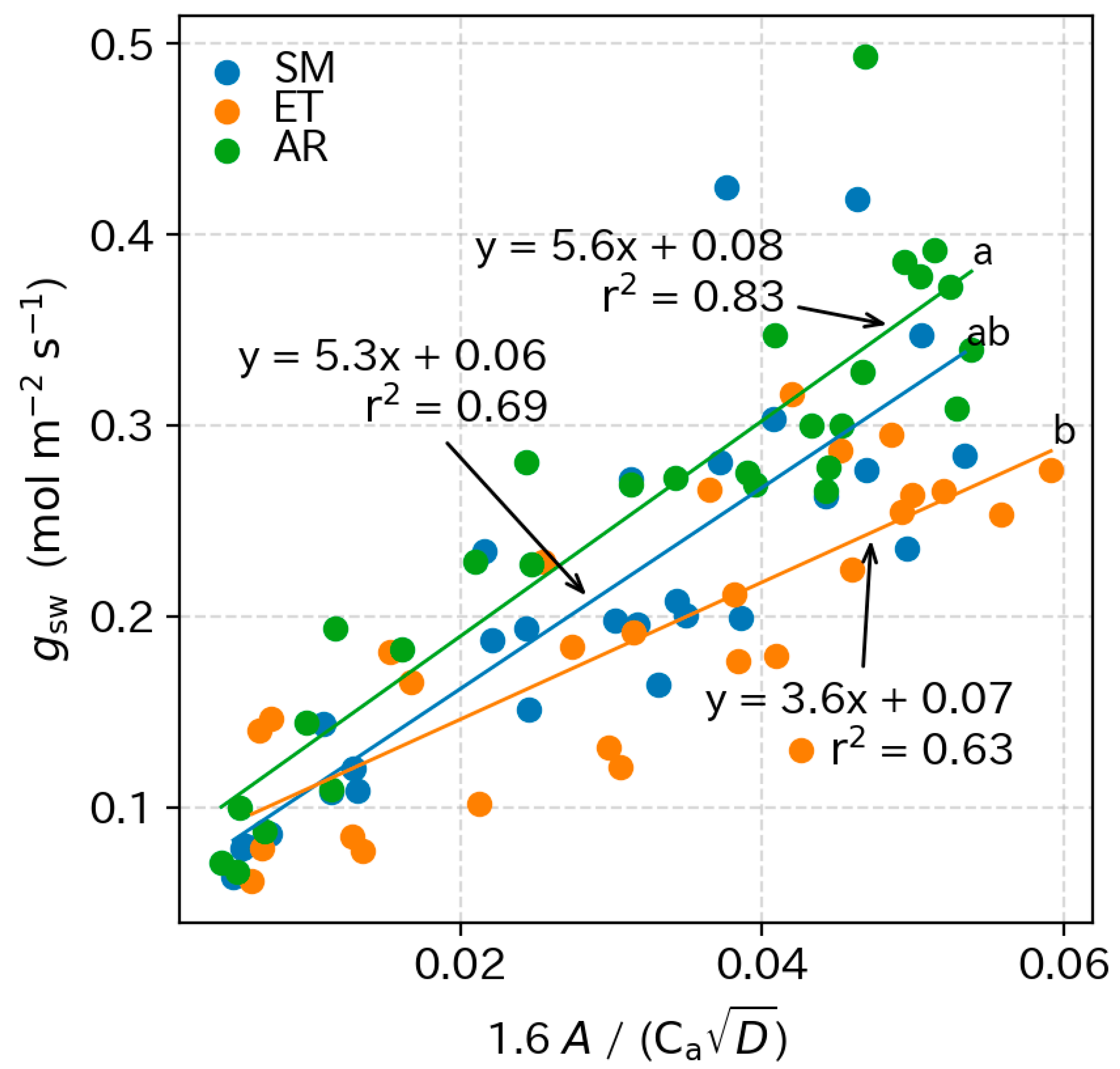
3.5. Photosynthetic Parameters
4. Discussion
4.1. Advantages and Disadvantages of the Different Fertigation Methods
4.2. Optimum Moisture for Chinese Chive Cultivation
4.3. Photosynthetic Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Neural Network Model

Appendix A.2. Equations of Leaf Photosynthesis
References
- Fernández, J.E.; Alcon, F.; Diaz-Espejo, A.; Hernandez-Santana, V.; Cuevas, M.V. Water Use Indicators and Economic Analysis for On-Farm Irrigation Decision: A Case Study of a Super High Density Olive Tree Orchard. Agric. Water Manag. 2020, 237, 106074. [Google Scholar] [CrossRef]
- Li, H.; Mei, X.; Wang, J.; Huang, F.; Hao, W.; Li, B. Drip Fertigation Significantly Increased Crop Yield, Water Productivity and Nitrogen Use Efficiency with Respect to Traditional Irrigation and Fertilization Practices: A Meta-Analysis in China. Agric. Water Manag. 2021, 244, 106534. [Google Scholar] [CrossRef]
- Sinha, I.; Buttar, G.S.; Brar, A.S. Drip Irrigation and Fertigation Improve Economics, Water and Energy Productivity of Spring Sunflower (Helianthus annuus L.) in Indian Punjab. Agric. Water Manag. 2017, 185, 58–64. [Google Scholar] [CrossRef]
- Xiukang, W.; Yingying, X. Evaluation of the Effect of Irrigation and Fertilization by Drip Fertigation on Tomato Yield and Water Use Efficiency in Greenhouse. Int. J. Agron. 2016, 2016, 3961903. [Google Scholar] [CrossRef]
- Azad, N.; Behmanesh, J.; Rezaverdinejad, V.; Abbasi, F.; Navabian, M. An Analysis of Optimal Fertigation Implications in Different Soils on Reducing Environmental Impacts of Agricultural Nitrate Leaching. Sci. Rep. 2020, 10, 7797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Q.; Tan, C.S.; Liu, K.; Drury, C.F.; Papadopoulos, A.P.; Warner, J. Yield and Economic Assessments of Fertilizer Nitrogen and Phosphorus for Processing Tomato with Drip Fertigation. Agron. J. 2010, 102, 774–780. [Google Scholar] [CrossRef]
- Incrocci, L.; Thompson, R.B.; Fernandez-Fernandez, M.D.; De Pascale, S.; Pardossi, A.; Stanghellini, C.; Rouphael, Y.; Gallardo, M. Irrigation Management of European Greenhouse Vegetable Crops. Agric. Water Manag. 2020, 242, 106393. [Google Scholar] [CrossRef]
- García, L.; Parra, L.; Jimenez, J.M.; Lloret, J.; Lorenz, P. IoT-Based Smart Irrigation Systems: An Overview on the Recent Trends on Sensors and Iot Systems for Irrigation in Precision Agriculture. Sensors 2020, 20, 1042. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, G.; Neocleous, D.; Katsoulas, N.; Kittas, C. Irrigation of Greenhouse Crops. Horticulturae 2019, 5, 7. [Google Scholar] [CrossRef]
- Gu, Z.; Qi, Z.; Burghate, R.; Yuan, S.; Jiao, X.; Xu, J. Irrigation Scheduling Approaches and Applications: A Review. J. Irrig. Drain. Eng. 2020, 146, 04020007. [Google Scholar] [CrossRef]
- Jones, H.G. Irrigation Scheduling: Advantages and Pitfalls of Plant-Based Methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef]
- Lemay, I.; Caron, J. Defining Irrigation Set Points Based on Substrate Properties for Variable Irrigation and Constant Matric Potential Devices in Greenhouse Tomato. HortScience 2012, 47, 1141–1152. [Google Scholar] [CrossRef]
- Choi, K.Y.; Choi, E.Y.; Kim, I.S.; Lee, Y.B. Improving Water and Fertilizer Use Efficiency during the Production of Strawberry in Coir Substrate Hydroponics Using a FDR Sensor-Automated Irrigation System. Hortic. Environ. Biotechnol. 2016, 57, 431–439. [Google Scholar] [CrossRef]
- Pereira, L.S.; Paredes, P.; Jovanovic, N. Soil Water Balance Models for Determining Crop Water and Irrigation Requirements and Irrigation Scheduling Focusing on the FAO56 Method and the Dual Kc Approach. Agric. Water Manag. 2020, 241, 106357. [Google Scholar] [CrossRef]
- Morille, B.; Migeon, C.; Bournet, P.E. Is the Penman-Monteith Model Adapted to Predict Crop Transpiration under Greenhouse Conditions? Application to a New Guinea Impatiens Crop. Sci. Hortic. 2013, 152, 80–91. [Google Scholar] [CrossRef]
- Vereecken, H.; Kasteel, R.; Vanderborght, J.; Harter, T. Upscaling Hydraulic Properties and Soil Water Flow Processes in Heterogeneous Soils: A Review. Vadose Zone J. 2007, 6, 1–28. [Google Scholar] [CrossRef]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic Limitations in Response to Water Stress and Recovery in Mediterranean Plants with Different Growth Forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Maroco, J.P.; Rodrigues, M.L.; Lopes, C.; Chaves, M.M. Limitations to Leaf Photosynthesis in Field-Grown Grapevine under Drought—Metabolic and Modelling Approaches. Funct. Plant Biol. 2002, 29, 451–459. [Google Scholar] [CrossRef]
- Wei, Z.; Yanling, J.; Feng, L.; Guangsheng, Z. Responses of Photosynthetic Parameters of Quercus Mongolica to Soil Moisture Stresses. Acta Ecol. Sin. 2008, 28, 2504–2510. [Google Scholar] [CrossRef]
- Albert, K.R.; Mikkelsen, T.N.; Michelsen, A.; Ro-Poulsen, H.; van der Linden, L. Interactive Effects of Drought, Elevated CO2 and Warming on Photosynthetic Capacity and Photosystem Performance in Temperate Heath Plants. J. Plant Physiol. 2011, 168, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Stefanski, A.; Bermudez, R.; Sendall, K.M.; Montgomery, R.A.; Reich, P.B. Surprising Lack of Sensitivity of Biochemical Limitation of Photosynthesis of Nine Tree Species to Open-Air Experimental Warming and Reduced Rainfall in a Southern Boreal Forest. Glob. Chang. Biol. 2020, 26, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Sperlich, D.; Barbeta, A.; Ogaya, R.; Sabaté, S.; Peñuelas, J. Balance between Carbon Gain and Loss under Long-Term Drought: Impacts on Foliar Respiration and Photosynthesis in Quercus ilex L. J. Exp. Bot. 2016, 67, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Clarke, V. The Nitrogen Cost of Photosynthesis. J. Exp. Bot. 2018, 70, 7–15. [Google Scholar] [CrossRef]
- Muñoz-Carpena, R.; Li, Y.C.; Klassen, W.; Dukes, M. Field Comparison of Tensiometer and Granular Matrix Sensor Automatic Drip Irrigation on Tomato. Horttechnology 2005, 15, 584–590. [Google Scholar] [CrossRef]
- Bonelli, L.; Montesano, F.F.; D’Imperio, M.; Gonnella, M.; Boari, A.; Leoni, B.; Serio, F. Sensor-Based Fertigation Management Enhances Resource Utilization and Crop Performance in Soilless Strawberry Cultivation. Agronomy 2024, 14, 465. [Google Scholar] [CrossRef]
- Schattman, R.E.; Jean, H.; Faulkner, J.W.; Maden, R.; McKeag, L.; Nelson, K.C.; Grubinger, V.; Burnett, S.; Erich, M.S.; Ohno, T. Effects of Irrigation Scheduling Approaches on Soil Moisture and Vegetable Production in the Northeastern U.S.A. Agric. Water Manag. 2023, 287, 108428. [Google Scholar] [CrossRef]
- Takagi, H. Vegetables Peculiar to Japan. In Horticulture in Japan; XXIVth International Horticultural Congress Publication Committee, Ed.; Asakura Publishing Co.: Tokyo, Japan, 1994; pp. 111–112. [Google Scholar]
- Imahori, Y.; Suzuki, Y.; Kawagishi, M.; Ishimaru, M.; Ueda, Y.; Chachin, K. Physiological Responses and Quality Attributes of Chinese Chive Leaves Exposed to CO2-Enriched Atmospheres. Postharvest Biol. Technol. 2007, 46, 160–166. [Google Scholar] [CrossRef]
- Topp, G.C.; Davis, J.L.; Annan, A.P. Electromagnetic Determination of Soil Water Content: Measurements in Coaxial Transmission Lines. Water Resour. Res. 1980, 16, 574–582. [Google Scholar] [CrossRef]
- Kochi Prefectural Government Guidlines for Fertilization in Kochi Prefecture. Available online: https://www.nogyo.tosa.pref.kochi.lg.jp/download/?t=LD&id=5581&fid=29558 (accessed on 7 July 2024).
- Yasuoka, Y.; Itokawa, S. Relationship between water absorption volume of Chinese chive in glasshouse and its growth and solar radiation under carbon dioxide application. Bull. Kochi Agric. Res. Cent. 2018, 27, 9–14. (In Japanese) [Google Scholar]
- Jacovides, C.P.; Tymvios, F.S.; Assimakopoulos, V.D.; Kaltsounides, N.A. The Dependence of Global and Diffuse PAR Radiation Components on Sky Conditions at Athens, Greece. Agric. For. Meteorol. 2007, 143, 277–287. [Google Scholar] [CrossRef]
- Nomura, K.; Saito, M.; Tada, I.; Iwao, T.; Yamazaki, T.; Kira, N.; Nishimura, Y.; Mori, M.; Baeza, E.; Kitano, M. Estimation of Photosynthesis Loss Due to Greenhouse Superstructures and Shade Nets: A Case Study with Paprika and Tomato Canopies. HortScience 2022, 57, 464–471. [Google Scholar] [CrossRef]
- Kaneko, T.; Nomura, K.; Yasutake, D.; Iwao, T.; Okayasu, T.; Ozaki, Y.; Mori, M.; Hirota, T.; Kitano, M. A Canopy Photosynthesis Model Based on a Highly Generalizable Artificial Neural Network Incorporated with a Mechanistic Understanding of Single-Leaf Photosynthesis. Agric. For. Meteorol. 2022, 323, 109036. [Google Scholar] [CrossRef]
- Nomura, K.; Kaneko, T.; Iwao, T.; Kitayama, M.; Goto, Y.; Kitano, M. Hybrid AI Model for Estimating the Canopy Photosynthesis of Eggplants. Photosynth. Res. 2023, 155, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.S.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; De Angelis, P.; Freeman, M.; Wingate, L. Reconciling the Optimal and Empirical Approaches to Modelling Stomatal Conductance. Glob. Chang. Biol. 2011, 17, 2134–2144. [Google Scholar] [CrossRef]
- Gaastra, P. Photosynthesis of Crop Plants as Influenced by Light, Carbon Dioxide, Temperature, and Stomatal Diffusion Resistance; Wageningen University: Wageningen, The Netherlands, 1959. [Google Scholar]
- Jones, H.G. Plants and Microclimate; Cambridge University Press: Cambridge, UK, 2013; ISBN 9780511845727. [Google Scholar]
- Collatz, G.J.; Ball, J.T.; Grivet, C.; Berry, J.A. Physiological and Environmental Regulation of Stomatal Conductance, Photosynthesis and Transpiration: A Model That Includes a Laminar Boundary Layer. Agric. For. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- Nomura, K.; Wada, E.; Saito, M.; Yamasaki, H.; Yasutake, D.; Iwao, T.; Tada, I.; Yamazaki, T.; Kitano, M. Estimation of the Leaf Area Index, Leaf Fresh Weight, and Leaf Length of Chinese Chive (Allium tuberosum) Using Nadir-Looking Photography in Combination with Allometric Relationships. HortScience 2022, 57, 777–784. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas Exchange Measurements, What Can They Tell Us about the Underlying Limitations to Photosynthesis? Procedures and Sources of Error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef]
- Newville, M.; Stensitzki, T.; Allen, D.B.; Ingargiola, A. LMFIT: Non-Linear Least-Square Minimization and Curve-Fitting for Python. 2014. Zendo. Available online: https://ui.adsabs.harvard.edu/abs/2016ascl.soft06014N/abstract (accessed on 23 July 2024).
- von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; CSIRO Publishing: Clayton, Australia, 2000; ISBN 9780643103405. [Google Scholar]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R., Jr.; Long, S.P. Improved Temperature Response Functions for Models of Rubisco-Limited Photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28–30 June 2010; pp. 92–96. [Google Scholar] [CrossRef]
- Shina, K.; Nonaka, S. The Moisture of the Capillary Bonds Rupture. J. Jpn. Soc. Soil Phys. 1971, 24, 14–16. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 23 July 2024).
- Nomura, K.; Yasutake, D.; Kaneko, T.; Takada, A.; Okayasu, T.; Ozaki, Y.; Mori, M.; Kitano, M. Long-Term Compound Interest Effect of CO2 Enrichment on the Carbon Balance and Growth of a Leafy Vegetable Canopy. Sci. Hortic. 2021, 283, 110060. [Google Scholar] [CrossRef]
- Denmead, O.T. Plant Physiological Methods for Studying Evapotranspiration: Problems of Telling the Forest from the Trees. Agric. Water Manag. 1984, 8, 167–189. [Google Scholar] [CrossRef]
- Garcia, R.L.; Norman, J.M.; McDermitt, D.K. Measurements of Canopy Gas Exchange Using an Open Chamber System. Remote Sens. Rev. 1990, 5, 141–162. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat Stress in Cultivated Plants: Nature, Impact, Mechanisms, and Mitigation Strategies—A Review. Plant Biosyst. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Huber, S.; Midmore, D.J. Aerated Subsurface Irrigation Water Gives Growth and Yield Benefits to Zucchini, Vegetable Soybean and Cotton in Heavy Clay Soils. Ann. Appl. Biol. 2004, 144, 285–298. [Google Scholar] [CrossRef]
- Friedman, S.P.; Naftaliev, B. A Survey of the Aeration Status of Drip-Irrigated Orchards. Agric. Water Manag. 2012, 115, 132–147. [Google Scholar] [CrossRef]
- Bakker, D.M.; Hamilton, G.J.; Houlbrooke, D.J.; Spann, C. The Effect of Raised Beds on Soil Structure, Waterlogging, and Productivity on Duplex Soils in Western Australia. Soil Res. 2005, 43, 575. [Google Scholar] [CrossRef]
- Hirooka, Y.; Shoji, K.; Watanabe, Y.; Izumi, Y.; Awala, S.K. Soil & Tillage Research Ridge Formation with Strip Tillage Alleviates Excess Moisture Stress for Drought-Tolerant Crops. Soil Tillage Res. 2019, 195, 104429. [Google Scholar] [CrossRef]
- Kay, B.D.; Hajabbasi, M.A.; Ying, J.; Tollenaar, M. Optimum versus Non-Limiting Water Contents for Root Growth, Biomass Accumulation, Gas Exchange and the Rate of Development of Maize (Zea mays L.). Soil Tillage Res. 2006, 88, 42–54. [Google Scholar] [CrossRef]
- Osroosh, Y.; Peters, R.T.; Campbell, C.S.; Zhang, Q. Comparison of Irrigation Automation Algorithms for Drip-Irrigated Apple Trees. Comput. Electron. Agric. 2016, 128, 87–99. [Google Scholar] [CrossRef]
- Lena, B.P.; Bondesan, L.; Pinheiro, E.A.R.; Ortiz, B.V.; Morata, G.T.; Kumar, H. Determination of Irrigation Scheduling Thresholds Based on HYDRUS-1D Simulations of Field Capacity for Multilayered Agronomic Soils in Alabama, USA. Agric. Water Manag. 2022, 259, 107234. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; p. 300. [Google Scholar]
- Cassel, D.K.; Nielsen, D.R. Field Capacity and Available Water Capacity. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Soil Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 901–926. [Google Scholar] [CrossRef]
- Gallardo, M.; Thompson, R.B.; Fernández, M.D. Water Requirements and Irrigation Management in Mediterranean Greenhouses: The Case of the Southeast Coast of Spain. In Good Agricultural Practices for Greenhouse Vegetable Crops; Plant Production and Protection Paper; FAO: Rome, Italy, 2013; Volume 217, pp. 109–136. ISBN 9789251076491. [Google Scholar]
- Thompson, R.B.; Gallardo, M.; Valdez, L.C.; Fernández, M.D. Using Plant Water Status to Define Threshold Values for Irrigation Management of Vegetable Crops Using Soil Moisture Sensors. Agric. Water Manag. 2007, 88, 147–158. [Google Scholar] [CrossRef]
- Soulis, K.X.; Elmaloglou, S.; Dercas, N. Investigating the Effects of Soil Moisture Sensors Positioning and Accuracy on Soil Moisture Based Drip Irrigation Scheduling Systems. Agric. Water Manag. 2015, 148, 258–268. [Google Scholar] [CrossRef]
- Shen, X.; Liang, J.; Zeleke, K.T.; Liang, Y.; Wang, G.; Duan, A.; Mi, Z.; Ning, H.; Gao, Y.; Zhang, J. Optimizing the Positioning of Soil Moisture Monitoring Sensors in Winter Wheat Fields. Water 2018, 10, 1707. [Google Scholar] [CrossRef]
- Héroult, A.; Lin, Y.S.; Bourne, A.; Medlyn, B.E.; Ellsworth, D.S. Optimal Stomatal Conductance in Relation to Photosynthesis in Climatically Contrasting Eucalyptus Species under Drought. Plant Cell Environ. 2013, 36, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Miner, G.L.; Bauerle, W.L. Seasonal Variability of the Parameters of the Ball–Berry Model of Stomatal Conductance in Maize (Zea mays L.) and Sunflower (Helianthus annuus L.) under Well-Watered and Water-Stressed Conditions. Plant Cell Environ. 2017, 40, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Kingma, D.P.; Ba, J.L. Adam: A Method for Stochastic Optimization. In 3rd International Conference on Learning Representations. In Proceedings of the ICLR 2015—Conference Track Proceedings, San Diego, CA, USA, 7–9 May 2015; pp. 1–15. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, K.; Wada, E.; Saito, M.; Itokawa, S.; Mizobuchi, K.; Yamasaki, H.; Tada, I.; Iwao, T.; Yamazaki, T.; Kitano, M. Effect of Different Fertigation Scheduling Methods on the Yields and Photosynthetic Parameters of Drip-Fertigated Chinese Chive (Allium tuberosum) Grown in a Horticultural Greenhouse. Horticulturae 2024, 10, 794. https://doi.org/10.3390/horticulturae10080794
Nomura K, Wada E, Saito M, Itokawa S, Mizobuchi K, Yamasaki H, Tada I, Iwao T, Yamazaki T, Kitano M. Effect of Different Fertigation Scheduling Methods on the Yields and Photosynthetic Parameters of Drip-Fertigated Chinese Chive (Allium tuberosum) Grown in a Horticultural Greenhouse. Horticulturae. 2024; 10(8):794. https://doi.org/10.3390/horticulturae10080794
Chicago/Turabian StyleNomura, Koichi, Eriko Wada, Masahiko Saito, Shuji Itokawa, Keisuke Mizobuchi, Hiromi Yamasaki, Ikunao Tada, Tadashige Iwao, Tomihiro Yamazaki, and Masaharu Kitano. 2024. "Effect of Different Fertigation Scheduling Methods on the Yields and Photosynthetic Parameters of Drip-Fertigated Chinese Chive (Allium tuberosum) Grown in a Horticultural Greenhouse" Horticulturae 10, no. 8: 794. https://doi.org/10.3390/horticulturae10080794
APA StyleNomura, K., Wada, E., Saito, M., Itokawa, S., Mizobuchi, K., Yamasaki, H., Tada, I., Iwao, T., Yamazaki, T., & Kitano, M. (2024). Effect of Different Fertigation Scheduling Methods on the Yields and Photosynthetic Parameters of Drip-Fertigated Chinese Chive (Allium tuberosum) Grown in a Horticultural Greenhouse. Horticulturae, 10(8), 794. https://doi.org/10.3390/horticulturae10080794





