Effect of Menthol Treatment on the Sprouting and Quality of Potato Tuber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatments
2.3. Determination of Sprouting Percentage and Sprouting Index
2.4. Determination of Glucoside Alkaloids Content
2.5. Determination of Moisture Content and Weight Loss
2.5.1. Determination of Moisture Content
2.5.2. Determination of Weight Loss
2.6. Determination of Browning
2.6.1. Determination of Browning Index
2.6.2. Determination of Total Phenolic Content
2.6.3. Determination of PPO Activity
2.7. Determination of Respiration Intensity and MDA Content
2.7.1. Determination of Respiration Intensity
2.7.2. Determination of MDA Content
2.8. Determination of Reducing Sugar and Starch Content
2.8.1. Determination of Reducing Sugar Content
2.8.2. Determination of Starch Content
2.9. Determination of Soluble Protein and Amino Acid Content
2.9.1. Determination of Soluble Protein Content
2.9.2. Determination of Amino Acid Content
2.10. Data Analysis
3. Results and Analysis
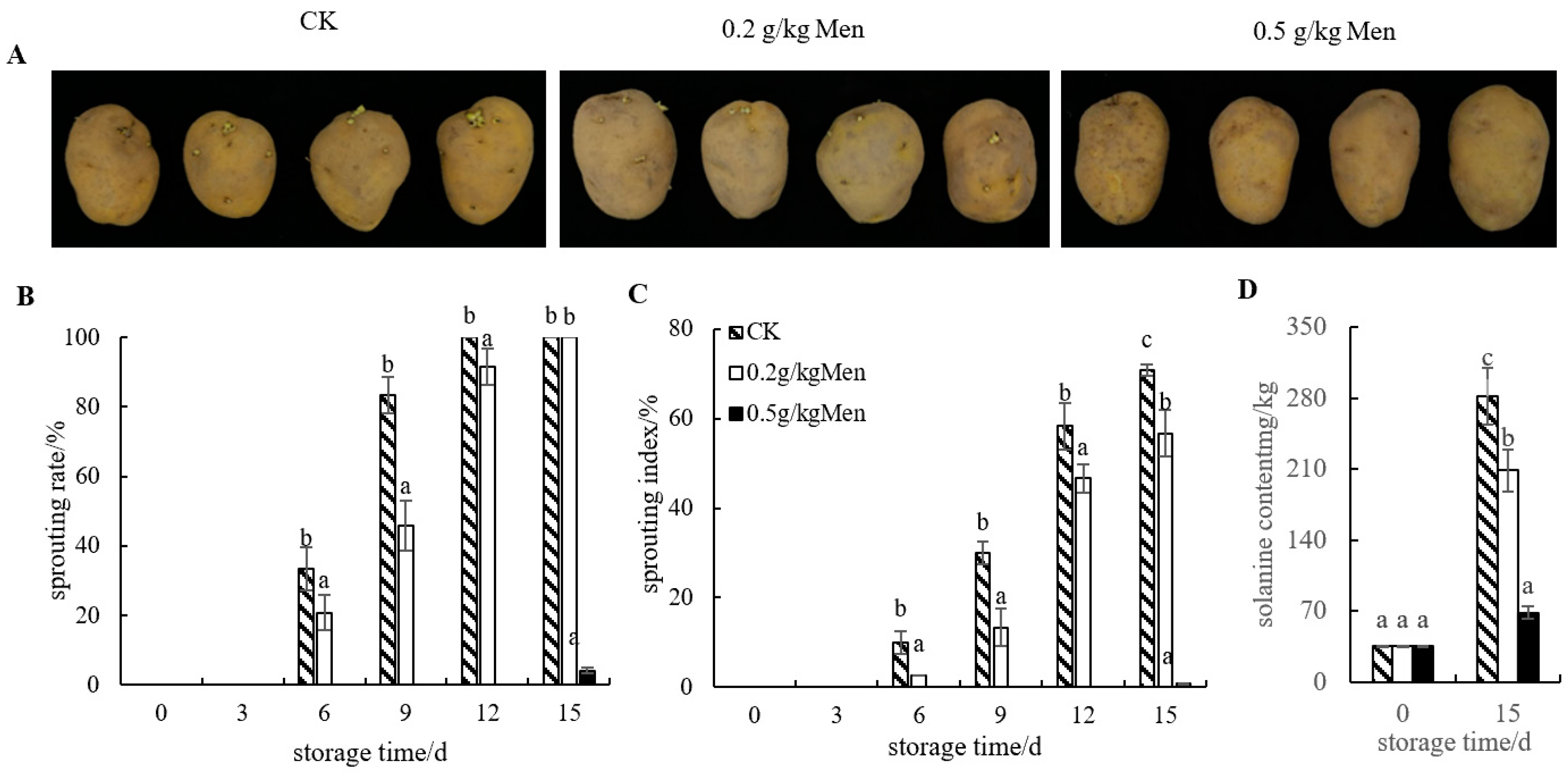
3.1. Effect of Menthol Treatment on Potato Tuber Sprouting
3.2. Impact of Menthol Treatment on Glucoside Alkaloid Content in Potatoes
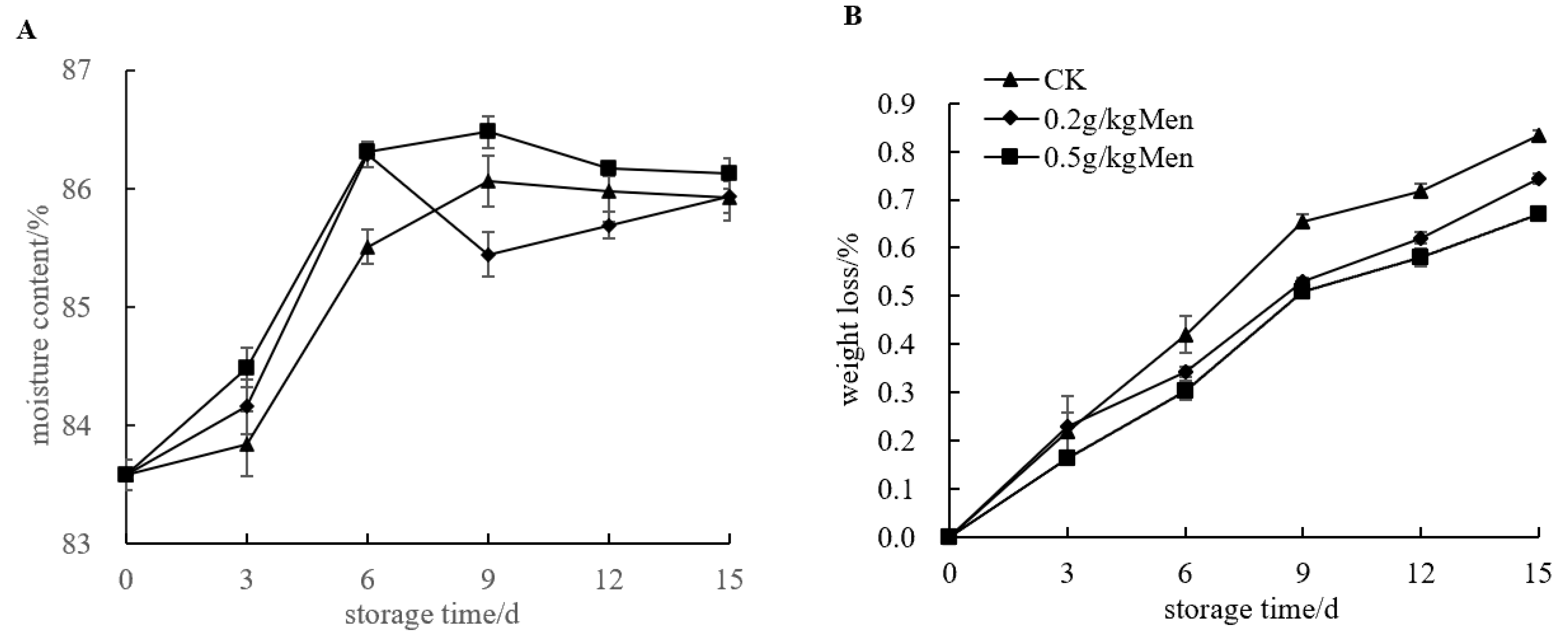
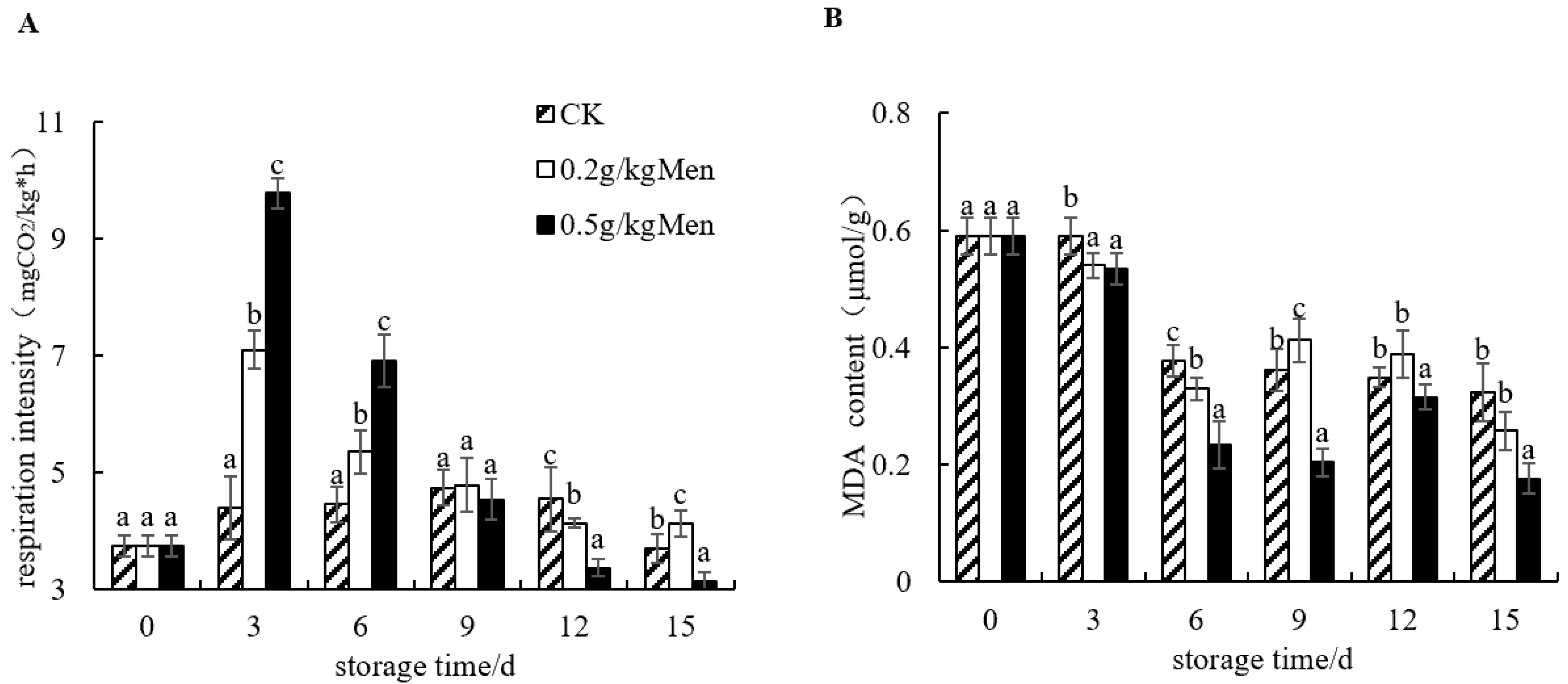
3.3. Impact of Menthol Treatment on Potato Moisture Content, Weight Loss, Respiration Intensity, and MDA Content
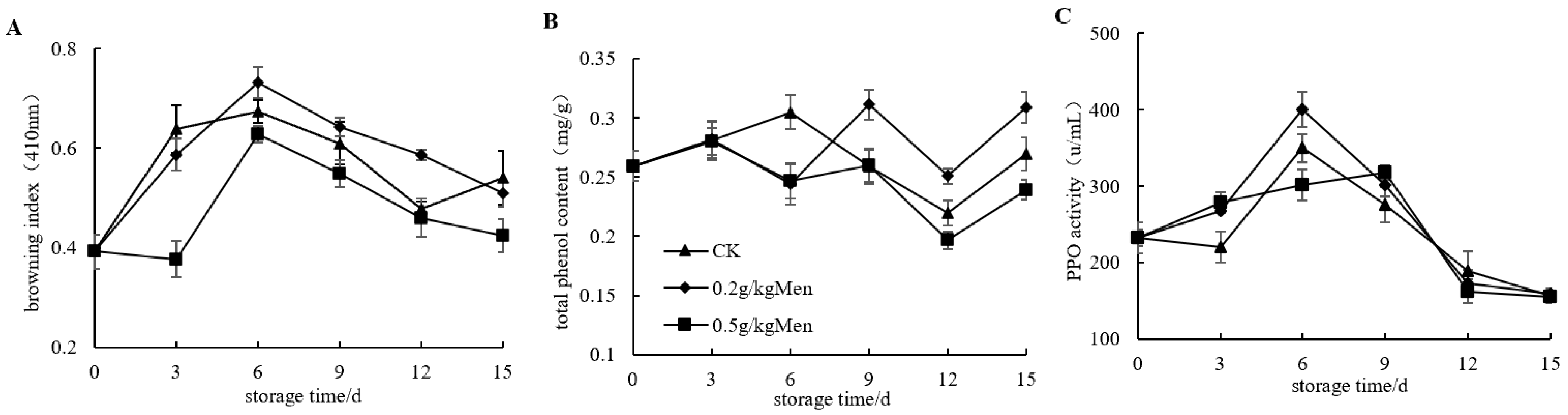
3.4. Impact of Menthol Treatment on Browning Index, Total Phenol Content, and PPO Activity in Potatoes
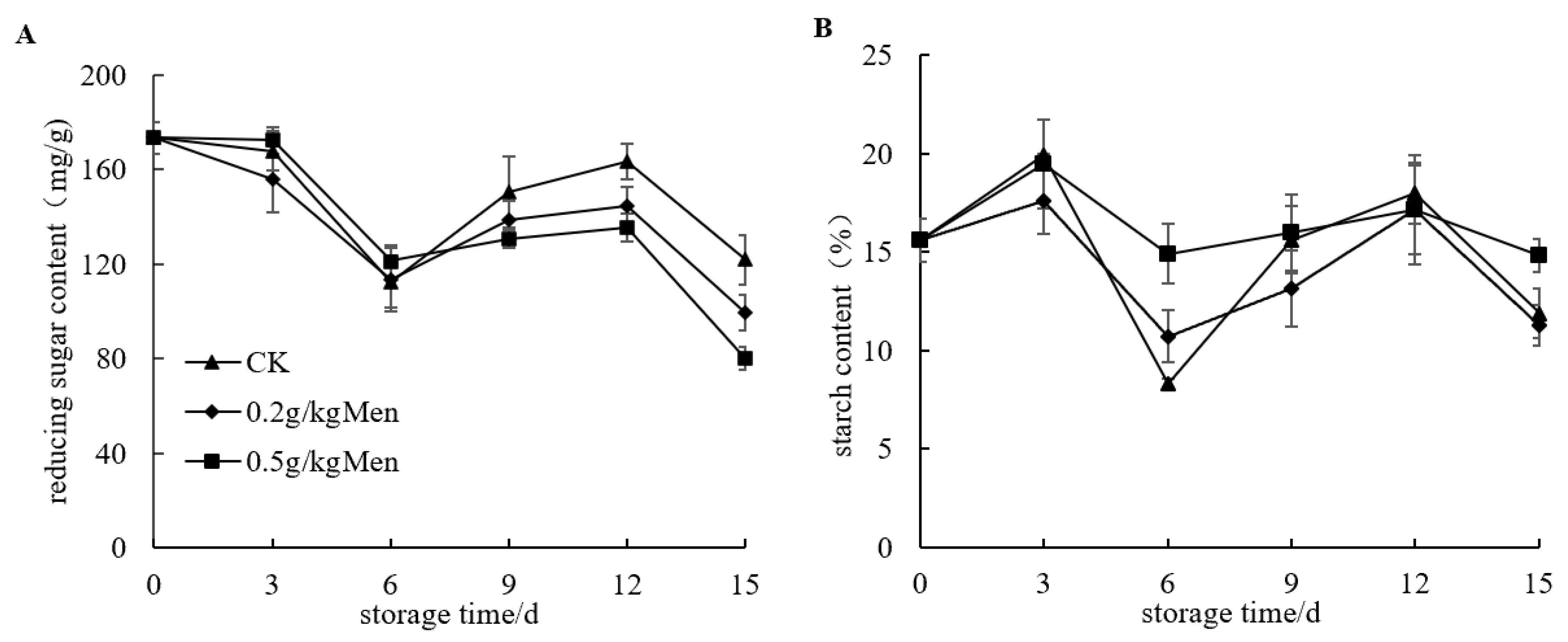
3.5. Effect of Menthol Treatment on Reducing Sugar and Starch Content of Potatoes
3.6. Effect of Menthol Treatment on Soluble Protein and Amino Acid Content of Potatoes
3.7. Correlation Analysis of Potato Germination and Quality Indicators
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gumbo, N.; Magwaza, L.S.; Ngobese, N.Z. Evaluating ecologically acceptable sprout suppressants for enhancing dormancy and potato storability: A review. Plants 2021, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C. Potato peels as sources of functional compounds for the food industry: A review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Ahmadu, T.; Abdullahi, A.; Ahmad, K.; Ahmadu, T.; Abdullahi, A.; Ahmad, K. The role of crop protection in sustainable potato (Solanum tuberosum L.) production to alleviate global starvation problem: An overview. In Solanum tuberosum—A Promising Crop for Starvation Problem; Intech Open: London, UK, 2021; pp. 19–51. [Google Scholar]
- Sharma, N.; Sucheta; Dangi, S.; Yadav, S.K. Long-term storability of potato tubers in aspect of biochemical changes and overall quality index affected by different packaging materials in refrigerated and non-refrigerated storage. Potato Res. 2020, 63, 303–321. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, S.S.; Zheng, X. Antimicrobial activity against phytopathogens and inhibitory activity on glucoside alkaloids in potatoes of the endophytic bacteria isolated from potato tubers. Front. Microbiol. 2020, 11, 570926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fu, M.R.; Liu, X.H. Research progress of potato sprout inhibition technology. Food Sci. 2013, 17, 338–343. [Google Scholar]
- Alamar, M.C.; Tosetti, R.; Landahl, S.; Bermejo, A.; Terry, L.A. Assuring potato tuber quality during storage: A future perspective. Front. Plant Sci. 2017, 8, 2034. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Ezekiel, R.; Pandey, R. Sprout suppression on potato: Need to look beyond CIPC for more effective and safer alternatives. J. Food Sci. Technol. 2016, 53, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Fu, M.; Yang, X.; Chen, Q. Ethylene inhibited sprouting of potato tubers by influencing the carbohydrate metabolism pathway. J. Food Sci. Technol. 2016, 53, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Levaj, B.; Pelaić, Z.; Galić, K.; Kurek, M.; Ščetar, M.; Poljak, M.; Repajić, M. Maintaining the quality and safety of fresh-cut potatoes (Solanum tuberosum): Overview of recent findings and approaches. Agronomy 2023, 13, 2002. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Hartmans, K.J.; Scheffer, J.J.C. Inhibition of potato sprout growth by carvone enantiomers and their bioconversion in sprouts. Potato Res. 1995, 38, 219–230. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Guan, Z.; Yue, F.; Wang, S.; Chen, Q.; Fu, M. Optimized preparation of methyl salicylate hydrogel and its inhibition effect on potato tuber sprouting. Horticulturae 2022, 8, 866. [Google Scholar] [CrossRef]
- Huang, T.; Ye, X.; Huang, X.; Li, X.; Mei, M.; Yu, L.; Wang, X. Effect of menthol and Jasmine essential oil on potato bud inhibition. J. Sichuan Agric. Univ. 2018, 618–625. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T.; Tonguc, M.; Baydar, H. Effects of caraway (Carum carvi L.) seed on sprouting of potato (Solanum tuberosum L.) tubers under different temperature conditions. Turk. J. Field Crops 2010, 15, 54–58. [Google Scholar]
- Oishi, A.; Nagatomi, Y.; Suzuki, K. Simultaneous LC-MS/MS Determination of 18 Plant Toxins in Beverages. Food Hyg. Saf. Sci. 2019, 60, 108–112. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.3-2016; Determination of Moisture in Food. China Standards Press: Beijing, China, 2016.
- Wang, T.; Yan, T.; Shi, J.; Sun, Y.; Wang, Q.; Li, Q. The stability of cell structure and antioxidant enzymes are essential for fresh-cut potato browning. Food Res. Int. 2023, 164, 112449. [Google Scholar] [CrossRef] [PubMed]
- Joly, N.; Souidi, K.; Depraetere, D.; Wils, D.; Martin, P. Potato by-products as a source of natural chlorogenic acids and phenolic compounds: Extraction, characterization, and antioxidant capacity. Molecules 2020, 26, 177. [Google Scholar] [CrossRef] [PubMed]
- Palamutoğlu, R. Antibrowning effect of commercial and acid-heat coagulated whey on potatoes during refrigerated storage. J. Food Sci. 2020, 85, 3858–3865. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lin, L.; Liu, T.; Ouyang, P.; He, B.; Liu, S. Reducing sugar content in hemicellulose hydrolysate by DNS method: A revisit. J. Biobased Mater. Bioenergy 2008, 2, 156–161. [Google Scholar] [CrossRef]
- Wegener, C.B.; Jansen, G.; Jurgens, H.U. Influence of drought and wounding stress on soluble phenols and proteins in potato tubers. Sustain. Agric. Res. 2014, 3, 1–15. [Google Scholar] [CrossRef][Green Version]
- GB5009.124-2016; Determination of Amino Acids in Food under National Food Safety Standards. China Standards Press: Beijing, China, 2016.
- Lee, S.K.; Jeon, J.S. Crucial role of inorganic pyrophosphate in integrating carbon metabolism from sucrose breakdown to starch synthesis in rice endosperm. Plant Sci. 2020, 298, 110572. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Wellard, R.M.; Nagy, S.A.; Joardder, M.U.H.; Karim, M.A. Experimental investigation of bound and free water transport process during drying of hygroscopic food material. Int. J. Therm. Sci. 2017, 117, 266–273. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Zhao, W.; Zheng, Y.; Wang, Y.; Wang, P.; Ma, Y.; Zhao, X. Low frequency ultrasound treatment enhances antibrowning effect of ascorbic acid in fresh-cut potato slices. Food Chem. 2022, 380, 132190. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Guan, Y.; Ji, Y.; Yang, X. Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes. Food Biosci. 2021, 44, 101435. [Google Scholar] [CrossRef]
- He, X.; Pu, Y.; Chen, L.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. A comprehensive review of intelligent packaging for fruits and vegetables: Target responders, classification, applications, and future challenges. Compr. Rev. Food Sci. Food Saf. 2023, 22, 842–881. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.M.; Chellappan, S.; Sabu, A. Enzymes in fruit and vegetable processing. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–110. [Google Scholar]
- Nath, P.; Pandey, N.; Samota, M.; Sharma, K.; Kale, S.; Kannaujia, P.; Chauhan, O.P. Browning reactions in foods. In Advances in Food Chemistry; Springer Nature: Singapore, 2022; pp. 117–159. [Google Scholar]
- Kasnak, C. Evaluation of the anti-browning effect of quercetin on cut potatoes during storage. Food Packag. Shelf Life 2022, 31, 100816. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.X.; Zeng, L.; Suo, H.C.; Li, C.C.; Shan, J.W.; Xiong, X.Y. Characteristics and differences of polyphenol oxidase, peroxidase activities and polyphenol content in different potato (solanum tuberosum) tubers. Appl. Ecol. Environ. Res. 2020, 18, 8171–8187. [Google Scholar] [CrossRef]
- Dobránszki, J.; Hidvégi, N.; Gulyás, A.; Tóth, B.; Teixeira da Silva, J.A. Transcription profile of potato (Solanum tuberosum L.) growing in vitro. J. Plant Growth Regul. 2021, 40, 749–760. [Google Scholar] [CrossRef]
- Cui, P.; Li, Y.; Cui, C.; Huo, Y.; Lu, G.; Yang, H. Proteomic and metabolic profile analysis of low-temperature storage responses in Ipomoea batata Lam. tuberous roots. BMC Plant Biol. 2020, 20, 435. [Google Scholar] [CrossRef]
- Gikundi, E.N. Physico-Chemical Properties and Storability of Selected Irish Potato Varieties Grown in Kenya. Doctoral Dissertation, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2021. [Google Scholar]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.; Saleh, M.A. The role of various amino acids in enzymatic browning process in potato tubers, and identifying the browning products. Food Chem. 2016, 192, 879–885. [Google Scholar] [CrossRef]

| Bud Length/mm | Sprouting Grade | Bud Length/mm | Sprouting Grade |
|---|---|---|---|
| 0~2 | 0 | 15~20 | 4 |
| 2~5 | 1 | 20~25 | 5 |
| 5~10 | 2 | 25~30 | 6 |
| 10~15 | 3 | >30 | 7 |
| Soluble Protein Content (mg/g) | Treatment Groups | ||
|---|---|---|---|
| CK | 0.2 g/kg Men | 0.5 g/kg Men | |
| 0 d | 3.18 ± 0.07 a | 3.18 ± 0.07 a | 3.18 ± 0.07 a |
| 3 d | 3.21 ± 0.05 a | 3.62 ± 0.05 b | 3.69 ± 0.04 b |
| 6 d | 2.99 ± 0.12 a | 3.38 ± 0.11 b | 3.43 ± 0.11 b |
| 9 d | 3.55 ± 0.17 a | 3.78 ± 0.09 a | 3.63 ± 0.10 a |
| 12 d | 3.73 ± 0.07 a | 3.71 ± 0.03 a | 4.06 ± 0.08 b |
| 15 d | 3.87 ± 0.05 a | 3.86 ± 0.06 a | 4.06 ± 0.08 b |
| Amino Acid Content (mg/g) | 0 d | 15 d | ||
|---|---|---|---|---|
| Control Value | CK | 0.2 g/kg Men | 0.5 g/kg Men | |
| glycine | 0.43 ± 0.015 | 0.43 ± 0.017 a | 0.43 ± 0.020 a | 0.39 ± 0.016 a |
| methionine | 0.03 ± 0.0025 | 0.026 ± 0.0021 b | 0.025 ± 0.0025 b | 0.021 ± 0.018 a |
| proline | 0.39 ± 0.011 | 0.49 ± 0.031 b | 0.45 ± 0.034 a | 0.43 ± 0.027 a |
| total amino acid | 14.67 ± 0.105 | 14.256 ± 0.162 b | 14.005 ± 0.128 a | 13.891 ± 0.131 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Qin, Y.; Hou, Q.; Niu, D.; Chen, Q. Effect of Menthol Treatment on the Sprouting and Quality of Potato Tuber. Horticulturae 2024, 10, 528. https://doi.org/10.3390/horticulturae10050528
Xu Y, Qin Y, Hou Q, Niu D, Chen Q. Effect of Menthol Treatment on the Sprouting and Quality of Potato Tuber. Horticulturae. 2024; 10(5):528. https://doi.org/10.3390/horticulturae10050528
Chicago/Turabian StyleXu, Ye, Yang Qin, Qianqian Hou, Defu Niu, and Qingmin Chen. 2024. "Effect of Menthol Treatment on the Sprouting and Quality of Potato Tuber" Horticulturae 10, no. 5: 528. https://doi.org/10.3390/horticulturae10050528
APA StyleXu, Y., Qin, Y., Hou, Q., Niu, D., & Chen, Q. (2024). Effect of Menthol Treatment on the Sprouting and Quality of Potato Tuber. Horticulturae, 10(5), 528. https://doi.org/10.3390/horticulturae10050528




