Recent Advances in Understanding and Controlling Fusarium Diseases of Alliums
Abstract
1. Introduction
| Fusarium Species Causing Rot | Affected Allium Species | References |
|---|---|---|
| Fusarium oxysporum f. sp. cepae (FOC) | Allium cepa, Allium sativum, Allium cepa Aggregatum group, Allium fistulosum | [19,21,24,28,32,33,34,38] |
| Fusarium proliferatum | Allium cepa, Allium sativum, Allium fistulosum | [20,21,24,29,32,34,35,36,37] |
| Fusarium solani | Allium cepa, Allium sativum, Allium fistulosum | [20,24,29,34] |
| Fusarium acuminatum | Allium sativum | [29] |
| Fusarium equiseti | Allium cepa | [22] |
| Fusarium culmorum | Allium porrum | [30,31] |
| Fusarium falciforme | Allium cepa | [23,34] |
| Fusarium brachygibbosum | Allium cepa | [23] |
| Fusarium redolens | Allium cepa | [20,21] |
| Fusarium verticillioides | Allium sativum | [29] |
| Fusarium acutatum | Allium cepa | [32,33,34] |
| Fusarium anthophilium | Allium cepa | [32] |
2. Detection of Fusarium Diseases in Alliums
2.1. Conventional Detection
2.2. Molecular Detection
2.3. Biochemical Detection
2.4. Imaging and Spectroscopic Techniques
2.5. Biosensors
3. Recent Research on Methods to Control Fusarium Diseases in Alliums
3.1. Chemical Methods
3.2. Biological Methods
3.3. Cultural Methods
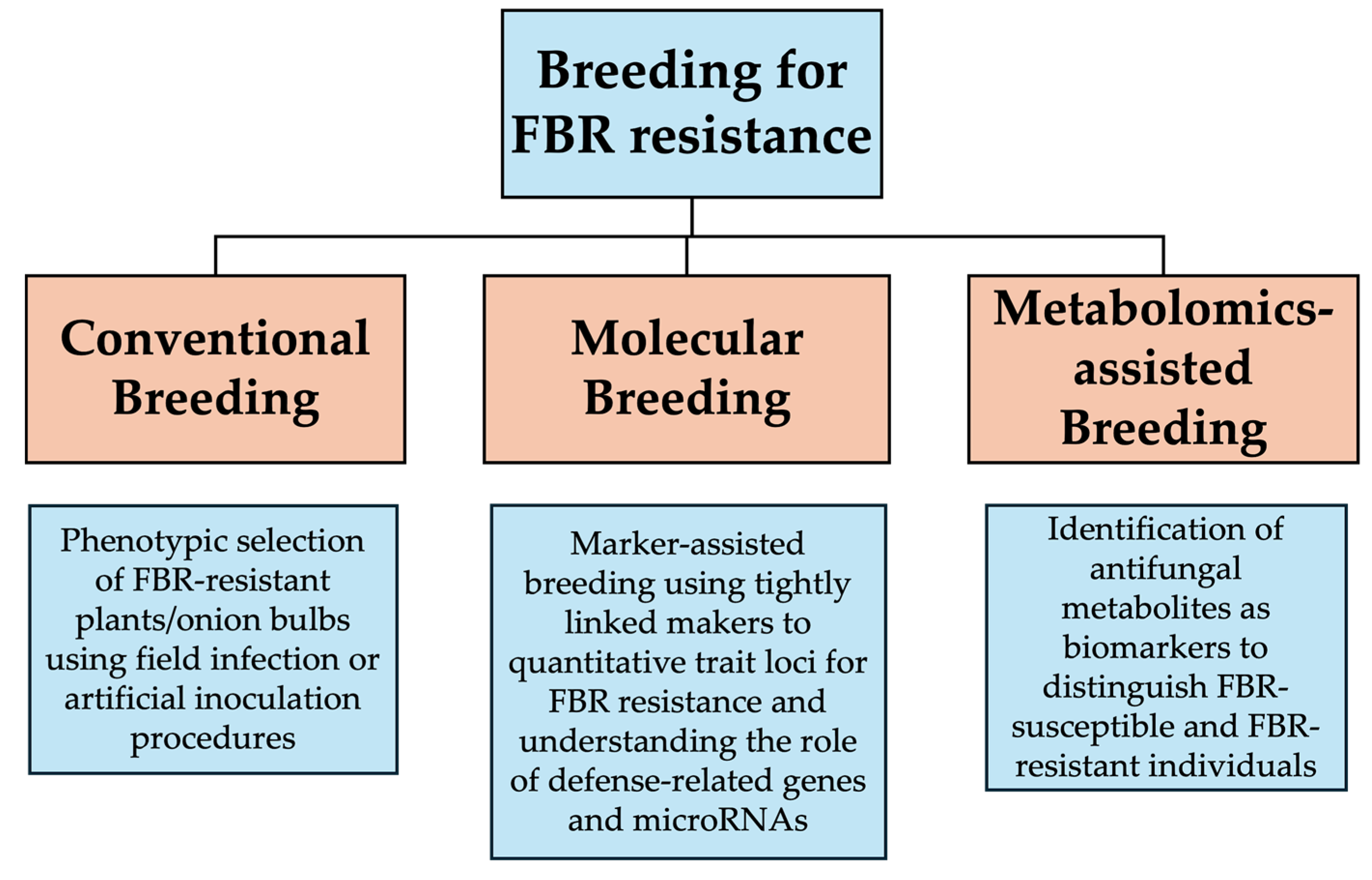
3.4. Breeding for FBR Resistance
3.4.1. Conventional Breeding
3.4.2. Molecular Breeding
3.4.3. Metabolomics-Assisted Breeding
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical Constituents and Medicinal Properties of Allium Species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Puizina, J. Edible Allium Species: Chemical Composition, Biological Activity and Health Effects. Ital. J. Food Sci. 2019, 31. [Google Scholar] [CrossRef]
- Havey, M.J. Onion and Other Cultivated Alliums. Evol. Crop Plants 1995, 2, 344–350. [Google Scholar]
- Fritsch, R.M.; Friesen, N. Evolution, Domestication and Taxonomy. In Allium Crop Science: Recent Advances; CABI Digital Library: Wallingford, UK, 2002; pp. 5–30. [Google Scholar]
- Kamenetsky, R.; Fritsch, R.M. Ornamental Alliums. In Allium Crop Science: Recent Advances; CABI Digital Library: Wallingford, UK, 2002; pp. 459–491. [Google Scholar]
- Finkers, R.; van Kaauwen, M.; Ament, K.; Burger-Meijer, K.; Egging, R.; Huits, H.; Kodde, L.; Kroon, L.; Shigyo, M.; Sato, S.; et al. Insights from the First Genome Assembly of Onion (Allium cepa). G3 Genes Genomes Genet. 2021, 11, jkab243. [Google Scholar] [CrossRef]
- Gross, M. All about Allium. Curr. Biol. 2021, 31, R1449–R1452. [Google Scholar] [CrossRef]
- Simin, N.; Orcic, D.; Cetojevic-Simin, D.; Mimica-Dukic, N.; Anackov, G.; Beara, I.; Mitic-Culafic, D.; Bozin, B. Phenolic Profile, Antioxidant, Anti-Inflammatory and Cytotoxic Activities of Small Yellow Onion (Allium flavum L. Subsp. flavum, Alliaceae). LWT-Food Sci. Technol. 2013, 54, 139–146. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial Properties of Allium Species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef]
- Moon, J.; Do, H.-J.; Kim, O.Y.; Shin, M.-J. Antiobesity Effects of Quercetin-Rich Onion Peel Extract on the Differentiation of 3T3-L1 Preadipocytes and the Adipogenesis in High Fat-Fed Rats. Food Chem. Toxicol. 2013, 58, 347–354. [Google Scholar] [CrossRef]
- Tang, X.; Olatunji, O.J.; Zhou, Y.; Hou, X. Allium Tuberosum: Antidiabetic and Hepatoprotective Activities. Food Res. Int. 2017, 102, 681–689. [Google Scholar] [CrossRef]
- Sup Lee, W.; Mi Yi, S.; Won Yun, J.; Hyun Jung, J.; Hoon Kim, D.; Jung Kim, H.; Chang, S.-H.; Kim, G.; Ho Ryu, C.; Chul Shin, S.; et al. Polyphenols Isolated from Allium cepa L. Induces Apoptosis by Induction of P53 and Suppression of Bcl-2 through Inhibiting PI3K/Akt Signaling Pathway in AGS Human Cancer Cells. J. Cancer Prev. 2014, 19, 14–22. [Google Scholar]
- Lee, K.H.; Park, E.; Lee, H.J.; Kim, M.O.; Cha, Y.J.; Kim, J.M.; Lee, H.; Shin, M.J. Effects of Daily Quercetin-Rich Supplementation on Cardiometabolic Risks in Male Smokers. Nutr. Res. Pract. 2011, 5, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Lee, C.H.; Yoo, K.-Y.; Choi, J.H.; Park, O.K.; Lim, S.S.; Kang, I.-J.; Kwon, D.Y.; Park, J.; Yi, J.-S. Neuroprotective Effects of Onion Extract and Quercetin against Ischemic Neuronal Damage in the Gerbil Hippocampus. J. Med. Food 2009, 12, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Elberry, A.A.; Mufti, S.; Al-Maghrabi, J.; Abdel Sattar, E.; Ghareib, S.A.; Mosli, H.A.; Gabr, S.A. Immunomodulatory Effect of Red Onion (Allium cepa Linn) Scale Extract on Experimentally Induced Atypical Prostatic Hyperplasia in Wistar Rats. Mediat. Inflamm. 2014, 2014, 640746. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khar, A.; Khosa, J.S.; Mandal, S.; Malla, S. Recent Advances in Molecular Genetics of Onion. Horticulturae 2024, 10, 256. [Google Scholar] [CrossRef]
- Sharma, S.; Cramer, C.S. Reduced Iris Yellow Spot Symptom Expression in the Selected Onion Germplasm. Veg. Res. 2023, 3, 26. [Google Scholar] [CrossRef]
- Khar, A.; Galván, G.A.; Singh, H. Allium Breeding against Biotic Stresses. In Genomic Designing for Biotic Stress Resistant Vegetable Crops; Springer: Berlin/Heidelberg, Germany, 2022; pp. 233–259. [Google Scholar]
- Cramer, C.S. Breeding and Genetics of Fusarium Basal Rot Resistance in Onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Mohammadi Goltapeh, E.; Safaie, N. Identification of Fusarium Species Causing Basal Rot of Onion in East Azarbaijan Province, Iran and Evaluation of Their Virulence on Onion Bulbs and Seedlings. Arch. Phytopathol. Plant Prot. 2014, 47, 1050–1062. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Kuivainen, E.; Qiu, Y.; Segerstedt, M.; Hannukkala, A.O. Fusarium oxysporum, F. proliferatum and F. redolens Associated with Basal Rot of Onion in Finland. Plant Pathol. 2016, 65, 1310–1320. [Google Scholar] [CrossRef]
- Romero-Arenas, O.; Martínez-Salgado, S.J.; Rivera-Tapia, A.; Huerta-Lara, M.; Laug-Garcia, B.; Villa-Ruano, N. First Report of Basal Rot Caused by Fusarium equiseti in Onion Crops from Puebla, Mexico. Trop. Subtrop. Agroecosyst. 2022, 25. [Google Scholar] [CrossRef]
- Tirado-Ramirez, M.A.; López-Urquídez, G.A.; Amarillas-Bueno, L.A.; Retes-Manjarrez, J.E.; Vega-Gutiérrez, T.A.; López Avendaño, J.E.; López-Orona, C.A. Identification and Virulence of Fusarium falciforme and Fusarium brachygibbosum as Causal Agents of Basal Rot on Onion in Mexico. Can. J. Plant Pathol. 2021, 43, 722–733. [Google Scholar] [CrossRef]
- Le, D.; Ameye, M.; De Boevre, M.; De Saeger, S.; Audenaert, K.; Haesaert, G. Population, Virulence, and Mycotoxin Profile of Fusarium spp. Associated with Basal Rot of Allium Spp. In Vietnam. Plant Dis. 2021, 105, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Galván, G.A.; Koning-Boucoiran, C.F.S.; Koopman, W.J.M.; Burger-Meijer, K.; González, P.H.; Waalwijk, C.; Kik, C.; Scholten, O.E. Genetic Variation among Fusarium Isolates from Onion, and Resistance to Fusarium Basal Rot in Related Allium Species. Eur. J. Plant Pathol. 2008, 121, 499–512. [Google Scholar] [CrossRef]
- Geiser, D.M.; Al-Hatmi, A.M.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.; Blomquist, C.L.; Bowden, R.L.; et al. Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani species complex. Phytopathology 2021, 111, 1064–1079. [Google Scholar] [PubMed]
- O’Donnell, K.; Al-Hatmi, A.M.; Aoki, T.; Brankovics, B.; Cano-Lira, J.F.; Coleman, J.J.; De Hoog, G.S.; Di Pietro, A.; Frandsen, R.J.; Geiser, D.M.; et al. No to Neocosmospora: Phylogenomic and practical reasons for continued inclusion of the Fusarium solani species complex in the genus Fusarium. mSphere 2020, 5, 10-1128. [Google Scholar] [CrossRef]
- Sakane, K.; Akiyama, M.; Ando, A.; Shigyo, M.; Ito, S.; Sasaki, K. Identification of a Novel Effector Gene and Its Functional Tradeoff in Fusarium oxysporum f. sp. cepae That Infects Welsh Onion. Physiol. Mol. Plant Pathol. 2023, 123, 101939. [Google Scholar] [CrossRef]
- Delgado-Ortiz, J.C.; Ochoa-Fuentes, Y.M.; Cerna-Chávez, E.; Beltrán-Beache, M.; Rodríguez-Guerra, R.; Aguirre-Uribe, L.A.; Vázquez-Martínez, O. Patogenicidad de Especies de Fusarium Asociadas a La Pudrición Basal Del Ajo En El Centro Norte de México. Rev. Argent. Microbiol. 2016, 48, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Armengol, J.; Vicent, A.; Sales, R.; Garcia-Jimenez, J.; Rodríguez, J.M. First Report of Basal Rot of Leek Caused by Fusarium culmorum in Spain. Plant Dis. 2001, 85, 679. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.T.; Gordon, T.R.; Aegerter, B.J. Root and Basal Rot of Leek Caused by Fusarium culmorum in California. Plant Dis. 2003, 87, 601. [Google Scholar] [CrossRef]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Harel, Y.M.; Degani, O. Isolation and Identification of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot in Northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef]
- Degani, O.; Dimant, E.; Gordani, A.; Graph, S.; Margalit, E. Prevention and Control of Fusarium Spp., the Causal Agents of Onion (Allium cepa) Basal Rot. Horticulturae 2022, 8, 1071. [Google Scholar] [CrossRef]
- Sogoba, K.H.; Koita, K.; Ouattara, A.; Nana, T.A.; Soura, B.H.; Campa, C. Distribution, Diversity and Identification of Hot Spot of Fusarium Spp. Associated with Onion (Allium cepa L.) in Burkina Faso. J. Agric. Crop Res. 2021, 9, 241–249. [Google Scholar]
- Mondani, L.; Chiusa, G.; Battilani, P. Fungi Associated with Garlic During the Cropping Season, with Focus on Fusarium proliferatum and F. oxysporum. Plant Health Prog. 2021, 22, 37–46. [Google Scholar] [CrossRef]
- Gálvez, L.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł.; Palmero, D. Fusarium proliferatum—Causal Agent of Garlic Bulb Rot in Spain: Genetic Variability and Mycotoxin Production. Food Microbiol. 2017, 67, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, P.L.; Laurent, S.; Bornard, I.; Troulet, C.; El Maâtaoui, M.; Leyronas, C. Unraveling the Infection Process of Garlic by Fusarium proliferatum, the Causal Agent of Root Rot. Phytopathol. Mediterr. 2020, 59, 285–293. [Google Scholar]
- Sintayehu, A.; Sakhuja, P.K.; Fininsa, C.; Ahmed, S. Management of Fusarium Basal Rot (Fusarium oxysporum f. sp. cepae) on Shallot through Fungicidal Bulb Treatment. Crop Prot. 2011, 30, 560–565. [Google Scholar] [CrossRef]
- Abawi, G.S.; Lorbeer, J.W. Several Aspects of the Ecology and Pathology of Fusarium oxysporum f. sp. cepae. Phytopathology 1972, 62, 870–876. [Google Scholar] [CrossRef]
- Le, D.; Audenaert, K.; Haesaert, G. Fusarium Basal Rot: Profile of an Increasingly Important Disease in Allium spp. Trop. Plant Pathol. 2021, 46, 241–253. [Google Scholar] [CrossRef]
- Lucas, P. Diseases Caused by Soil-Borne Pathogens. In The Epidemiology of Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2006; pp. 373–386. [Google Scholar]
- Grünwald, N.J.; Hu, S.; Van Bruggen, A.H.C. Short-Term Cover Crop Decomposition in Organic and Conventional Soils: Characterization of Soil C, N, Microbial and Plant Pathogen Dynamics. Eur. J. Plant Pathol. 2000, 106, 37–50. [Google Scholar] [CrossRef]
- Stirling, G.R. The Impact of Farming Systems on Soil Biology and Soilborne Diseases: Examples from the Australian Sugar and Vegetable Industries—The Case for Better Integration of Sugarcane and Vegetable Production and Implications for Future Research. Australas. Plant Pathol. 2008, 37, 1–18. [Google Scholar] [CrossRef]
- van Bniggen, A.H.C.; Termorskuizen, A.J. Integrated Approaches to Root Disease Management in Organic Farming Systems. Australas. Plant Pathol. 2003, 32, 141–156. [Google Scholar]
- del Mar Jiménez-Gasco, M.; Jiménez-Díaz, R.M. Development of a Specific Polymerase Chain Reaction-Based Assay for the Identification of Fusarium oxysporum f. sp. ciceris and Its Pathogenic Races 0, 1A, 5, and 6. Phytopathology 2003, 93, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Lievens, B.; Rep, M.; Thomma, B.P.H.J. Recent Developments in the Molecular Discrimination of Formae Speciales of Fusarium oxysporum. Pest Manag. Sci. 2008, 64, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Lager, S. Survey of Fusarium Species on Yellow Onion (Allium cepa) on Öland. Master’s Thesis, Swedish University of Agricultural Science, Uppsala, Sweden, 2011. [Google Scholar]
- Cramer, C.S.; Mandal, S.; Sharma, S.; Nourbakhsh, S.S.; Goldman, I.; Guzman, I. Recent Advances in Onion Genetic Improvement. Agronomy 2021, 11, 482. [Google Scholar] [CrossRef]
- Palmero, D.; De Cara, M.; Nosir, W.; Gálvez, L.; Cruz, A.; Woodward, S.; Teresa González-Jaén, M.; Tello, J.C. Fusarium proliferatum Isolated from Garlic in Spain: Identification, Toxigenic Potential and Pathogenicity on Related Allium Species. Phytopathol. Mediterr. 2012, 51, 207–218. [Google Scholar]
- Latvala, S.; Haapalainen, M.; Kivijärvi, P.; Suojala-Ahlfors, T.; Iivonen, S.; Hannukkala, A. Sampling and PCR Method for Detecting Pathogenic Fusarium oxysporum Strains in Onion Harvest. Lett. Appl. Microbiol. 2020, 70, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Nakahara, K.; Shigyo, M.; Tanaka, S.; Ito, S.I. Detection and Quantification of Onion Isolates of Fusarium oxysporum f. sp. cepae in Onion Plant. J. Gen. Plant Pathol. 2015, 81, 232–236. [Google Scholar] [CrossRef]
- Dimant, E.; Degani, O. Molecular Real-Time PCR Monitoring of Onion Fusarium Basal Rot Chemical Control. J. Fungi 2023, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Haapalainen, M.; Latvala, S.; Edelenbos, M.; Johansen, A. Discriminant Analysis of Volatile Organic Compounds of Fusarium oxysporum f. sp. cepae and Fusarium proliferatum Isolates from Onions as Indicators of Fungal Growth. Fungal Biol. 2018, 122, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Infantino, A.; Taiti, C.; Grottoli, A.; Mancuso, S.; Costa, C.; Garzoli, S. Examination of Volatile Signatures of Fusarium Bulb Rot in Garlic Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry. Separations 2023, 10, 556. [Google Scholar] [CrossRef]
- Wang, A.; Islam, M.N.; Johansen, A.; Haapalainen, M.; Latvala, S.; Edelenbos, M. Pathogenic Fusarium oxysporum f. sp. cepae Growing inside Onion Bulbs Emits Volatile Organic Compounds That Correlate with the Extent of Infection. Postharvest Biol. Technol. 2019, 152, 19–28. [Google Scholar] [CrossRef]
- Mandal, S.; Cramer, C.S. Comparing Visual and Image Analysis Techniques to Quantify Fusarium Basal Rot Severity in Mature Onion Bulbs. Horticulturae 2021, 7, 156. [Google Scholar] [CrossRef]
- Tamburini, E.; Mamolini, E.; De Bastiani, M.; Marchetti, M.G. Quantitative Determination of Fusarium proliferatum Concentration in Intact Garlic Cloves Using Near-Infrared Spectroscopy. Sensors 2016, 16, 1099. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Soylu, E.M. Identification of Alternaria, Aspergillus, Fusarium and Penicillium spp. on Onion Plant (Allium cepa L.) Growing in Hatay, Amasya and Tokat Provinces Using MALDI-TOF Mass Spectrometry. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 2525–2529. [Google Scholar] [CrossRef]
- Makarichian, A.; Chayjan, R.A.; Ahmadi, E.; Zafari, D. Early Detection and Classification of Fungal Infection in Garlic (A. Sativum) Using Electronic Nose. Comput. Electron. Agric. 2022, 192, 106575. [Google Scholar] [CrossRef]
- Labanska, M.; van Amsterdam, S.; Jenkins, S.; Clarkson, J.P.; Covington, J.A. Preliminary Studies on Detection of Fusarium Basal Rot Infection in Onions and Shallots Using Electronic Nose. Sensors 2022, 22, 5453. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumari, K.; Thiruvudainambi, S.; Ebenezar, E.; Senthil, N. Efficacy of Some Fungicides and Oil Cake Extracts against Basal Rot of Onion Caused by Fusarium oxysporum. Int. J. Farm Sci. 2019, 9, 93. [Google Scholar] [CrossRef]
- Behrani, G.Q.; Syed, R.N.; Abro, M.A.; Jiskani, M.M.; Khanzada, M.A. Pathogenicity and chemical control of basal rot of onion caused by Fusarium oxysporum f. sp. cepae. Pak. J. Agric. Agric. Eng. Vet. Sci. 2015, 31, 60–70. [Google Scholar]
- Patón, L.G.; Marrero, M.D.R.; Llamas, D.P. In Vitro and Field Efficacy of Three Fungicides against Fusarium Bulb Rot of Garlic. Eur. J. Plant Pathol. 2017, 148, 321–328. [Google Scholar] [CrossRef]
- Mondani, L.; Chiusa, G.; Battilani, P. Chemical and Biological Control of Fusarium Species Involved in Garlic Dry Rot at Early Crop Stages. Eur. J. Plant Pathol. 2021, 160, 575–587. [Google Scholar] [CrossRef]
- Bunbury-Blanchette, A.L.; Walker, A.K. Trichoderma Species Show Biocontrol Potential in Dual Culture and Greenhouse Bioassays against Fusarium Basal Rot of Onion. Biol. Control. 2019, 130, 127–135. [Google Scholar] [CrossRef]
- Astiko, W.; Made Sudantha, I. The Effect of Legundi (Vitex trifolia) Biofungicide Doses Fermented with Trichoderma on Fusarium Wilt Disease in Several Shallot Varieties (Allium ascalonicum L.). Intl. J. Innov. Sci. Res. Tech. 2023, 8, 19–26. [Google Scholar]
- M Ahmed, H.A.; Soliman, Z.; Khalil, M.A.; M Fawaz, S.B. Efficacy of Biological Therapies against Onion Basal Rot Caused by Fusarium oxysporum f. sp. cepae under Field and Storage Conditions. J. Phytopathol. Pest Manag. 2021, 8, 92–105. [Google Scholar]
- Rajeswari, E.; Latha, P.; Kamalakannan, A. Eco-Friendly Management of Onion Basal Rot Disease Using Trichoderma viride and AM Fungi. J. Pharmacogn. Phytochem. 2019, 8, 892–896. [Google Scholar]
- Rajamohan, K.; Udhayakumar, R.; Sanjaygandhi, S.; Vengadesh Kumar, L.; Thamarai Selvi, M.; Sudhasha, S.; Yuvarani, R. Management of Basal Rot of Onion Caused by Fusarium oxysporum f. sp. cepae Using Bioregulators. J. Biopestic. 2019, 12, 239–247. [Google Scholar]
- Asrul, A. Potential of Local Bacillus spp. Isolates as Wilt Disease Biocontrol Agents for Fusarium oxysporum f. sp. cepae on Allium × Wakegi. Biodiversitas 2023, 24, 4989–4997. [Google Scholar] [CrossRef]
- Anton Ciptady, M.; Nisa, C. Control of Fusarium Disease in Onion with Plant Growth Promoting Rhizobacteria (PGPR) and Mycorrhizae and Its Effect on Growth and Yield. J. Wetl. Environ. Manag. 2019, 7, 18–32. [Google Scholar] [CrossRef]
- Bektas, I.; Kusek, M. Biological Control of Onion Basal Rot Disease Using Phosphate Solubilizing Rhizobacteria. Biocontrol Sci. Technol. 2021, 31, 190–205. [Google Scholar] [CrossRef]
- Kara, M.; Soylu, S.; Gümüş, Y.; Soylu, E.M.; Uysal, A.; Kurt, Ş. Determination of in Vitro Biocontrol Potentials of Antagonist Bacterial Isolates Against Onion Basal and Root Rot Disease Agent Fusarium proliferatum. Int. J. Innov. Approaches Agric. Res. 2023, 7, 487–497. [Google Scholar] [CrossRef]
- Zhou, M. Management of Fusarium Basal Rot Disease of Onion (Allium cepa L.) By Using Plant Growth Promoting Rhizobacteria in Seaweed Formulation. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 2023. [Google Scholar]
- Sintayehu, A.; Ahmed, S.; Fininsa, C.; Sakhuja, P.K. Evaluation of Green Manure Amendments for the Management of Fusarium Basal Rot (Fusarium oxysporum f. sp. cepae) on Shallot. Int. J. Agron. 2014, 2014, 150235. [Google Scholar] [CrossRef]
- Javaid, A.; Rauf, S. Management of Basal Rot Disease of Onion with Dry Leaf Biomass of Chenopodium album as Soil Amendment. Int. J. Agric. Biol. 2015, 17, 142–148. [Google Scholar]
- Deng, Y.; Ning, Y.; Yang, D.L.; Zhai, K.; Wang, G.L.; He, Z. Molecular Basis of Disease Resistance and Perspectives on Breeding Strategies for Resistance Improvement in Crops. Mol. Plant 2020, 13, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Bacher, J.W. Inheritance of Resistance to Fusarium oxysporum f. sp. cepae in Cultivated Onions; Michigan State University: East Lansing, MI, USA, 1989; ISBN 9798641845807. [Google Scholar]
- Tsutsui, K. Inheritance of Resistance to Fusarium oxysporum in Onion; University of Wisconsin: Madison, WI, USA, 1991. [Google Scholar]
- Straley, E.; Marzu, J.C.; Havey, M.J. Genetic Analyses of Resistance to Fusarium Basal Rot in Onion. Horticulturae 2021, 7, 538. [Google Scholar] [CrossRef]
- Rout, E.; Tripathy, P.; Nanda, S.; Nayak, S.; Joshi, R.K. Evaluation of Cultivated and Wild Allium Accessions for Resistance to Fusarium oxysporum f. sp. cepae. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 643–649. [Google Scholar] [CrossRef]
- Mandal, S.; Cramer, C.S. Screening of USDA Onion Germplasm for Fusarium Basal Rot Resistance. Horticulturae 2021, 7, 174. [Google Scholar] [CrossRef]
- Sharma, S.; Cramer, C.S. Selection Progress for Resistance to Fusarium Basal Rot in Short-Day Onions Using Artificial Inoculation Mature Bulb Screening. Horticulturae 2023, 9, 99. [Google Scholar] [CrossRef]
- Mahanty, B.; Mishra, R.; Joshi, R.K. A Global Study of MiRNAome Dynamics in Response to Fusarium Basal Rot Infection in Onion (Allium cepa L.). Physiol. Mol. Plant Pathol. 2023, 128, 102157. [Google Scholar] [CrossRef]
- Black, L.; Chan, E.K.; Colcol, J.F.; Jones, R.; Kramer, C.; Xiang, W. QTLs Conferring Resistance to Fusarium Basal Rot, Pink Root and Complementary Pinks in Onions. U.S. Patent 11,457,582, 4 October 2022. [Google Scholar]
- Taylor, A.; Teakle, G.R.; Walley, P.G.; Finch-Savage, W.E.; Jackson, A.C.; Jones, J.E.; Hand, P.; Thomas, B.; Havey, M.J.; Pink, D.A.C.; et al. Assembly and Characterisation of a Unique Onion Diversity Set Identifies Resistance to Fusarium Basal Rot and Improved Seedling Vigour. Theor. Appl. Genet. 2019, 132, 3245–3264. [Google Scholar] [CrossRef]
- Le, D.; Ameye, M.; Landschoot, S.; Audenaert, K.; Haesaert, G. Phenology-Regulated Defence Mechanisms as Drivers for Fusarium Basal Rot in Onion (Allium cepa). Plant Pathol. 2022, 71, 1440–1453. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Naghavi, M.R. Phyto-MiRNAs-Based Regulation of Metabolites Biosynthesis in Medicinal Plants. Gene 2019, 682, 13–24. [Google Scholar] [CrossRef]
- Khansarinejad, B.; Dashti, F.; Buratti, E.; Mirzaie-asl, A.; Zafari, D.; Romano, M. Changes in the Expression of COI1, TIR1, and ERF1 Genes and Respective MiRNAs in Fusarium Basal Rot-Stressed Onion. Gene 2024, 905, 148212. [Google Scholar] [CrossRef]
- Rout, E.; Nanda, S.; Nayak, S.; Joshi, R.K. Molecular Characterization of NBS Encoding Resistance Genes and Induction Analysis of a Putative Candidate Gene Linked to Fusarium Basal Rot Resistance in Allium sativum. Physiol. Mol. Plant Pathol. 2014, 85, 15–24. [Google Scholar] [CrossRef]
- Chand, S.K.; Nanda, S.; Mishra, R.; Joshi, R.K. Multiple Garlic (Allium sativum L.) MicroRNAs Regulate the Immunity against the Basal Rot Fungus Fusarium oxysporum f. sp. cepae. Plant Sci. 2017, 257, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Wishart, D.S.; Wani, S.H.; Hameed, M.K.; Mubin, M.; Saleem, F. Advances in Metabolomics-Driven Diagnostic Breeding and Crop Improvement. Metabolites 2022, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a Tool to Investigate Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Matsuda, S.; Tejedor, M.L.; Esaki, T.; Sakane, I.; Mizuno, H.; Tsuyama, N.; Masujima, T. Direct Metabolomics for Plant Cells by Live Single-Cell Mass Spectrometry. Nat. Protoc. 2015, 10, 1445–1456. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Hirata, S.; Sawada, Y.; Hirai, M.Y.; Sato, S.; Hirakawa, H.; Mine, Y.; Tanaka, K.; Shigyo, M. Widely Targeted Metabolome and Transcriptome Landscapes of Allium Fistulosum–A. cepa Chromosome Addition Lines Revealed a Flavonoid Hot Spot on Chromosome 5A. Sci. Rep. 2019, 9, 3541. [Google Scholar] [CrossRef]
- Karban, R.; Agrawal, A.A.; Mangel, M. The Benefits of Induced Defenses against Herbivores. Ecology 1997, 78, 1351–1355. [Google Scholar] [CrossRef]
- Simms, E.L. Costs of Plant Resistance to Herbivory. In Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics; University of Chicago Press: Chicago, IL, USA, 1992; Volume 3, pp. 392–425. [Google Scholar]
- Khan, T.; Abbasi, B.H.; Zeb, A.; Ali, G.S. Carbohydrate-Induced Biomass Accumulation and Elicitation of Secondary Metabolites in Callus Cultures of Fagonia indica. Ind. Crops Prod. 2018, 126, 168–176. [Google Scholar] [CrossRef]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical Evaluation, Antimicrobial Activity, and Determination of Bioactive Components from Leaves of Aegle marmelos. Biomed. Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- VanEtten, H.D.; Mansfield, J.W.; Bailey, J.A.; Farmer, E.E. Two Classes of Plant Antibiotics: Phytoalexins versus Phytoanticipins. Plant Cell 1994, 6, 1191. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.P.; Osbourn, A.E. Fungal Resistance to Plant Antibiotics as a Mechanism of Pathogenesis. Microbiol. Mol. Biol. Rev. 1999, 63, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Sreij, R.; Dargel, C.; Hannappel, Y.; Jestin, J.; Prévost, S.; Dattani, R.; Wrede, O.; Hellweg, T. Temperature Dependent Self-Organization of DMPC Membranes Promoted by Intermediate Amounts of the Saponin Aescin. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.-i.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaal, F.F.; El-Sayed, M.A. α-Tomatine, the Major Saponin in Tomato, Induces Programmed Cell Death Mediated by Reactive Oxygen Species in the Fungal Pathogen Fusarium oxysporum. FEBS Lett. 2007, 581, 3217–3222. [Google Scholar] [CrossRef]
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic Cyclization of Squalene and Oxidosqualene to Sterols and Triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Lanzotti, V.; Romano, A.; Lanzuise, S.; Bonanomi, G.; Scala, F. Antifungal Saponins from Bulbs of White Onion, Allium cepa L. Phytochemistry 2012, 74, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; El-Sayed, M.; Sato, S.; Hirakawa, H.; Ito, S.-i.; Tanaka, K.; Mine, Y.; Sugiyama, N.; Suzuki, M.; Yamauchi, N.; et al. RNA-Sequencing-Based Transcriptome and Biochemical Analyses of Steroidal Saponin Pathway in a Complete Set of Allium fistulosum—A. cepa Monosomic Addition Lines. PLoS ONE 2017, 12, e0181784. [Google Scholar] [CrossRef]
- Lanzotti, V. Bioactive Saponins from Allium and Aster Plants. Phytochem. Rev. 2005, 4, 95–110. [Google Scholar] [CrossRef]
- Lanzotti, V.; Bonanomi, G.; Scala, F. What Makes Allium Species Effective against Pathogenic Microbes? Phytochem. Rev. 2013, 12, 751–772. [Google Scholar] [CrossRef]
- Adão, C.R.; Da Silva, B.P.; Parente, J.P. A New Steroidal Saponin from Allium ampeloprasum var. porrum with Antiinflammatory and Gastroprotective Effects. Phytochem. Lett. 2011, 4, 306–310. [Google Scholar] [CrossRef]
- Barile, E.; Bonanomi, G.; Antignani, V.; Zolfaghari, B.; Sajjadi, S.E.; Scala, F.; Lanzotti, V. Saponins from Allium minutiflorum with Antifungal Activity. Phytochemistry 2007, 68, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Zolfaghari, B.; Senatore, M.; Lanzotti, V. Spirostane, Furostane and Cholestane Saponins from Persian Leek with Antifungal Activity. Food Chem. 2013, 141, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal Saponins from the Genus Allium. Phytochem. Rev. 2016, 15, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Hirata, S.; Ito, S.I.; Yamauchi, N.; Shigyo, M. Compartmentation and Localization of Bioactive Metabolites in Different Organs of Allium roylei. Biosci. Biotechnol. Biochem. 2014, 78, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Teshima, Y.; Ikeda, T.; Imada, K.; Sasaki, K.; El-Sayed, M.A.; Shigyo, M.; Tanaka, S.; Ito, S.I. Identification and Biological Activity of Antifungal Saponins from Shallot (Allium cepa L. Aggregatum Group). J. Agric. Food Chem. 2013, 61, 7440–7445. [Google Scholar] [CrossRef]
- Mostafa, A.; Sudisha, J.; El-Sayed, M.; Ito, S.I.; Ikeda, T.; Yamauchi, N.; Shigyo, M. Aginoside Saponin, a Potent Antifungal Compound, and Secondary Metabolite Analyses from Allium nigrum L. Phytochem. Lett. 2013, 6, 274–280. [Google Scholar] [CrossRef]
- Ariyanti, N.A.; Hoa, V.Q.; Khrustaleva, L.I.; Hirata, S.; Abdelrahman, M.; Ito, S.I.; Yamauchi, N.; Shigyo, M. Production and Characterization of Alien Chromosome Addition Lines in Allium fistulosum Carrying Extra Chromosomes of Allium roylei Using Molecular and Cytogenetic Analyses. Euphytica 2015, 206, 343–355. [Google Scholar] [CrossRef]
- Vu, H.Q.; El-Sayed, M.A.; Ito, S.I.; Yamauchi, N.; Shigyo, M. Discovery of a New Source of Resistance to Fusarium oxysporum, Cause of Fusarium Wilt in Allium fistulosum, Located on Chromosome 2 of Allium cepa Aggregatum Group. Genome 2012, 55, 797–807. [Google Scholar] [CrossRef]
| Biological Control Agents Alone or in Combinations | References |
|---|---|
| Trichoderma hamatum, Trichoderma atroviride, Trichoderma gamsii, and Trichoderma harzianum | [65] |
| Trichoderma harzianum + fermented Legundi leaf (Vitex trifolia) | [66] |
| Saccharomyces cerevisiae and Candida tropicalis | [67] |
| Trichoderma viride + arbuscular mycorrhizal fungi (AMF) | [68] |
| Trichoderma viride + Pseudomonas fluorescens + Glomus mosseae | [69] |
| Several rhizospheric bacterial isolates of Bacillus species | [70] |
| Local plant growth-promoting rhizobacteria (PGPR) + mycorrhizae, and phosphate-solubilizing bacteria (PSB) | [71,72] |
| Bacillus cereus, Enterobacter xiangfangensis, Bacillus thuringiensis, Alcaligenes faecalis, Pseudomonas putida, and Citrobacter freundii | [73] |
| Pseudomonas protegens, Bacillus subtilis, and Enterobacter cloacae | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Mandal, S.; Cramer, C.S. Recent Advances in Understanding and Controlling Fusarium Diseases of Alliums. Horticulturae 2024, 10, 527. https://doi.org/10.3390/horticulturae10050527
Sharma S, Mandal S, Cramer CS. Recent Advances in Understanding and Controlling Fusarium Diseases of Alliums. Horticulturae. 2024; 10(5):527. https://doi.org/10.3390/horticulturae10050527
Chicago/Turabian StyleSharma, Suman, Subhankar Mandal, and Christopher S. Cramer. 2024. "Recent Advances in Understanding and Controlling Fusarium Diseases of Alliums" Horticulturae 10, no. 5: 527. https://doi.org/10.3390/horticulturae10050527
APA StyleSharma, S., Mandal, S., & Cramer, C. S. (2024). Recent Advances in Understanding and Controlling Fusarium Diseases of Alliums. Horticulturae, 10(5), 527. https://doi.org/10.3390/horticulturae10050527






