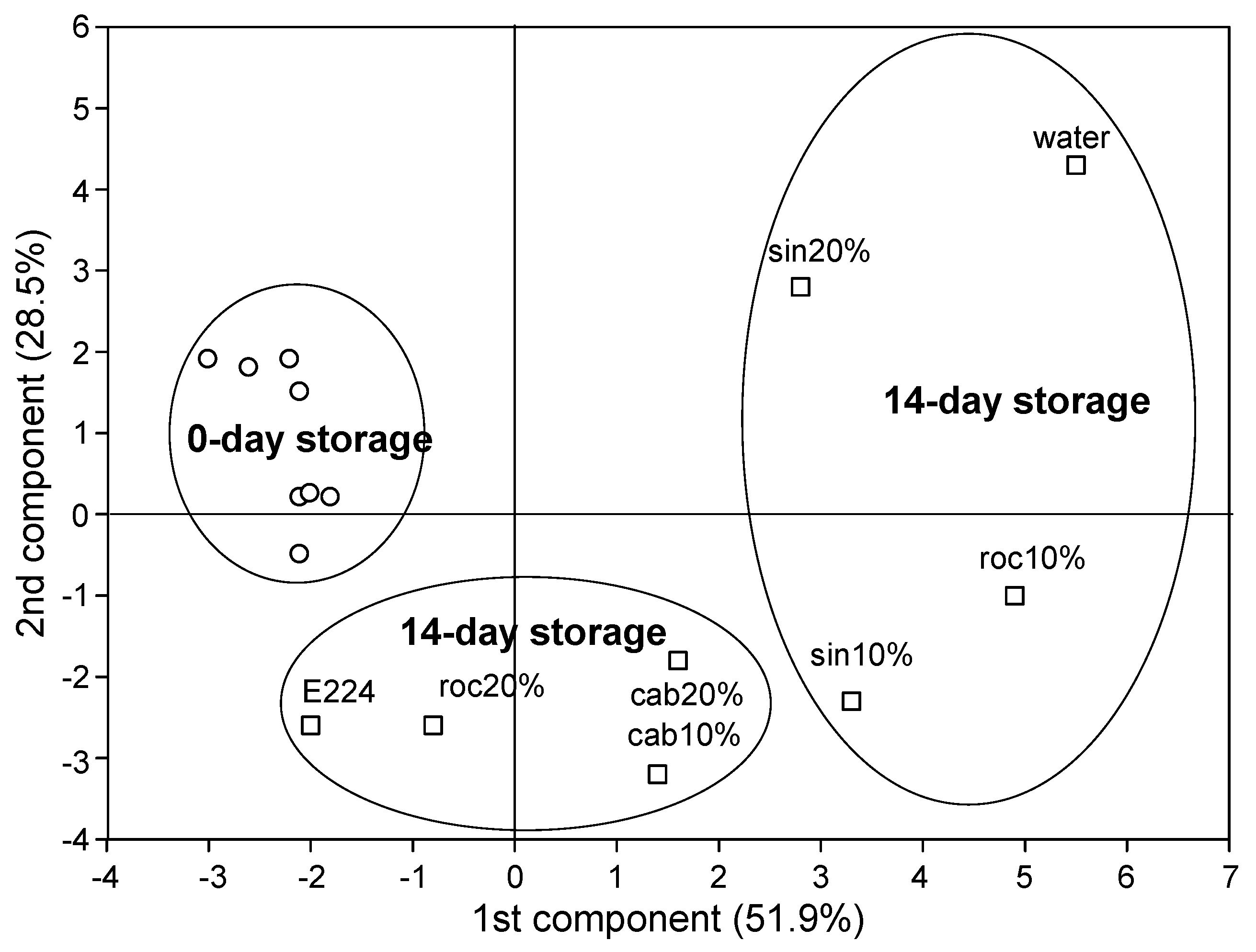Abstract
Enzymatic browning, occurring on the cut surfaces of many popular fresh-cut fruit and vegetables due to wounding and the activity of endogenous polyphenyloxidase enzymes, is considered as the main reason for their rejection by consumers. In this study, water extracts were obtained from seeds of cabbage, sinapis, and wild rocket at 10 and 20% w/w seed:water ratios (SWE) and analyzed for total phenolic compounds (TPC) and antioxidant capacity (AC). The extract was then applied on cut surfaces of mid rib segments of lettuce leaves for 1 or 3 min. The segments were stored at 7 °C for 14 days. The SWE’s inhibitory capacity on enzymatic browning were measured by CIELAB color coordinates L* a* and b* and expressed as second derivatives, their % inhibition and different indices. An additional visual acceptance measurement and calculation of shelf life was also performed. The seed extracts of cabbage at 10–20% and wild rocket at 20% showed the highest anti-browning efficacy (comparable to 25 mM potassium metabisulfite control) along with TPC and AC. A high % of seed:water extract and increased exposure time led to a considerable increase in shelf life, visual score, % inhibition of browning or whitening index of the extracts of all seed sources. Chromatometric outcome data clearly showed that the visual data were more accurate than the chromatometric procedure (L*, a*, b* values, their derives ΔE, h°, C, Δh° and ΔC or calculated indices), although the latter could detect the differing degrees of browning development or its inhibition in treated and control segments during storage.
1. Introduction
Fresh-cut fruit and vegetables suffer from tissue browning as a result of processing (cutting or other mechanical treatment involving breaking of cells) promoted physiological deterioration, biochemical changes and microbial degradation. It is well known that browning and its intensity in the fruit and vegetable tissues is predominately influenced by polyphenoloxidase (PPO) and secondarily by peroxidase (POD) which are wound-response enzymes that oxidize phenolic substrates into brown substances after tissue is damaged [1,2,3], in addition to phenylalanine ammonia lyase (PAL) which catalyzes phenylalanine into cinnamic acid, generating phenolic substrates [3,4].
The control of browning is one of the most important issues in the fresh-cut fruit and vegetable industry, since color significantly influences consumer decisions and browned foods are perceived as spoiled [5]. Anti-browning agents like sodium bisulfite, L-cysteine, 4-hexylresorcinol, glutathione and ascorbic acid have been extensively studied for the prevention of enzymatic browning [6,7,8]. Narváez-Cuenca [9] reported that sodium hydrogen sulfite reacts with o-quinones generated by PPO, and the sulfo-adducts formed terminate the browning reactions. Sulfites, used as food additives, are industrially employed to inhibit PPO-induced browning reactions for a wide range of products [10]. However, the use of sulfites is often discouraged due to the associated side effects or toxicity [11].
The dramatic growth of the fresh-cut produce market is a result of consumer demand for fresh, healthy, convenient, and additive-free foods that are safe and nutritious [12]. Currently, other than chemical approaches to prevent the browning of fresh-cut fruit and vegetable tissues, other methods are being implemented to inhibit the activity of PPO and preserve the intrinsic quality of produce, such as physical treatment [12], mild heat treatment [13,14] and different storage temperatures or atmosphere composition [15]. The use of high CO2 modified atmosphere storage (80% N2, 20% CO2) combined with appropriate wrapping films for retail packages has been successfully implemented to fresh-cut lettuce salads [16]; however, recently, a new disorder indicating tissue collapse has been reported in certain lettuce cultivars [17].
Lately, natural anti-browning preserving agents are favored by consumers since they are non-toxic and have no known adverse side effects even though some of these methods are generally constrained by their single-action or organoleptic-impairing properties. All natural anti-browning preserving agents are characterized by increased phenolic compounds content and/or antioxidant activity. Different sources of natural anti-browning agents include pineapple juice [18], green tea extract [19], whey protein concentrate [20], phytoncide essential oil derived from pine leaves [1], onion extract [21], rice bran extracts [22], allium plant [22,23], purslane extract [24], coconut [25], hawthorn leaf extract [26], clove essential oil and eugenol [27] or basil leaves and wheat bran water extracts [28], among others. Lately, diacetyl treatment repressed the browning of fresh-cut stem lettuce by regulating the phenylpropanoid metabolism pathway and antioxidant ability [29]. Decreased PPO activity and therefore extension of the shelf life of fresh-cut produce could be achieved by the integration of mild technologies with synergistic effects [30,31].
Cruciferous vegetable by-products have also been the focus of some research that investigated their potential as natural anti-browning agents in different matrices. Landi [32] reported that the PPO activity of cut rocket was much lower than that in lettuce and that cutting induced an increase in PPO activity only in lettuce. Zocca [33], using extract prepared by cooking young cruciferous leaves with water, found that it has the capacity to inhibit both commercial and grape polyphenol oxidases when applied with ascorbic acid. Broccoli seed supercritical CO2 extract, when tested with the passive staining of apples, had a better ΔE value compared to that of sodium bisulfite reference solution [34]. Further, cruciferous and allium extracts exerted anti-browning, anti-radical and reducing capacity [35,36], which has been utilized to stabilize refrigerated avocado pulp [37].
Due to PPO-induced browning’s impact on the food industry, research into potential inhibitory compounds continues. In this respect, the goal of this study was to investigate the potential of the inhibition of the enzymic browning of wild rocket, cabbage and sinapis seed extracts at two seed:water ratios using a lettuce mid rib segment as model. This approach was expected to increase our understanding on the potential anti-browning properties of the water extracts of these cruciferous seeds, since to the best of our knowledge at this time, no relevant data were reported.
2. Materials and Methods
2.1. Chemicals and Reagents
Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid), DPPH (2,2-diphenyl-picrylhydrazyl) and gallic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). ABTS (2,2′-azino-bis(3-ethylben-zothiazoline-6-sulfonic acid)) and TPTZ (2,4,6-tripyridyl-s-triazine) were purchased from TCI (Chuo-ku, Tokyo, Japan). Folin–Ciocalteu’s phenol reagent, sodium carbonate, sodium acetate, potassium persulfate, ferric chloride hexahydrate, and glacial acetic acid, as well as HPLC grade methanol, were purchased from Chem-Lab (Zedelgen, Belgium).
2.2. Plant Material Handling and Storage
The romaine-type lettuce (Lactuca sativa L., var. longifolia Lam.) used in this study was purchased from the local market. The lettuce head outer leaves were removed and discarded, and the remaining leaves were washed with tap water and cut with a sharp stainless-steel knife to obtain the mid rib. Only the white part of the cut ribs was used, and the ribs were further cut into 1 cm segments before being subjected to treatments. To create units for treatments, 12–16 segments were placed in a Petri dish. Four Petri dish replications per each treatment were created. All dishes were stored at 7 °C for 14 days in the dark.
2.3. Seed Water Extracts
Wild sinapis, cabbage and wild rocket seeds were selected to produce water extracts as anti-browning agents of lettuce mid rib segments based on previous pre-experimentation screening. Sinapis arvensis seeds were collected from the Aristotle University farm; the local variety of cabbage (Brassica oleracea, cv. Kilkis) and wild rocket (Diplotaxis tenuifolia (L.) DC. cv. Celebries) were obtained from the local market.
Pre-experimentation also indicated that the maximum and intermediate level of seed:water ratio (w/v) were 20 and 10%, respectively. To obtain water extracts, seeds were weighed and washed in tap water; preparations of 5 or 10 g of seeds were combined with 50 mL deionized water, resulting in proportions of 10% and 20% (w/w), respectively. These mixtures were then homogenized using a T18 Digital Ultra TURRAX (IKA, Staufen, Germany) homogenizer at 7000 rpm for 1.5 min in a water-ice bath (5 °C). The homogenate was then filtered through a 0.5 mm plastic sieve and centrifuged at 6000× g for 20 min (at 25 °C) using a Sigma 3–15 (Sigma Laborzentrifugen GmbH, Osterode, Germany) centrifuge (to result in seed water extracts—SWE) used thereafter. Besides SWEs, two controls were also used; a distilled water control and 25 mΜ potassium metabisulfite (Ε224), resulting in 8 final treatment solutions.
2.4. Exposure Time
Lettuce mid rib segments, prepared as previously described, were grouped in random in 15–20 pieces and were submerged in SWE (four pooled batches) or control solutions for 1 or 3 min then rinsed with 100 mL of deionized water, before being placed in Petri dishes. A drop of water was added to each Petri dish to establish a uniform relative humidity environment. The Petri dishes were placed in a refrigerator at 7 °C for 14 days. The chromatometric color as well as the visual estimation of enzymatic browning were recorded at the 0, 3, 7, 10 and 14th day of storage.
2.5. Determination of SWE Phenolics and Antioxidant Capacity
The SWE, comprising four batch-replications per treatment, were produced and utilized as anti-browning treatments on lettuce mid rib segments, and were analyzed for their phenolic compound content as well as antioxidant capacity.
The phenolic compounds (PC) of SWE were determined using Folin–Ciocalteu (FC) reagent according to AOCS [38] and Scalbert [39], with some modifications. A total of 0.6 mL of SWE, or water used as a control, was mixed with Folin–Ciocalteu reagent after 3 min with 1.5 mL of Na2CO3 (20% w/v) and kept in a dark environment at room temperature (at 20 °C) for 60 min. The absorbance of the solution was monitored at 760 nm using a Genesys 80 UV-VIS (Thermo Fisher Scientific, Waltham, MA, USA) spectrophotometer. To the control, a 0.6 mL of distilled water was added. The phenolic compounds (PC) of SWE were determined from the linear regression equation of a standard curve (y = 0.025x − 0.0707, R2 = 0.995), and the results were expressed as mg of gallic acid equivalent (GAE) per L of SWE.
The non-enzymatic antioxidant activity of SWE was evaluated using ferric-reducing antioxidant power (FRAP) assay by Benzie and Strain [40] with some modifications. SWE and samples, of 120 μL, were mixed with 3 mL of freshly prepared FRAP working solution; this comprised 0.3 M acetate buffer (pH 3.6) containing 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) and 40 mM FeCl3 6H2O. The mixture was incubated at 37 °C for 4 min and the absorbance was measured at 593 nm using a Genesys 80 UV-VIS (Thermo Fisher Scientific, MA, USA) spectrophotometer. Trolox was used as a standard and results were expressed in mmol L−1.
The antioxidant activity of the SWE samples was also determined using DPPH as a free radical according to Brand-Williams [41] and Nenadis [42]. The 0.6 mL of SWE or methanol used as control were added to 2960 μL of DPPH methanolic solution (100 μΜ) in a test tube. The tubes were then vortexed and kept in a dark environment at room temperature (at 20 °C) for 30 min. The absorbance of the solution was monitored at 517 nm using a Genesys 80 UV-VIS (Thermo Fisher Scientific, MA, USA) spectrophotometer. The antioxidant activity of SWE (%) values (%RSA) were determined by using the formula %RSA = [Abs515(t = 0) − Abs515(t)] × 100/Abs515(t = 0) after correction with appropriate blanks. Trolox equivalents were obtained using the linear regression equation of a calibration curve (y = 82,239x − 0.3069, R2 = 0.995) and results were expressed in mmol L−1.
ABTS•+ scavenging activity (ABTS) was evaluated according to Re [43] using 10 or 20 μL of diluted SWE (1:10, v/v, with distilled water). The degree of quenching of the ABTS•+ radical, measured as inhibition in percent (% Inh), was calculated using the formula % Inh = [Abs734(t = 0) − Abs734(t)] × 100/Abs734(t = 0) after correction with an appropriate blank [44]. Trolox equivalents were obtained using the linear regression equation of the calibration curve (y = 2.8165x − 0.1907, R2 = 0.999) and results were expressed in mmol L−1.
2.6. Chromatometric Color Measurement
The surface color of cut mid rib tissue was measured using a chromameter (Minolta CR-400, Minolta, Osaka, Japan), equipped with an 8 mm measuring head and a D65 illuminant. The instrument was calibrated with a white reference tile (L* = 97.52, a* = −5.06, b* = 3.57) prior to measurements.
Four color measurements were taken at four locations of each Petri dish; four Petri dish-replications were employed for each treatment, amounting to 16 readings per treatment at every sampling time.
The L* (0 = black, 100 = white), a* (+red, −green) and b* (+yellow, −blue) color coordinates were determined according to the CIELAB coordinate color space system [45]. For color change during storage, ΔE (total change in color) and the % inhibition of ΔL (percentage change in brightness), Δa (percentage change in green color to red), and Δb (percentage change in blue color to yellow), were calculated to provide normalized indicators for eliminating the heterogeneity among the samples [46]. The method of calculation is shown below:
where tristimulus color values at day 0 were L*start, a*start and b*start and the color values at sampling day were L*end, a*end, b*end.
Positive values of the percentage change (0 and 100) would indicate treatment effectiveness in browning inhibition or even bleaching for values greater than 100%. Negative values would indicate ineffectiveness and even the promotion of browning.
Second derivative parameters of the L*, a*, b* model, hue angle (h°) and color saturation (C) as well as their % inhibition, Δh° (percentage change in hue angle) and ΔC (percentage change in color saturation C) were also calculated, as follows:
When a* < 0 and b* ≥ 0: 90° < Hue < 180°),
When a* > 0 and b* ≥ 0: 0° < Hue < 90°)
where h° and C color values at day 0 were h°start and Cstart and the color values at sampling day were h°end and Cend.
Further, were calculated the browning index (BI), whiteness index (WI) saturation index (SI) and color index (CI), as follows [47]:
where [48]
2.7. Visual Evaluation of Browning and Shelf-Life
The degree of browning of the mid rib leaf cut surface was evaluated by three referees using a scale of 1 to 5, where 1 = none, 3 = moderate-limit of marketability, 5 = severe browning. The grading index is shown in Table 1 [17]. A visual score index (VSI) was obtained for each treatment through averaging the three scores (obtained by the three referees) per replication (four replications per treatment). VSI was employed as a reference for the development of browning during storage and was compared to chromatometric raw or processed data.

Table 1.
Visual color evaluation scores of browning and acceptability of cut mid rib lettuce leaves.
The shelf-life was defined as the total time it took a unit of segments to reach grade 3 for which browning has developed to an unacceptable level. Shelf-life was obtained from four Petri dish/replications, producing the average shelf life for each treatment based on VSI.
2.8. Statistical Analysis
The statistical analysis followed a completely randomized design. An ANOVA with four replications per treatment with 3 independent variables (water seed extract, exposure time and storage time) and 15 dependent variables (L*, a*, b*, ΔL, Δa, Δb, ΔE, h° Δh°, C, ΔC, BI, WI, SI and VSI) was employed. The effect of each factor was evaluated using partial eta squared (η2) calculated as follows: partial eta2 = SSeffect/(SSeffect + SSerror), where SS = sum of squares. All dependent variables were tested for normality using the Anderson–Darling test; Pearson’s correlation coefficient and principal component analysis (PCA) analyses was additionally performed to indicate variable interrelations and the effective expression of browning during storage. The mean separation of data was based on Duncan’s multiple range test or Least Significant Difference (LSD) at p < 0.05. All analyses were performed using the SPSS statistic software for Windows (version 29).
3. Results and Discussion
3.1. Phenolics and Antioxidant Capacity of SWE
Total phenolic compounds (TPC) of SWE were different in all samples. SWE obtained from 20% seed to water (w/w) level were higher in TPC than at the 10% level; highest TPC in all SWE were observed in cabbage (1748 mg GA L−1), followed by wild rocket and sinapis (Table 2). Similar was the pattern in antioxidant capacity evaluation; cab20% showed maximum FRAP antioxidant capacity, followed by cab10% and roc20%, while in DPPH or ABTS, maximum capacity was followed by roc20% and cab10% (Table 2).

Table 2.
Total phenolics and antioxidant capacity of seed:water (w/w) extracts (SWE%) of sinapis (sin10%, sin20%), wild rocket (roc10%, roc20%) or cabbage (cab10%, cab20%).
Broccoli seed supercritical CO2 extract solutions (1%) have been reported to contain 13.25 mg/100 mL TPC (as caffeic acid equivalents) and 15.34 mM Trolox equivalents antioxidant capacity [34]. These values are close to the ones obtained for cabbage or wild rocket seed water extracts. Further, extracts of cruciferous as well as allium vegetables exerted anti-radical and reducing capacity [35,36] and concluded that all natural anti-browning preserving agents are characterized by an increase in phenolic compounds content and antioxidant activity.
3.2. Analysis of Variance
The analysis of variance was performed, using the seed water extract (SWE) including water and sodium metabisulfite (E224) controls, the exposure time of the model plant material (lettuce leaf mid rib segments) to SWE, as well as storage time as the main factors. These main factors are known to affect browning and shelf-life of cut produce, since the development of brown color on cut surfaces during the storage of cut mid rib lettuce segments is mainly limiting self-life [17].
Variables related to color attributes (L*, a*, b*) and their % inhibition, (ΔL, Δa, Δb) or second derivatives (h°, Δh°, C, ΔC) as well as calculated indexes (BI, WI, SI, CI) including visual score index (VSI) were predominantly influenced by the SWE or the storage time factor (Table 3); this was indicated by an increased η2 accounted for these variables. Color variables L* and a*, ΔE, ΔL, Δa and WI were highly influenced (η2 = 0.44–0.62) by both SWE and storage time factors, while CI was mainly influenced only by SWE and DE, DC, BI and VSI only by storage time.

Table 3.
Analysis of variance for the variables L*, a*, b* hue angle (h°), saturation (C), total change in color (ΔE), % inhibition (ΔL, Δb, Δa, Δh°, ΔC) as well as indices for browning (BI), whitening (WI), saturation (SI), color (CI) and visual score (VSI) obtained during 14 day/7 °C storage of cut mid rib segments of leaf lettuce previously exposed to seed:water (w/w) extracts (SWE%) of sinapis (sin10%, sin20%), wild rocket (roc10%, roc20%) and cabbage (cab10%, cab20%), in addition to including water or bisulfite controls for 1 or 3 min.
SWE exerted a moderate influence (η2 = 0.19–0.39) on C, Db, Δh°, ΔC and SI and storage time on CI (Table 3). The factor of exposure time influenced most variables very poorly (very small, though statistically significant, η2 values were accounted for them). A significant interaction of exposure time and storage time was observed; all variables except for DE, h° and VSI were moderately influenced by this interaction (η2 = 0.32−0.22).
The main effects of SWE treatment including water and E224 controls showed the greatest overall average differences among them in most color variables except for b*. Roc10% and sin20% had increased browning and variable values that were similar or close to water-control while roc20% and cab10% exhibited little browning and variable values that were similar or close to the E224-control (Table 3). Bustos [37] reported that crucifer vegetables, such as cauliflower and Brussels sprout extracts were highly effective as PPO inhibitors, extending the shelf life of refrigerated avocado pulp by up to two weeks. Further, Wessels [34] reported the partial inhibition of the browning of treated fresh-cut apples using 1% broccoli seed extract among many different plant sources. It is well known that cruciferous vegetables contain organosulfur glucosinolates [49,50], to which is attributed the effective inhibition of PPO. However, the precise mechanisms for the enzymatic anti-browning effects have not been yet fully elucidated.
Exposure for 3 min was more effective than 1 min for all treatments as shown by increased L*, a*, Δa, ΔE, ΔL and WI and lower C, BI and VSI. The exposure time of cut lettuce mid ribs with both the extracts and control solutions had a positive effect on browning inhibition due to the increased probability of the interaction of molecules remaining in physical motion in a common space. It thus appears that 1 min was not sufficient time for successful inhibition of browning. Therefore, the longer exposure (of 3 min) of cut segments to SWE resulted in significant inhibition as expressed by most variables (Table 4).

Table 4.
Pearson linear correlation coefficients of the variables L*, a*, b* hue angle (h°), saturation (C), total change in color (ΔE), % inhibition (ΔL, Δb, Δa, Δh°, ΔC) as well as indices for browning (BI), whitening (WI), saturation (SI), color (CI) and visual score (VSI).
Storage time factor decreased variables L*, h°, ΔE, Δa, or WI and increased a*, C, ΔL, Δa, Δh°, ΔC, BI, SI, CI and VSI. Enzymatic browning is a time-evolving catalytic reaction and studies focusing on its kinetic characteristics are often conducted, e.g., in evaluating eggplant polyphenyloxidase enzymatic activity [51]. The time dependence of the effect is justified and explains why the duration of exposure accumulated the largest percentage of variability in the analysis of variance of the overall experiment (Table 3).
3.3. Pearson Linear Correlation and Principal Component Analysis
The processing of collected data was given the possibility to identify and focus on the chromatometric variable(s) which were meaningful while ignoring others that do not render the enzymic browning as consumers perceive it. For this reason, Pearson’s linear correlation coefficient was used; of particular interest was the correlation of the chromatometric variables obtained objectively with the organoleptic variable VSI, which results from a subjective visual evaluation. Table 4 shows the analysis of Pearson coefficients; significant strong to weak correlations have been shown among color variables (L*, a*, b*), their % inhibition (ΔLab) or second derivatives and their % inhibition (h°, Δh°, C, ΔC), as well as calculated indexes (BI) and VSI. VSI, a variable based on visual scoring, strongly correlated with a*, ΔL, Δa, Δh° and BI or a medium with L*. Both correlations were statistically significant (p < 0.05).
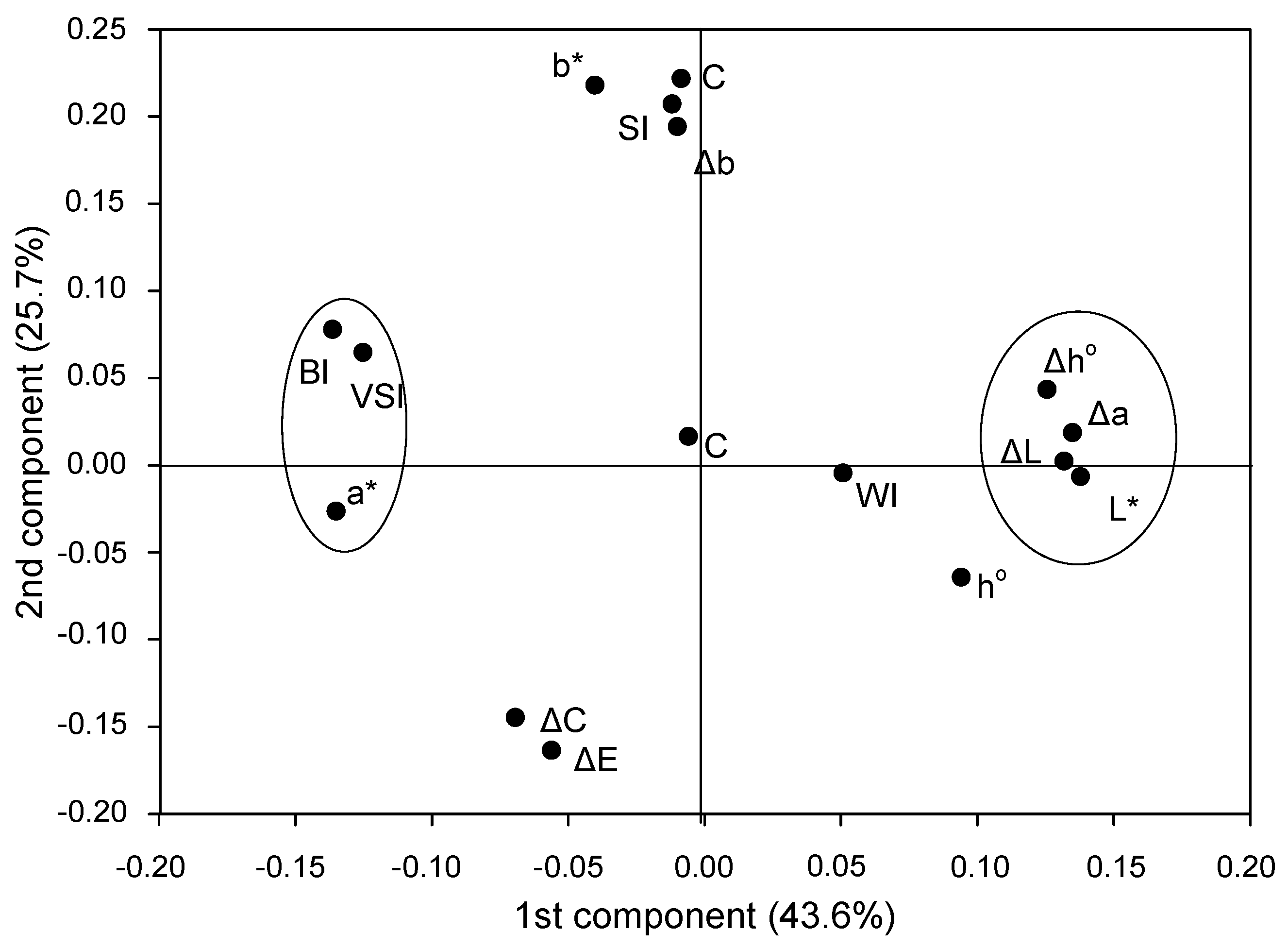
Variables with high eta2 were discriminated by principal component analysis (PCA) analysis into two separate groups (Figure 1); group one with VSI, BI and color attribute a* and group two with L*, ΔL and Δa values including a variable Δh° with medium eta2 values for factors treatment (SWE) and storage time (Table 3). The remaining variables were either grouped differently or remained scattered (Figure 1).
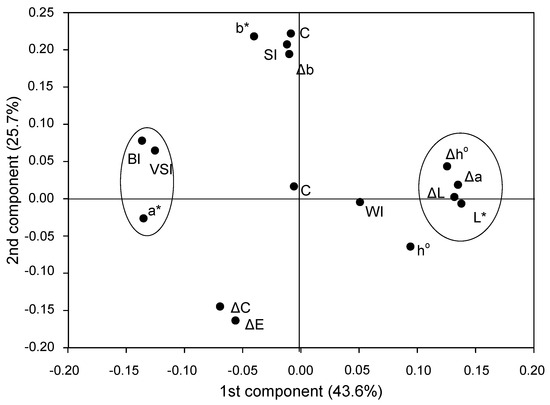
Figure 1.
PCA analysis of the variables L*, a*, b* hue angle (h°), total change in color (ΔE), saturation (C), % inhibition (ΔL, Δb, Δa, Δh°, ΔC) as well as indices for browning (BI), whitening (WI), saturation (SI), color (CI) and visual score (VSI).
Based on the eta2 values (Table 3), the Pearson coefficient (Table 4) and PCA (Figure 1), two groups of variables were selected to express browning as an interaction of SWE treatments with storage time: VSI, BI and ΔL, Δa and Δh°.
Further, the PCA of SWEs in day 0 and day 14 of storage revealed two distinct groups within day 14 (Figure 2): the E224-control group which included treatments cab10%, cab20%, roc20% and E224-control, and the water-control group which included sin10%, sin20%, roc10% and water-control). Sin10% was somehow in the border of the two groups, and depending on the variable examined, it occasionally might participate in either the E224-control or the water-control group.
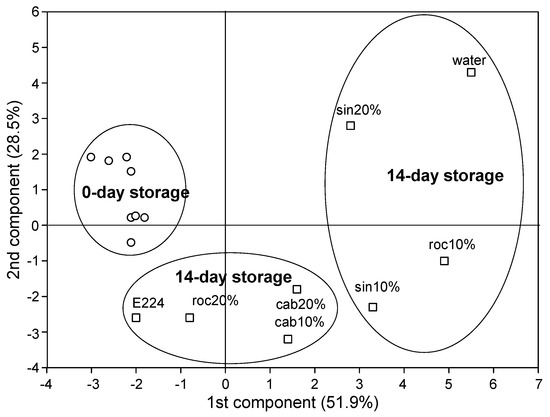
Figure 2.
PCA analysis of extracts (SWE) of sinapis (sin10%, sin20%), rocket (roc10%, roc20%) and cabbage (cab10%, cab20%), water or E224 at day 0 and day 14 of storage at 7 °C of cut mid rib segments of leaf lettuce.
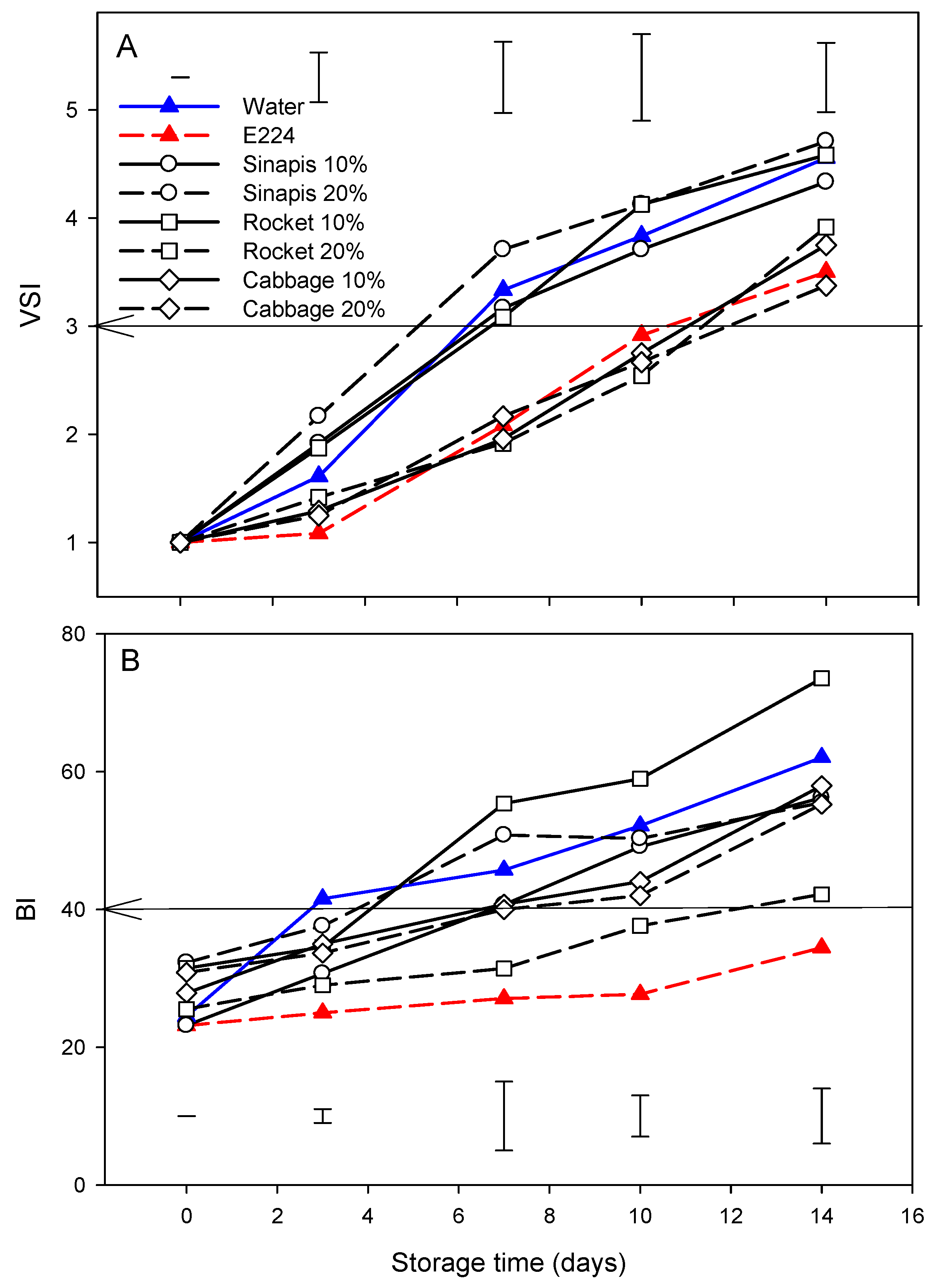
3.4. VSI and Browning Index
Figure 3A shows the development of browning, expressed by the VSI, in cut mid rib segments of leaf lettuce for the controls and each SWE during storage for 14 days at 7 °C. The browning of E224 preservative solution-control remained stable until the third day, whereas it increased in water-control or other SWE treatments. This increase continued until the end of the period of storage, but this was lower in E224 compared to the water-control. The differences between E224 and water-controls were found to be statistically significant (p < 0.05). The cab10%, cab20% and roc20% group showed a similar pattern of development during storage to that of the E224-control, while the sin10%, sin20% and roc10% group showed a similar pattern to that of the water-control; the VSI increased above the 3 points of the acceptable sales limit between days 10–12 and 4–6 of storage for the two groups, respectively. The demand for fresh-cut lettuce, among other vegetables, reflects the consumer preferences towards appearance and freshness at the time of purchase as among the main quality criteria [52]. However, the browning of cut edges of romaine lettuce leaves is considered as the most important disorder that limits its shelf life [53]. In this study, the treatment group of cab10%, cab20% and roc20% on fresh-cut lettuce retained the quality for the first 10 days of storage compared to the E224-control.
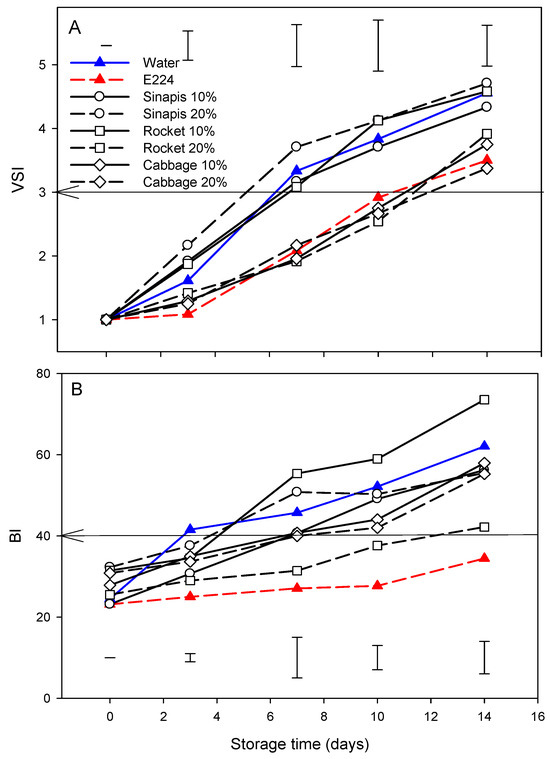
Figure 3.
Development of browning based on visual score-VSI (A) and browning index-BI (B) of cut mid rib segments of leaf lettuce during storage for 14 days at 7 °C, previously exposed to seed:water (w/w) extracts (SWE) of sinapis (sin10%, sin20%), wild rocket (roc10%, roc20%) and cabbage (cab10%, cab20%), water or bisulfite for 3 min. Vertical bars in each figure represent Least Significant Difference method (LSD) at each sampling date and confidence level of 0.05. Arrows indicate borderline of acceptable/unacceptable color.
A similar pattern of browning development in cut mid rib segments of leaf lettuce for SWE during storage for 14 days at 7 °C was also shown for variables BI (Figure 3B). The correlation of VSI with BI (R2 = 0.833, p < 0.05) indicated that the acceptable sales limit (VSI score point 3) corresponded to a value of 40. The BI was more sensitive than VSI; for BI (Figure 3B), the SWE treatments that formed a group with the water-control, roc10% and sin 20% increased above the acceptable sales limit as early as day 3, sin10%, cab10% and cab20% at day 14, and roc20% as late as day 14, while the E224 preservative solution-control did not reach a critical level even by day 14th. E224 (sodium hydrogen sulfite) is considered as an anti-browning effective agent since it reacts with o-quinones yielding non-browning sulfo-adducts [54]. Although sulfites have been used in the food industry to inhibit PPO-induced browning of cut produce for a wide range of products [10], its use is associated with side effects or toxicity [11].
The decreased BI value of lettuce cut segments was concomitant with the increments of Δa and ΔL values during storage. BI has been used to describe the browning development of fresh-cut lotus roots treated with ultrasound and/or cysteine following storage at 4 °C for 12 days [55] or fresh-cut carrots with carvacrol-loaded chitosan nanoparticles [56] and cut green beans [57].
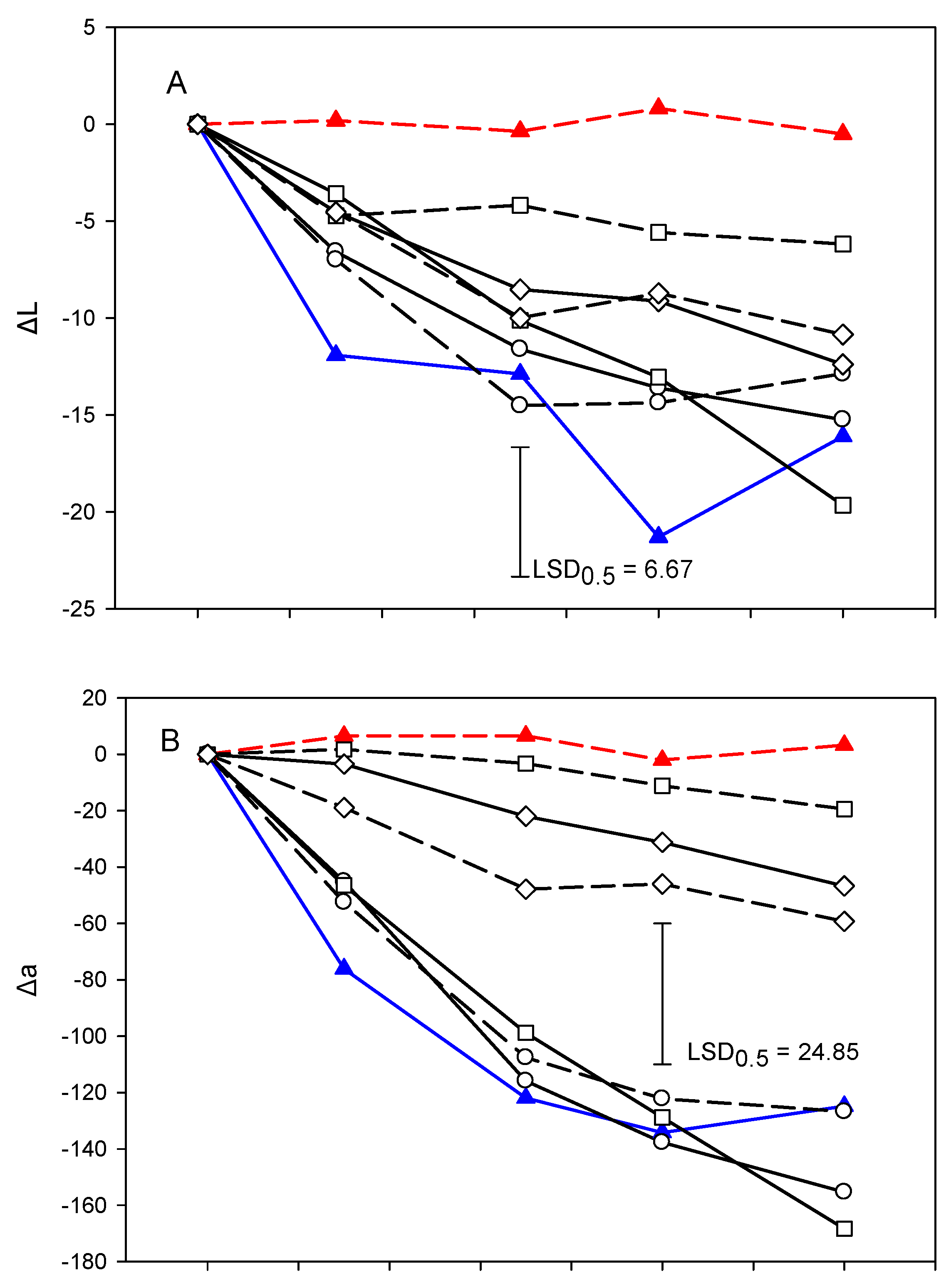
3.5. ΔL and Δa and Δh° Values
The lightness % inhibition (ΔL) of fresh-cut lettuce samples in all treatments decreased during storage (Figure 4A). This decrease, however, was lower in the E244-control and roc20% than the other treatments or the water-control and this difference was statistically significant (p < 0.05). Both lightness and its % inhibition (ΔL) has been used by researchers as an indicator of vegetable deterioration [58]. In this study, a decrease in the ΔL values of fresh-cut lettuce in all SWE treatments was observed during storage and this was correlated with a decrease in BI or VSI (Table 4).
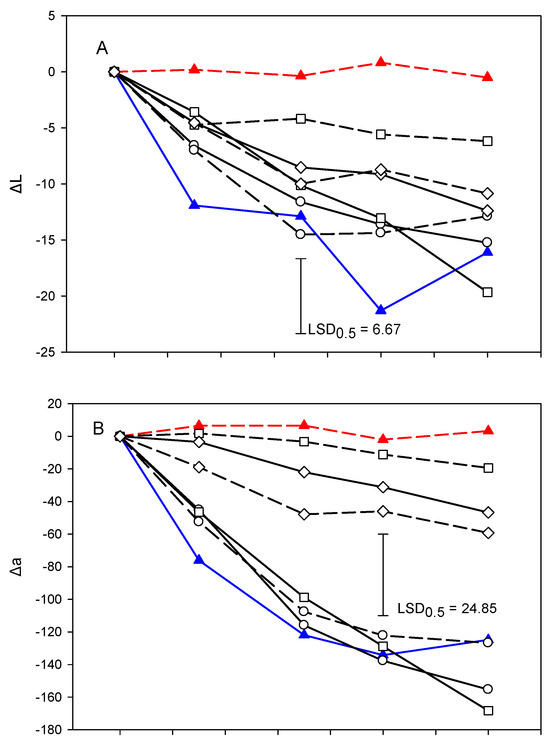
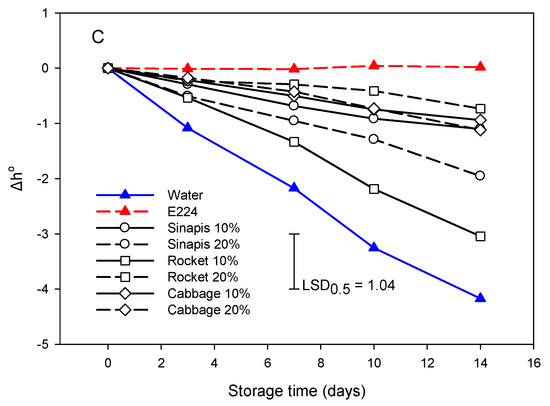
Figure 4.
Development of browning based on ΔL (A), Δa (B) and Δh° (C) of cut mid rib segments of leaf lettuce during storage for 14 days at 7 °C, previously exposed to seed:water (w/w) extracts (SWE) of sinapis (sin10%, sin20%), wild rocket (roc10%, roc20%) and cabbage (cab10%, cab20%), water or bisulfite for 3 min. Mean comparison was performed using the Least Significant Difference method (LSD), at a confidence level of 0.05.
The Δa values (Figure 4B) of fresh-cut lettuce were increased during storage following a pattern similar to VSI in two distinct groups (the E224 group: cab10%, cab20%, roc20% and E224-control, and the water-control group: sin10%, sin20%, roc10% and water-control) to show statistically significant differences at the end of storage (p < 0.05). Rocculi [59] used only L* and a to evaluate browning in potato pieces by choosing the parameters they used. Lante [60] used only the total color change, ΔE, of the L*a*b color model to evaluate the effectiveness of UV radiation in the preservation of cut apple and pear varieties.
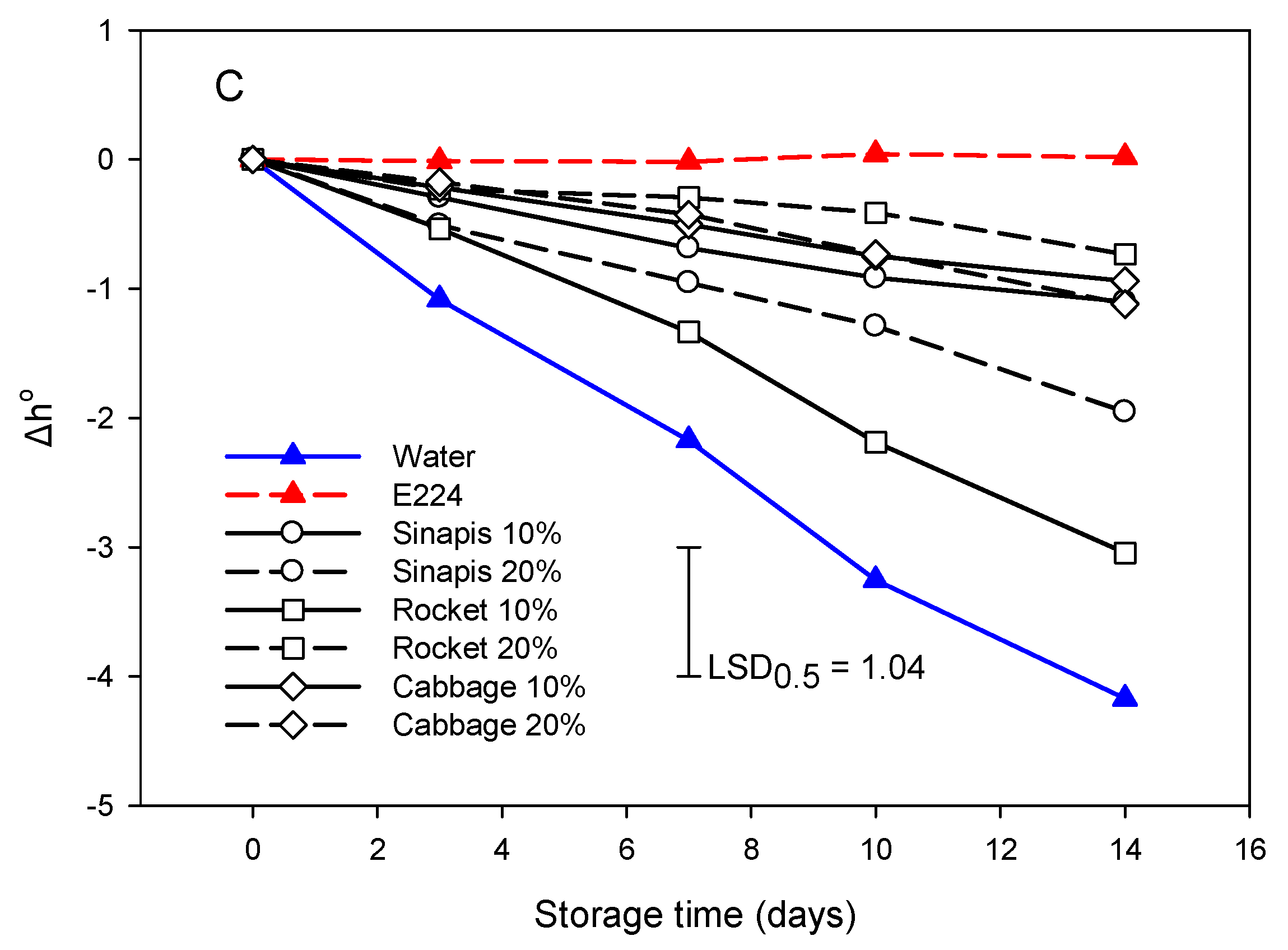
The pattern for the evolution of SWE treatments was similar in regard to Δh° values during storage (Figure 4C). However, sin10% was grouped with the E224-control group. Hue value reduction indicated that the color change in cut lettuce segments was moved to more red tones.
The SWE treatments and the E224-control group (cab10%, cab20% and roc 20%) could not ameliorate the color attributes of lettuce cut segments during storage. However, it maintained a WI value and delayed the increments of both BI, Δa, and ΔL values compared to the water-control group (sin10%, sin20% and roc10%).
3.6. Shelf-Life
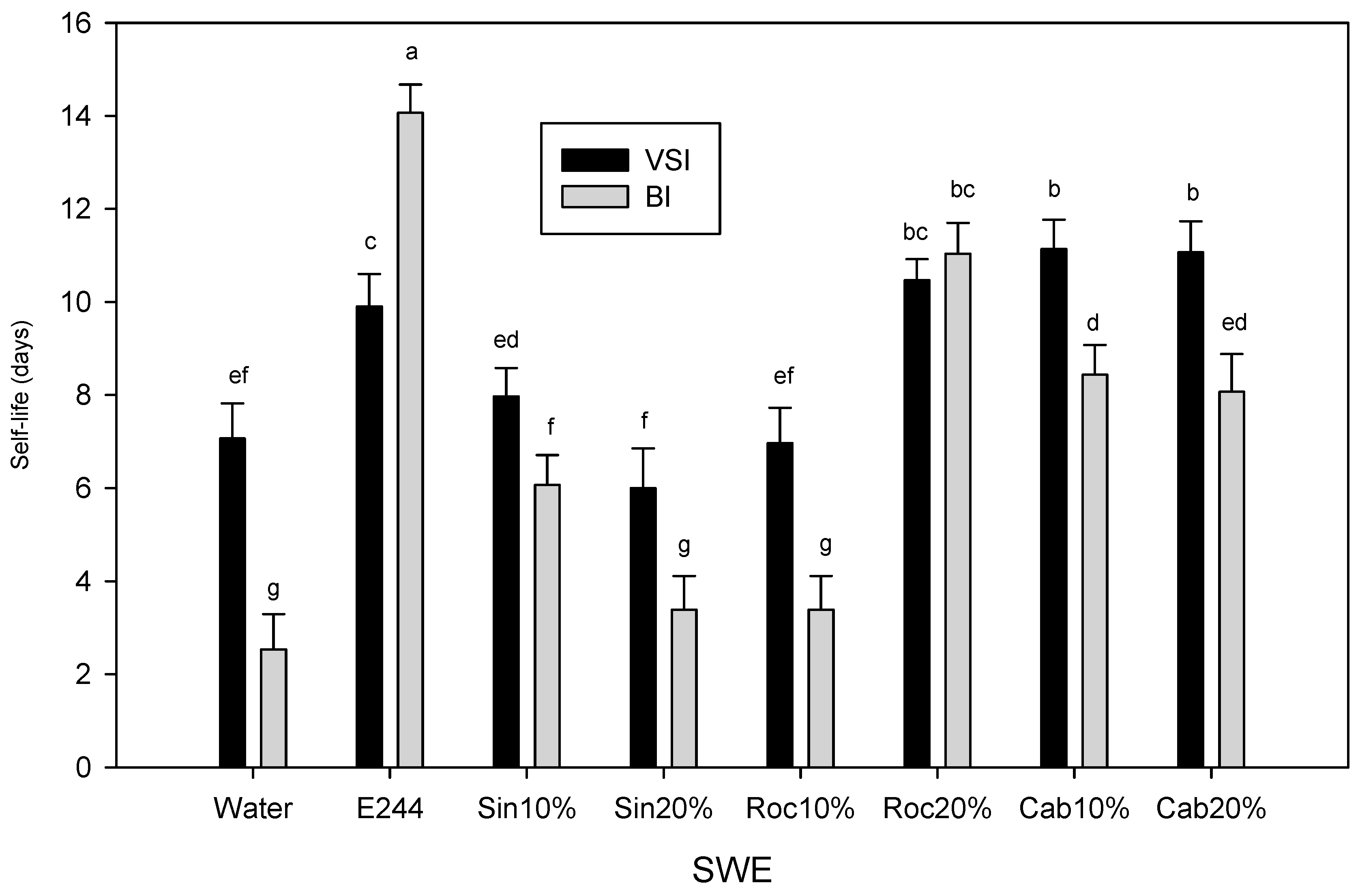
Shelf-life was calculated as the time that VSI or BI (following the correlation of VSI with BI) for browning crossed the threshold of 3 and 40, respectively (Figure 3A,B). Shelf-life based on Δa, Δh° and ΔL were similar to BI. The SWE that showed the highest performance were the E224-control group which included cab10%, cab20% and roc20% with 11.5, 11.3 and 10.4 days of effective acceptable browning levels based on VSI and 9, 8.5 and 11.5 days based on BI, respectively. It should be noted that the shelf-life for the preservative-control was about 10 and 14 days based on VSI and VI, respectively (Figure 5).
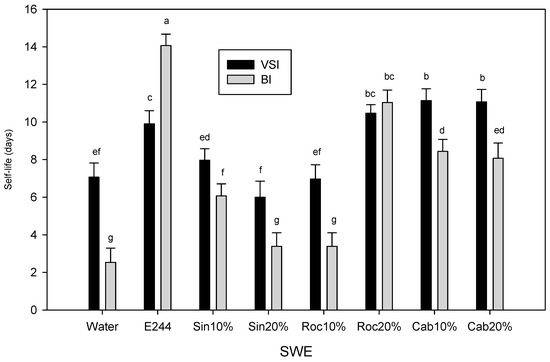
Figure 5.
Shelf life (average of 4 replications ± S.D.) of cut mid rib segments of leaf lettuce previously exposed to seed:water (w/w) extracts (SWE) of sinapis (sin10%, sin20%), rocket (roc10%, roc20%) and cabbage (cab10%, cab20%), water or bisulfite for 3 min during storage for 14 day at 7 °C based on visual score (VSI) and browning index (BI). Different letters on VSI or BI bars represent a statistically significant difference according to Least Significant Difference method at a confidence level of 0.05 (LSD0.05 = 1.1546).
All SWE possessing an anti-browning preserving capacity were characterized by increased phenolic compounds content and antioxidant activity (Table 1). The TPC of SWE correlated with the reducing antioxidant capacity content (R2 = 0.72–0.839, p < 0.01) (Table 5). All SWEs had similar polyphenol content, although lettuce mid ribs treated with these extracts showed differences in browning rate delay and therefore in shelf life (Figure 5). Weak and statistically non-significant correlations were observed between the TPC content of SWE and the shelf life based on VSI or BI (Table 5), indicating that phenolic compounds of SWE may contribute only partially to their anti-browning properties. However, strong and statistically significant correlations were observed between the antioxidant activity (DPPH, ABTS and FRAP) of SWE and shelf life based on VSI (R2 = 0.751–0.766, p < 0.01) or BI (R2 = 0.545–0.638), p < 0.01) (Table 5). Rojas-Graü [54] reported that natural sulfur compounds may delay the browning of fresh-cut fruit. Cabbage and wild rocket SWE anti-browning properties might be attributed to glucosinolates, which are natural sulfur- and nitrogen-containing compounds reported to be found in cruciferous vegetables [33,34,61,62]. Further experimentation and analysis of the phenolic and glucosinolate profile of SWE is needed to establish the effects of either or both groups of phytochemicals on the enzymatic browning of fresh cut lettuce or other produce.

Table 5.
Pearson linear correlation coefficients of the variables TPC, DPPH, ABTS, FRAP, as well as shelf life based on VSI and BI.
4. Conclusions
This study examined the effect of a water extract of cruciferous seeds to discover whether it possesses a comparable inhibitory activity to a classical sulfate preservatives reference solution when used in a dipping system. With the experimental conditions being as close as possible to an industrial application, the results of the study indicated that the cruciferous seeds under study contain ingredients that inhibit the action of polyphenoloxidase on the cut surfaces of fresh mid rib lettuce segments. Water seed extraction is an efficient method for extracting the above components, and dipping lettuce cut mid rib segments for 3 min in the resulting extract could exert an anti-browning effect.
The independent variables used in our experimental set-up (L*, a*, b*, ΔL, Δa, Δb, ΔE, h° Δh°, C, ΔC, BI, WI, SI, CI and VSI) did not serve well in their role as indicators of developing browning in lettuce mid rib pieces equally. Cabbage or wild rocket seed water extracts had the highest TPC and AC among SWEs and exhibited the highest inhibitory capacity of the browning rate, which was equivalent to 25 mM potassium metabisulfite solution. Sinapis seed extracts had little or no blocking ability.
The research carried out demonstrated the contribution of the constituents contained in the cruciferous seeds to the inhibition of browning reactions in cut mid rib of lettuce leaves. However, the potential anti-browning properties of the water extracts of cabbage, wild rocket or even sinapis seeds among other cruciferous seeds need to be attributed to specific compounds in future studies.
Author Contributions
Conceptualization, D.G.; methodology, A.K. and A.G.; project execution, E.A. and A.G.; formal analysis, A.G. and E.A.; writing—original draft preparation, D.G. and A.K.; writing—review and editing, A.S.S.; supervision, D.G.; project administration, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article. Additional data can be obtained by contacting the corresponding author of the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, D.H.; Kim, H.B.; Chung, H.S.; Moon, K.D. Browning Control of Fresh-Cut Lettuce by Phytoncide Treatment. Food Chem. 2014, 159, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, L.; Wu, Y.W.; Fan, J.F.; Ouyang, J. Salicylic Acid Inhibits Enzymatic Browning of Fresh-Cut Chinese Chestnut (Castanea mollissima) by Competitively Inhibiting Polyphenol Oxidase. Food Chem. 2015, 171, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zeng, S.; Yuan, S.; Yue, X.; Tian, T.; Zhu, X.; Zheng, S.; Xu, X.; Zuo, J.; et al. Browning Mechanism in Stems of Fresh-Cut Lettuce. Food Chem. 2023, 405, 134575. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Espin, J.C. Phenolic Compounds and Related Enzymes as Determinants of Quality in Fruits and Vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol Oxidases in Plants and Fungi: Going Places? A Review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kim, D.; Park, J. Various Antibrowning Agents and Green Tea Extract During Processing and Storage. J. Food Proc. Preserv. 2003, 27, 213–225. [Google Scholar] [CrossRef]
- Pace, B.; Capotorto, I.; Ventura, M.; Cefola, M. Evaluation of L-Cysteine as Anti-Browning Agent in Fresh-Cut Lettuce Processing. J. Food Proc. Preserv. 2014, 201439, 985–993. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The Combined and Single Effect of Marjoram Essential Oil, Ascorbic Acid, and Chitosan on Fresh-Cut Lettuce Preservation. Foods 2021, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Cuenca, C.E.; Kuijpers, T.F.M.; Vincken, J.P.; de Waard, P.; Gruppen, H. New Insights into an Ancient Antibrowning Agent: Formation of Sulfophenolics in Sodium Hydrogen Sulfite-Treated Potato Extracts. J. Agric. Food Chem. 2011, 59, 10247–10255. [Google Scholar] [CrossRef]
- McEvily, A.J.; Iyengar, R.; Otwell, W.S. Inhibition of Enzymatic Browning in Foods and Beverages. Crit. Rev. Food Sci. 1992, 32, 253–273. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and Synthetic Tyrosinase Inhibitors as Anti-Browning Agents: An Update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Zhan, L.; Li, Y.; Hu, J.; Pan, L.; Fan, H. Browning Inhibition and Quality Preservation of Fresh-Cut Romaine Lettuce Exposed to High Intensity Light. Innov. Food Sci. Emerg. Technol. 2012, 14, 70–76. [Google Scholar] [CrossRef]
- Loaiza-Velarde, J.G.; Tomás-Barberán, F.A.; Saltveit, M.E. Effect of Intensity and Duration of Heats Hock Treatments on Wound-Induced Phenolic Metabolism in Iceberg Lettuce. J. Amer. Soc. Hort. Sci. 1997, 122, 873–877. [Google Scholar] [CrossRef]
- Vanden Abeele, C.; Raes, K.; Samperset, I. Effect of Mild Heat Treatment on Browning-Related Parameters in Fresh-Cut Iceberg Lettuce. J. Food Biochem. 2019, 43, e12906. [Google Scholar] [CrossRef] [PubMed]
- Soliva, R.C.; Elez, P.; Sebastian, M.; Martin, O. Evaluation of Browning Effect on Avocado Puree Preserved by Combined Methods. Innov. Food Sci. Emerg. Technol. 2001, 1, 216–268. [Google Scholar] [CrossRef]
- Escalona, V.H.; Verlinden, B.E.; Geysen, S.; Nicolai, B.M. Changes in Respiration of Fresh-Cut Butterhead Lettuce Under Controlled Atmospheres Using Low and Superamospheric Oxygen Conditions with Different Carbon Dioxide Levels. Postharvest Biol. Technol. 2006, 39, 48–55. [Google Scholar] [CrossRef]
- Koukounaras, A.; Siomos, A.S.; Gerasopoulos, D.; Papachristodoulou, M. Active modified Atmosphere Package Induced Collapse of Minimally Processed Romaine Lettuce Leaves. Food Pack. Shelf Life 2019, 22, 100411. [Google Scholar] [CrossRef]
- Chaisakdanugull, C.; Theerakulkait, C.; Wrolstad, R.E. Pineapple Juice and Its Fractions in Enzymatic Browning Inhibition of Banana [Musa (AAA Group) Gros Michel]. J. Agric. Food Chem. 2007, 55, 4252–4257. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Rico, D.; Barry-Ryan, C. Green Tea Extract As a Natural Antioxidant to Extend The Shelf-Life of Fresh-Cut Lettuce. Innov. Food Sci. Emerg. Technol. 2008, 9, 593–603. [Google Scholar] [CrossRef]
- Altunkaya, A. Effect of Whey Protein Concentrate on Phenolic Profile and Browning of Fresh-Cut Lettuce (Lactuca Sativa). Food Chem. 2011, 128, 754–760. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, C.Y.; Park, I. Prevention of Enzymatic Browning of Pear by Onion Extract. Food Chem. 2008, 89, 181–184. [Google Scholar] [CrossRef]
- Sukhontha, S.; Kunwadee, K.; Chockchai, T. Inhibitory Effect of Rice Bran Extracts and Its Phenolic Compounds on Polyphenol Oxidase Activity and Browning in Potato and Apple Puree. Food Chem. 2016, 190, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K. Inhibitory Effect of Banana Polyphenol Oxidase During Ripening of Banana by Onion Extract and Maillard Reaction Products. Food Chem. 2007, 102, 146–149. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of purslane (Portulaca oleracea L.) extract on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 283, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Supapvanich, S.; Yimpong, A.; Srisuwanwichan, J. Browning Inhibition on Fresh-Cut Apple by the Immersion of Liquid Endosperm from Mature Coconuts. J. Food Sci. Technol. 2020, 57, 4424–4431. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Wang, H.; Shao, J.; Lu, L.; Tian, J.; Liu, X. A Novel Mitigator of Enzymatic Browning—Hawthorn Leaf Extract and its Application in the Preservation of Fresh-Cut Potatoes. Food Qual. Safety 2021, 5, 1399–2399. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of Clove Essential Oil and Eugenol on Quality and Browning Control of Fresh-Cut Lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Złotek, U.; Świeca, M. Effect of Basil Leaves and Wheat Bran Water Extracts on Enzymatic Browning of Shredded Storage Iceberg Lettuce. Int. J. Food Sci. Technol. 2020, 55, 1318–1325. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Wang, Q.; Dong, T. Diacetyl Inhibits the Browning of Fresh-Cut Stem Lettuce by Regulating the Metabolism of Phenylpropane and Antioxidant Ability. Foods 2023, 12, 740. [Google Scholar] [CrossRef]
- Nogales-Delgado, S. Polyphenoloxidase (PPO): Effect, Current Determination and Inhibition Treatments in Fresh-Cut Produce. Appl. Sci. 2021, 11, 7813. [Google Scholar] [CrossRef]
- Peng, H.; Simko, I. Extending Lettuce Shelf Life through Integrated Technologies. Curr. Opin. Biotechnol. 2023, 81, 102951. [Google Scholar] [CrossRef]
- Landi, M.; Degl’Innocenti, E.; Guglielminetti, L.; Guidi, L. Role of Ascorbic Acid in the Inhibition of Polyphenol Oxidase and the Prevention of Browning in Different Browning-Sensitive Lactuca sativa var. capitata (L.) and Eruca sativa (Mill.) Stored as Fresh-Cut Produce. J. Sci. Food Agric. 2013, 93, 1814–1819. [Google Scholar] [CrossRef]
- Zocca, F.; Lomolino, G.; Lante, A. Antibrowning Potential of Brassicacaea Processing Water. Biores. Technol. 2010, 101, 3791–3795. [Google Scholar] [CrossRef]
- Wessels, B.; Damm, S.; Kunz, B.; Schulze-Kaysers, N. Effect of Selected Plant Extracts on the Inhibition of Enzymatic Browning in Fresh-Cut Apple. J. Applied Bot. Food Qual. 2014, 87, 16–23. [Google Scholar] [CrossRef]
- Bustos, M.C.; Agudelo-Laverde, L.M.; Mazzobre, F.; Buera, M.P. The Relationship between Antibrowning, Anti-Radical and Reducing Capacity of Brassica and Allium Extracts. Int. J. Food Stud. 2014, 3, 82–92. [Google Scholar] [CrossRef]
- Bustos, M.C.; Pérez, G.; León, A.E. Structure and Quality of Pasta Enriched with Functional Ingredients. RSC Adv. 2015, 5, 30780–30792. [Google Scholar] [CrossRef]
- Bustos, M.C.; Mazzobre, M.F.; Buera, M.P. Stabilization of Refrigerated Avocado Pulp: Chemometrics-Assessed Antibrowning Allium and Brassica Extracts as Effective Lipid Oxidation Retardants. Food Bioprocess Technol. 2017, 10, 1142–1153. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1990. [Google Scholar]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nenadis, N.; Kyriakoudi, A.; Tsimidou, M.Z. Impact of Alkaline or Acid Digestion to Antioxidant Activity, Phenolic Content and Composition of Rice Hull Extracts. LWT—Food Sci. Technol. 2013, 54, 207–215. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for Measuring Antioxidant Activity and Its Application to Monitoring the Antioxidant Capacity of Wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Lister, C.E.; Reay, P.F.; Triggs, C.M. Influence of Pigment Composition on Skin Color in a Wide Range of Fruit and Vegetables. J. Am. Soc. Hortic. Sci. 1997, 122, 594–598. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S.; Raghavan, G.S.V. Kinetics of Astaxanthin Degradation and Color Changes of Dried Shrimp During Storage. J. Food Engin. 2008, 87, 591–600. [Google Scholar] [CrossRef]
- Palou, E.; Lopez-Malo, A.; Barbosa-Canovas, G.V.; Welti-Chanes, J.; Swanson, G.B. Polyphenoloxidase Activity and Colour of Balanced and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Bolin, H.R.; Huxsoll, C.C. Control of Minimally Process Carrot (Daucus carota) Surface Discoloration Caused by Abrasion Peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of Thermal Treatment on Glucosinolates and Antioxidant-Related Parameters in Red Cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Hansen, M.; Wicklund, T.; Bengtsson, G.B. Processing (Blanching, Boiling, Steaming) Effects on the Content of Glucosinolates and Antioxidant-Related Parameters in Cauliflower (Brassica oleracea L. ssp. botrytis). LWT—Food Sci. Technol. 2009, 42, 63–73. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Free phenolics and polyphenol oxidase (PPO): The Factors Affecting Post-Cut Browning in Eggplant (Solanum melongena). Food Chem. 2013, 139, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Martín-Diana, A.B.; Barat, J.B.; Barry-Ryan, C. Extending and Measuring the Quality of Fresh-Cut Fruit and Vegetables; A Review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Saltveit, M.E. Wound Induced Changes in Phenolic Metabolism and Tissue Browning are Altered by Heat Shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martin-Belloso, O. Effect of Natural Antibrowning Agents on Color and Related Enzymes in Fresh-Cut Fuji Apples as an Alternative to the Use of Ascorbic Acid. J. Food Sci. 2008, 73, S267–S272. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Li, D.; Tang, D.; Huang, Z.; Kedbanglai, P.; Ge, Z.; Du, X.; Supapvanich, S. Effects of Simultaneous Ultrasonic and Cysteine Treatment on Antibrowning and Physicochemical Quality of Fresh-Cut Lotus Roots during Cold Storage. Postharvest Biol. Technol. 2020, 168, 111294. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Carvacrol-Loaded Chitosan Nanoparticles Maintain Quality of Fresh-Cut Carrots. Innov. Food Sci. Emerg. Technol. 2017, 41, 56–63. [Google Scholar] [CrossRef]
- Kasım, R.; Kasım, M.U. Biochemical Changes and Color Properties of Fresh-Cut Green Bean (Phaseolus vulgaris L. cv. gina) Treated with Calcium Chloride During Storage. Food Sci. Technol. 2015, 35, 266–272. [Google Scholar] [CrossRef]
- Mastrocola, D.; Lerici, C.R. Colorimetric measurements of enzymatic and non enzymatic browning in apple purees. Ital. J. Food Sci. 1991, 3, 219–229. [Google Scholar]
- Rocculi, P.; Galindo, F.; Mendoza, F.; Wadsö, L.; Romani, S.; Dalla Rosa, M.; Sjöholm, I. Effect of the Application of Anti-Browning Substances on the Metabolic Activity and Sugar Composition of Fresh-Cut Potatoes. Postharvest Biol. Technol. 2007, 43, 151–157. [Google Scholar] [CrossRef]
- Lante, A.; Tinello, F.; Nicoletto, M. UV-A Light Treatment for Controlling Enzymatic Browning of Fresh-Cut Fruits. Innov. Food Sci. Emerg. Technol. 2016, 34, 141–147. [Google Scholar] [CrossRef]
- Verkerk, R.; Dekker, M. Glucosinolates. In Bioactive Compounds in Foods; Gilbert, J., Şenyuva, H.Z., Eds.; Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, L-Ascorbic Acid, Total Phenols, Anthocyanins, Antioxidant Capacities and Colour in Cauliflower (Brassica oleracea L. ssp. botrytis); Effects of Long-Term Freezer Storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).