Abstract
A greenhouse experiment was realized to investigate the effects of plant-growth-promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on soil quality and the growth, nutrient uptake, and physiological performance of Batavia lettuce (Lactuca sativa L. var. longifolia). For this purpose, six fertilization treatments were applied: (i) inorganic fertilization (I.F.—control, i.e., usual/conventional fertilization), (ii) I.F. + PGPR, (iii) I.F. + PGPR + AMF, (iv) manure (MAN), (v) MAN + PGPR, and (vi) MAN + PGPR + AMF. Soil fertility was influenced by the treatments, and soil respiration (CO2 flux) was significantly increased after applications of PGPR and AMF. Following MAN fertilization in particular, an approximately sixfold higher soil respiration value was recorded following the MAN + PGPR + AMF treatment compared to the control (I.F.). Root, leaf, and total biomass dry weights were significantly affected by the PGPR and AMF applications, mainly following the I.F. treatment. In contrast, K, Mg, and Fe uptake was significantly influenced by PGPR and AMF application following the MAN treatment. The SPAD value, performance index (PI), photosynthetic rate, and stomatal conductance were significantly higher in the I.F. + PGPR + AMF-treated plants compared to the control plants. Overall, these data prove the beneficial roles of PGPR and AMF in soil quality and fertility and the nutrient uptake and physiological performance of lettuce plants. However, further clarification is needed in the near future to test the interaction effects between PGPR, AMF, and the type of fertilizer used (organic or inorganic).
1. Introduction
Lactuca sativa L. is one of the most economically important leafy vegetable crops; it belongs, to the Asteraceae family and contains polyphenols, flavonoids, vitamins A, C, and E, carotenoids, Ca, Fe, and other nutrients. It is traditionally consumed fresh [1,2,3,4]. It is widely cultivated in temperate areas, and more than 21 million tons are produced annually [3]. Lettuce plants have a quick and short growing period and require high fertilization rates to satisfy their urgent nutritional needs and support their growth rate.
In recent years, there has been an increasing interest in the benefits of sustainable agriculture and a demand for high-quality, healthier fruits and vegetables. Sustainable agriculture not only preserves environmental resources, producing high-quality products and protecting human health [5], but also addresses major environmental and agronomic challenges [6], such as decreases in high inorganic fertilization rates and the enhancement of sustainable crop management [7,8]. In this direction, the use of biofertilizers or microbial fertilizers (e.g., the use of Rhizobium leguminosarum, isolated from Phaseolus vulgaris nodules, or the production of rock biofertilizer that is mixed with organic matter, inoculated with diazotrophic bacteria and the fungus Cunninghamella elegans), proved to be a promising tool for improving nutrient uptake (mainly N and P) and plant growth for some vegetable species, such as lettuce and carrot crops [9,10]. Similarly, the inoculation of lettuce plants with N2-fixing plant-growth-promoting rhizobacteria led to a reduction in crop N fertilizer requirements [11], while the use of native phosphate-solubilizing rhizobacteria enhanced lettuce and tomato crop productivity and sustainability [12].
In the context of sustainable agriculture, plant-growth-promoting rhizobacteria (PGPR), biostimulants, and arbuscular mycorrhizal fungi (AMF) were used in an L. sativa crop and were found to play a crucial role in (i) boosting plant biomass and yield, as well as improving nutritional quality and impact on human health [13,14,15,16], (ii) increasing nutrient (especially N, P, Mg) [15,16] and water uptake (by also ensuring plant adaptation to drought conditions) [17], (iii) increasing plant tolerance to severe salt stress [18,19,20,21], and limiting the detrimental effects of salinity on soil structural stability [22]. The colonization of greenhouse lettuce plants by AMF may induce the accumulation of secondary metabolites, vitamins, and minerals in leaves; thus, AMF could allow the intake of minerals and compounds with antioxidant properties to be enhanced, without increasing the consumption of lettuce by humans [14]. In another study, the inoculation of lettuce plants with AMF was found to increase their yields (by an average of 186%), but resulted in no substantial change in lettuce nutritional quality; in addition, the AMF inoculants did not alter natural soil communities [22]. In contrast, other biostimulants (based on Trichoderma) were found to modulate rhizosphere microbial populations and improve the nutritional quality of leafy vegetables [16].
Apart from AMF and PGPR, the organic production of lettuce has relied on the application of organic materials, such as manures, humus-rich composts, and seaweed extracts (in foliar applications), in order to boost plant growth and yield, increase nutrient uptake and mycorrhizal colonization, and improve the antioxidant responses of L. sativa [23,24,25]. In addition, urban waste compost application improved soil properties and increased lettuce production [26]. In another comparative study, organic amendments applied to lettuce and orchardgrass were shown to supply adequate levels of N while resulting in good productivity [27]. Other studies focused on the mineral nutrition of lettuce plants grown under hydroponic conditions in a recirculating nutrient solution [28].
Despite the significant effects of manure and PGPR application and AMF inoculation on the stimulation of the growth, nutrient uptake, and nutritional quality of L. sativa plants, to the best of our knowledge, no attention has been systematically paid to their combined application until now, and almost no comparative studies have been conducted on the inorganic and organic fertilization of Batavia lettuce. In addition, the influence of AMF inoculation and PGPR application on lettuce productivity and physiological performance, in relation to soil health and fertility, has not been studied.
Thus, the aims of our research were as follows: (i) to investigate the effects of PGPR and AMF on soil health and Batavia lettuce productivity, nutrient uptake, and plant physiology under inorganic and cow manure regimes and (ii) to perform a comparative study on the effects of inorganic and organic fertilization on soil respiration and lettuce growth, nutrient uptake, photosystem II activity (Fv/Fm ratio; performance index), photosynthetic and transpiration rates, and intrinsic water use efficiency (WUEi). Batavia (also known as French) lettuce was chosen for study since it is one of the most economically important leafy green genotypes for salads with good tolerance to heat and contains significant quantities of vitamins A and C, antioxidants, and K, Ca, and Fe.
2. Materials and Methods
2.1. Plant Material and Conditions of Experimental Greenhouse
The plant material consisted of Batavia lettuce plants (L. sativa L. var. longifolia), which were grown inside a plastic-covered experimental greenhouse at Perrotis College of the American Farm School (AFS) in Thessaloniki (latitude, 40.341134°; longitude, 22.592796°; altitude, 65 m above sea level), Macedonia, Northern Greece. The plants were obtained as seedlings and randomized based on the initial number and surface of leaves. They were divided into six groups coinciding with the six fertilization treatments described below and were transplanted into 0.60 × 3.30 m plots containing 65 plants. The lettuce plants were grown inside the experimental greenhouse under natural light conditions for 65 days (from the middle of October to the middle of December 2019). During the experiment, the lettuce plants were irrigated with an automated drip irrigation system (for one hour to reach a soil moisture level of approximately 70% of its water-holding capacity) twice per week, providing them with high-quality tap water (0.72 mS/cm). The minimum and maximum temperatures during the experiment were 7 °C (in early December) and 24 °C (in early November), respectively, while the average temperature for the whole experimental period was approximately 17 °C. Finally, the relative humidity inside the greenhouse ranged from 65% to 77%.
2.2. Treatments and Methodology of PGPR and AMF Application
Six treatments were applied: (i) inorganic fertilization (I.F.—control, i.e., usual/conventional fertilization), (ii) I.F. + PGPR, (iii) I.F. + PGPR + AMF, (iv) manure (MAN), (v) MAN + PGPR, and (vi) MAN + PGPR + AMF. Each treatment consisted of 65 plants with an area of 2 m2. In the I.F. treatments, a 25-10-0 (N-P-K) fertilizer (300 kg/ha N and 120 kg/ha P) was combined with a 0-0-30 (N-P-K) fertilizer (275 kg/ha K) in each of the treatments receiving inorganic fertilizer; the fertilizers were mixed with soil and incorporated at a 20 cm depth before the initiation of the experiment. For the organic fertilizer applications, well-digested and composted cow manure (MAN) (2 ton/ha), was incorporated at a 20 cm depth before the initiation of the experiment. The nutrient composition of the cow manure (MAN), as well as the initial and final fertility of the greenhouse soil (GS) are described in Table 1.

Table 1.
The properties of cow manure and greenhouse soil (initial fertility) before the beginning of the experiment (i.e., before manure, PGPR, and AMF applications), as well as at the end of the experimentation (final fertility, i.e., after applying the treatment).
In order to study the influence of PGPR and AMF on the nutrition and physiological performance of the Batavia lettuce plants, the following commercial products were used: (a) Micoseeds Plus, 1 kg (powder of mycorrhiza fungi, containing the mycelium and seeds of the AMF Glomus sp. enriched with beneficial microorganisms, Trichoderma sp., Bacillus spp., Streptomyces sp., and Pseudomonas sp.), provided by Comerco Agrotechnology (Athens, Greece); (b) Clonotri, 0.25 mL (a commercial liquid product provided by Comerco Agrotechnology containing rhizospheric bacteria, PGPR, and other beneficial microorganisms;; (c) Strepse, 0.25 mL (provided by Comerco Agrotechnology; a liquid product, containing rhizospheric bacteria, PGPR, and other beneficial microorganisms); and (d) Nutryaction, 1 L (an activator of microorganisms and a biostimulant of plant growth; provided by Comerco Agrotechnology).
The PGPR containing the commercial products Clonotri, Strepse, and Nutryaction were prepared as follows: 50 mL of Clonotri, 50 mL of Strepse, and 150 mL of Nutryaction were mixed with a limited volume of water (2–3 L) at room temperature (20–25 °C). The three products were stirred very well and allowed to stand for 2 h in order for the microorganisms to be activated. The ratio of Clonotri, Strepse, and Nutryaction in the final volume solution was 2:2:6 (i.e., 2 mL/L Clonotri, 2 mL/L Strepse, and 6 mL/L Nutryaction). Τhe rhizosphere of each lettuce seedling was soaked in this final solution for a few seconds before the establishment of the experiment. The same solution was prepared and added by fertigation 7 days after the initiation of the experiment. To investigate the additional effects of AMF, the lettuce seedlings were first soaked in the PGPR solution, and a small quantity (1–2 g) of granular Micoseeds Plus was added on the rhizosphere of each seedling prior to their transplantation.
2.3. Soil Sampling, Manure Nutrient Composition, and Lab Analyses
A composite soil sample from the upper 20 cm of the soil surface of the experimental area was collected and analyzed, and its chemical properties are shown in Table 1. A composite sample from well-digested and composted cow manure, produced at the American Farm School, was analyzed. Its nutrient composition is presented in Table 1. Soil samples were dried at room temperature, stones were removed, and the samples were sieved to pass through a 10-mesh screen; the screened samples were sent to the Soil and Water Resources Institute’s lab (ELGO DIMITRA) for chemical analyses of the following parameters: pH, organic matter, % CaCO3 content, NO3-N, available P, exchangeable cations (Ca, Mg, and K) and micronutrients (Fe, Mn, Zn, Cu, and B). The parameters were determined as follows: pH was determined in a 1:1 soil—distilled water paste [29]; particle size was analyzed according to the Bouyoucos method [30]; organic matter was determined using potassium dichromate [31]; the NO3-N concentration was determined according to the VCl3/Griess method [32]; and available P was determined according to the Olsen method [33]. The exchangeable cations were extracted using CH3COONH4 [34]; Fe, Mn, Zn, and Cu were extracted using a DTPA solution at a pH of 7.3 [35]; and the B concentration was determined according to the method described by Wolf [36]. Finally, the % CaCO3 content was determined according to the acid neutralization method [37]. Potassium, Ca, Mg, Fe, Mn, Zn, and Cu concentrations were measured by ICP spectrometry (OPTIMA 2100 DV optical emission spectrometer, Perkin Elmer, Waltham, MA, USA) [38].
2.4. Methodology of Soil Respiration Rate Measurement
Soil respiration (Rs) was measured in situ in each of the six treatments using a LI-COR LI-6800 soil CO2 flux chamber (LI-COR, Lincoln, NE, USA). A PVC collar (with a 19.5 cm inner diameter and a height of 11.5 cm) was inserted into the soil to a depth of 5 cm in each location at least 24 h prior to the measurements, and it was kept in place for the duration of the experiment. Each measurement was obtained in triplicate and comprised a 90 s measurement, including a 25 s dead-band measurement and a 45 s post-purge measurement. For each location soil temperature and soil moisture were simultaneously measured using a Li-COR Stevens probe (LICOR, Lincoln, NE, USA).
2.5. Plant Growth Measurements
At the end of the experiment, all plants were harvested and separated into root systems and leaves to determine the total plant, root, and leaf biomass values and leaf/root ratio. After weighting all the plant tissues separately and determining their fresh weight (F.W.), they were washed twice (with high-quality tap water followed by distilled water) and dried at 75 °C for 72 h to determine their dry weight (D.W.) values. By adding the leaf and root dry weights, the total plant D.W., i.e., the total plant biomass, was calculated.
2.6. Nutrient Uptake and Tissue Nutrient Concentrations
The dried vegetal tissues were ground to a fine powder and passed through a 30-mesh screen. A 0.5 g. portion of the fine powder of each sample was dry-ashed in a muffle furnace at 515 °C for 5 h. The ash was dissolved in 3 mL of 6 N HCl and diluted with double-distilled water up to 50 mL. Phosphorus, K, Ca, Mg, Fe, Mn, Zn, and Cu concentrations were determined by ICP spectrometry (OPTIMA 2100 DV optical emission spectrometer, Perkin Elmer, Waltham, MA, USA) [38], while those of N and B were determined by the Kjeldahl and azomethine-H methods, respectively [39,40]. Macronutrient concentrations were expressed in % D.W., while those of micronutrients were expressed in mg kg−1.
2.7. Chlorophyll Fluorescence, Gas Exchange Measurements, and Intrinsic Water Use Efficiency (WUEi)
At the end of the experiment, the following parameters were determined using a PAM-2000 fluorometer (HeinzWalz GmbH, Effeltrich, Germany): Fv/Fm, the maximum quantum yield of primary photochemistry; F0, the minimum fluorescence; Fm, the maximum fluorescence; and Fv = Fm − F0, the variable fluorescence. In addition, the performance index (PI) was determined, reflecting the functionality of both photosystems I and II and providing quantitative information on the current state of plant performance under stress conditions [41]. The above-mentioned parameters were determined on the youngest mature, fully expanded plant leaves. Before performing the measurements with the fluorometer, the leaves were preconditioned under dark conditions for 20 min [42].
For the gas exchange measurements, i.e., photosynthetic and transpiration rates, stomatal conductance, and the intercellular CO2 concentration, an LC PRO portable gas-exchange-measuring system (ADC Bioscientific Ltd., Hoddesdon, UK) was used. All the measurements were carried out during the time period from 10:00 to 12:00 a.m. (under natural light intensity) on the youngest mature, fully expanded leaves. Finally, the intrinsic water use efficiency (WUEi, showing the water efficiency at the plant level) was determined as the net photosynthetic rate per stomatal conductance.
2.8. Statistical Analysis
The experimental design consisted of a 6 × 1 completely randomized factorial with six treatments and one plant species. In each of the six treatments, 10 plant replicates were randomly selected for analyses (thus, the total number of experimental plants was 60). For the determination of final soil fertility, three sample replicates were included per treatment. The data were statistically analyzed via a one-way ANOVA using the SPSS statistical program (version 28, IBM, Armonk, NY, USA); for the comparison of mean values among the treatments, Duncan’s multiple range test, with p ≤ 0.05, was used.
3. Results
3.1. Initial and Final Soil Fertility
The properties and nutrient contents of the cow manure used, as well as the initial and final soil fertility, are shown in Table 1.
As indicated by the results in Table 1, the MAN was a good source of nutrients (particularly NO3-N, P, K, and micronutrients), contributing highly to the enrichment of the GS with organic C. At the end of the experiment (final fertility), high amounts of nutrients were removed (compared to the initial soil fertility), as indicated in Table 1. Significantly higher NO3-N concentrations were determined in the IF treatments compared to the organic (MAN, MAN + PGPR, and MAN + PGPR + AMF) ones. Similarly, for the Olsen-determined P, significantly higher concentrations were recorded following the IF and IF + PGPR + AMF treatments compared to the MAN and MAN + PGPR treatments. The highest exchangeable K concentration was found in soil receiving the IF treatment (298 mg kg−1), and it was significantly higher than those determined following the other two IF treatments, MAN and MAN + PGPR + AMF (Table 1). Regarding micronutrients, the highest Fe concentration (1.74 mg kg−1) was recorded for the MAN + PGPR + AMF treatment, followed by the IF treatment (1.04 mg kg−1). These concentrations were significantly higher than those found in soil following the MAN, MAN + PGPR, and IF + PGPR + AMF treatments (Table 1). Similar to Fe, the highest Mn and Zn concentrations were determined in the MAN + PGPR + AMF treated soil (4.39 mg kg−1 and 2.20 mg kg−1, respectively). Finally, the B concentration was significantly higher in the MAN + PGPR-treated soil (1.17 mg kg−1) compared to the inorganic treatments (IF, IF + PGPR, and IF + PGPR + AMF) (Table 1).
3.2. Soil Respiration Data
Figure 1 presents soil respiration data among the six treatments. As shown for the organic fertilization (MAN) treatments, significantly higher CO2 emissions were recorded compared to the relevant (with or without PGPR and AMF application) inorganic treatments. For both fertilization regimes (either inorganic or organic), PGPR and AMF significantly and synergistically boosted CO2 emissions; the highest CO2 emission was determined for the MAN + PGPR + AMF treatment (Figure 1).
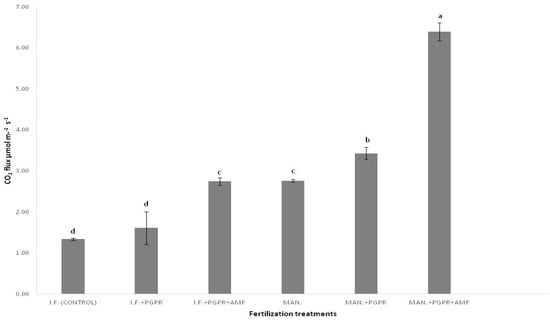
Figure 1.
Soil respiration (μmol m−2s−1 CO2 flux) under different fertilization treatments. Different letters on bars (means ± standard deviations) indicate statistically significant differences among treatments.
3.3. Plant Growth
From Table 2, it can be concluded that the addition of PGPR and AMF to inorganic fertilizer (I.F.) positively influenced the dry weights of the roots, leaves, and total biomass. In particular, between IF and the IF + PGPR + AMF treatment, significant differences in the three dry weights were recorded. In the ΜAΝ treatments, only PGPR application (i.e., MAN + PGPR) positively influenced the dry weights of the roots, leaves, and total biomass, while AMF addition (i.e., MAN + PGPR + AMF) had a negative effect (Table 2).

Table 2.
Fresh and dry weights of different plant tissues, total biomass, and leaf/root ratio values of L. sativa L. (var. longifolia) plants among 6 treatments.
3.4. Plant Tissue Nutrient Concentrations
Leaf and root nutrient concentrations among treatments are shown in Table 3 and Table 4. Nitrogen and P concentrations were not significantly influenced among the treatments, whereas significant differences in K concentration were found in the organic fertilization regime between the MAN and MAN + PGPR + AMF treatments (Table 3). The lowest leaf Mg concentration was observed in the MAN treatment (0.39% D.W.); significant differences in Mg uptake were only found between the (i) MAN and (ii) MAN + PGPR, MAN + PGPR + AMF treatments (Table 3).

Table 3.
Leaf nutrient concentrations of L. sativa L. (var. longifolia) plants among 6 treatments.

Table 4.
Root nutrient concentrations of L. sativa L. (var. longifolia) plants among 6 treatments.
In both the I.F. and organic (MAN) fertilization regimes, PGPR and AMF application significantly increased leaf Fe concentrations (Table 3). Despite the beneficial role of PGRP and AMF in Fe uptake, a similar influence was not found for the other micronutrients. In the IF regime, for example, foliar Mn, Zn, Cu, and B concentrations were significantly decreased after PGPR and/or AMF application, while in the MAN regime, the addition of PGPR and AMF did not significantly affect the uptake of these micronutrients (Table 3).
3.5. Physiological Performance (SPAD, Performance Index-PI, Photosynthetic Rate, Intercellular CO2, and Water Use Efficiency—WUEi) of Lettuce Plants
As shown in Figure 2, significantly higher values of SPAD were determined for the combined application of PGPR and AMF in the I.F. regime compared to the control (inorganic fertilization only). Similarly, the highest value for the performance index (PI) was recorded for the I.F. + PGPR + AMF treatment, and it was significantly higher than the values determined for the single I.F. and MAN applications (Figure 3B). The photosynthetic rate was significantly higher in the MAN + PGPR treatment group compared to the single MAN and I.F. applications as well as the I.F. + PGPR treatment (Figure 4A). Stomatal opening was significantly higher following the combined application of PGPR and AMF in the I.F. regime compared to the single applications of I.F. and MAN as well as the I.F. + PGPR and MAN + PGPR treatments (Figure 4B). Water use efficiency (WUEi) was significantly higher following the single MAN application compared to the MAN + PGPR + AMF treatment (Figure 4E).
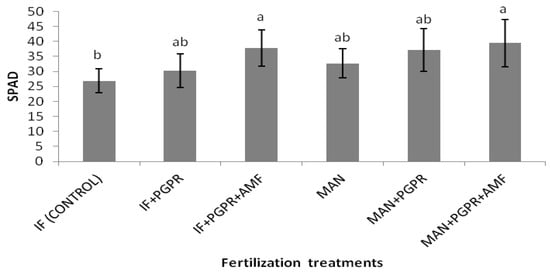
Figure 2.
SPAD values of Batavia lettuce plants under different fertilization treatments. Different letters on bars (means ± standard deviations) indicate statistically significant differences among treatments.
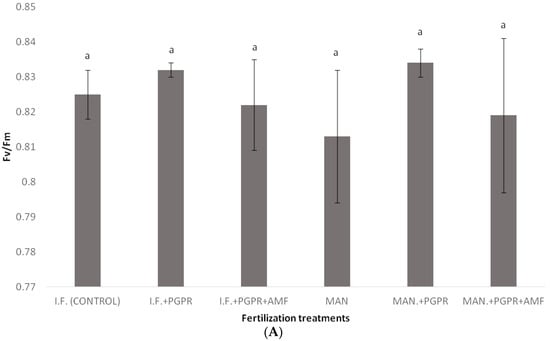
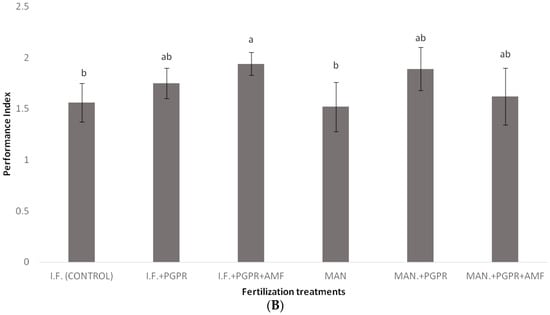
Figure 3.
Photosystem II (PSII) activity of Batavia lettuce plants, as indicated by parameters Fv/Fm (A) and performance index (PI) (B), following different fertilization treatments. Different letters on bars (means ± standard deviations) indicate statistically significant differences among treatments.
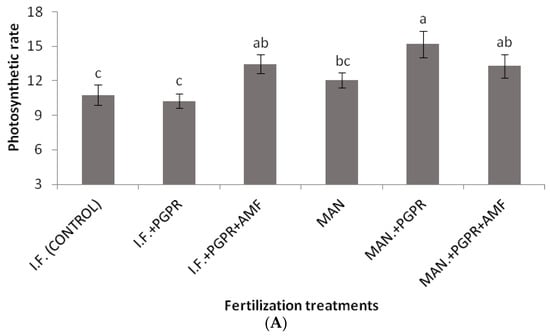
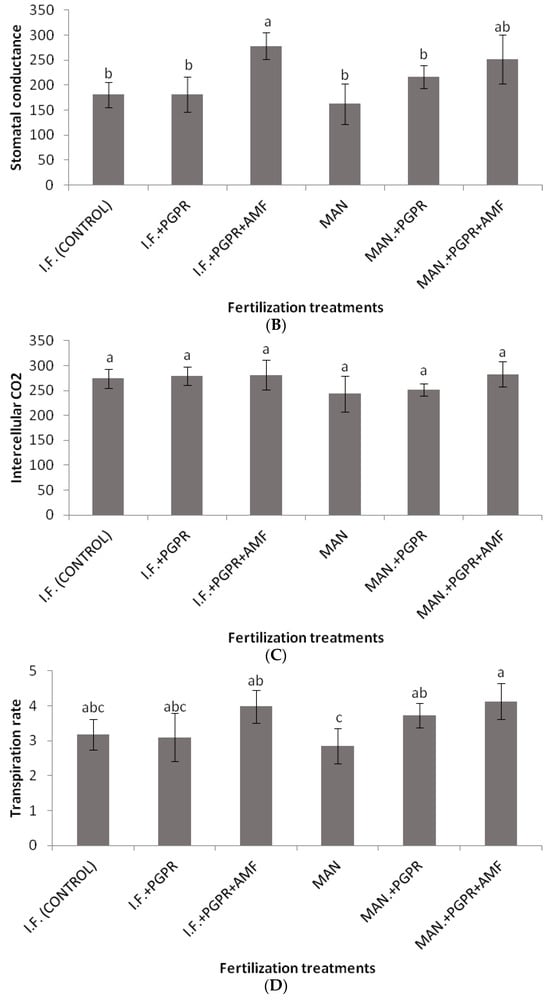
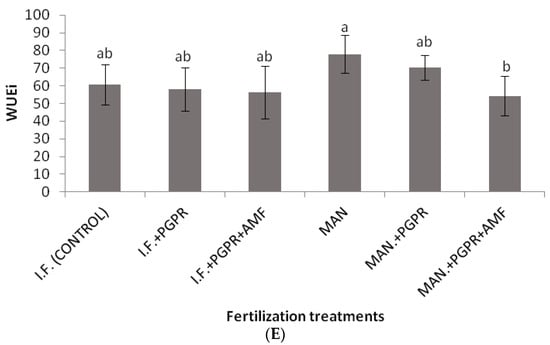
Figure 4.
Photosynthetic rate (μmol CO2 m−2s−1) (A), stomatal conductance (mmol m−2s−1) (B), intercellular CO2 concentration (μmol CO2 mol air−1) (C), transpiration rate (mmol H2O m−2s−1) (D), and intrinsic water use efficiency (WUEi) (μmol CO2 mol H2O−1) (E) of Batavia lettuce plants following different fertilization treatments. Different letters on bars (means ± standard deviations) indicate statistically significant differences among treatments.
4. Discussion
As shown in Table 1, it is clear that cow manure (MAN) was a good organic source of nutrients (particularly NO3-N, P, K, and micronutrients), contributing highly to the enrichment of the greenhouse soil (GS) with organic C. In terms of final soil fertility, significantly higher NO3-N concentrations were determined for the IF treatments compared to the organic ones (MAN, MAN + PGPR, and MAN + PGPR + AMF) (Table 1). This could be ascribed to the slow N mineralization rate of organic matter and agrees with other published data [43]. Similar conclusions can be drawn for the Olsen-determined P (lower concentrations were determined in the organic treatments compared to the IF ones) (Table 1). The differences in the Olsen-determined P concentrations between the inorganic and organic (MAN) regimes are probably due to the direct application of the 25-10-0 fertilizer in the IF treatment before the experimentation combined with the slow organic P mineralization rate in the MAN treatments, something which is also in agreement with other published data [44].
PGPR and AMF applications boosted soil respiration (CO2 flux); in the organic fertilization (MAN) regime in particular, differences among the three treatments were significant, and the highest CO2 flux was recorded for the simultaneous application (co-application) of PGPR and AMF (the MAN + PGPR + AMF treatment) (Figure 1). This is in agreement with other studies, which concluded that PGPR application boosted soil respiration [45,46]. Both the above-mentioned studies concluded that the combined application (co-application) of PGPR and organic fertilizers had a positive impact on soil quality. The boost in soil respiration after the combined application of biochar and PGPR was found to be dependent on soil type (a 100% increase in soil respiration in acidic soils and an approximately 50% increase in calcareous soils) [45]. In our study, approximately three- and sixfold higher soil respiration values were determined after the combined application of PGPR and AMF in the IF and MAN fertilization regimes, respectively, compared to the control (Figure 1), which is quite impressive. In contrast to the above data, it was found that PGPR application did not significantly influence soil respiration in Ginger (Zingiber officinale Rosc.) plantations compared to the application of a simple NPK fertilizer [47]. The differences in the effect of PGPR on soil respiration data in the above studies could possibly be ascribed to the different genera/species of PGPR used for experimentation.
PGPR and AMF additions in the inorganic fertilization (IF) regime positively influenced root, leaf, and total biomass dry weights. In particular, between the IF and IF + PGPR + AMF treatments, significant differences in the three dry weights were recorded (Table 2). Although other studies concluded that the simultaneous application of PGPR and organic fertilizers positively influenced plant growth and biomass [45,46], there seems to be a lack of published data on the co-application of inorganic fertilizers and PGPR as well as on the co-application of IF with PGPR and AMF. From this point of view and to the best of our knowledge, our study is the first one showing that the combined application of inorganic fertilizer with PGPR and AMF can have a significant impact on the leaf d.w. and total biomass of lettuce plants (Table 2). This was not observed in the organic fertilization (MAN) regime, in which the combined application effect of MAN, PGPR, and AMF was not significant compared to the MAN application only (Table 2). In contrast, significantly lower root, leaf, and total biomass dry weights were recorded for the MAN + PGPR + AMF treatment compared to the MAN + PGPR treatment (Table 2), indicating that the single application of PGPR with MAN was more beneficial than the one with PGPR and AMF combined. This result is quite interesting and needs to be further investigated in the near future. Other studies have shown that treatments involving biochar applications (at doses of 3 t ha−1) resulted in a 70% decrease in AMF infection [45]. It seems possible that a similar mechanism could be responsible in our case, i.e., MAN fertilization could have impeded root system infection by AMF. Of course, this hypothesis needs to be further investigated in the near future.
Significant differences in K uptake were found in the organic fertilization regime between the MAN and MAN + PGPR + AMF treatments (Table 3). The increase in K uptake after the simultaneous application of PGPR and AMF led to an increase in stomatal opening (Figure 4B), photosynthetic (Figure 4A) and transpiration (Figure 4D) rates. Potassium nutrition affects stomatal opening and photosynthetic and transpiration rates, as shown by other researchers [48,49]. In addition, significant differences between the (i) MAN treatment and the (ii) MAN + PGPR and MAN + PGPR + AMF treatments were found in Mg uptake (Table 3). Magnesium is part of the chlorophyll molecule; thus, the differences in Mg concentrations among the three organic (MAN) treatments could have led to differences in chlorophyll content, which could possibly explain the differences in the photosynthetic rate (Figure 4A). This hypothesis is also confirmed by the significantly higher SPAD values determined following the co-application of the PGPR and AMF treatments in the IF regime (Figure 2). Thus, the previous data may explain the beneficial effect of PGPR and AMF co-application on K and Mg uptake (in the organic (MAN) fertilization regime), as well as on SPAD (Figure 2), the photosynthetic rate (Figure 4A), and stomatal opening (Figure 4B). Of course, further research is needed to test this hypothesis regarding the influence of PGPR and AMF on Mg uptake by Batavia lettuce plants, as well as on their chlorophyll content and photosynthetic rates. In other studies with L. sativa [15,16], applications of AMF and Trichoderma sp. biostimulants showed increased N, P, and Mg uptake, which is in partial disagreement with our data. Maybe the differences between our results and those of the other researchers [15,16] could be ascribed to differences in the growth experimental conditions among the three studies (greenhouse soil experimentation with organic or inorganic fertilization and PGPR and AMF applications, a soilless system with sub-optimal P, and greenhouse experimentation with sub-optimal, optimal, and supra-optimal levels of N, respectively).
Τhe significant increase in Fe uptake after PGPR and AMF addition could influence SPAD values (Figure 2) since Fe plays a crucial role in chlorophyll synthesis without being part of its molecule. The enhanced SPAD values following the PGPR and AMF treatments could influence the photosynthetic rate of lettuce plants (Figure 4A); however, further investigation is needed into the roles of PGPR and AMF in Fe uptake and its subsequent effect on chlorophyll content and photosynthesis. The co-application of Azolla compost, Azolla biochar, and PGPR (P. fluorescens) has been found to increase most nutrient concentrations in rosemary plant tissues (shoot and root nutrient concentrations), while the highest shoot and root nutrient concentrations were determined in plants following the Azolla biochar + PGPR treatment [46], which is in partial agreement with our results, especially those concerning Fe (Table 3 and Table 4).
Although non-significant differences were found among the treatments in the Fv/Fm ratio, the performance index (PI) was a more reliable index, showing quicker reactions of PSII to various stresses, as also shown in previous studies [50]. In our study, the co-application of PGPR and AMF in the IF regime positively influenced the PI, causing significant a difference in its values between the IF and IF + PGPR + AMF treatments (Figure 3B). In contrast, in the MAN fertilization regime, the addition of PGPR and AMF did not significantly increase the PI (Figure 3B). Thus, it seems that PGPR and AMF co-application did not work as efficiently in the MAN fertilization regime as it worked in the IF regime, something that also confirms our plant growth data (Table 2). Similar to the PI, the photosynthetic rate showed a significant increase after PGPR and AMF co-application only in the IF regime and not in the MAN fertilization regime; in that case, only PGPR addition positively influenced the photosynthetic rate of the plants (Figure 4A). The conclusions for stomatal conductance, in which the influence of PGPR and AMF co-application was significant only in the IF and not in the MAN fertilization regime, were similar (although the addition of both PGPR and AMF increased stomatal opening, this tendency was non-significant) (Figure 4B). Thus, further research is needed to investigate and clarify the effects of PGPR and AMF on PI, the photosynthetic rate, and stomatal conductance in lettuce and other species. The intrinsic water use efficiency (WUEi), which shows how efficiently water is used at the plant level, showed a significant decline in plants in the MAN fertilization regime after simultaneous PGPR + AMF application (Figure 4E). Other studies have shown that PGPR (AG-54-Pseudomonas sp. and AG-70-Bacillus sp., applied separately or combined) improved water stress tolerance in wheat plants [51], while the WUEi was influenced by both AMF inoculation and by N and K fertilization [52,53].
5. Conclusions and Future Perspectives
The application of PGPR and AMF positively influenced soil quality and fertility as well as plant growth, nutrient uptake, SPAD, performance index, photosynthetic rate, and stomatal opening in Batavia lettuce plants under both inorganic (IF) and organic (MAN) fertilization conditions. In some cases, the results were more promising in the IF treatment; however, this tendency needs further investigation in the near future in order to clarify the effect(s) of the type of fertilization (organic or inorganic) on the effects of PGPR and AMF. Thus, their roles as soil improvers and plant growth promoters should be further examined in the frame of sustainable agriculture with the aim of decreasing high conventional fertilization rates.
Author Contributions
Conceptualization, T.C. and A.C.; methodology, T.C., K.Z., C.V., and A.C.; software: T.C.; validation, T.C. and A.C.; formal analysis, T.C.; investigation, T.C., K.Z., C.V., A.A., A.B., and A.E.G.; resources, T.C., K.Z., and C.V.; data curation, T.C., K.Z., C.V., A.E.G. and A.B.; writing—original draft preparation, T.C.; writing—review and editing, T.C., A.C., and C.V.; visualization, T.C. and K.Z.; supervision, T.C.; project administration, T.C.; funding acquisition, T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by small-scale projects of the Institute of Soil and Water Resources (ELGO DIMITRA).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors are thankful to the enterprise “COMERCO AGROTECHNOLOGY” for providing them with the commercial products “MICOSEEDS PLUS”, “CLONOTRI”, “STREPSE”, and “NUTRYACTION” for the needs of this research.
Conflicts of Interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mou, B. Nutritional quality of lettuce. Curr. Nutr. Food Sci. 2012, 8, 177–187. [Google Scholar] [CrossRef]
- Ahmed, Z.F.; Alnuaimi, A.K.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) production under hydroponic system: Nutrient solution derived from fish waste vs. inorganic nutrient solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- Shatilov, M.V.; Razin, A.F.; Ivanova, M.I. Analysis of the world lettuce market. IOP Conf. Ser. Earth Environ. Sci. 2019, 395, 012053. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; Garcia-Estevez, I.; Jimenez-Gomez, A.; Flores-Felix, J.D.; Escribano-Bailón, M.T.; Rivas, R. Rhizobium laguerreae Improves Productivity and Phenolic Compound Content of Lettuce (Lactuca sativa L.) under Saline Stress Conditions. Foods 2020, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Brahmachari, K.; Shankar, T.; Bhadra, P.; Palai, J.B.; et al. Intercropping—A low input agricultural strategy for food and environmental security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Gupta, L.M.; Gupta, M. Effect of integrated nutrient management on growth and yield of Aloe barbadensis. Indian J. Agric. Sci. 2016, 86, 91–95. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Tzanakakis, V.; Giannakoula, A.; Psoma, P. Inorganic and organic amendments affect soil fertility, nutrition, photosystem II activity and fruit weight and may enhance the sustainability of Solanum lycopersicon L. (cv. ‘Mountain Fresh’) crop. Sustainability 2020, 12, 9028. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Tsaniklidis, G.; Papaioannou, A.; Giannakoula, A.; Koukounaras, A. Comparative approach on the effects of soil amendments and a Controlled release fertilizer application on the growth, nutrient uptake, physiological performance and fruit quality of pepper (Capsicum annuum L.) plants. Agronomy 2022, 12, 1935. [Google Scholar] [CrossRef]
- Flores-Felix, J.D.; Menendez, E.; Rivera, L.P.; Marcos-Garcia, M.; Martinez-Hidalgo, P.; Mateos, P.F.; Martinez-Molina, E.; De la Encarnacion Velazquez, M.; Garcia-Fraile, P.; Rivas, R. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J. Plant Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar] [CrossRef]
- Stamford, N.P.; Felix, F.; Oliveira, W.; Silva, E.; Carolina, S.; Arnaud, T.; Dolores Freitas, A. Interactive effectiveness of microbial fertilizer enriched in N on lettuce growth and on characteristics of an Ultisol of the rainforest region. Sci. Hortic. 2019, 247, 242–246. [Google Scholar] [CrossRef]
- Kaymak, H.C.; Aksoy, A.; Kotan, R. Inoculation with N2 fixing plant growth promoting rhizobacteria to reduce nitrogen fertilizer requirement of lettuce. Acta Sci. Pol. Hortorum Cultus 2020, 19, 23–35. [Google Scholar] [CrossRef]
- Maldonado, S.; Rodriguez, A.; Avila, B.; Morales, P.; Gonzalez, M.P.; Araya Angel, J.P.A.; Olalde, V.; Bravo, J.; Jana, C.; Sierra, C.; et al. Enhanced crop productivity and sustainability by using native phosphate solubilizing rhizobacteria in the agriculture of arid zones. Front. Sustain. Food Syst. 2020, 4, 607355. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. The arbuscular mycorrhizal symbiosis can overcome reductions in yield and nutritional quality in greenhouse-lettuces cultivated at inappropriate growing seasons. Sci. Hortic. 2013, 164, 145–154. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Enhanced accumulation of vitamins, nutraceuticals and minerals in lettuces associated with arbuscular mycorrhizal fungi (AMF): A question of interest for both vegetables and humans. Agriculture 2013, 3, 188–209. [Google Scholar] [CrossRef]
- Cela, F.; Avio, L.; Giordani, T.; Vangelisti, A.; Cavallini, A.; Turrini, A.; Sbrana, C.; Pardossi, A.; Incrocci, L. Arbuscular mycorrhizal fungi increase nutritional quality of soilless grown lettuce, while overcoming low phosphorus supply. Foods 2022, 11, 3612. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Glola, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Azcon, R.; Gomez, M.; Tobar, R. Physiological and nutritional responses by Lactuca sativa L. to nitrogen sources and mycorrhizal fungi under drought conditions. Biol. Fertil. Soils 1996, 22, 156–161. [Google Scholar] [CrossRef]
- Kohler, J.; Hernandez, J.A.; Caravaca, F.; Roldan, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Roldan, A. An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol. Biochem. 2010, 42, 429–434. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of salt stress by plant growth-promoting bacteria in hydroponic leaf lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- Epelde, L.; Urra, J.; Anza, M.; Gamboa, J.; Garbisu, C. Inoculation of arbuscular mycorrhizal fungi increases lettuce yield without altering natural soil communities. Arch. Agron. Soil Sci. 2022, 68, 413–430. [Google Scholar] [CrossRef]
- Brito, L.M.; Pinto, R.; Mourao, I.; Coutinho, J. Organic lettuce, rye/vetch, and Swiss chard growth and nutrient uptake response to lime and horse manure compost. Org. Agric. 2012, 2, 163–171. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and antioxidant responses of lettuce (Lactuca sativa L.) to arbuscular mycorrhiza inoculation and seaweed extract foliar application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Yang, H.; Archdeacon, D.; Tippett, O.; Tibi, M.; Whiteley, A.S. Humus-rich compost increases lettuce growth, nutrient uptake, mycorrhizal colonization and soil fertility. Pedosphere 2019, 29, 170–179. [Google Scholar] [CrossRef]
- De Nobile, F.B.; Calero Hurtado, A.; De Melo Prado, R.; De Souza, H.A.; Anunciacao, M.G.; Palaretti, L.F.; Sizuki Nociti Dezem, L.A. A novel technology for processing urban waste compost as a fast-releasing nitrogen source to improve soil properties and broccoli and lettuce production. Waste Biomass Valorization 2021, 12, 6191–6203. [Google Scholar] [CrossRef]
- Hammermeister, A.M.; Astatkie, T.; Jeliazkova, E.A.; Warman, P.R.; Martin, R.C. Nutrient supply from organic amendments applied to unvegetated soil, lettuce and orchardgrass. Can. J. Soil Sci. 2006, 86, 21–33. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. The effects of phosphorus supply limitation on photosynthesis, biomass production, nutritional quality and mineral nutrition in lettuce grown in a recirculating nutrient solution. Sci. Hortic. 2019, 252, 379–387. [Google Scholar] [CrossRef]
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA; SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Gee, G.; Bauder, J. Particle-size analysis. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; Klute, A., Ed.; ASA; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA; SSSA: Madison, WI, USA, 1982; pp. 539–547. [Google Scholar]
- Hood-Nowotny, R.; Umana, N.H.-N.; Inselbacher, E.; Oswald-Lachouani, P.; Wanek, W. Alternative Methods for Measuring Inorganic, Organic, and Total Dissolved Nitrogen. Soil Sci. Soc. Am. J. 2010, 74, 1018–1027. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA; SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Thomas, G.W. Exchangeable cations methods of soil analysis. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA; SSSA: Madison, WI, USA, 1982; pp. 159–166. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Wolf, B. The determination of boron in soil extracts, plant materials, composts, manures, water and nutrient solutions. Com. Soil Sci. Plant Anal. 1971, 2, 363–374. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Centre: Wageningen, The Netherlands, 2002. [Google Scholar]
- Hansen, T.H.; De Bang, T.C.; Laursen, K.H.; Pedas, P.; Husted, S.; Schjoerring, J.K. Multielement plant tissue analysis using ICP spectrometry. In Plant Mineral Nutrients. Methods in Molecular Biology (Methods and Protocols); Maathuis, F., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 953. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; Division of Agricultural Sciences, University of California: Riverside, CA, USA, 1961; p. 309. [Google Scholar]
- Gaines, T.P.; Mitchell, G.A. Boron determination in plant tissue by the azomethine-H method. Commun. Soil Sci. Plant Anal. 1979, 10, 1099–1108. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G., Govindjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Chatzistathis, T.; Papadakis, I.E.; Papaioannou, A.; Chatzissavvidis, C.; Giannakoula, A. Comparative study effects between manure application and a Controlled-release fertilizer on the growth, nutrient uptake, photosystem II activity and photosynthetic rate of Olea europaea L. (cv. ‘Koroneiki’). Sci. Hortic. 2020, 264, 109176. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Monokrousos, N.; Psoma, P.; Tziachris, P.; Metaxa, I.; Strikos, G.; Papadopoulos, F.H.; Papadopoulos, F.H. How fully productive olive trees (Olea europaea L., cv. ‘Chondrolia Chalkidikis’) manage to over-satisfy their P nutritional needs under low Olsen P availability in soils? Sci. Hortic. 2020, 265, 109251. [Google Scholar] [CrossRef]
- Rekasi, Μ.; Szili-Kovacs, T.; Takacs, T.; Bernhadrt, B.; Puspan, I.; Kovacs, R.; Kutasi, J.; Draskovits, E.; Molnar, S.; Molnar, M.; et al. Improving the fertility of sandy soils in the temperate region by combined biochar and microbial inoculants treatments. Arch. Agron. Soil Sci. 2019, 65, 44–57. [Google Scholar] [CrossRef]
- Sadegh-Kasmaei, L.; Yasrebi, J.; Zarei, M.; Ronaghi, A.; Ghasemi, R.; Saharkhiz, M.J.; Ahmadabadi, Z.; Schnug, E. Influence of plant growth promoting rhizobacteria, compost and biochar of Azolla on Rosemary (Rosmarinus officinalis L.) growth and some soil quality indicators in a calcareous soil. Commun. Soil Sci. Plant Anal. 2019, 50, 119–131. [Google Scholar] [CrossRef]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Srinivasan, V.; Bini, Y.K.; Subila, K.P.; Aravind, R.; Hamza, S. Effects of plant growth-promoting rhizobacteria and NPK fertilizers on biochemical and microbial properties of soils under ginger (Zingiber officinale) cultivation. Agric. Res. 2013, 2, 346–353. [Google Scholar] [CrossRef]
- Arquero, O.; Barranco, D.; Benlloch, M. Potassium starvation increases stomatal conductance in olive trees. HortScience 2006, 41, 433–436. [Google Scholar] [CrossRef]
- Therios, I. Olives. In Crop Production Science in Horticulture; CAB: International, UK, 2009; pp. 156–163. [Google Scholar]
- Chatzistathis, T.; Papadakis, I.E.; Papaioannou, A.; Dichala, O.; Giannakoula, A.; Kostas, S.; Tziachris, P. Genotypic tolerance of two Punica granatum L. cultivars (Wonderful and Acco) to serpentine stress. Sci. Hortic. 2019, 247, 344–355. [Google Scholar] [CrossRef]
- Mutumba, F.A.; Zagal, E.; Gerding, M.; Castillo-Rosales, D.; Paulino, L.; Schoebitz, M. Plant growth promoting rhizobacteria for improved water stress tolerance in wheat genotypes. J. Soil Sci. Plant Nutr. 2018, 18, 1080–1096. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhang, H.; Jing, Q.; Wu, Y.X.; Xiao, S.Z.; Wang, M.M. Effect of mycorrhiza fungi inoculation and N fertilization on physiological characteristics, growth and nitrogen and phosphorus uptake of wheat under two distinct water regimes. Agric. Res. Arid Areas 2019, 37, 214–220. [Google Scholar]
- Zhu, L.D.; Shao, X.H.; Zhang, Y.C.; Zhang, H.; Hou, M.M. Effects of K fertilizer application on photosynthesis and seedling growth of sweet potato under drought stress. J. Food Agric. Environ. 2012, 10, 487–491. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).