Variation in Fruit Traits and Seed Nutrient Compositions of Wild Camellia oleifera: Implications for Camellia oleifera Domestication
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Collection
2.2. Data Collection (or Evaluated Traits) for Wild C. oleifera
2.2.1. Fruit Trait Measurement
2.2.2. Determination of Chemical Composition of Fruits
Determination of the Oil Content of the Kernel
Determination of Fatty Acid Composition
Determination of the Tocopherol Content
Measurement of Squalene
Reagents and Equipment
2.3. Data Collection (or Evaluated Traits) of the Cultivated C. oleifera
2.4. Statistical Analysis
3. Results
3.1. Comprehensive Evaluation of Yield Traits and Seed Chemical Composition of Wild C. oleifera Fruits from Different Seed Sources
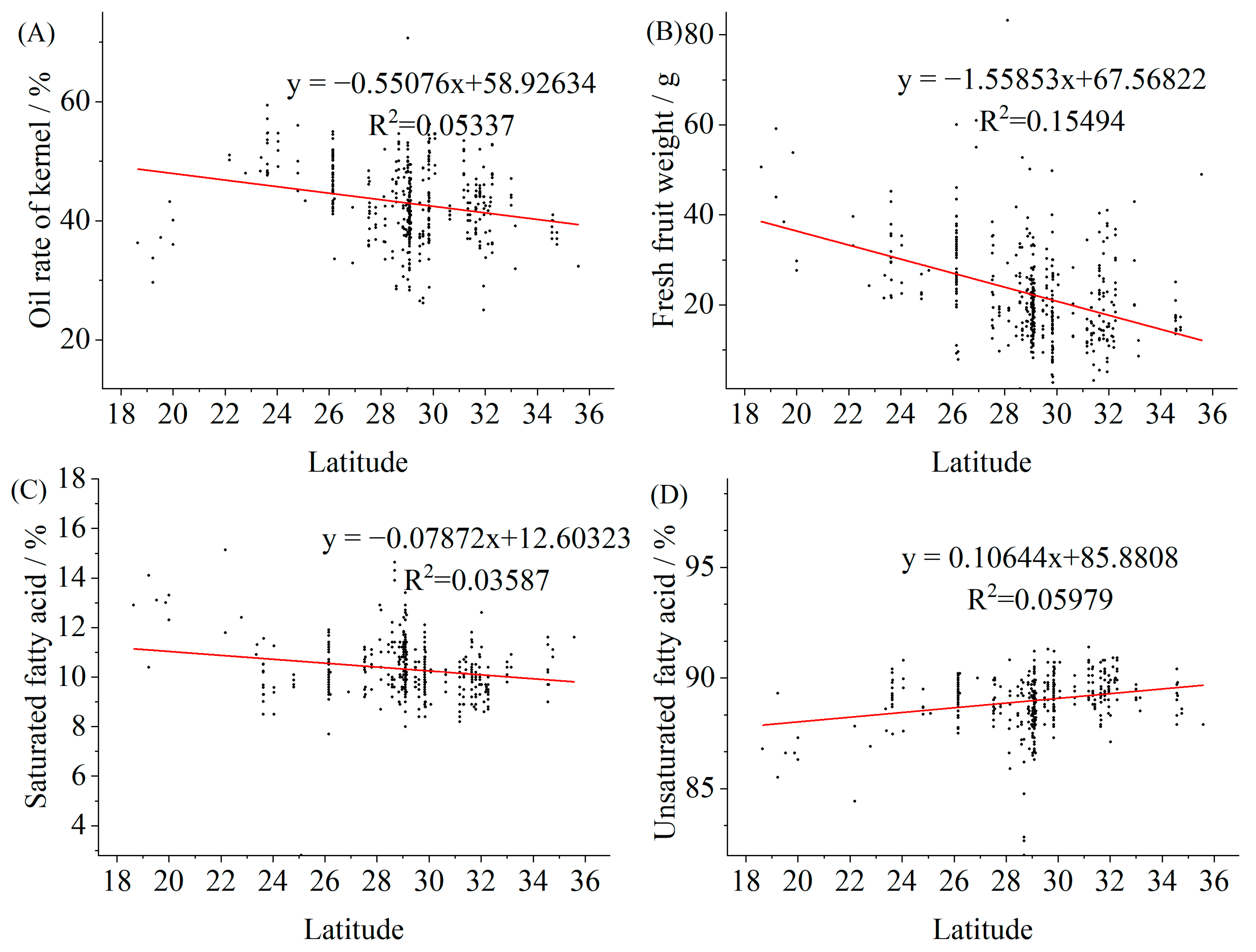
3.2. Correlations between 25 Quantitative Traits of Wild C. oleifera and Meteorological Factors
3.3. Trait Characteristics of 434 Cultivated C. oleifera Varieties
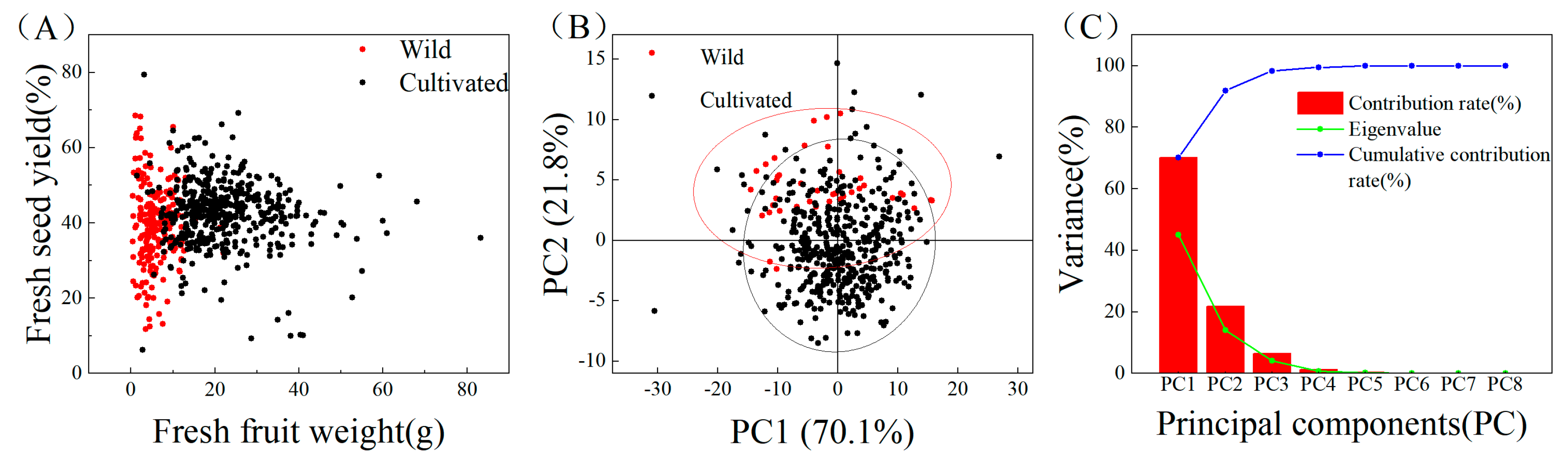
3.4. Principal Component Analysis
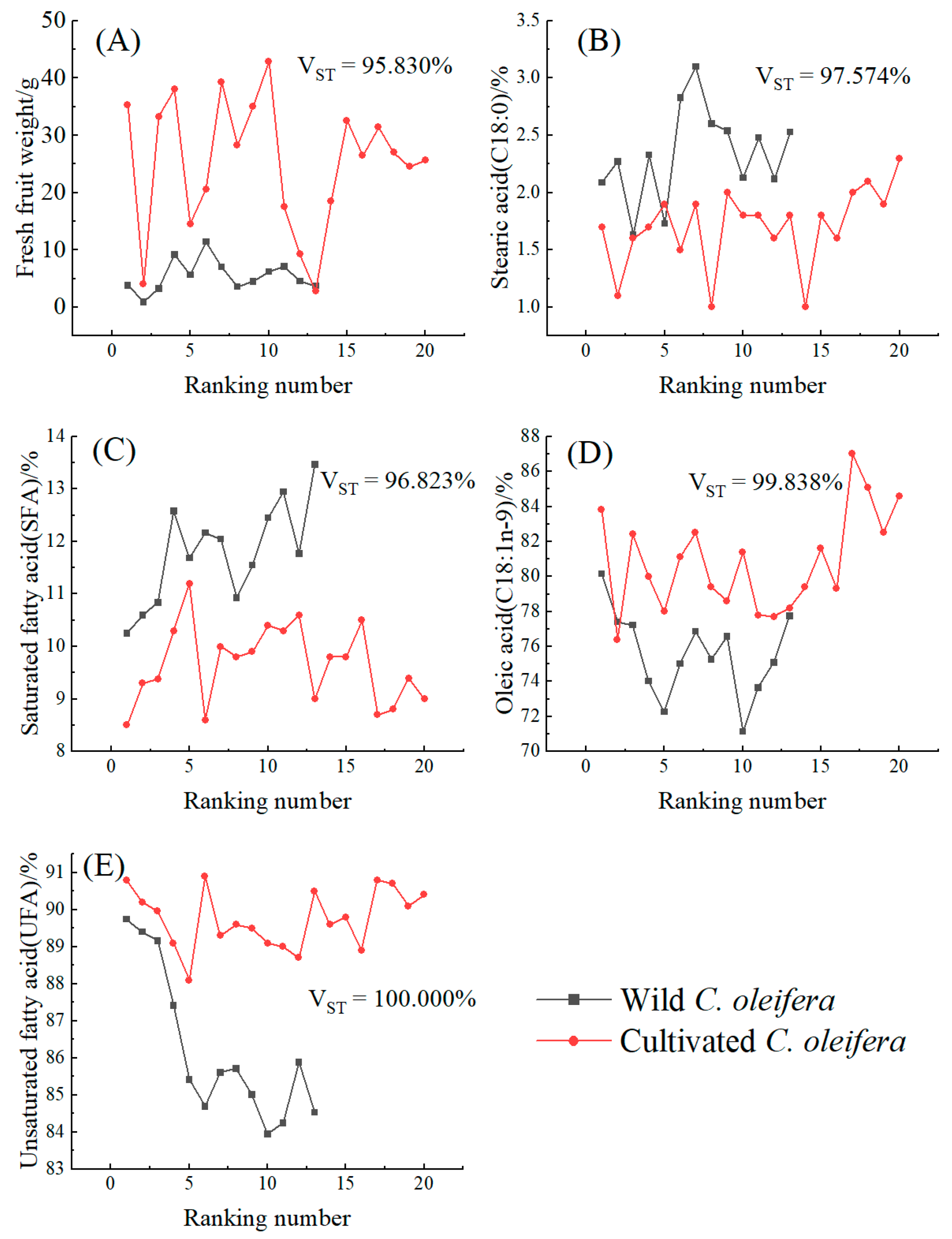
3.5. Comparative Analysis of Fruit Yield Traits and Seed Chemical Composition between Wild C. oleifera and Cultivars
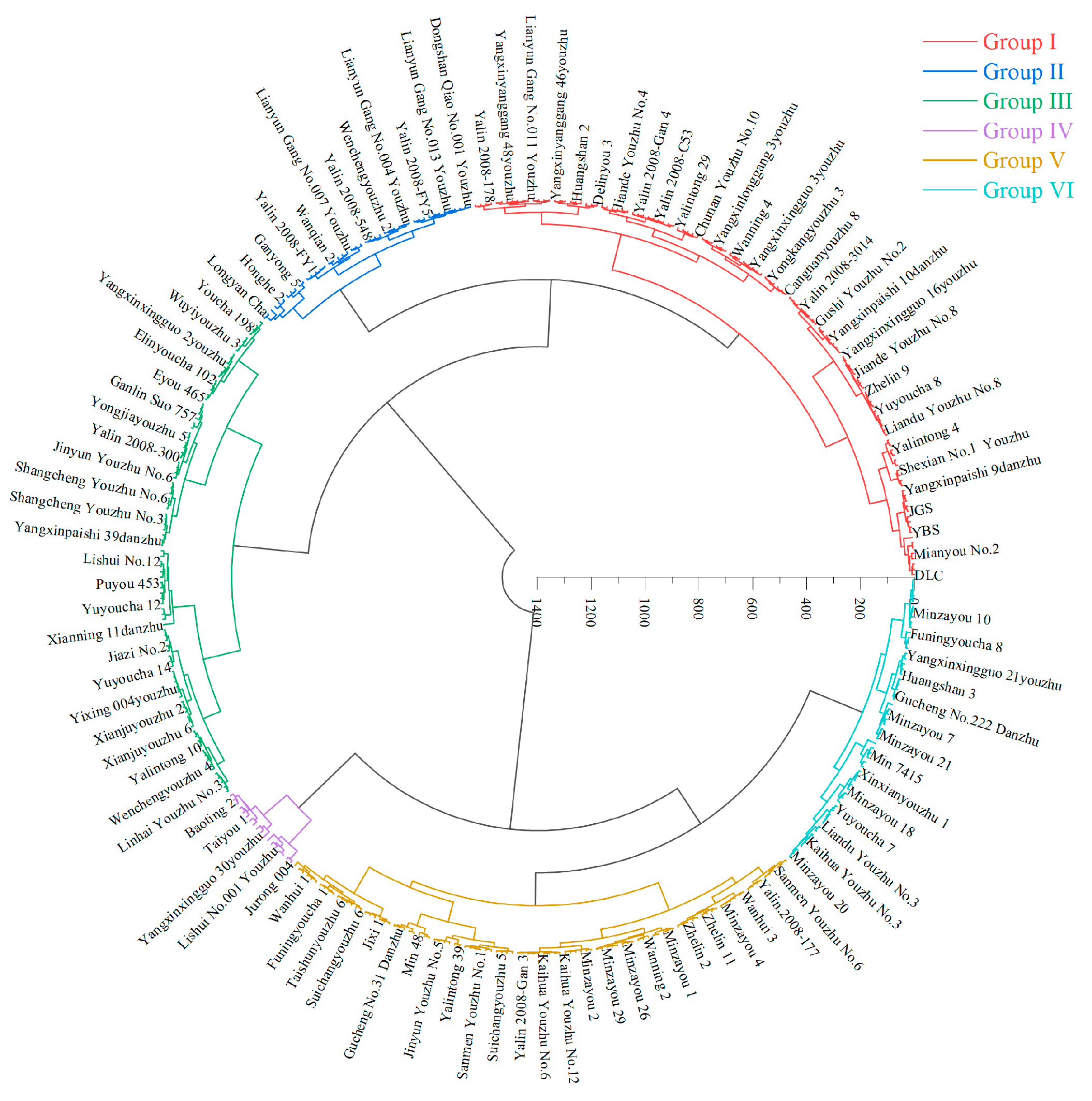
3.6. Cluster Analysis
3.7. TOPSIS Comprehensive Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Qin, S.; Rong, J.; Zhang, W.; Chen, J. Cultivation history of Camellia oleifera and genetic resources in the Yangtze River Basin. Biodivers. Sci. 2018, 26, 384–395. [Google Scholar] [CrossRef]
- Ma, J.; Ye, H.; Rui, Y.; Chen, G.; Zhang, N. Fatty acid composition of Camellia oleifera oil. J. Verbrauch Lebensm. 2011, 6, 9–12. [Google Scholar] [CrossRef]
- Kong, Q.; Jiang, H.; Guo, C.; Chen, T.; Feng, S.; Ding, C.; Zhou, L. Research Progress on Main Chemical Constituents and Pharmacological Activities of Camellia oleifera. J. Chin. Cereals Oils Assoc. 2022, 37, 194–202. [Google Scholar]
- Gong, W.; Song, Q.; Ji, K.; Gong, S.; Wang, L.; Chen, L.; Zhang, J.; Yuan, D. Full-Length Transcriptome from Camellia oleifera Seed Provides Insight into the Transcript Variants Involved in Oil Biosynthesis. J. Agric. Food Chem. 2020, 68, 14670–14683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Wang, A.; Gao, C.; Li, C. Applications of Chinese Camellia oleifera and its By-Products: A Review. Front. Chem. 2022, 10, 921246. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, L.; Ma, J.; Xia, S.; Gong, S.; Yin, Y.; Chen, Y. Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids. Nutrients 2022, 14, 2424. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qiu, S.; Yang, R.; Tian, H.; Tang, T.; Liao, D.; Li, J.; Li, S. Research Progress of Edible and Medicinal Value of Camellia Oleifera Seeds. Mod. Tradit. Chin. Med. Mater. Medica-World Sci. Technol. 2019, 21, 2770–2774. [Google Scholar]
- Cui, X.; Wang, W.; Yang, X.; Li, S.; Qin, S.; Rong, J. Potential distribution of wild Camellia oleifera based on ecological niche modeling. Biodivers. Sci. 2016, 24, 1117–1128. [Google Scholar] [CrossRef]
- Cui, X.; Li, C.; Qin, S.; Huang, Z.; Gan, B.; Jiang, Z.; Huang, X.; Yang, X.; Li, Q.; Xiang, X.; et al. High-throughput sequencing-based microsatellite genotyping for polyploids to resolve allele dosage uncertainty and improve analyses of genetic diversity, structure and differentiation: A case study of the hexaploid Camellia oleifera. Mol. Ecol. Resour. 2022, 22, 199–211. [Google Scholar] [CrossRef]
- Miller, A.J.; Gross, B.L. From forest to field: Perennial fruit crop domestication. Am. J. Bot. 2011, 98, 1389–1414. [Google Scholar] [CrossRef] [PubMed]
- Purugganan, M.D. Evolutionary Insights into the Nature of Plant Domestication. Curr. Biol. 2019, 29, R705–R714. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kantar, M.B.; Moxley, D.; Ortiz-Barrientos, D.; Rieseberg, L.H. Crop Adaptation to Climate Change: An Evolutionary Perspective. Mol. Plant 2023, 16, 1518–1546. [Google Scholar] [CrossRef]
- Song, B.; Ning, W.; Wei, D.; Jiang, M.; Zhu, K.; Wang, X.; Edwards, D.; Odeny, D.A.; Cheng, S. Plant genome resequencing and population genomics: Current status and future prospects. Mol. Plant 2023, 16, 1252–1268. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Genetics of domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef]
- Li, G.; Ma, L.; Yan, Z.; Zhu, Q.; Cai, J.; Wang, S.; Yuan, Y.; Chen, Y.; Deng, S. Extraction of Oils and Phytochemicals from Camellia oleifera Seeds: Trends, Challenges, and Innovations. Processes 2022, 10, 1489. [Google Scholar] [CrossRef]
- Lesack, K.; Naugler, C. An open-source software program for performing Bonferroni and related corrections for multiple comparisons. J. Pathol. Inform. 2011, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, Z.; Ma, Q.; Liu, J.; Liu, Y.; Liang, W.; Zhang, T.; Yin, D.; Liu, W.; Qiao, Q. A study on the phenotypic diversity of Sinopodophyllum hexandrum (Royle) ying. Pak. J. Bot. 2022, 54, 2291–2302. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.; Zhou, Y.; Zheng, Q.; Luo, S.; Xiang, T.; Zhou, L.; Feng, S.; Yang, H.; Ding, C. Characterization and comprehensive evaluation of phenotypic characters in wild Camellia oleifera germplasm for conservation and breeding. Front. Plant Sci. 2023, 14, 1052890. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil authentication: A comparative analysis and recent analytical techniques developed for its assessment. A review. Trends Food Sci. Technol. 2020, 97, 88–99. [Google Scholar] [CrossRef]
- Milla, R.; Osborne, C.P.; Turcotte, M.M.; Violle, C. Plant domestication through an ecological lens. Trends Ecol. Evol. 2015, 30, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Breton, C.; Terral, J.-F.; Pinatel, C.; Médail, F.; Bonhomme, F.; Bervillé, A. The origins of the domestication of the olive tree. CR Biol. 2009, 332, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zou, Y.; Guo, X.; Li, H.; Lu, H. Fatty acid desaturases (FADs) modulate multiple lipid metabolism pathways to improve plant resistance. Mol. Biol. Rep. 2022, 49, 9997–10011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Barg, R.; Yin, M.G.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant, J. 2005, 44, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Murata, N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid Res. 2003, 42, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, J.; Cheng, J.; Zhao, S.; Wen, Q.; Kong, P.; Zhao, Y.; Xiang, X.; Rong, J. Field plus lab experiments help identify freezing tolerance and associated genes in subtropical evergreen broadleaf trees: A case study of Camellia oleifera. Front. Plant Sci. 2023, 14, 1113125. [Google Scholar] [CrossRef] [PubMed]
- Zeder, M.A. Core questions in domestication research. Proc. Natl. Acad. Sci. USA 2015, 112, 3191–3198. [Google Scholar] [CrossRef]
- Chen, Y.H.; Gols, R.; Benrey, B. Crop Domestication and Its Impact on Naturally Selected Trophic Interactions. Annu. Rev. Entomol. 2015, 60, 35–58. [Google Scholar] [CrossRef]
- Levis, C.; Costa, F.R.C.; Bongers, F.; Peña-Claros, M.; Clement, C.R.; Junqueira, A.B.; Neves, E.G.; Tamanaha, E.K.; Figueiredo, F.O.G.; Salomao, R.P.; et al. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 2017, 355, 925–931. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Turcotte, M.M.; Poveda, K. Domestication impacts on plant-herbivore interactions: A meta-analysis. Philos. Trans. R Soc. B Biol. Sci. 2017, 372, 20160034. [Google Scholar] [CrossRef]
- Kassa, A.; Konrad, H.; Geburek, T. Molecular diversity and gene flow within and among different subspecies of the wild olive (Olea europaea L.): A review. Flora 2019, 250, 18–26. [Google Scholar] [CrossRef]
- Besnard, G.; Terral, J.-F.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef]
- Gaut, B.S.; Diez, C.M.; Morrell, P.L. Genonics and the Contrasting Dynamics of Annual and Perennial Domestication. Trends Genet. 2015, 31, 709–719. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Xu, J.; Korban, S.S.; Fei, Z.; Tao, S.; Ming, R.; Tai, S.; Khan, A.M.; Postman, J.D.; et al. Diversification and independent domestication of Asian and European pears. Genome Biol. 2018, 19, 77. [Google Scholar] [CrossRef]
- Jahed, K.R.; Hirst, P.M. Fruit growth and development in apple: A molecular, genomics and epigenetics perspective. Front. Plant Sci. 2023, 14, 1122397. [Google Scholar] [CrossRef]
- Ye, C.; He, Z.; Peng, J.; Wang, R.; Wang, X.; Fu, M.; Zhang, Y.; Wang, A.; Liu, Z.; Jia, G.; et al. Genomic and genetic advances of oiltea-camellia (Camellia oleifera). Front. Plant Sci. 2023, 14, 1101766. [Google Scholar] [CrossRef]
- Yan, C.; Lin, P.; Lyu, T.; Hu, Z.; Fan, Z.; Li, X.; Yao, X.; Li, J.; Yin, H. Unraveling the Roles of Regulatory Genes during Domestication of Cultivated Camellia: Evidence and Insights from Comparative and Evolutionary Genomics. Genes 2018, 9, 488. [Google Scholar] [CrossRef]
- La, T.C.; Pathan, S.M.; Vuong, T.; Lee, J.D.; Scaboo, A.M.; Smith, J.R.; Gillen, A.M.; Gillman, J.; Ellersieck, M.R.; Nguyen, H.T.; et al. Effect of High-Oleic Acid Soybean on Seed Oil, Protein Concentration, and Yield. Crop Sci. 2014, 54, 2054–2062. [Google Scholar] [CrossRef]
- Bachleda, N.; Grey, T.; Li, Z.L. Effects of high oleic acid soybean on seed yield, protein and oil contents, and seed germination revealed by near-isogeneic lines. Plant Breed 2017, 136, 539–547. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, B.; Ye, J.; He, Y.; Tang, S.; Wang, H.; Wen, Q. Comparative transcriptomic analysis reveals genes related to the rapid accumulation of oleic acid in Camellia chekiangoleosa, an oil tea plant with early maturity and large fruit. Plant Physiol. Biochem. 2022, 171, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Guo, M.; Wang, Y.; Duan, Z.; Yang, K.; Luan, X. Recent Advances in Research on Polyphenol Compounds in Camellia oleifera Seed Oil. Food Sci. 2021, 42, 311–320. [Google Scholar]
- Yang, J.; Chen, B.; Manan, S.; Li, P.; Liu, C.; She, G.; Zhao, S.; Zhao, J. Critical metabolic pathways and SAD/FADs, WRI1s, and DGATs cooperate for high-oleic acid oil production in developing oil tea (Camellia oleifera) seeds. Hortic Res. 2022, 9, uhac087. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ruan, C.; Han, P.; Ruan, D.; Xiong, C.; Ding, J.; Liu, S. Comparative transcriptomic analysis of high- and low-oil Camellia oleifera reveals a coordinated mechanism for the regulation of upstream and downstream multigenes for high oleic acid accumulation. 3 Biotech 2019, 9, 257. [Google Scholar] [CrossRef]
- Lin, P.; Wang, K.; Zhou, C.; Xie, Y.; Yao, X.; Yin, H. Seed Transcriptomics Analysis in Camellia oleifera Uncovers Genes Associated with Oil Content and Fatty Acid Composition. Int. J. Mol. Sci. 2018, 19, 118. [Google Scholar] [CrossRef]


| Fruit Characters and Nutrients | Among Provenances | Within Provenances | Random Error | Phenotypic Differentiation Coefficient/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Square | F Value | Component/% | Mean Square | F Value | Component/% | Mean Square | Component/% | ||
| Fresh fruit weight | 36604.016 | 475.468 ** | 95.637 | 1592.856 | 20.69 ** | 4.162 | 76.985 | 0.201 | 95.830 |
| Fresh seed yield | 1754.040 | 19.957 ** | 82.193 | 292.123 | 3.324 | 13.689 | 87.893 | 4.119 | 85.723 |
| Oil rate of kernel | 214.558 | 5.062 * | 45.778 | 211.747 | 4.996 * | 45.179 | 42.384 | 9.043 | 50.330 |
| Palmitic acid (C16:0) | 5.015 | 14.588 ** | 84.272 | 0.592 | 1.723 | 9.948 | 0.344 | 5.781 | 89.442 |
| Stearic acid (C18:0) | 18.061 | 14.402 ** | 91.383 | 0.449 | 0.358 | 2.272 | 1.254 | 6.345 | 97.574 |
| Saturated fatty acid (SFA) | 63.088 | 49.85 ** | 94.978 | 2.070 | 1.636 | 3.116 | 1.266 | 1.906 | 96.823 |
| Oleic acid (C18:1n-9) | 853.657 | 106.001 ** | 98.906 | 1.386 | 0.172 | 0.161 | 8.053 | 0.933 | 99.838 |
| Linoleic acid (C18:2n-6) | 27.434 | 4.628 * | 76.644 | 2.432 | 0.410 | 6.794 | 5.928 | 16.561 | 91.857 |
| Linolenic acid (C18:3n-3) | 0.336 | 16.782 ** | 66.142 | 0.152 | 7.597 ** | 29.921 | 0.020 | 3.937 | 68.852 |
| Unsaturated fatty acid (UFA) | 185.992 | 111.317 ** | 99.110 | 0.000 | 0.000 | 0.000 | 1.671 | 0.890 | 100.000 |
| Mean | 3972.620 | 81.806 | 83.504 | 210.381 | 4.091 | 11.524 | 22.580 | 4.972 | 87.627 |
| Groups | Fresh Fruit Weight (g) | Fresh Seed Yield (%) | Oil Rate of Kernel (%) | Fatty Acid Composition of C. oleifera Oils(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stearic Acid (C18:0) | Palmitic Acid (C16:0) | Saturated Fatty Acid (SFA) | Oleic Acid (C18:1n-9) | Linoleic Acid (C18:2n-6) | Linolenic Acid (C18:3n-3) | Unsaturated Fatty Acid (UFA) | ||||
| I | 15.175 ± 0.535 d | 43.781 ± 0.357 b | 38.777 ± 0.469 c | 1.892 ± 0.044 bc | 8.568 ± 0.085 b | 10.483 ± 0.085 b | 79.710 ± 0.266 c | 8.516 ± 0.209 ab | 0.331 ± 0.016 | 88.714 ± 0.114 a |
| II | 15.979 ± 0.604 d | 55.272 ± 0.947 a | 42.816 ± 0.719 b | 1.811 ± 0.093 c | 8.710 ± 0.163 b | 10.521 ± 0.172 b | 80.341 ± 0.450 bc | 7.939 ± 0.459 bc | 0.309 ± 0.021 | 88.589 ± 0.249 ab |
| III | 15.708 ± 0.532 d | 35.030 ± 0.680 d | 43.556 ± 0.628 b | 2.058 ± 0.060 ab | 8.460 ± 0.106 bc | 10.523 ± 0.112 b | 81.162 ± 0.243 ab | 7.300 ± 0.205 cd | 0.282 ± 0.011 | 88.782 ± 0.136 bc |
| IV | 50.387 ± 3.117 a | 28.946 ± 3.372 e | 38.781 ± 1.853 c | 2.158 ± 0.263 a | 9.143 ± 0.291 a | 11.301 ± 0.379 a | 78.487 ± 1.038 d | 9.338 ± 0.975 a | 0.328 ± 0.029 | 88.153 ± 0.374 a |
| V | 25.896 ± 0.460 c | 44.541 ± 0.544 b | 44.385 ± 0.513 b | 1.943 ± 0.048 abc | 8.059 ± 0.130 cd | 10.002 ± 0.117 c | 81.897 ± 0.262 a | 7.062 ± 0.212 cd | 0.281 ± 0.015 | 89.240 ± 0.094 c |
| VI | 34.531 ± 0.747 b | 41.179 ± 0.641 c | 49.165 ± 0.623 a | 2.173 ± 0.079 a | 7.893 ± 0.114 d | 10.065 ± 0.131 c | 82.161 ± 0.325 a | 6.809 ± 0.257 d | 0.285 ± 0.016 | 89.255 ± 0.131 c |
| Cultivated | Wild | ||||||
|---|---|---|---|---|---|---|---|
| Variety | Cluster Group | Comprehensive Score | Rank | Population | Cluster Group | Comprehensive Score | Rank |
| Yunyoucha 9 | VI | 0.690 | 1 | LS | III | 0.440 | 260 |
| Xianning 15youzhu | I | 0.681 | 2 | LFS | I | 0.428 | 285 |
| Yunyoucha 14 | VI | 0.644 | 3 | JGS | I | 0.398 | 335 |
| Minzayou 22 | VI | 0.636 | 4 | NL | I | 0.354 | 387 |
| Yangxintongcha 208 | III | 0.628 | 5 | DLC | I | 0.335 | 401 |
| Shihe Youzhu No.2 | I | 0.623 | 6 | TK | I | 0.328 | 407 |
| Yongkangyouzhu 7 | VI | 0.616 | 7 | HK | I | 0.327 | 409 |
| Wanning 2 | V | 0.607 | 8 | HLT | I | 0.322 | 415 |
| Minzayou 25 | VI | 0.606 | 9 | DCP | I | 0.321 | 416 |
| Nanzheng 1 | VI | 0.604 | 10 | EM | III | 0.317 | 421 |
| Yangxinyanggang 48youzhu | I | 0.604 | 11 | BR | I | 0.312 | 428 |
| Minlong No.31 | II | 0.602 | 12 | QL | I | 0.309 | 430 |
| Xianning 11danzhu | III | 0.601 | 13 | YBS | I | 0.290 | 438 |
| Wanhui 2 | I | 0.600 | 14 | ||||
| Minzayou 24 | VI | 0.599 | 15 | ||||
| Minzayou 1 | V | 0.598 | 16 | ||||
| Yuyoucha 7 | VI | 0.596 | 17 | ||||
| Wanqi 2 | V | 0.595 | 18 | ||||
| Minzayou 9 | V | 0.591 | 19 | ||||
| Wanqi 3 | V | 0.589 | 20 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, K.-F.; Zou, Y.-J.; Xie, H.-X.; Chen, S.; Zhou, J.; Luo, X.-T.; Chen, G.-H.; Zhao, Y.; Deng, Z.-Y.; Rong, J.; et al. Variation in Fruit Traits and Seed Nutrient Compositions of Wild Camellia oleifera: Implications for Camellia oleifera Domestication. Horticulturae 2024, 10, 450. https://doi.org/10.3390/horticulturae10050450
Xing K-F, Zou Y-J, Xie H-X, Chen S, Zhou J, Luo X-T, Chen G-H, Zhao Y, Deng Z-Y, Rong J, et al. Variation in Fruit Traits and Seed Nutrient Compositions of Wild Camellia oleifera: Implications for Camellia oleifera Domestication. Horticulturae. 2024; 10(5):450. https://doi.org/10.3390/horticulturae10050450
Chicago/Turabian StyleXing, Kai-Feng, Yu-Jing Zou, Hao-Xing Xie, Shang Chen, Jun Zhou, Xie-Tian Luo, Gong-Hu Chen, Yao Zhao, Ze-Yuan Deng, Jun Rong, and et al. 2024. "Variation in Fruit Traits and Seed Nutrient Compositions of Wild Camellia oleifera: Implications for Camellia oleifera Domestication" Horticulturae 10, no. 5: 450. https://doi.org/10.3390/horticulturae10050450
APA StyleXing, K.-F., Zou, Y.-J., Xie, H.-X., Chen, S., Zhou, J., Luo, X.-T., Chen, G.-H., Zhao, Y., Deng, Z.-Y., Rong, J., Li, J., & Zhang, J. (2024). Variation in Fruit Traits and Seed Nutrient Compositions of Wild Camellia oleifera: Implications for Camellia oleifera Domestication. Horticulturae, 10(5), 450. https://doi.org/10.3390/horticulturae10050450





