Longevity and Potential Mechanisms of Fenpropathrin Resistance in Asian Citrus Psyllid, Diaphorina citri Kuwayama
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects, Plants, and Chemicals
2.2. Stability of Fenpropathrin Resistance
2.3. Nucleic Acid Extraction and cDNA Synthesis
2.4. Real-Time Quantitative PCR for Expression
2.5. Statistical Analysis
3. Results
3.1. Stability of Fenpropathrin Resistance among D. citri Populations
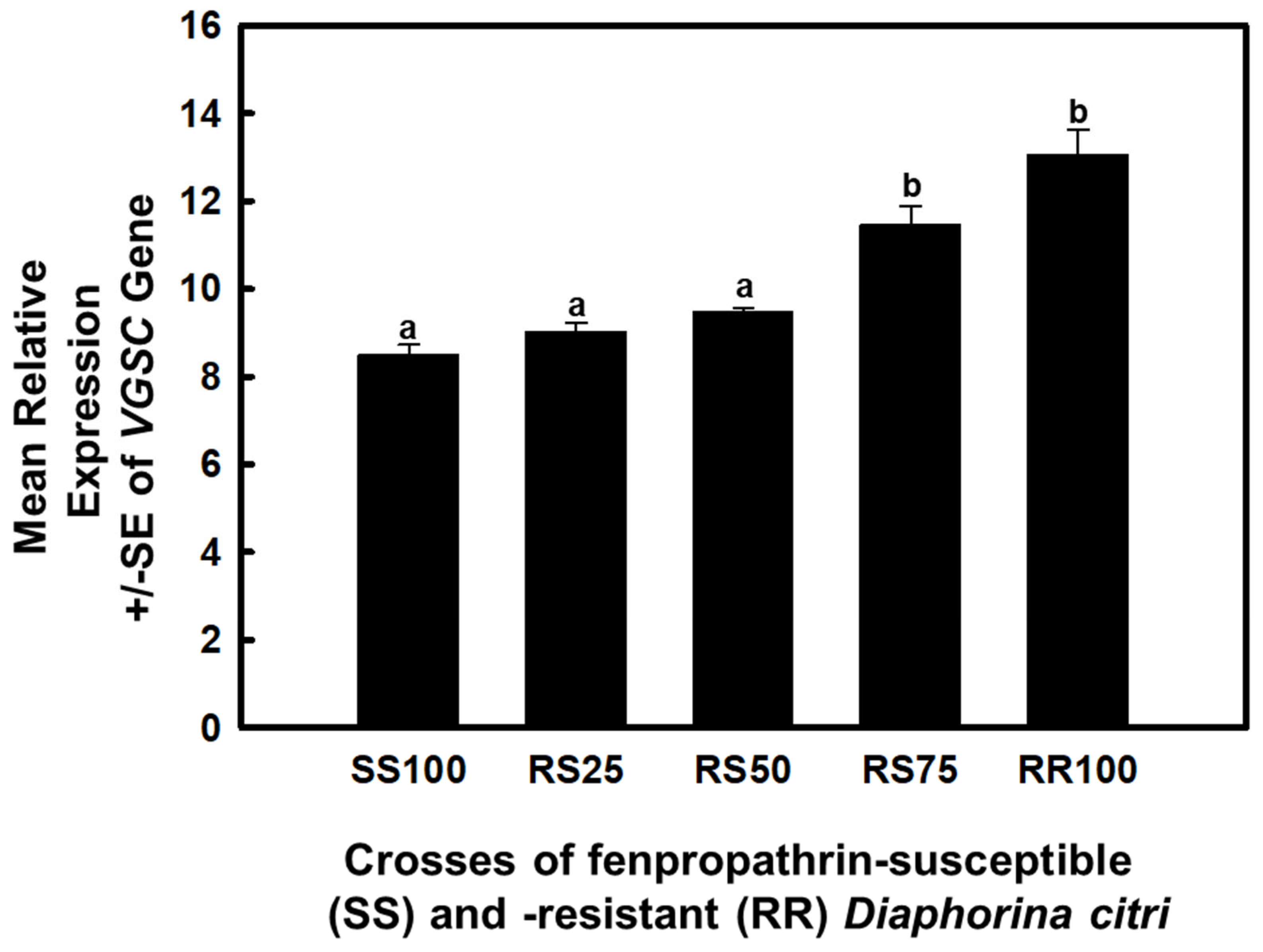
3.2. Expression of VGSC in Insecticide-Resistant, Susceptible and Crossed Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bove, J.M. Huanglongbing: A Destructive, Newly-Emerging, Century-Old Disease of Citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and Management of Asian Citrus Psyllid, Vector of the Huanglongbing Pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Kanga, L.H.B.; Eason, J.; Haseeb, M.; Qureshi, J.; Stansly, P. Monitoring for Insecticide Resistance in Asian Citrus Psyllid (Hemiptera: Psyllidae) Populations in Florida. J. Econ. Entomol. 2016, 109, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide Resistance in Field Populations of Asian Citrus Psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Mo, X.; Rizvi, S.A.H.; Li, C.; Zeng, X. Detection and Biochemical Characterization of Insecticide Resistance in Field Populations of Asian Citrus Psyllid in Guangdong of China. Sci. Rep. 2018, 8, 12587. [Google Scholar] [CrossRef] [PubMed]
- Coy, M.R.; Bin, L.; Stelinski, L.L. Reversal of Insecticide Resistance in Florida Populations of Diaphorina citri (Hemiptera: Liviidae). Fla. Entomol. 2016, 99, 26–32. [Google Scholar] [CrossRef]
- Vázquez-García, M.; Velázquez-Monreal, J.; Medina-Urrutia, V.M.; Cruz-Vargas, C.D.J.; Sandoval-Salazar, M.; Virgen-Calleros, G.; Torres-Morán, J.P. Insecticide Resistance in Adult Diaphorina citri Kuwayama1 from Lime Orchards in Central West Mexico. Southwest. Entomol. 2013, 38, 579–596. [Google Scholar] [CrossRef]
- Pardo, S.; Martínez, A.M.; Figueroa, J.I.; Chavarrieta, J.M.; Viñuela, E.; Rebollar-Alviter, Á.; Miranda, M.A.; Valle, J.; Pineda, S. Insecticide Resistance of Adults and Nymphs of Asian Citrus Psyllid Populations from Apatzingán Valley, Mexico. Pest Manag. Sci. 2018, 74, 135–140. [Google Scholar] [CrossRef]
- Naeem, A.; Freed, S.; Jin, F.L.; Akmal, M.; Mehmood, M. Monitoring of Insecticide Resistance in Diaphorina citri Kuwayama (Hemiptera: Psyllidae) from Citrus Groves of Punjab, Pakistan. Crop Prot. 2016, 86, 62–68. [Google Scholar] [CrossRef]
- Chen, X.D.; Sanada-Morimura, S.; Yanagi, S.I.; Nakasuji, F. Rapid Recovery of Susceptibility under Harsh Environmental Conditions in Fenvalerate-Resistant Strains of the Diamondback Moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 2006, 41, 641–650. [Google Scholar] [CrossRef]
- Afzal, M.B.S.; Shad, S.A.; Abbas, N.; Ayyaz, M.; Walker, W.B. Cross-Resistance, the Stability of Acetamiprid Resistance and Its Effect on the Biological Parameters of Cotton Mealybug, Phenacoccus solenopsis (Homoptera: Pseudococcidae), in Pakistan. Pest Manag. Sci. 2015, 71, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Saddiq, B.; Shahzad Afzal, M.B.; Shad, S.A. Studies on Genetics, Stability and Possible Mechanism of Deltamethrin Resistance in Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) from Pakistan. J. Genet. 2016, 95, 1009–1016. [Google Scholar] [CrossRef]
- Bielza, P.; Quinto, V.; Grávalos, C.; Fernández, E.; Abellán, J.; Contreras, J. Stability of Spinosad Resistance in Frankliniella occidentalis (Pergande) under Laboratory Conditions. Bull. Entomol. Res. 2008, 98, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.C.; Smith, L.B.; Silva, J.J.; Fan, Y.; Sun, H.; Scott, J.G. Fitness Studies of Insecticide Resistant Strains: Lessons Learned and Future Directions. Pest Manag. Sci. 2021, 77, 3847–3856. [Google Scholar] [CrossRef] [PubMed]
- Basit, M.; Sayyed, A.H.; Saleem, M.A.; Saeed, S. Cross-Resistance, Inheritance and Stability of Resistance to Acetamiprid in Cotton Whitefly, Bemisia tabaci Genn (Hemiptera: Aleyrodidae). Crop Prot. 2011, 30, 705–712. [Google Scholar] [CrossRef]
- Inoue, K. The Change of Susceptibility of Mite Population to Dicofol and Genetic Analysis of Dicofol-Resistance in the Citrus Red Mite, Panonychus citri (McG). J. Pestic. Sci. 1979, 4, 337–344. [Google Scholar] [CrossRef]
- Needham, P.H.; Sawicki, R.M. Diagnosis of Resistance to Organophosphorus Insecticides in Myzus persicae (Sulz). Nature 1971, 230, 125–126. [Google Scholar] [CrossRef]
- Keiding, J. Resistance in the Housefly in Denmark and Elsewhere Kriding Pesticide Management and Insecticide Resistance. In Pesticide Management and Insecticide Resistance; Watson, D.L., Brown, A.W.A., Eds.; Academic Press: New York, NY, USA, 1977; pp. 261–302. [Google Scholar]
- Scott, J.G.; Georghiou, G.P. Mechanisms Responsible for High Levels of Permethrin Resistance in the House Fly. Pestic. Sci. 1986, 17, 195–206. [Google Scholar] [CrossRef]
- Gajendiran, A.; Abraham, J. An Overview of Pyrethroid Insecticides. Front. Biol. 2018, 13, 79–90. [Google Scholar] [CrossRef]
- Catterall, W.A. From Ionic Currents to Molecular Mechanisms: The Structure and Function of Voltage-Gated Sodium Channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular Biology of Insect Sodium Channels and Pyrethroid Resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Coy, M.R.; Wang, J.J.; Stelinski, L.L. Characterization of the Voltage-Gated Sodium Channel of the Asian Citrus Psyllid, Diaphorina citri. Insect Sci. 2017, 24, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.S.; Martinez-Torres, D.; Hick, C.A.; Devonshire, A.L. Identification of Mutations in the Housefly Para-Type Sodium Channel Gene Associated with Knockdown Resistance (Kdr) to Pyrethroid Insecticides. Mol. Gen. Genet. 1996, 252, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M.; Knipple, D.C. The Molecular Biology of Knockdown Resistance to Pyrethroid Insecticides. Insect Biochem. Mol. Biol. 2003, 33, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T. Neuroreceptors and Ion Channels as the Basis for Drug Action: Past, Present, and Future. J. Pharmacol. Exp. Ther. 2000, 294, 1–26. [Google Scholar] [PubMed]
- Chen, X.D.; Sandoval-Mojica, A.F.; Bonilla, S.I.; Ebert, T.A.; Gossett, H.; Pelz-Stelinski, K.S.; Stelinski, L.L. Fenpropathrin Resistance in Asian Citrus Psyllid, Diaphorina citri Kuwayama: Risk Assessment and Changes in Expression of CYP and GST Genes Associated with Resistance. Int. J. Pest Manag. 2023, 69, 54–63. [Google Scholar] [CrossRef]
- Chen, X.D.; Stelinski, L.L. Resistance Management for Asian Citrus Psyllid, Diaphorina citri Kuwayama, in Florida. Insects 2017, 8, 103. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS Institute (2002–2012) User’s Manual, Version 9.4; SAS Institute: Cary, NC, USA, 2016.
- Robertson, J.L.; Preisler, H.K. Pesticide Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar] [CrossRef]
- Liu, Y.H.; Tsai, J.H. Effects of temperature and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Ann. Appl. Biol. 2000, 137, 201–206. [Google Scholar] [CrossRef]
- Robb, K.L. Analysis of Frankliniella occidentalis (Pergande) as a Pest of Floricultural Crops in California 405 Greenhouses. Ph.D. Thesis, University of California, Riverside, CA, USA, 1989. [Google Scholar]
- Kontsedalov, S.; Weintraub, P.G.; Horowitz, A.R.; Ishaaya, I. Effects of Insecticides on Immature and Adult Western Flower Thrips (Thysanoptera: Thripidae) in Israel. J. Econ. Entomol. 1998, 91, 1067–1071. [Google Scholar] [CrossRef]
- Chen, X.D.; Ebert, T.A.; Pelz-Stelinski, K.S.; Stelinski, L.L. Fitness Costs Associated with Thiamethoxam and Imidacloprid Resistance in Three Field Populations of Diaphorina citri (Hemiptera: Liviidae) from Florida. Bull. Entomol. Res. 2020, 110, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Roush, R.T.; Croft, B.A. Experimental population genetics and ecological studies of pesticide resistance in insects and mites. In Pesticide Resistance Strategies and Tactics for Management; National Academy Press: Washington, DC, USA, 1986; pp. 257–270. [Google Scholar]
- Denholm, I.; Rowland, M.W. Tactics for Managing Pesticide Resistance in Arthropods: Theory and Practice. Annu. Rev. Entomol. 1992, 37, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Nkya, T.E.; Akhouayri, I.; Kisinza, W.; David, J.P. Impact of Environment on Mosquito Response to Pyrethroid Insecticides: Facts, Evidences and Prospects. Insect Biochem. Mol. Biol. 2013, 43, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Amichot, M.; Castella, C.; Cuany, A.; Berge, J.B.; Pauron, D. Target Modification as a Molecular Mechanism of Pyrethroid Resistance in Drosophila melanogaster. Pestic. Biochem. Physiol. 1992, 44, 183–190. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, H.; Zhang, L.; Liu, N. Sodium Channel Gene Expression Associated with Pyrethroid Resistant House Flies and German Cockroaches. Gene 2006, 379, 62–67. [Google Scholar] [CrossRef]
- Sonoda, S.; Igaki, C.; Ashfaq, M.; Tsumuki, H. Pyrethroid-Resistant Diamondback Moth Expresses Alternatively Spliced Sodium Channel Transcripts with and without T929I Mutation. Insect Biochem. Mol. Biol. 2006, 36, 904–910. [Google Scholar] [CrossRef]
| Gene | Forward/Reverse | Sequence (Forward/Reverse) | Reference |
|---|---|---|---|
| F | AGCGGAAAATTACACGTGGG | ||
| VGSC | R | CGGATACCTTTGGCCCCTTT | [23] |
| F | CCCTGGACTTTGAACAGGAA | ||
| Actin | R | CTCGTGGATACCGCAAGATT | [27] |
| Line | Month | Slope ± SE | LC50 (ng/µL) (95 % Confidence Interval) | LC90 (ng/µL) (95 % Confidence Interval) |
|---|---|---|---|---|
| 100 RR + 00 SS | 0 | 0.78 ± 0.17 | 18.57 (3.31–161.99) | 912.09 (107.592–3966) |
| 2 | 0.69 ± 0.15 | 10.70 (1.52–134.51) | 781.53 (76.23–547546) | |
| 4 | 0.82 ± 0.12 | 11.90 (5.01–30.69) | 436.53 (131.87–3111) | |
| 6 | 0.82 ± 0.13 | 14.75 (5.324–3.67) | 532.98 (142.90–5757.00) | |
| 8 | 0.69 ± 0.12 | 5.02 (1.67–15.54) | 249.68 (61.72–3256.98) | |
| 75 RR + 25 SS | 2 | 0.88 ± 0.31 | 4.84 (2.21–11.13) | 570.42 (174.23–3169) |
| 4 | 0.81 ± 0.14 | 6.54 (1.50–35.52) | 329.94 (54.15–18627) | |
| 6 | 0.61 ± 0.09 | 7.50 (3.06–19.77) | 389.60 (59.00–1082) | |
| 8 | 0.89 ± 0.09 | 3.31 (1.42–7.54) | 91.38 (33.59–418) | |
| 50 RR + 50 SS | 2 | – | – | – |
| 4 | 0.60 ± 0.20 | 5.57 (1.18–35.54) | 303.57 (46.09–22548) | |
| 6 | 0.51 ± 0.07 | 2.51 (0.81–8.43) | 794.56 (144.00–13207) | |
| 8 | 0.88 ± 0.13 | 1.99 (0.80–5.12) | 102.19 (30.70–671.50) | |
| 25 RR + 75 SS | 2 | 0.42 ± 0.06 | 0.91 (0.30–2.63) | 314.73 (70.41–3281) |
| 4 | 0.56 ± 0.09 | 0.85 (0.15–5.34) | 164.11 (19.18–16330) | |
| 6 | 0.67 ± 0.09 | 0.81 (0.33–2.00) | 33.76 (13.13–235.75) | |
| 8 | 0.89 ± 0.13 | 0.77 (0.42–1.42) | 64.23 (27.35–196.74) | |
| 00 RR + 100 SS | 0 | 0.89 ± 0.12 | 0.35 (0.16–0.78) | 9.73 (3.803–8.46) |
| 2 | 1.11 ± 0.14 | 0.40 (0.22–0.77) | 5.71 (2.75–16.59) | |
| 4 | 0.76 ± 0.16 | 0.40 (0.06–3.62) | 19.36 (2.71–475.16) | |
| 6 | 0.70 ± 0.10 | 0.74 (0.29–1.90) | 48.70 (14.41–317.51) | |
| 8 | 0.79 ± 0.11 | 0.43 (0.27–0.67) | 16.67 (9.84–31.78) |
| Line | Month | RR50 | RR90 |
|---|---|---|---|
| 100 RR + 00 SS | 0 | 60.14 (16.99–212.83) | 951.00 (82.00–10941.00) |
| 2 | 34.00 (11.73–100.09) | 80.32 (13.00–472.00) | |
| 4 | 32.85 (13.63–79.14) | 31.07 (7.3–7131.01) | |
| 6 | 19.96 (5.64–70.63) | 10.95 (1.37–87.25) | |
| 8 | 10.83 (2.91–40.34) | 20.14 (3.16–128.36) | |
| 75 RR + 25 SS | 2 | 13.92 (4.56–42.48) | 58.60 (9.61–357.47) |
| 4 | 18.06 (7.38–44.17) | 23.48 (5.37–102.64) | |
| 6 | 10.15 (2.78–37.09) | 35.20 (0.93–69.70) | |
| 8 | 7.61 (2.20–26.32) | 5.48 (1.04–28.81) | |
| 50 RR + 50 SS | 2 | – | – |
| 4 | 15.85 (6.46–38.95) | 22.57 (5.14–99.17) | |
| 6 | 3.39 (0.78–14.75) | 16.32 (1.20–221.31) | |
| 8 | 2.69 (0.88–8.27) | 2.10 (0.34–12.84) | |
| 25 RR + 75 SS | 2 | 3.89 (0.84–18.09) | 30.45 (3.69–251.60) |
| 4 | 2.35 (0.77–7.20) | 11.68 (1.75–77.59) | |
| 6 | 1.15 (0.2–83.94) | 1.32 (0.16–11.15) | |
| 8 | 1.97 (0.51–7.57) | 1.41 (0.22–9.16) | |
| 00 RR + 100 SS | 0 | 1 (0.34–2.98) | 1 (0.19–5.47) |
| 2 | 1 (0.35–2.83) | 1 (0.18–5.47) | |
| 4 | 1 (0.40–2.47) | 1 (0.23–4.26) | |
| 6 | 1 (0.27–3.75) | 1 (0.11–9.23) | |
| 8 | 1 (0.22–4.55) | 1 (0.14–7.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Stockton, D.G.; Gill, T.A.; Gossett, H.; Qureshi, J.A.; Pelz-Stelinski, K.S.; Stelinski, L.L. Longevity and Potential Mechanisms of Fenpropathrin Resistance in Asian Citrus Psyllid, Diaphorina citri Kuwayama. Horticulturae 2024, 10, 448. https://doi.org/10.3390/horticulturae10050448
Chen X, Stockton DG, Gill TA, Gossett H, Qureshi JA, Pelz-Stelinski KS, Stelinski LL. Longevity and Potential Mechanisms of Fenpropathrin Resistance in Asian Citrus Psyllid, Diaphorina citri Kuwayama. Horticulturae. 2024; 10(5):448. https://doi.org/10.3390/horticulturae10050448
Chicago/Turabian StyleChen, Xuedong, Dara G. Stockton, Torrence A. Gill, Hunter Gossett, Jawwad A. Qureshi, Kirsten S. Pelz-Stelinski, and Lukasz L. Stelinski. 2024. "Longevity and Potential Mechanisms of Fenpropathrin Resistance in Asian Citrus Psyllid, Diaphorina citri Kuwayama" Horticulturae 10, no. 5: 448. https://doi.org/10.3390/horticulturae10050448
APA StyleChen, X., Stockton, D. G., Gill, T. A., Gossett, H., Qureshi, J. A., Pelz-Stelinski, K. S., & Stelinski, L. L. (2024). Longevity and Potential Mechanisms of Fenpropathrin Resistance in Asian Citrus Psyllid, Diaphorina citri Kuwayama. Horticulturae, 10(5), 448. https://doi.org/10.3390/horticulturae10050448








