Evaluation of Quality and Microbial Communities in Fermented Chinese Mustard Greens from Guangdong Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Evaluation of Colour and Texture
2.3. DNA Extraction and PCR Amplification
2.4. Illumina MiSeq Sequencing and Processing
2.5. Statistical Analysis
3. Results
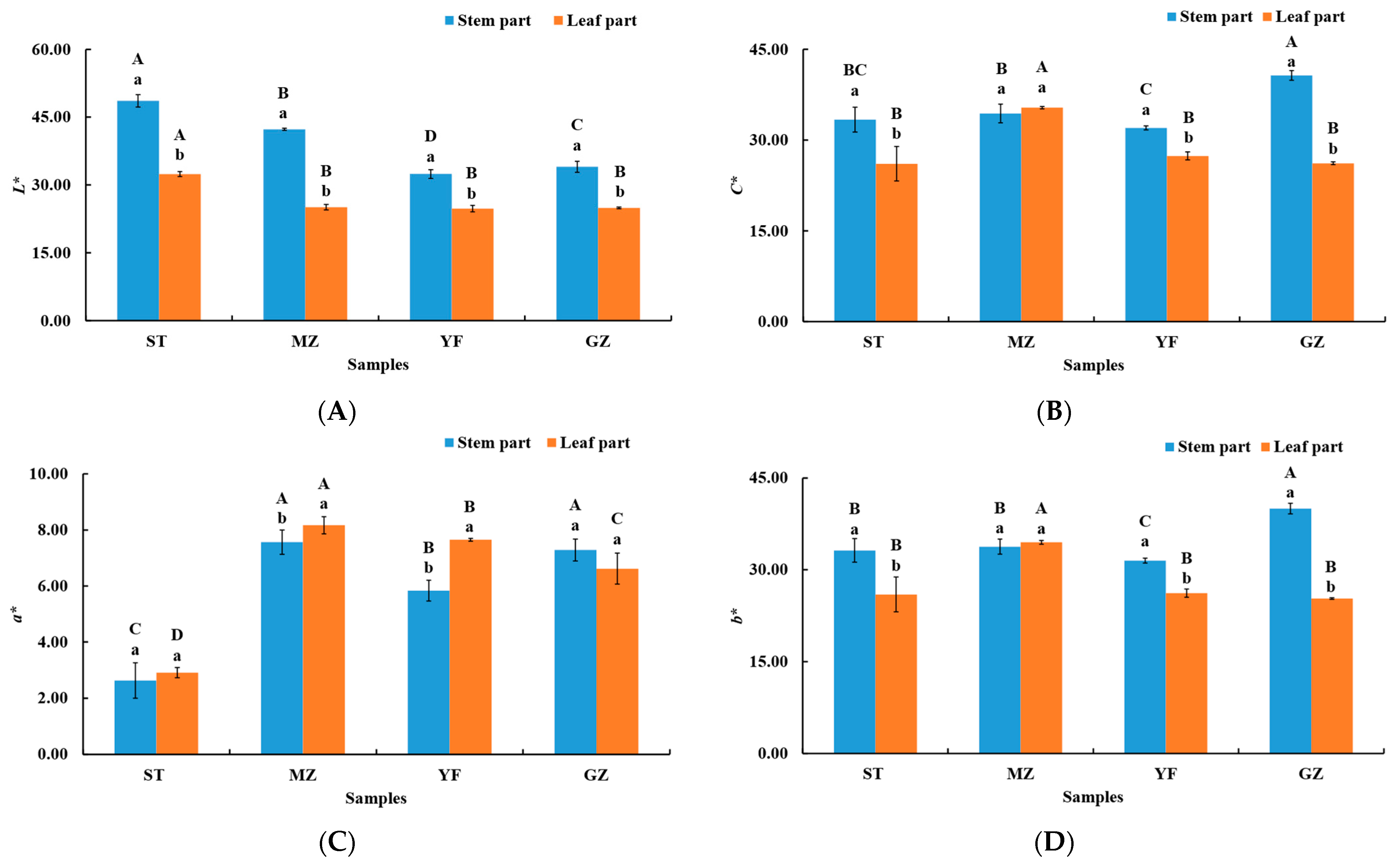
3.1. Evaluation of Colour in Fermented Chinese Mustard Greens
3.2. Analysis of Textures in Fermented Chinese Mustard Greens
3.3. Comparison of the Diversity Indices of Fermented Chinese Mustard Greens
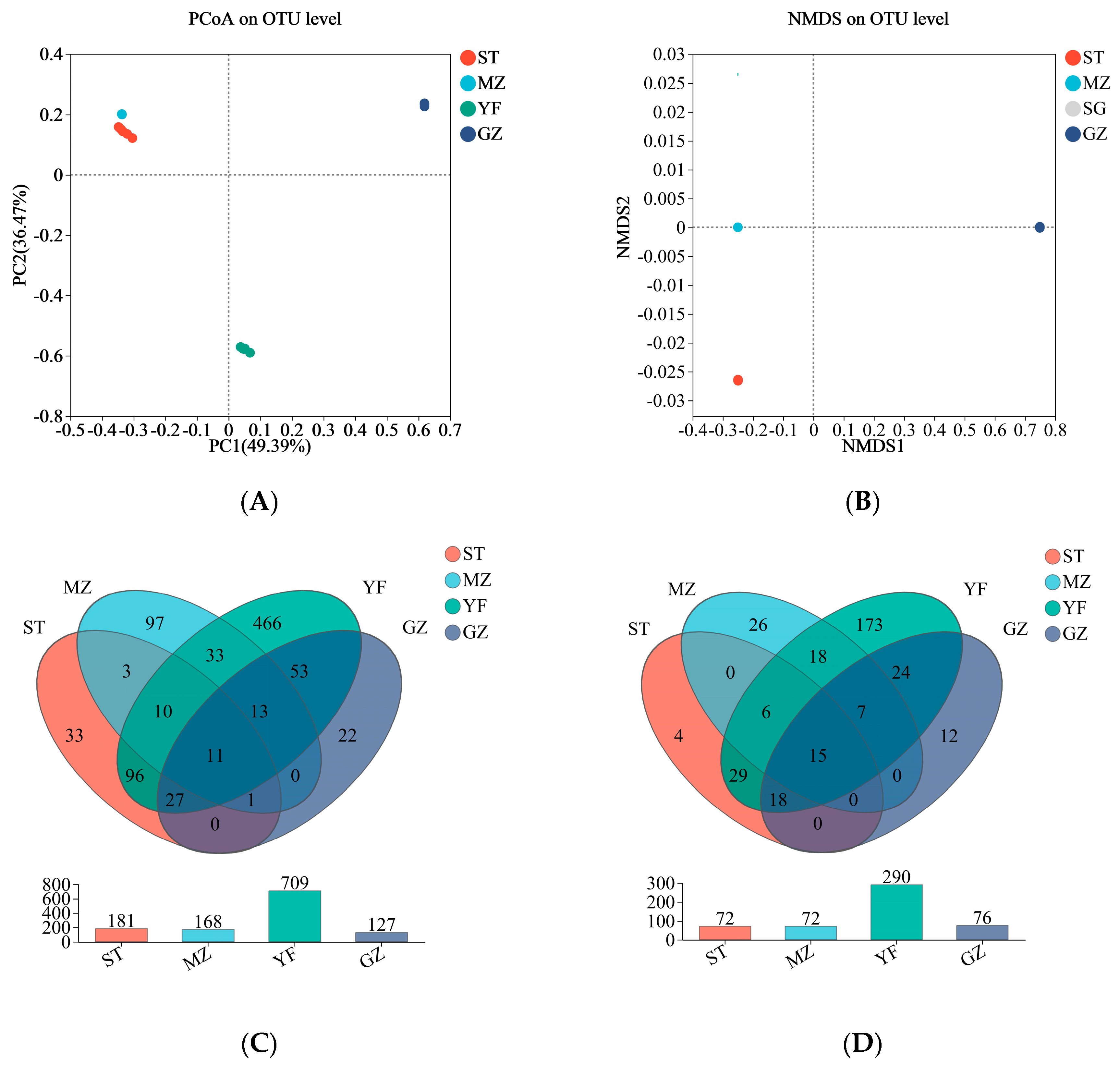
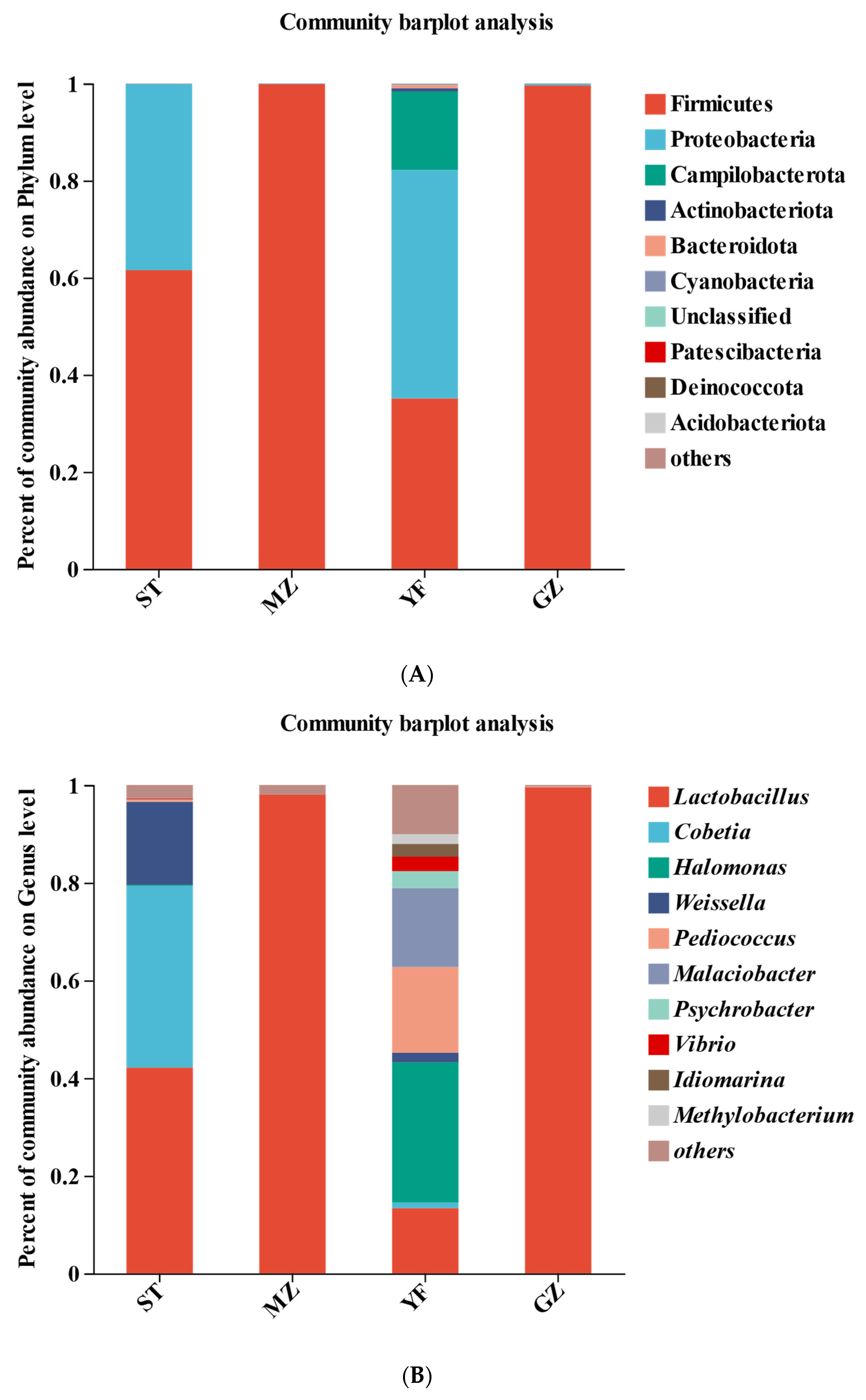
3.4. Microbial Community Comparisons
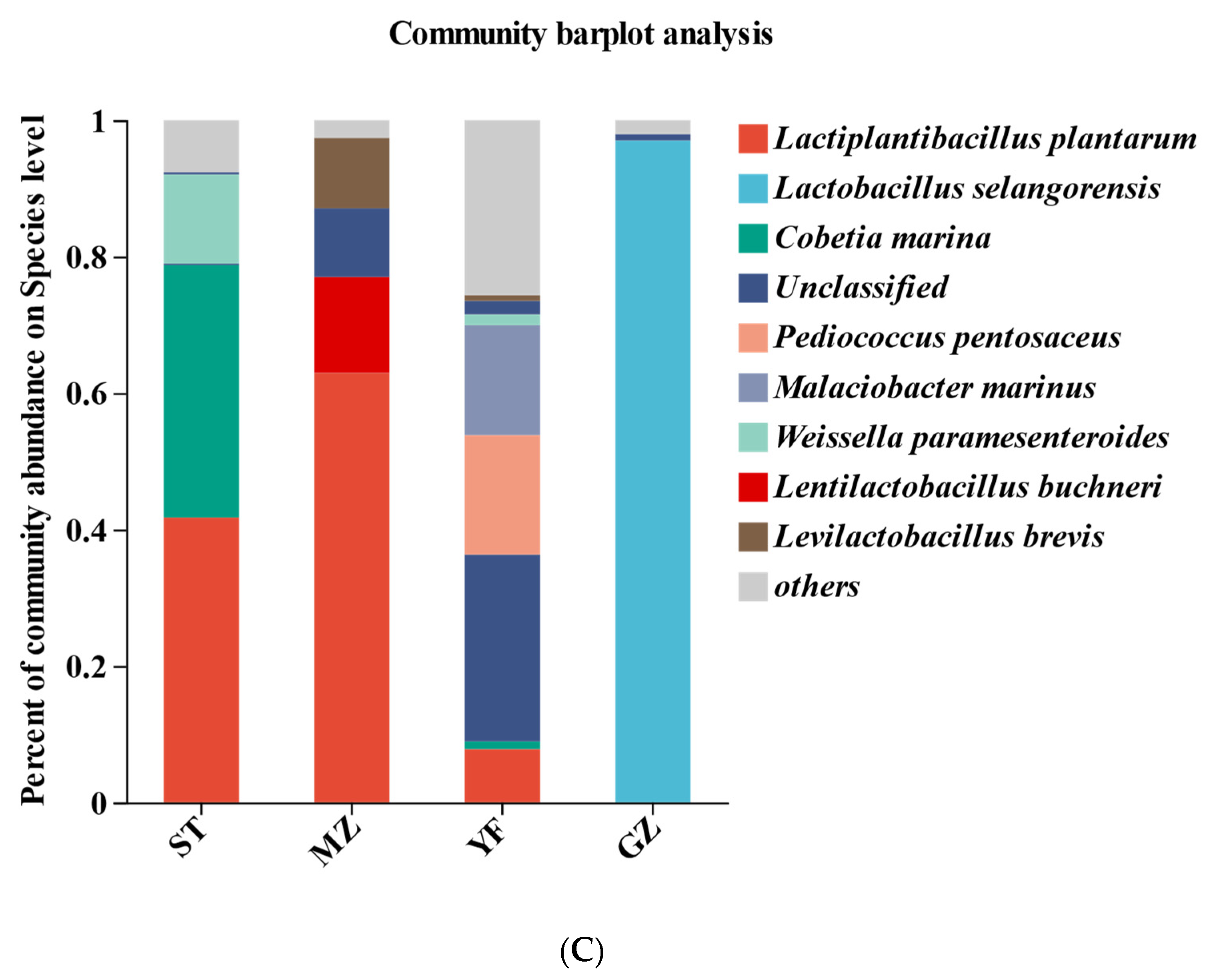
3.5. Bacterial Profiles of Fermented Chinese Mustard Greens
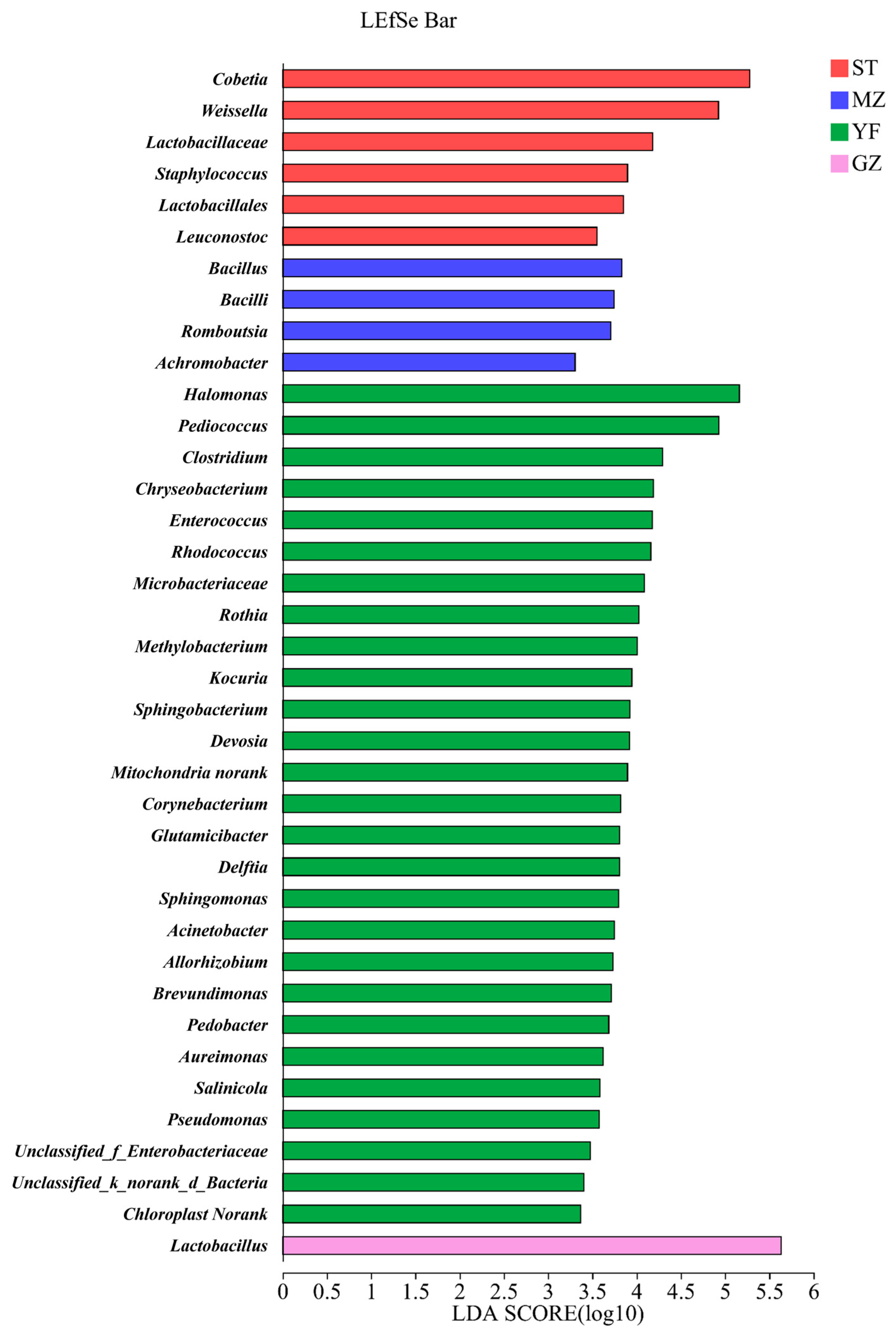
3.6. Differential Bacteria in the Fermented Chinese Mustard Greens
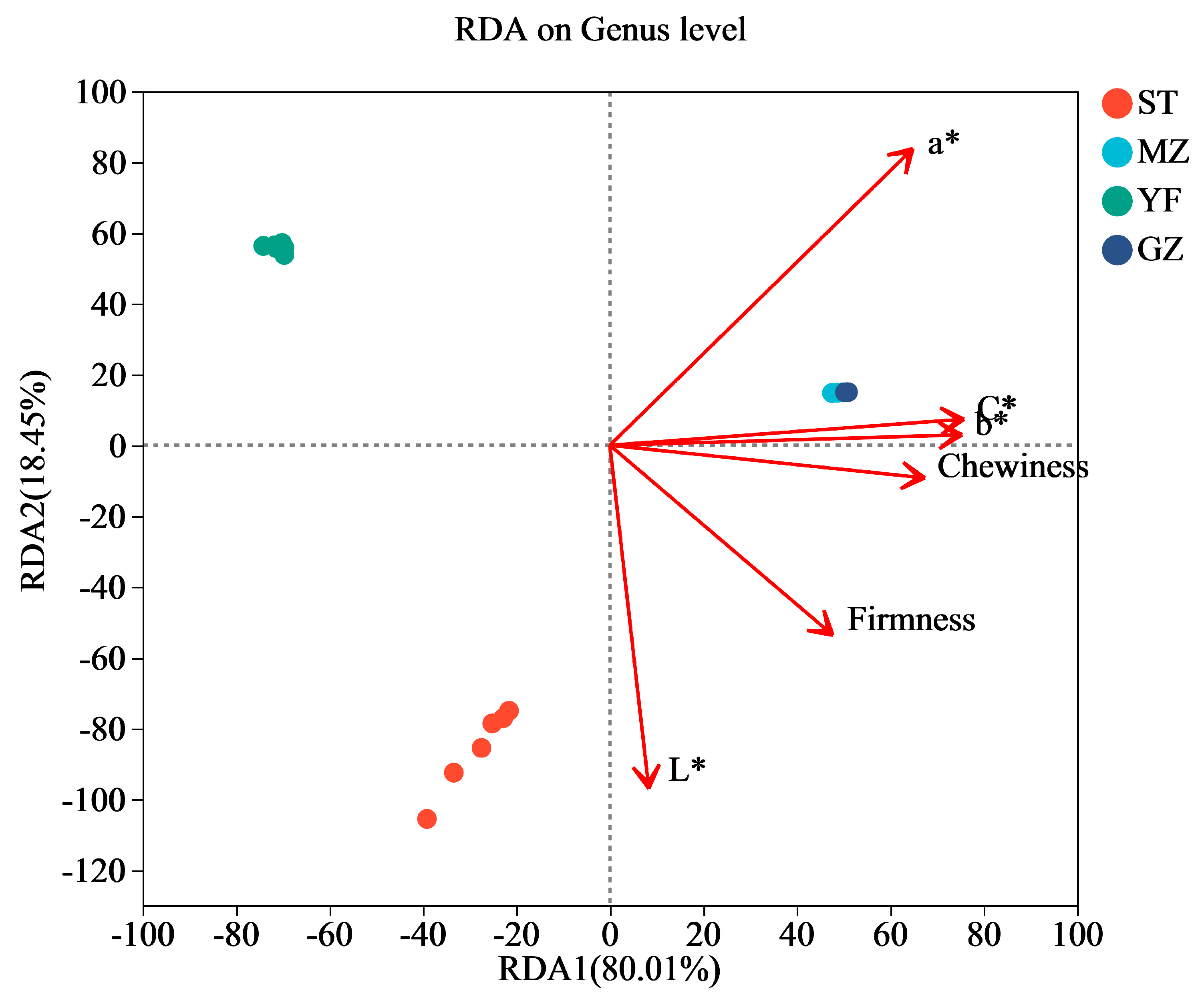
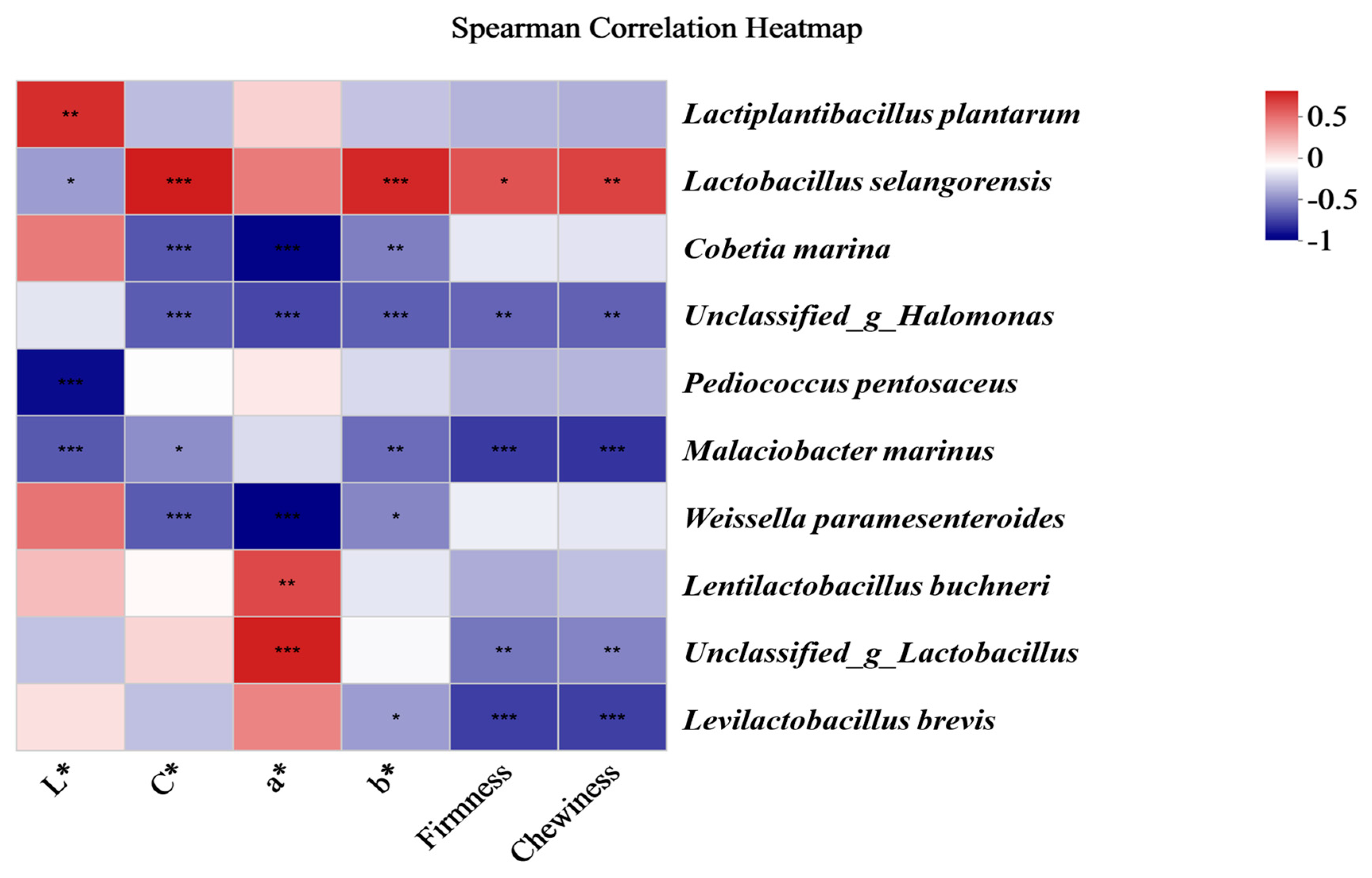
3.7. Correlation of Bacterial Communities and the Quality Indices of Fermented Chinese Mustard Greens
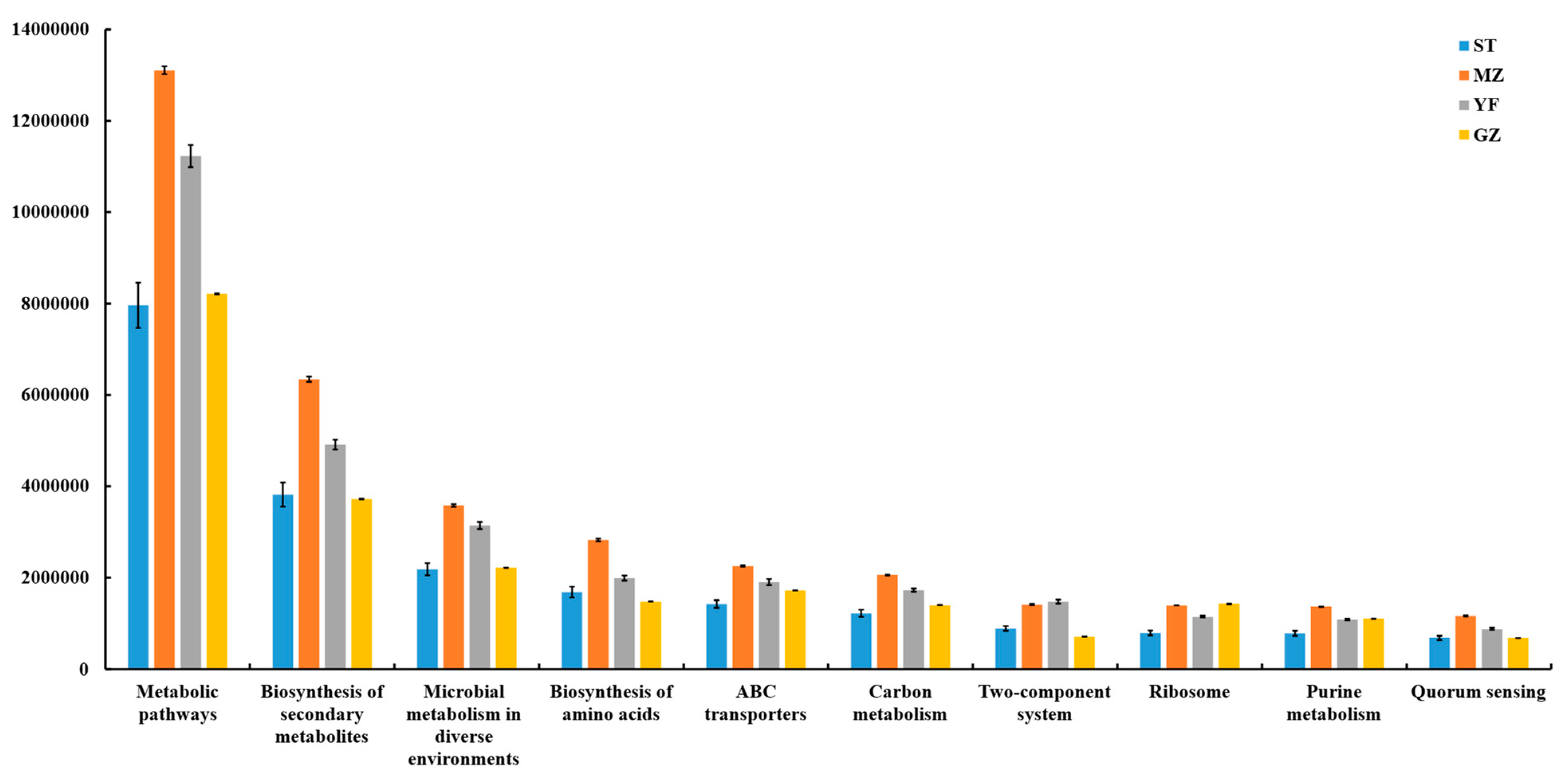
3.8. Predicted Functions of Microbial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hutkins, R.W. Fermented vegetables. Microbiol. Technol. Fermented Foods 2006, 5, 223–259. [Google Scholar]
- Qian, Y.; Tao, Y.; Li, Y.; Guo, S.; Xiang, W.; Liu, G.; Rao, Y. Microbiota succession and chemical composition involved in the radish fermentation process in different containers. Front. Microbiol. 2020, 11, 445. [Google Scholar]
- Hanelt, P.; Buttner, R. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops: Except Ornamentals; Springer: Berlin/Heidelberg, Germany, 2001; Volume 1. [Google Scholar]
- Tian, Y.; Deng, F. Phytochemistry and biological activity of mustard (Brassica juncea): A review. CyTA-J. Food 2020, 18, 704–718. [Google Scholar] [CrossRef]
- Fan, Y.; Shen, J.; Dong, D. Development status and research prospect of mustard vegetables industry. Agric. Sci. Technol. 2017, 18, 556–564. [Google Scholar]
- Zhang, C.; Zhang, J.; Liu, D. Biochemical changes and microbial community dynamics during spontaneous fermentation of Zhacai, a traditional pickled mustard tuber from China. Int. J. Food Microbiol. 2021, 347, 109199. [Google Scholar] [CrossRef]
- Lingjuan, J.; Yu, C.; Zeyuan, D.; Bing, Z.; Hongyan, L. Evaluation and comparison of physicochemical properties, volatile substances, and microbial communities of leaf mustard (Brassica juncea var. multiceps) under natural and inoculated fermentation. J. Food Sci. 2023, 88, 3255–3273. [Google Scholar]
- Liu, D.; Zhang, C.; Zhang, J.; Xin, X.; Liao, X. Metagenomics reveals the formation mechanism of flavor metabolites during the spontaneous fermentation of potherb mustard (Brassica juncea var. multiceps). Food Res. Int. 2021, 148, 110622. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Li, X.; Liu, D. Bacterial community and quality characteristics of the fermented potherb mustard (Brassica juncea var. multiceps) under modified atmospheres. Food Res. Int. 2019, 116, 266–275. [Google Scholar] [PubMed]
- Zhao, D.; Ding, X. Studies on the low-salt Chinese potherb mustard (Brassica juncea, Coss.) pickle. I—The effect of a homofermentative l (+)-lactic acid producer Bacillus coagulans on starter culture in the low-salt Chinese potherb mustard pickle fermentation. LWT-Food Sci. Technol. 2008, 41, 474–482. [Google Scholar] [CrossRef]
- Zhao, D.; Tang, J.; Ding, X. Analysis of volatile components during potherb mustard (Brassica juncea, Coss.) pickle fermentation using SPME–GC-MS. LWT-Food Sci. Technol. 2007, 40, 439–447. [Google Scholar] [CrossRef]
- Xiao, M.; Huang, T.; Huang, C.; Hardie, J.; Peng, Z.; Xie, M.; Xiong, T. The microbial communities and flavour compounds of Jiangxi yancai, Sichuan paocai and Dongbei suancai: Three major types of traditional Chinese fermented vegetables. LWT-Food Sci. Technol. 2020, 121, 108865. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Kim, J.; Moon, B. Effect of main vegetable ingredient on the glucosinolate, carotenoids, capsaicinoids, chlorophylls, and ascorbic acid content of kimchis. J. Food Compos. Anal. 2022, 110, 104523. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, B.K.; Park, K.Y. Antimutagenic and anticancer effects of leaf mustard and leaf mustard kimchi. J. Food Sci. Nutr. 2007, 12, 84–88. [Google Scholar] [CrossRef]
- Lee, M.A.; Choi, J.H.; Choi, Y.S.; Han, D.J.; Kim, H.Y.; Shim, S.Y.; Chung, H.-K.; Kim, C.-J. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extracts on refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010, 84, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Tsukamoto, C.; Kim, K.; Choi, M. Investigation of glucosinolates, and the antioxidant activity of D olsan leaf mustard kimchi extract using HPLC and LC-PDA-MS/MS. J. Food Biochem. 2017, 41, e12366. [Google Scholar] [CrossRef]
- Lim, H.S.; Yoo, E.J.; Choi, M.R. Changes of physiological activity of mustard leaf during its fermentation period. J. Microbiol. Biotechnol. 2000, 10, 43–47. [Google Scholar]
- Wang, Z.; Shao, Y. Effects of microbial diversity on nitrite concentration in pao cai, a naturally fermented cabbage product from China. Food Microbiol. 2018, 72, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Saren, G.; Ji, Y.; Guan, Y.; Feng, K. Microbial community dynamics and metabolome changes during spontaneous fermentation of northeast sauerkraut from different households. Front. Microbiol. 2020, 11, 1878. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Jiang, S.; Chen, J.; Ma, C.; Huo, D.; Shao, Y.; Zhang, J. Unique microbial diversity and metabolic pathway features of fermented vegetables from Hainan, China. Front. Microbiol. 2018, 9, 399. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Sanz, T.; Hernando, I.; Llorca, E.; Fiszman, S.M.; Quiles, A. New formulations of functional white sauces enriched with red sweet pepper: A rheological, microstructural and sensory study. Eur. Food Res. Technol. 2015, 240, 1187–1202. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Saren, G.; Ji, Y.; Feng, K. Microbial dynamics and volatilome profiles during the fermentation of Chinese northeast sauerkraut by Leuconostoc mesenteroides ORC 2 and Lactobacillus plantarum HBUAS 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef] [PubMed]
- Gaowa, S.; Feng, K.; Li, Y.; Long, Y.; Hu, W. Effect of alginate-based edible coating containing thyme essential oil on quality and microbial safety of fresh-cut potatoes. Horticulturae 2023, 9, 543. [Google Scholar] [CrossRef]
- Sarengaowa; Wang, L.; Liu, Y.; Yang, C.; Feng, K.; Hu, W. Screening of Essential Oils and Effect of a chitosan-based edible coating containing cinnamon oil on the quality and microbial safety of fresh-cut potatoes. Coatings 2022, 12, 1492. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berglyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. Msystems 2016, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.; Huang, C.H.; Wang, C.L.; Lee, A.Y.; Mori, K.; Tamura, T.; Watanabe, M.; Blom, J.; Huang, L.; Watanabe, K. Lactobacillus suantsaii sp. nov., isolated from suan-tsai, a traditional Taiwanese fermented mustard green. Int. J. Syst. Evol. Microbiol. 2019, 69, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, J.; Hou, Q.; Xu, H.; Zheng, Y.; Zhang, H.; Zhang, L. Assessment of bacterial profiles in aged, home-made Sichuan paocai brine with varying titratable acidity by PacBio SMRT sequencing technology. Food Control 2017, 78, 14–23. [Google Scholar] [CrossRef]
- Liu, A.; Liu, G.; Huang, C.; Shen, L.; Li, C.; Liu, Y.; Liu, S.; Hu, B.; Chen, H. The bacterial diversity of ripened Guang’yuan Suancai and in vitro evaluation of potential probiotic lactic acid bacteria isolated from Suancai. LWT-Food Sci. Technol. 2017, 85, 175–180. [Google Scholar] [CrossRef]
- Liu, X.; Kuda, T.; Takahashi, H.; Kimura, B. Bacterial and fungal microbiota of spontaneously fermented Chinese products, Rubing milk cake and Yan-cai vegetable pickles. Food Microbiol. 2018, 72, 106. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zheng, W.; Huang, T.; Xiao, Y.; Xiong, T. Comparison of microbial communities and physiochemical characteristics of two traditionally fermented vegetables. Food Res. Int. 2020, 128, 108755. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef]
- Liang, T.; Xie, X.; Wu, L.; Li, L.; Li, H.; Xi, Y.; Feng, Y.; Xue, L.; Chen, M.; Chen, X.; et al. Microbial communities and physiochemical properties of four distinctive traditionally fermented vegetables from North China and their influence on quality and safety. Foods 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, H.; Wang, X.; Lin, X.; Ji, C.; Li, S.; Liang, H. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. LWT-Food Sci. Tech. 2020, 118, 108773. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.S.; Zhang, C.; Kim, Y.; Roh, S.W.; Liu, D. Culture-independent analysis of the bacterial community in Chinese fermented vegetables and genomic analysis of lactic acid bacteria. Arch. Microbiol. 2021, 203, 4693–4703. [Google Scholar] [CrossRef]
- Tsuji, A.; Takei, Y.; Nishimura, T.; Azuma, Y. Identification of new Halomonas strains from food-related environments. Microbes Environ. 2022, 37, ME21052. [Google Scholar] [CrossRef] [PubMed]
- Korena, K.; Krzyzankova, M.; Florianova, M.; Karasova, D.; Babak, V.; Strakova, N.; Juricova, H. Microbial succession in the cheese ripening process—Competition of the starter cultures and the microbiota of the cheese plant environment. Microorganisms 2023, 11, 1735. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Wu, B.B.; Zhao, W.T.; Lao, F.; Chen, F.; Liao, X.; Wu, I.H. Shifts in autochthonous microbial diversity and volatile metabolites during the fermentation of chili pepper (Capsicum frutescens L.). Food Chem. 2020, 335, 127512. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xue, W.J.; Ding, H.; An, C.; Ma, S.J.; Liu, Y. Probiotic potential of lactobacillus strains isolated from fermented vegetables in Shaanxi, China. Front. Microbiol. 2022, 12, 774903. [Google Scholar] [CrossRef] [PubMed]
- Larissa, B.; Anna, P.; Lubov, S.; Marina, E.; Yulia, N.; Olga, N.; Oksana, S.; Liudmila, T.; Valery, R. Nucleolytic enzymes from the marine bacterium Cobetia amphilecti KMM 296 with antibiofilm activity and biopreservative effect on meat products. Food Control 2017, 78, 270–278. [Google Scholar]
- Carolina, N.; Nestor, G.; Karen, A.; Ana, P.; Dely, R.C.; Martha, Y.L. Texture properties of miniature chihuahua-type cheese manufactured with different strains of Lactococcus lactis isolated from plants and raw milk cheese. J. Texture Stud. 2014, 45, 487–494. [Google Scholar]
- Skonberg, D.I.; Fader, S.; Perkins, L.B.; Perry, J.J. Lactic acid fermentation in the development of a seaweed sauerkraut-style product: Microbiological, physicochemical, and sensory evaluation. J. Food Sci. 2021, 86, 334–342. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Niamah, A.K. Bacterial viability, antioxidant stability, antimutagenicity and sensory properties of onion types fermentation by using probiotic starter during storage. Nutr. Food Sci. 2022, 52, 901–916. [Google Scholar] [CrossRef]

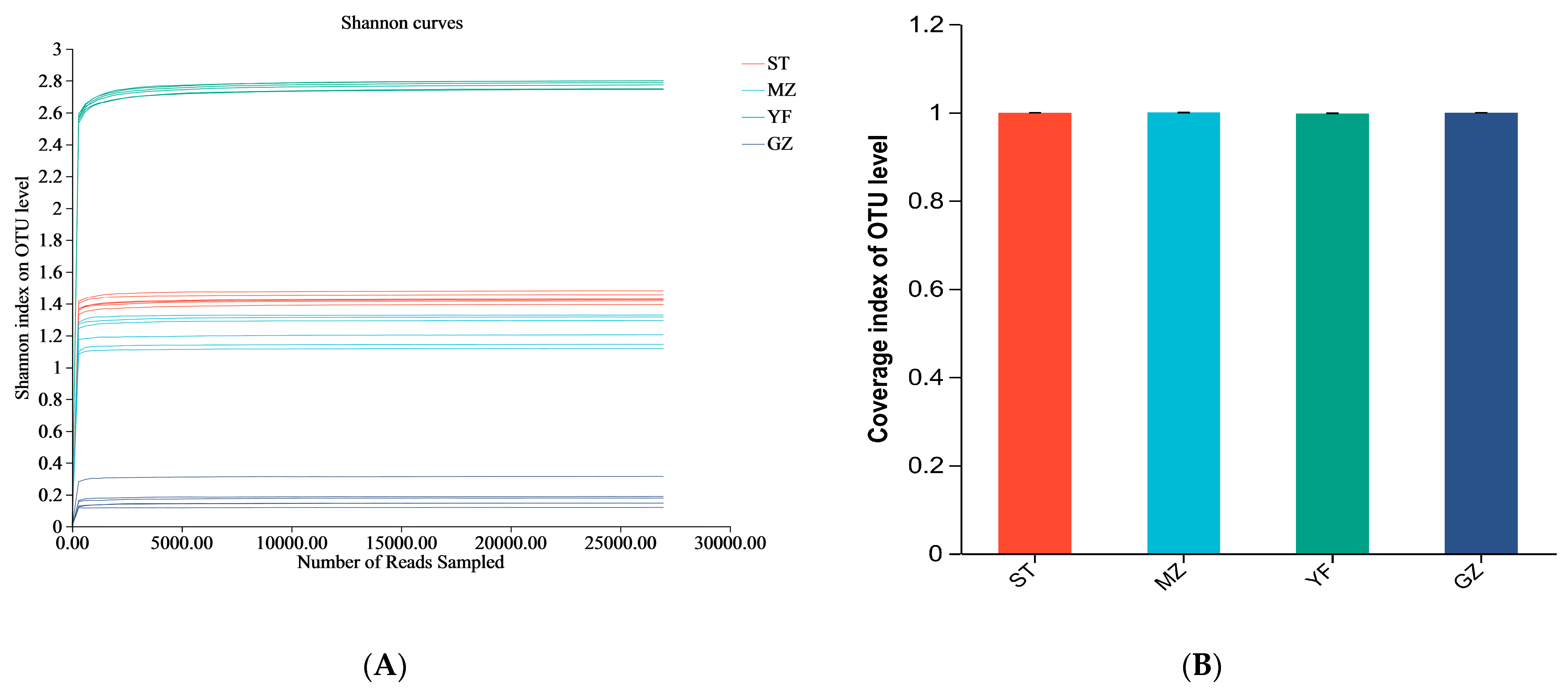
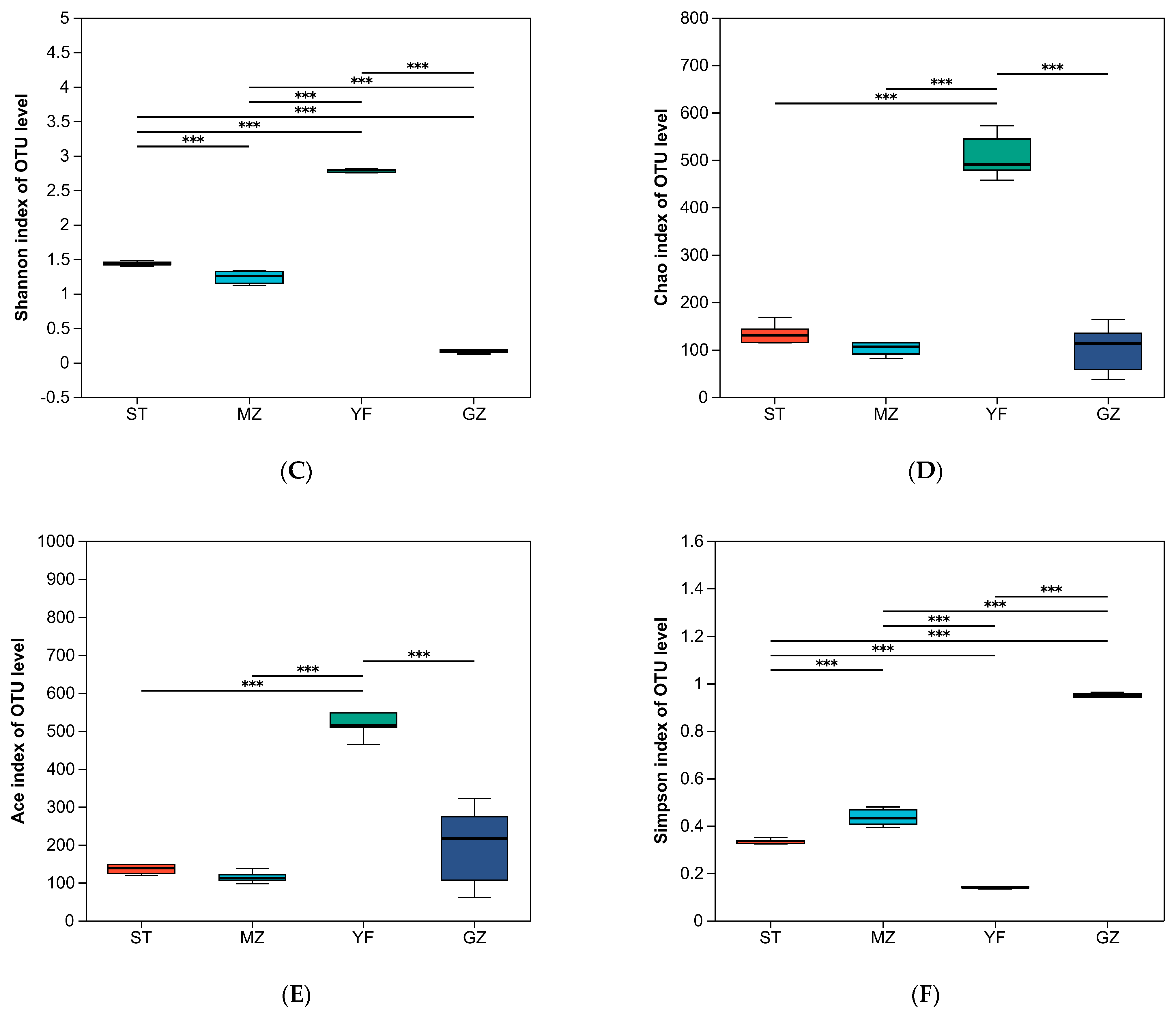
| Sample Name | Seq_Num | Average Length (bp) | OUT | Shannon | Simpson | Ace | Chao | Coverage | |
|---|---|---|---|---|---|---|---|---|---|
| ST | C1 | 36108 | 429 | 110 | 1.419 | 0.335 | 142.906 | 143.214 | 0.999 |
| C2 | 29802 | 429 | 112 | 1.477 | 0.324 | 147.435 | 143.059 | 0.999 | |
| C3 | 30169 | 429 | 97 | 1.455 | 0.322 | 124.651 | 136.000 | 0.999 | |
| C4 | 33044 | 429 | 99 | 1.425 | 0.335 | 159.028 | 126.188 | 0.999 | |
| C5 | 36898 | 429 | 107 | 1.394 | 0.350 | 128.868 | 127.313 | 0.999 | |
| C6 | 38158 | 429 | 104 | 1.422 | 0.338 | 168.485 | 139.429 | 0.999 | |
| MZ | E1 | 69701 | 429 | 106 | 1.295 | 0.415 | 126.277 | 121.789 | 1 |
| E2 | 51736 | 429 | 106 | 1.317 | 0.409 | 145.387 | 151 | 0.999 | |
| E3 | 152149 | 429 | 121 | 1.148 | 0.468 | 140.437 | 133.5 | 1 | |
| E4 | 34585 | 429 | 83 | 1.329 | 0.393 | 102.897 | 97.882 | 0.999 | |
| E5 | 59649 | 429 | 103 | 1.215 | 0.447 | 174.507 | 132.526 | 0.999 | |
| E6 | 119244 | 429 | 87 | 1.112 | 0.482 | 133.938 | 114 | 1 | |
| YF | H1 | 29989 | 424 | 390 | 2.811 | 0.132 | 511.462 | 486.397 | 0.996 |
| H2 | 84887 | 424 | 569 | 2.749 | 0.143 | 645.960 | 614.124 | 0.999 | |
| H3 | 41615 | 423 | 439 | 2.784 | 0.139 | 512.510 | 492.647 | 0.998 | |
| H4 | 41674 | 424 | 415 | 2.797 | 0.137 | 543.110 | 546.483 | 0.997 | |
| H5 | 46715 | 424 | 420 | 2.754 | 0.143 | 552.843 | 589.333 | 0.997 | |
| H6 | 49099 | 424 | 458 | 2.786 | 0.138 | 559.101 | 555.225 | 0.998 | |
| GZ | J1 | 33784 | 429 | 51 | 0.147 | 0.956 | 120.774 | 99.333 | 0.999 |
| J2 | 39876 | 429 | 57 | 0.187 | 0.940 | 149.415 | 105.333 | 0.999 | |
| J3 | 83583 | 429 | 72 | 0.147 | 0.955 | 110.231 | 114.273 | 1.000 | |
| J4 | 32054 | 429 | 69 | 0.179 | 0.948 | 201.595 | 136.364 | 0.999 | |
| J5 | 43058 | 429 | 82 | 0.315 | 0.886 | 216.753 | 168.000 | 0.999 | |
| J6 | 90210 | 429 | 60 | 0.123 | 0.962 | 91.875 | 87.083 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarengaowa; Kuang, Y.; Ding, Y.; Xie, H.; Tong, X.; Hu, W.; Feng, K. Evaluation of Quality and Microbial Communities in Fermented Chinese Mustard Greens from Guangdong Province, China. Horticulturae 2024, 10, 399. https://doi.org/10.3390/horticulturae10040399
Sarengaowa, Kuang Y, Ding Y, Xie H, Tong X, Hu W, Feng K. Evaluation of Quality and Microbial Communities in Fermented Chinese Mustard Greens from Guangdong Province, China. Horticulturae. 2024; 10(4):399. https://doi.org/10.3390/horticulturae10040399
Chicago/Turabian StyleSarengaowa, Yongxi Kuang, Yun Ding, Hao Xie, Xinyang Tong, Wenzhong Hu, and Ke Feng. 2024. "Evaluation of Quality and Microbial Communities in Fermented Chinese Mustard Greens from Guangdong Province, China" Horticulturae 10, no. 4: 399. https://doi.org/10.3390/horticulturae10040399
APA StyleSarengaowa, Kuang, Y., Ding, Y., Xie, H., Tong, X., Hu, W., & Feng, K. (2024). Evaluation of Quality and Microbial Communities in Fermented Chinese Mustard Greens from Guangdong Province, China. Horticulturae, 10(4), 399. https://doi.org/10.3390/horticulturae10040399





