Abstract
Late-season varieties of mandarin (Citrus reticulata Blanco) have a high economic value, so their study, characterization, and comparison among different commercial varieties is of great interest for agriculture. Detailed metabolomic analysis of mandarin leaves can provide valuable information on agronomic characteristics, vegetative development, and tree response to abiotic and biotic stresses. In this study, an analysis of the main metabolites presents in the leaves of three late-season mandarin orange varieties (‘Afourer’, ‘Orri’ and ‘Tango’), cultivated under homogeneous conditions, was carried out using nuclear magnetic resonance (1H NMR) and multivariate statistical analysis techniques. The results show that organic acids and sugars are the metabolites with the highest presence in mandarin leaves, especially malate and sucrose. Ten amino acids and other metabolites such as choline and trigonelline were also detected. Metabolites such as asparagine and isoleucine were widely implicated in the metabolic pathways of the detected compounds. The ‘Orri’ variety showed significantly more differences in metabolite concentrations compared to the other two varieties studied. Malate and sucrose were shown to be the metabolites with the greatest significant differences between the varieties compared. From an agronomic point of view, the ‘Orri’ variety differs from the other two varieties because it has concentrations of metabolites that provide good resistance to abiotic and biotic stresses and fruits of higher quality and sweetness.
1. Introduction
Mandarins (Citrus reticulata Blanco) are one of the most economically important citrus fruits in terms of tons produced and exported in the world [1]. A large number of mandarin varieties and hybrids are cultivated, which have very different agronomic, morphological, and biochemical properties [2]. Late-season mandarin varieties are characterized by a high economic value due to the small number of varieties that are harvested in the winter season and the high production of these varieties [3]. Some of the most outstanding late-season mandarin varieties that can be found in the market today are ‘Afourer’, ‘Orri’, and ‘Tango’.
In terms of production extension, it is estimated that there are 3 million trees of the ‘Afourer’ variety cultivated in Spain with the breeder’s permission [according to unpublished data provided by Agrupación de Viveristas de Agrios S.A. (AVASA)]. Regarding the ‘Orri’ variety, the number of trees that can be cultivated in Spain is limited to 1.3 million and with the condition that this variety cannot be commercialized outside the European Union (according to unpublished data provided by AVASA). As for the ‘Tango’ variety, for the moment, there is no limitation on the size of the plantation and, although there is no information available on the number of trees grown in Spain, it is known that the cultivation of this variety has experienced growth (according to unpublished data provided by AVASA).
Regarding the origins of the variety ‘Afourer’, also known as ‘Nadorcott’, it was first cultivated in Morocco in 1988; it’s the origin of this variety could have occurred from the hybridization of the variety ‘Murcott’, which is also known as ‘Nadorcott’ [Citrus reticulata Blanco × Citrus sinensis (L.) Osbeck] [4]. On the other hand, the ‘Orri’ variety is derived from irradiated cultivars of the ‘Orah’ variety (Citrus temple hort. ex Y. Tanaka) × (Citrus tangerina hort. ex Tanaka) in Israel [5]. The ‘Tango’ mandarin variety was developed at the University of California from hybridization via irradiation of the “Murcott” variety [6].
Previous studies of the fruits of the ‘Afourer’ variety indicate that it has a larger size and weight and a greater orange color if we compare them with ‘Orri’ and ‘Tango’ varieties [3]. Regarding the fruits of the ‘Orri’ variety, previous studies have identified that these are characterized by having a higher amount of juice and sugars than the other two varieties; in addition, it was identified that the main organic acid was malic acid and not citric acid as is usually common [3]. Finally, ‘Tango’ showed morphological and biochemical characteristics, very similar to the ‘Afourer’ variety [3].
Although few studies have been conducted on these varieties, they have also focused on showing the high quality and remarkable nutritional benefits of the mandarin fruit [7,8,9]. However, studies focused on other plant tissues, such as mandarin leaves, are much more limited. Leaves can provide us with information on their agronomic characteristics and their response mechanisms to different abiotic and biotic stresses [10,11]. Therefore, knowledge of these characteristics is of vital importance, since they are very important varieties and for which there are no previous publications that metabolomically analyze their leaves.
Metabolomic analysis techniques have improved significantly in recent years due to recent advances in more powerful and accurate instrumentation, as well as significant improvements in data processing and computational capacity [12,13]. Improvements in data analysis and statistical analysis techniques allow the results obtained to be analyzed and processed more efficiently, leading to a better understanding of the observed results [14]. Nuclear magnetic resonance is now a very powerful and accurate technique for the identification and quantification of primary and secondary metabolites in plant tissues [13,15]. Nuclear magnetic resonance is currently used in a large number of studies to accurately determine the metabolites present in fresh samples of any plant tissue [12,16,17,18,19].
The objective of this study is to determine and compare the metabolites present in the leaves of the three most important commercial late-season mandarin varieties (‘Afourer’, ‘Orri’, and ‘Tango’) in the Spanish market, under homogeneous growing conditions. Metabolite analysis was performed using nuclear magnetic resonance (1H NMR) and the results obtained were analyzed via multivariate data analysis.
2. Materials and Methods
2.1. Plant Material and Experimental Design
For this study on leaf metabolomics, the leaves of three late-season varieties of mandarin trees were used (Citrus reticulata Blanco)—’Afourer’, ‘Orri’, and ‘Tango’—cultivated on Citrus macrophylla rootstock. In a previous study, mandarins from these same trees were morphologically and biochemically characterized, showing their quality and good growing conditions [3]. All varieties were placed in homogeneous growing conditions, cultivated on plateaus, with a planting frame of 5.5 × 4 m; all trees were 12 years old, with an average height of 376 cm (‘Afourer’), 336 cm (‘Orri’), and 254 cm (‘Tango’), as well as a cup diameter of 316 cm (‘Afourer’), 350 cm (‘Orri’), and 216 cm (‘Tango’). The commercial plots where the varieties were grown are located in the southeast of Spain, in the municipality of Torre-Pacheco (Murcia, Spain, 37°46′11.2″ N 1°01′07.4″ W). The region in which the study was conducted is characterized by a semi-arid climate, with high inter-annual variability in rainfall and long periods of drought with short episodes of very intense torrential rainfall [20]. The average agrometeorological conditions recorded during the last 10 years in the study area were as follows: temperature of 18.43 ± 0.15 °C, humidity of 68.90 ± 0.92%, wind speed of 2.01 ± 0.03 m/s, rainfall of 330.07 ± 44.26 mm, and evapotranspiration of 1283.63 ± 20.38 mm [21].
These mandarin trees were grown following cultivation practices aimed at their commercialization both nationally and internationally. The irrigation system used is a drip irrigation system; each tree had 4 L/h drippers (four drippers per tree), which provided the ferti-irrigation needs. The water consumption in the plots where these mandarin trees were grown was between 5500 and 6000 m3/ha/year. Fertilization comprised between 180 and 200 units of NPK, 100 units of P2O5, and 50 units of K2O per year. The irrigation and fertilizer doses were based on those used by local citrus growers, adapted to the needs of the crop, and continuously monitored using substrate probes and periodic foliar analysis to ensure optimal agrometeorological conditions. The trees were pruned annually and phytosanitary products were applied according to the needs and the appearance of different pests in the crop.
A total of 25 adult leaves of each mandarin variety were collected in triplicate, taking 5 leaves for each orientation (north, south, east, and west), from 5 trees at random (different trees for each replicate), in the 5 × 5 m plot. The leaves collected presented a correct development, highlighting the absence of nutritional deficiencies as well as diseases or pests. The leaves had an average length with leafstalks of 107.94 cm, 123.18 cm, and 102.09 cm; an average leaf width of 46.30, 41.73, and 42.37 cm; and an average leaf surface of 36.20, 34.30, and 30.32 cm2 for ‘Afourer’, ‘Orri’, and ‘Tango’, respectively. The samples were quickly taken to the laboratory, to avoid their degradation, where they were cleaned with abundant distilled water to remove possible traces of dust, soil, and other elements. Then, all leaf samples were cut into homogeneous pieces and immediately frozen using liquid nitrogen. Once the samples were frozen, they were stored at −80 °C in propylene containers until lyophilization. The samples were freeze-dried (Christ Alpha 2-4 LSCplus, Martin Christ, Osterode am Harz, Germany) for 48 h. The samples were then ground (TSM6A013, Taurus, Oliana, Spain) and sieved to a fine homogeneous powder that was stored in 2 mL tubes at −20 °C until metabolomic analysis was performed.
2.2. Metabolomics Analysis of Leaves by 1H NMR
Metabolomic analysis was performed in triplicate via nuclear magnetic resonance (1H NMR) following the methodology described by Van der Sar et al. [22] and Melgarejo et al. [17]. A total of 0.5 mg of freeze-dried leaf sample was mixed with a hydromethanolic mixture (1:1, MeOH:H2O). The samples were then sonicated for 30 min, centrifuged at 11,000 rpm for 20 min, and the supernatant was extracted. It was then subjected to speed-vacuum until all the liquid phase was evaporated; the solid obtained was resuspended in 800 μL of potassium phosphate buffer together with 0.58 mM TPS. Finally, 600 μL of the sample was taken for quantitative analysis via 1H NMR (Ascend NMR Magnet 500 MHz, Bruker, Billerica, MA, USA). Subsequently, the identification and quantification of all detected metabolites were performed by integrating the peaks of the spectra obtained directly from the MNR (see Figure S1 in Supplementary Materials). For this purpose, chemical shifts obtained from an internal database of amino acids, organic acids, sugars, and other metabolites were used (Table 1).

Table 1.
1H NMR chemical shifts identified in the samples of the three mandarin varieties.
2.3. Statistical Analysis
A one-factor analysis of variance (ANOVA) was performed using the Statgraphics centurion 18 (StatPoint Technologies, Chicago, IL, USA), and Tukey’s HSD test (p ≤ 0.05) was applied for the contrast and separation of means. For the multivariate statistical analysis, MetaboAnalyst 5.0, an open-source JavaServer Faces Technology (Wishart Research Group, University of Alberta, Edmonton, AB, Canada), was employed. So, Principal Component Analysis (PCA), Partial Least Squares-Discriminant Analysis (PLSD-DA) and its Variable Importance in Projection (VIP), Significance Analysis of Microarray (SAM), hierarchical clustering heatmap, and Debiased Sparse Partial Correlation (DSPC) were calculated following the methodology described by Melgarejo et al. [23]. These statistical analyses are among the most effective for metabolomic studies in plants [24,25]. ANOVA with Tukey’s test allows us to see the variances between the means of each sample with high statistical precision. The PCA allows us to visualize the variances between the metabolites of the samples in a graph of small dimensions. Using the PLSD-DA, we can easily see which metabolites vary the most between samples. The hierarchical clustering heatmap allows easier graphical visualization due to a color scale within which metabolites are in higher concentrations in all samples. The SAM is similar to the previous one, but, in this analysis, the color scale shows the metabolites that are more differentiated among the samples. Finally, the DSPC is one of the most powerful tools in metabolomic analysis, since it allows us to see graphically and with great quality what the correlation is between all the detected metabolites and how strong this correlation is.
3. Results and Discussion
The main metabolites identified via nuclear magnetic resonance of mandarin leaves were amino acids, organic acids, and sugars (Table 2). First, significant differences were observed between the number of amino acids present in the leaves of the different varieties (Table 2). Ten different amino acids were identified in the leaves of the ‘Afourer’ and ‘Tango’ varieties and nine in the ‘Orri’ variety; asparagine was not detected in the latter. The most abundant amino acid in all samples analyzed was proline. In addition, five amino acids were identified in the leaves: citrate, formic, fumarate, malate, and quinate. Malate was the major organic acid in the varieties ‘Afourer’ and ‘Tango’, while quinate was the major organic acid in ‘Orri’. On the other hand, four sugars were detected: sucrose, glucose, myo-inositol, and maltose. The main sugar detected in all samples was sucrose, followed by glucose and myo-inositol; maltose was also detected in smaller amounts. Other metabolites detected were choline and trigonelline.

Table 2.
Metabolite concentration (mM) in the leaves of three late-season mandarins varieties grown under homogeneous conditions. Values are expressed as mean ± SE (n = 3).
A quantitative analysis of the different metabolites present in leaves of the three varieties studied (‘Afourer’, ‘Orri’, and ‘Tango’) provides a highly accurate characterization and comparison of the samples studied. The analysis of the metabolites provides valuable information on the agronomic characteristics, vegetative development, and resistance to abiotic and biotic stresses of mandarin trees [10,11]. Therefore, from an agronomic point of view, the results obtained can be interpreted as follows.
With the presence of proline as the main amino acid, choline, and trinonieline, the three varieties are widely adapted to cultivation in areas with high abiotic stresses of drought and salinity [26,27,28]. Such stresses include those found in the southeast of the Iberian Peninsula or, more generally, in the Mediterranean basin, areas where a large part of the world’s mandarin production is found [1]. The choice of a variety with greater resistance to abiotic factors is of great interest to growers because, in the scenario of climate change that we are facing, the cultivation of more resistant mandarins will save cultivation costs and ensure higher yields. Regarding this aspect, the cultivation of the varieties ‘Tango’ and ‘Orri’ could be of greater interest due to their ability to withstand abiotic stress due to the higher concentrations of GABA and isoleucine, respectively [29,30].
In parallel, it is also important for farmers’ crops to have resistance to biotic stresses, such as insect pests, which cause additional costs in pesticide treatment or loss of production. In this sense, the ‘Orri’ variety would be of great interest, since its higher concentrations of quinate and isoleucine give it greater protection against pests than the other two varieties [30]. Although, it should be noted that the variety ‘Afourer’ would also be of great interest due to its higher concentration of malate, a compound also involved in the protection against pests [31].
Vegetative development is another important factor to consider from an agronomic point of view. Greater growth of the trees will allow growers to have mature plantations sooner and, thus, achieve higher yields as soon as possible. In this respect, we highlight the ‘Afourer’ variety for its higher concentrations of asparagine, a metabolite closely linked to vegetative growth [32]. In comparison, the ‘Tango’ variety has lower concentrations; in the ‘Orri’ variety, it is not even detectable.
Finally, the final quality of the fruit is fundamental to citrus growers. The tastier and sweeter varieties will be more appreciated in the market, which will allow the growers to obtain greater economic profitability. According to the results obtained, the ‘Orri’ variety will stand out for its greater sweetness, since a much higher amount of sucrose and glucose was detected in the leaves than in the other two varieties, which correlates with the sugar that will be present in the fruit [33,34].
With this in mind, the results of the various statistical analyses used are analyzed and discussed in more detail below.
3.1. Analysis of Variance by ANOVA
3.1.1. Amino Acids
The most abundant amino acid in the three varieties was proline (Table 2), a metabolite closely related to citrus fruit ripening and responses to biotic and abiotic stresses [26,35]. Other amino acids also found in high concentrations were tyrosine, GABA, and aspartate.
However, of all the amino acids detected, there were only significant differences in four of them (GABA, asparagine, aspartate, and isoleucine). A significantly higher concentration of GABA was observed in the leaves of the ‘Tango’ variety, with up to 53% more of this amino acid than in the other two varieties. The presence of this amino acid in greater quantities provides us with information on the response of this variety to climatic conditions, influencing the response of the plants to drought [24,36]. This is of great interest due to the fact that plantations of this citrus are usually carried out in areas commonly affected by drought, such as southeastern Spain. In the same way, this amino acid provides us with information on the final quality of mandarins, since GABA concentrations in leaves are closely related to the conversion of citric acid Into sugars during the fruit ripening process [33].
On the other hand, the leaves of the ‘Afourer’ variety contain 28% more asparagine than those of the ‘Tango’ variety, while this amino acid was not detected in the ‘Orri’ variety. Asparagine is a metabolite that plays a crucial role in various plant biochemical processes [32]. In adult plant leaves, it primarily participates in the uptake and mobilization of inorganic nitrogen during plant growth, flowering, and senescence. Additionally, it facilitates the mobilization of nitrogen to the seeds of fruits [32]. A higher concentration of this amino acid in the leaves implies a higher mobilization of nitrogen, which contributes to higher vegetative growth of the tree [32,37,38]. The absence of this amino acid in the ‘Orri’ variety could be due to the fact that it had not been metabolized at the time of sampling, since precursors of this metabolite were detected, such as aspartate [37].
The ‘Tango’ variety also had 64% more aspartate. The function of this amino acid in plants is to form new amino acids such as lysine, threonine, isoleucine, methionine, and glycine to increase the efficiency of the Krebs cycle and, consequently, to intervene in the vegetative growth of plants [32,39,40]. This would indicate that the ‘Tango’ variety has less vegetative growth than the other two varieties studied [32,40].
On the other hand, the variety ‘Orri’ presented up to twice the amount of isoleucine than the rest of the varieties analyzed. The presence of isoleucine in the leaves is related to the biosynthesis of jasmonate, a compound involved in the physiological response of the leaves (stomata opening and closing) to biotic and abiotic stresses [30].
No significant differences were observed in the remaining amino acids detected in the samples of the three varieties, which showed values within the expected range for citrus fruits [18].
3.1.2. Organic Acids
Malate and quinate were the most abundant acids in all samples and showed significant differences between varieties (Table 2). The concentration of malate detected was up to 43% higher in the ‘Afourer’ variety than in the other two varieties. The presence of malate in plants contributes to a wide variety of metabolic processes affecting plant growth and development; it is one of the main compounds involved in photorespiration and is closely related to the mobilization of nitrogen and phosphorus within plants [41,42,43]. Some studies have shown that a higher amount of malic acid in leaves reduces the attack of some lepidopteran pests such as Helicoverpa armígera (Surekha Devi et al. [31] quoting Narayanamma et al. [44] and Yoshida et al. [45]).
Quinate is present in a significantly higher concentration in the ‘Orri’ variety, being its most important organic acid. Quinate is related to plant resistance to biotic stress, being a precursor of several organic acids that contribute to the protection of leaves against insect attack (Carrington et al. [46], quoting Leiss et al. [47]). In addition, this metabolite is involved in the metabolic pathways of Shikimate, which are essential in the biosynthesis of aromatic amino acids such as tyrosine or tryptophan in plants [46,48].
3.1.3. Sugars
As highlighted above, the main sugar detected in all samples was sucrose, followed by glucose and myo-inositol; maltose was also detected in smaller amounts (Table 2). This is in contrast to other studies conducted on lemon tree leaves, which show that maltose is one of the major sugars in lemon tree leaves [17].
Choline is a biomolecule synthesized by plants whose main function is usually to be the precursor of betaine, a compound linked to the response of plants to biotic and abiotic stresses [49]. Sucrose was significantly more present in the ‘Orri’ and ‘Tango’ varieties than in the ‘Afourer’ variety. Sucrose, the most abundant disaccharide in mandarins, is the main energy supplier for these plants, crucial for their metabolism and development [50]. The presence of this sugar is of great importance for the final quality of the fruit, since during the ripening process sucrose is mobilized from the leaves to the fruit, providing a large part of the characteristic sweet taste. [33,34,47,50].
On the other hand, the ‘Orri’ variety showed up to 2.3 times more glucose than the other two varieties studied. A higher concentration of glucose is also associated with a higher amount of sugar in the fruit and, therefore, higher sweetness [51]. A previous study carried out on the fruit of these three mandarin varieties shows that the fruit of the ‘Orri’ variety has a higher sugar content [3].
3.1.4. Other Metabolites
The concentrations of choline were significantly different among the three varieties, being higher in the leaves of the ‘Orri’ variety, followed by the ‘Afourer’ variety, and lower in the ‘Tango’ variety. The presence of trigonelline in the ‘Afourer’ and ‘Tango’ samples reached twice the concentration of the ‘Orri’ variety (Table 2).
On the other hand, trigolienin is a plant alkaloid that acts as an osmolyte and accumulates in plants under biotic and abiotic stress conditions [52]. Studies show that these two metabolites are involved in pathways related to the response of plants to salt stress [27,28,53]. This is noteworthy for agriculture because salinity stress in Mediterranean regions, caused mainly by frequent droughts and low irrigation water quality, is one of the main problems for citrus crops [54]. The problem of salinity is usually solved by choosing rootstocks better adapted to abiotic stresses [55,56]. However, this study shows how the choice of variety is also of great importance, since we observed significant differences in several of the metabolites present and involved in the response to stress, even when using the same rootstock (Citrus macrophylla).
3.2. Principal Component Analysis and Partial Least Squares-Discriminant Analysis
PCA (Principal Component Analysis) is a multivariate analysis method that allows us to visualize the data in smaller dimensions through a graphical representation, helping us to identify the variances and variations among the samples of the study [57]. The PCA of our study (Figure 1) shows that Component 1 represents 79.7% of the total variance among varieties, while Component 2 represents 19.3% of the total variability.
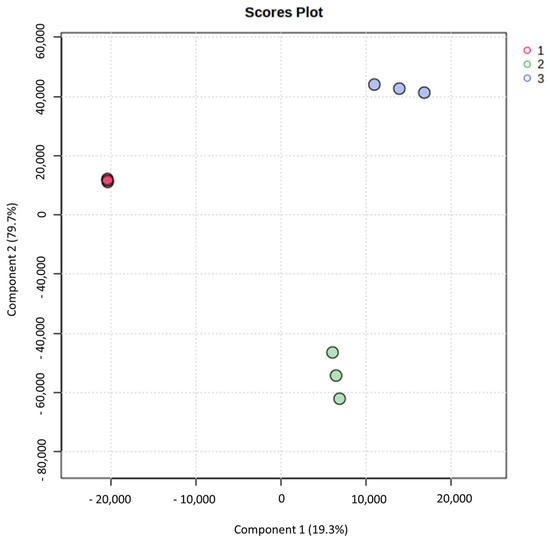
Figure 1.
Principal Component Analysis (PCA) graph of three late-season mandarin varieties (1. ‘Afourer’; 2. ‘Orri’; 3. ‘Tango’).
Using PLS-DA (Partial Least Squares-Discriminant Analysis) regression, the VIP (Variable Importance in Projection) was estimated, which shows us which metabolites are significant and differentiating among the three mandarin varieties [58,59]. The results (Figure 2) show that only malate, proline, and sucrose were the metabolites that had a VIP greater than 1, so these compounds had the highest significant differences among the three varieties.
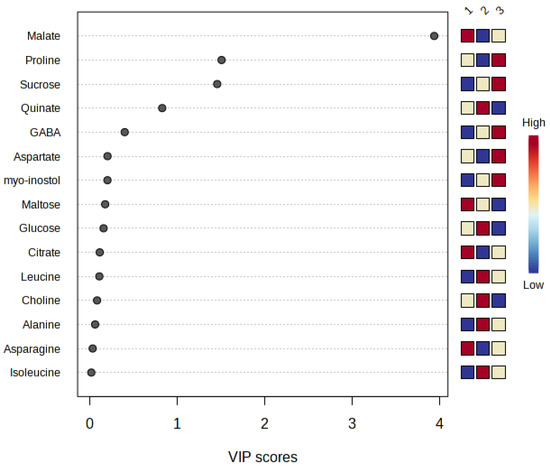
Figure 2.
Partial Least Squares-Discriminant Analysis (PLS-DA), graphically represented using a Variable Importance in Projection (VIP) plot, of the metabolites detected in the leaves of three late-season mandarin varieties (1. ‘Afourer’; 2. ‘Orri’; 3. ‘Tango’). The red color represents a higher concentration of a certain metabolite.
3.3. Hierarchical Clustering Heatmap
On the other hand, the hierarchical clustering heatmap shows visually with a color scale the relative concentrations of each of the detected metabolites [60]. The heatmap (Figure 3) showed that the ‘Orri’ variety was characterized by higher amounts of the metabolites choline, quinate, and formate acids, as well as the sugar glucose and the amino acids threonine, alanine, or isoleucine. The ‘Afourer’ variety was characterized by a higher concentration of malate and citrate acids, maltose sugar, and the amino acids asparagine and tyrosine. The ‘Tango’ variety was characterized by higher concentrations of the amino acids GABA, aspartate, and proline.
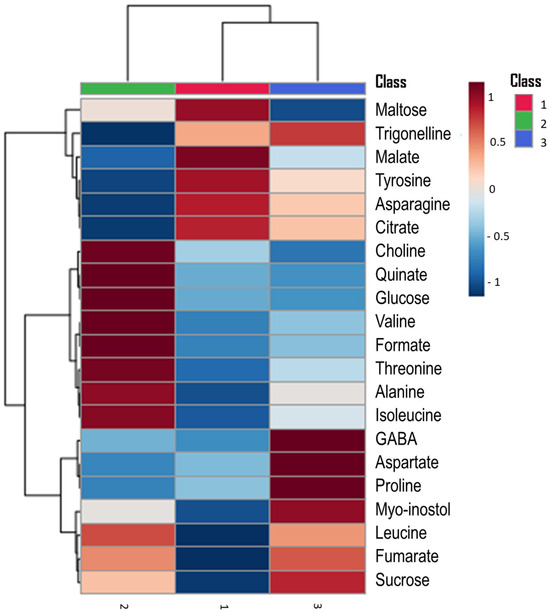
Figure 3.
Hierarchical clustering heatmap of metabolites detected in the leaves of three late-season mandarin orange varieties (1. ‘Afourer’; 2. ‘Orri’; 3. ‘Tango’). The red color represents a higher relative concentration of a given metabolite.
3.4. Significance Analysis of Microarray
Significance Analysis of Microarray (SAM) provides us with information on which metabolites have the greatest significant difference among all the samples analyzed [61]. SAM and cluster analysis (Figure 4) of our results show that malate and quinate to a greater extent and sucrose and proline to a lesser extent were the metabolites with the highest significance. Through the cluster, we also found that the varieties ‘Afourer’ and ‘Tango’ are more correlated with each other than the variety ‘Orri’, which is mainly due to the fact that the variety ‘Tango’ was obtained from the hybridization of the variety ‘Afourer’ [6].
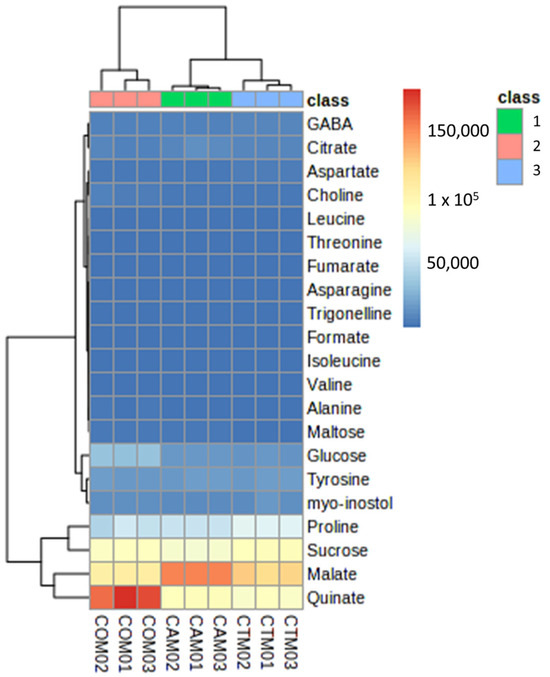
Figure 4.
Significance Analysis of Microarray (SAM) of metabolites detected in leaves of three late-season mandarin varieties (1. ‘Afourer’; 2. ‘Orri’; 3. ‘Tango’). The red color represents a higher significance of a given metabolite.
3.5. Deviated Scattered Partial Correlation
Analysis of the metabolic pathways was carried out by applying the Deviated Scattered Partial Correlation (DSPC) algorithm to the results obtained in 1H NMR. This algorithm was formulated by Janková and van de Geer [58] using graphical loop modeling. This analysis allows us to graphically identify the correlation between different metabolites present in the leaf samples to be analyzed [62]. The graphical model (Figure 5) represents the metabolites in a weighted node network, where the partial correlation coefficients and p-values of each pair of metabolites that are correlated by metabolic pathways are represented. Two nodes joined by a red line represent a positive relationship, while those joined by a blue line represent a negative relationship.
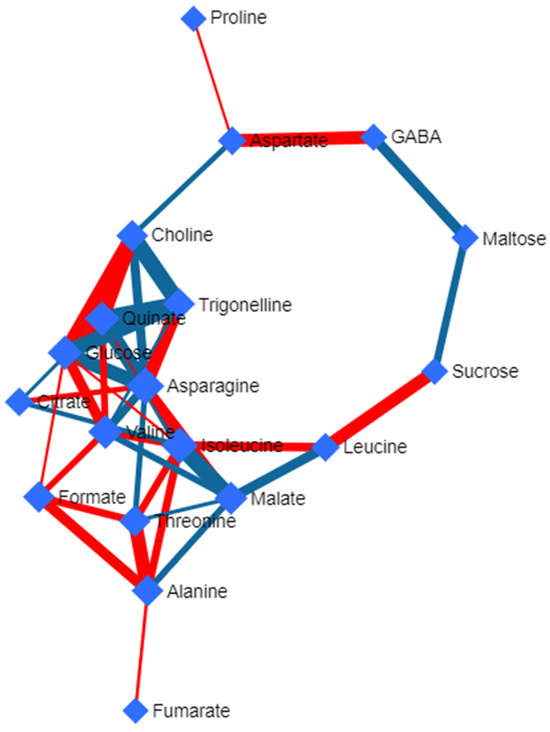
Figure 5.
Correlation network formed using the Debiased Sparse Partial Correlation (DSPC) algorithm of the metabolites detected via 1H NMR in the leaves of three late-season mandarin varieties (‘Afourer’, ‘Orri’, and ‘Tango’). Red lines represent positive relationships, while blue lines represent negative relationships. The size of the nodes shows the direction of change. The color of the lines is adjusted to a p-value < 0.05 and the false discovery rate (FDR) is adjusted to a p-value < 0.2.
The analysis of the metabolic pathways carried out on the mandarin varieties under study using the DSPC algorithm shows that asparagine has a grade 10 and an interrelation of 29.73, which shows that it is an important metabolite, as it is involved in many of the metabolic pathways of the rest of the detected compounds. Asparagine is positively related to trigonelline, malate, quinate, and citrate and negatively related to fumarate, valine, glucose, choline, isoleucine, and threonine. This amino acid in plants is involved in several metabolic pathways related to the process of photorespiration, in addition to being the main reservoir and transporter of nitrogen from the roots to the leaves [32,63].
Isoleucine also had an important presence in the metabolomic interrelationships with an interrelationship of 34.63 and a grade of 9. Most of its relationships were positive (leucine, alanine, threonine, asparagine, valine, and glucose), with only three being negative (malate, citrate, and quinate). Isoleucine is involved in the Krebs cycle, which implies that a higher amount of this compound is related to a lower amount of malate and citrate, while it is directly related to glucose [64].
Valine and glucose play direct and indirect roles in the Krebs cycle, which directly relates them to the synthesis and degradation of a wide variety of amino acids, acids, and sugars [65,66]. Quinate, on the other hand, is mainly involved in metabolic pathways for the synthesis of aromatic amino acids [46].
4. Conclusions
In this study, a metabolomic analysis of the leaves of three late-season mandarin varieties (‘Afourer’, ‘Orri’, and ‘Tango’) grown under homogeneous conditions on Citrus macrophylla rootstock was performed to characterize the metabolites present in the leaves of these mandarins and the differences between varieties. The most abundant metabolites found were organic acids and sugars, mainly malate, quinate, and sucrose. Ten amino acids were also identified in the leaves of these mandarins, with proline, tyrosine, and GABA standing out in terms of concentration, as well as other metabolites such as choline and trigonieline. Correlation analysis showed that asparagine and isoleucine were involved in many of the pathways of the other metabolites detected. The multivariate statistical analysis performed clearly shows that there are significant differences between the concentrations of metabolites present in the three varieties studied, especially in compounds such as malate, quinate, and sucrose. In summary, the results show that the ‘Orri’ variety has a more differentiated metabolomic profile compared to the other varieties studied. This indicates differentiated vegetative growth, different adaptation to different biotic and abiotic stresses (mainly pests and salt stress), and a direct correlation with the final quality of mandarins. Therefore, from an agronomic point of view, the variety ‘Afourer’ stands out from the other two varieties due to its greater vegetative growth. The variety ‘Tango’ stands out for its greater resistance to abiotic stress. But, above all, the variety ‘Orri’ stands out from the other two varieties because, although it has a lower vegetative growth than the other two varieties, it has a good resistance to both abiotic and biotic stresses and its fruits tend to be sweeter and of higher quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10040359/s1, Figure S1: 1H NMR spectra of the three varieties studied (‘Afourer’, ‘Orri’ and ‘Tango’).
Author Contributions
Conceptualization, J.J.M.-N., P.L., D.N.-G. and P.M.; Data curation, A.A.M.-V. and D.N.-G.; Formal analysis, A.A.M.-V.; Funding acquisition, P.M.; Investigation, A.A.M.-V.; Methodology, D.N.-G.; Resources, P.M.; Software, D.N.-G.; Supervision, J.J.M.-N., P.L., D.N.-G. and P.M.; Validation, D.N.-G. and P.M.; Writing—original draft, A.A.M.-V.; Writing—review and editing, A.A.M.-V., D.N.-G. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The data presented in this study are available in the present work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO Food and Agriculture Organization: Citrus. Available online: https://www.fao.org/economic/est/est-commodities/citrus-fruit/en/ (accessed on 27 January 2022).
- Shorbagi, M.; Fayek, N.M.; Shao, P.; Farag, M.A. Citrus reticulata Blanco (the Common Mandarin) Fruit: An Updated Review of Its Bioactive, Extraction Types, Food Quality, Therapeutic Merits, and Bio-Waste Valorization Practices to Maximize Its Economic Value. Food Biosci. 2022, 47, 101699. [Google Scholar] [CrossRef]
- Maciá-Vázquez, A.A.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Legua, P.; Melgarejo, P. Morphological and Biochemical Characterization of Late-Season Varieties of Mandarin Growing in Spain under Homogeneous Growing Conditions. Agronomy 2023, 13, 1825. [Google Scholar] [CrossRef]
- Nadori, E.B. Mandarin Tangerine Called Nadorcott. US 08/787,028, 7 July 1998. [Google Scholar]
- Vardi, A.; Spiegel-roy, P.; Frydman-shani, A.; Elchanati, A.; Neumann, H. Citrus Tree Named ‘Orri’. US 09/716,482, 4 March 2003. [Google Scholar]
- Roose, M.L.; Williams, T.E. Mandarin Variety Named “Tango”. US 11/220,875, 8 March 2007. [Google Scholar]
- Tarancón, P.; Giménez-Sanchis, A.; Aleza, P.; Besada, C. Selection of New Late-Season Mandarin Cultivars Based on Sensory Changes and Consumer Acceptance after Fruit Cold Storage. Agronomy 2021, 11, 116. [Google Scholar] [CrossRef]
- Simón-Grao, S.; Gimeno, V.; Simón, I.; Lidón, V.; Nieves, M.; Balal, R.M.; Carbonell-Barrachina, A.A.; Manera, F.J.; Hernández, F.; García-Sánchez, F. Fruit Quality Characterization of Eleven Commercial Mandarin Cultivars in Spain. Sci. Hortic. 2014, 165, 274–280. [Google Scholar] [CrossRef]
- Maciá-Vázquez, A.A.; Martínez-Nicolás, J.J.; Núñez-Gómez, D.; Melgarejo, P.; Legua, P. Influence of Rootstock on Yield, Morphological, Biochemical and Sensory Characteristics of “Afourer” Variety Mandarins. Sci. Hortic. 2024, 325, 112644. [Google Scholar] [CrossRef]
- Asai, T.; Matsukawa, T.; Kajiyama, S. Metabolomic Analysis of Primary Metabolites in Citrus Leaf during Defense Responses. J. Biosci. Bioeng. 2017, 123, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M. Synthesis versus Degradation: Directions of Amino Acid Metabolism during Arabidopsis Abiotic Stress Response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Brandizzi, F. High-Resolution Measurements in Plant Biology. Plant J. 2012, 70, 1–4. [Google Scholar] [CrossRef]
- Rolin, D.; Deborde, C.; Maucourt, M.; Cabasson, C.; Fauvelle, F.; Jacob, D.; Canlet, C.; Moing, A. High-Resolution 1H-NMR Spectroscopy and Beyond to Explore Plant Metabolome. Adv. Bot. Res. 2013, 67, 1–66. [Google Scholar]
- Okada, T.; Mochamad Afendi, F.; Altaf-Ul-Amin, M.; Takahashi, H.; Nakamura, K.; Kanaya, S. Metabolomics of Medicinal Plants: The Importance of Multivariate Analysis of Analytical Chemistry Data. Curr. Comput. Aided-Drug Des. 2010, 6, 179–196. [Google Scholar] [CrossRef]
- Kumar, D. Nuclear Magnetic Resonance (NMR) Spectroscopy For Metabolic Profiling of Medicinal Plants and Their Products. Crit. Rev. Anal. Chem. 2016, 46, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Gogna, N.; Hamid, N.; Dorai, K. Metabolomic Profiling of the Phytomedicinal Constituents of Carica papaya L. Leaves and Seeds by 1H NMR Spectroscopy and Multivariate Statistical Analysis. J. Pharm. Biomed. Anal. 2015, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Martínez-Font, R.; Lidón, V.; García-Sánchez, F.; Legua, P. Metabolomic Profile of Citrus Limon Leaves (‘Verna’ Variety) by 1H-NMR and Multivariate Analysis Technique. Agronomy 2022, 12, 1060. [Google Scholar] [CrossRef]
- Sousa, A.R.d.O.; Silva, E.M.d.A.; Filho, M.A.C.; Costa, M.G.C.; Filho, W.d.S.S.; Micheli, F.; Maserti, B.; Gesteira, A.d.S. Metabolic Responses to Drought Stress and Rehydration in Leaves and Roots of Three Citrus Scion/Rootstock Combinations. Sci. Hortic. 2022, 292, 110490. [Google Scholar] [CrossRef]
- Tristán, A.I.; Abreu, A.C.; Aguilera-Sáez, L.M.; Peña, A.; Conesa-Bueno, A.; Fernández, I. Evaluation of ORAC, IR and NMR Metabolomics for Predicting Ripening Stage and Variety in Melon (Cucumis melo L.). Food Chem. 2022, 372, 131263. [Google Scholar] [CrossRef] [PubMed]
- Castejón-Porcel, G.; Espín-Sánchez, D.; Ruiz-Álvarez, V.; García-Marín, R.; Moreno-Muñoz, D. Runoff Water as A Resource in the Campo de Cartagena (Region of Murcia): Current Possibilities for Use and Benefits. Water 2018, 10, 456. [Google Scholar] [CrossRef]
- SIAM Informe Agrometeorológico—TORRE PACHECO Station. Available online: http://siam.imida.es/apex/f?p=101:46:3131252762679599 (accessed on 29 March 2024).
- van der Sar, S.; Kim, H.K.; Meissner, A.; Verpoorte, R.; Choi, Y.H. Nuclear Magnetic Resonance Spectroscopy for Plant Metabolite Profiling. In The Handbook of Plant Metabolomics; Wiley: Hoboken, NJ, USA, 2013; pp. 57–76. [Google Scholar]
- Melgarejo, P.; Núñez-Gómez, D.; Hernández, F.; Martínez-Font, R.; Lidón Noguera, V.; Martínez-Nicolás, J.J.; Legua, P. Lemon Peel and Juice: Metabolomic Differentiation. Horticulturae 2023, 9, 510. [Google Scholar] [CrossRef]
- Huang, S.; Ying Lim, S.; Lau, H.; Ni, W.; Fong Yau Li, S. Effect of Glycinebetaine on Metabolite Profiles of Cold-Stored Strawberry Revealed by 1H NMR-Based Metabolomics. Food Chem. 2022, 393, 133452. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Rai, N.; Farag, M.A.; Maurya, S.; Yerasu, S.R.; Bisen, M.S.; Prabha, R.; Shukla, R.; Behera, T.K. Metabolic Diversity, Biosynthetic Pathways, and Metabolite Biomarkers Analysed via Untargeted Metabolomics and the Antioxidant Potential Reveal for High Temperature Tolerance in Tomato Hybrid. Plant Stress 2024, 11, 100420. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Yamada, N.; Promden, W.; Yamane, K.; Tamagake, H.; Hibino, T.; Tanaka, Y.; Takabe, T. Preferential Accumulation of Betaine Uncoupled to Choline Monooxygenase in Young Leaves of Sugar Beet—Importance of Long-Distance Translocation of Betaine under Normal and Salt-Stressed Conditions. J. Plant Physiol. 2009, 166, 2058–2070. [Google Scholar] [CrossRef]
- Cho, Y.; Lightfoot, D.A.; Wood, A.J. Trigonelline Concentrations in Salt Stressed Leaves of Cultivated Glycine Max. Phytochemistry 1999, 52, 1235–1238. [Google Scholar] [CrossRef]
- Cheng, P.; Yue, Q.; Zhang, Y.; Zhao, S.; Khan, A.; Yang, X.; He, J.; Wang, S.; Shen, W.; Qian, Q.; et al. Application of γ-Aminobutyric Acid (GABA) Improves Fruit Quality and Rootstock Drought Tolerance in Apple. J. Plant Physiol. 2023, 280, 153890. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D. Jasmonates: Key Players in Plant Stress Tolerance. In Emerging Plant Growth Regulators in Agriculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–192. [Google Scholar]
- Surekha Devi, V.; Sharma, H.C.; Arjuna Rao, P. Influence of Oxalic and Malic Acids in Chickpea Leaf Exudates on the Biological Activity of CryIAc towards Helicoverpa armigera. J. Insect Physiol. 2013, 59, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Gaufichon, L.; Reisdorf-Cren, M.; Rothstein, S.J.; Chardon, F.; Suzuki, A. Biological Functions of Asparagine Synthetase in Plants. Plant Sci. 2010, 179, 141–153. [Google Scholar] [CrossRef]
- Lourkisti, R.; Antoine, S.; Pailly, O.; Luro, F.; Gibon, Y.; Oustric, J.; Santini, J.; Berti, L. GABA Shunt Pathway Is Stimulated in Response to Early Defoliation-Induced Carbohydrate Limitation in Mandarin Fruits. Heliyon 2023, 9, e15573. [Google Scholar] [CrossRef]
- Katz, E.; Fon, M.; Lee, Y.J.; Phinney, B.S.; Sadka, A.; Blumwald, E. The Citrus Fruit Proteome: Insights into Citrus Fruit Metabolism. Planta 2007, 226, 989–1005. [Google Scholar] [CrossRef]
- Xiong, H.; Luo, Y.; Rennenberg, H.; Wang, J.; Hu, B.; Zhao, H.; Tang, X.; Zhang, Y.; Shi, X. Enhancing Proline Turnover Is the Key Physiological Response of Mature Citrus Leaves to Fruiting. Sci. Hortic. 2023, 315, 111979. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Y.; Wang, Y.; Li, M.; Han, D.; Gao, S.; Hu, Y.; Guo, J.; Zhang, T.; Shi, L. Integrated Transcriptomics and Metabolomics Reveals That Regulating Biosynthesis and Metabolism of HCN and GABA Plays a Key Role in Drought Resistance of Wild Soybean. Env. Exp. Bot. 2023, 215, 105505. [Google Scholar] [CrossRef]
- Potel, F.; Valadier, M.; Ferrario-Méry, S.; Grandjean, O.; Morin, H.; Gaufichon, L.; Boutet-Mercey, S.; Lothier, J.; Rothstein, S.J.; Hirose, N.; et al. Assimilation of Excess Ammonium into Amino Acids and Nitrogen Translocation in Arabidopsis thaliana—Roles of Glutamate Synthases and Carbamoylphosphate Synthetase in Leaves. FEBS J. 2009, 276, 4061–4076. [Google Scholar] [CrossRef]
- Antunes, F.; Aguilar, M.; Pineda, M.; Sodek, L. Nitrogen Stress and the Expression of Asparagine Synthetase in Roots and Nodules of Soybean (Glycine max). Physiol. Plant 2008, 133, 736–743. [Google Scholar] [CrossRef]
- Galili, G. The Aspartate-Family Pathway of Plants. Plant Signal Behav. 2011, 6, 192–195. [Google Scholar] [CrossRef]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis Seed Development and Germination Is Associated with Temporally Distinct Metabolic Switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar] [CrossRef]
- Schneidereit, J.; Häusler, R.E.; Fiene, G.; Kaiser, W.M.; Weber, A.P.M. Antisense Repression Reveals a Crucial Role of the Plastidic 2-oxoglutarate/Malate Translocator DiT1 at the Interface between Carbon and Nitrogen Metabolism. Plant J. 2006, 45, 206–224. [Google Scholar] [CrossRef]
- Lindén, P.; Keech, O.; Stenlund, H.; Gardeström, P.; Moritz, T. Reduced Mitochondrial Malate Dehydrogenase Activity Has a Strong Effect on Photorespiratory Metabolism as Revealed by 13C Labelling. J. Exp. Bot. 2016, 67, 3123–3135. [Google Scholar] [CrossRef]
- Dao, O.; Kuhnert, F.; Weber, A.P.M.; Peltier, G.; Li-Beisson, Y. Physiological Functions of Malate Shuttles in Plants and Algae. Trends Plant Sci. 2022, 27, 488–501. [Google Scholar] [CrossRef]
- Narayanamma, V.L.; Sharma, H.C.; Gowda, C.L.L.; Sriramulu, M. Incorporation of Lyophilized Leaves and Pods into Artificial Diets to Assess the Antibiosis Component of Resistance to Pod Borer Helicoverpa armigera (Lepidoptera: Noctuidae) in Chickpea. Int. J. Trop. Insect Sci. 2007, 27, 191. [Google Scholar] [CrossRef]
- Yoshida, M.; Cowgill, S.E.; Wightman, J.A. Roles of Oxalic and Malic Acids in Chickpea Trichome Exudate in Host-Plant Resistance to Helicoverpa armigera. J. Chem. Ecol. 1997, 23, 1195–1210. [Google Scholar] [CrossRef]
- Carrington, Y.; Guo, J.; Le, C.H.; Fillo, A.; Kwon, J.; Tran, L.T.; Ehlting, J. Evolution of a Secondary Metabolic Pathway from Primary Metabolism: Shikimate and Quinate Biosynthesis in Plants. Plant J. 2018, 95, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of Chlorogenic Acid as a Resistance Factor for Thrips in Chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. Introduction to the Analysis of Bioactive Compounds in Marine Samples. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–13. [Google Scholar]
- Chen, X.; Hao, R.; Chen, W.; Jia, H.; Qin, S.; Wang, Q.; Zhang, D.; Han, Z.; Li, Y. Effect of Choline Amino Acid Ionic Liquids on Maize Seed Germination and Endogenous Plant Hormone Levels. RSC Adv. 2024, 14, 382–389. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of Volatile, Phenolic, Organic Acid and Sugar Components in a Turkish Cv. Dortyol (Citrus sinensis L. Osbeck) Orange Juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Boo, K.H.; Kim, H.Y.; Eigenheer, R.A.; Phinney, B.S.; Shulaev, V.; Negre-Zakharov, F.; Sadka, A.; Blumwald, E. Label-Free Shotgun Proteomics and Metabolite Analysis Reveal a Significant Metabolic Shift during Citrus Fruit Development. J. Exp. Bot. 2011, 62, 5367–5384. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A Review on Biosynthesis, Analytical Techniques, and Pharmacological Activities of Trigonelline as a Plant Alkaloid. J. Diet. Suppl. 2018, 15, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, M.L.; McNeil, S.D.; Ziemak, M.J.; Hanson, A.D.; Jain, R.K.; Selvaraj, G. Choline Import into Chloroplasts Limits Glycine Betaine Synthesis in Tobacco: Analysis of Plants Engineered with a Chloroplastic or a Cytosolic Pathway. Metab. Eng. 2000, 2, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple Abiotic Stresses Occurring with Salinity Stress in Citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Primo-Millo, E.; Forner, J.B.; Forner-Giner, M.A. Citrus Rootstock Responses to Water Stress. Sci. Hortic. 2010, 126, 95–102. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.G.; Syvertsen, J.P.; Botía, P.; García-Sánchez, F. Leaf Water Relations and Net Gas Exchange Responses of Salinized Carrizo Citrange Seedlings during Drought Stress and Recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Janková, J.; van de Geer, S. Confidence Intervals for High-Dimensional Inverse Covariance Estimation. Electron. J. Stat. 2015, 9, 1205–1229. [Google Scholar] [CrossRef]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef]
- Wilkinson, L.; Friendly, M. The History of the Cluster Heat Map. Am. Stat. 2009, 63, 179–184. [Google Scholar] [CrossRef]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance Analysis of Microarrays Applied to the Ionizing Radiation Response. Proc. Natl. Acad. Sci. USA 2001, 98, 5116–5121. [Google Scholar] [CrossRef]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse Network Modeling and Metscape-Based Visualization Methods for the Analysis of Large-Scale Metabolomics Data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Ireland, R.J.; Lea, P.J. The Enzymes of Glutamine, Glutamate, Asparagine and Aspartate Metabolism; Dekker, M. Inc.: New York, NY, USA; Basel, Switzerland; Hong Kong, China, 1999. [Google Scholar]
- Gorissen, S.H.M.; Phillips, S.M. Branched-Chain Amino Acids (Leucine, Isoleucine, and Valine) and Skeletal Muscle. In Nutrition and Skeletal Muscle; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–298. [Google Scholar]
- Kumari, A. Citric Acid Cycle. In Sweet Biochemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 9–15. [Google Scholar]
- Duggleby, R.G.; McCourt, J.A.; Guddat, L.W. Structure and Mechanism of Inhibition of Plant Acetohydroxyacid Synthase. Plant Physiol. Biochem. 2008, 46, 309–324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).