‘Juxiangyuan’ Seedless Orange: A New Mutant with Male and Female Sterility
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fruit Quality Measurements
2.3. Pollen Morphology Observation and Male Fertility Analysis
2.4. Female Organs Observation
2.5. Artificial Hybridization
2.6. Statistical Analysis
3. Results
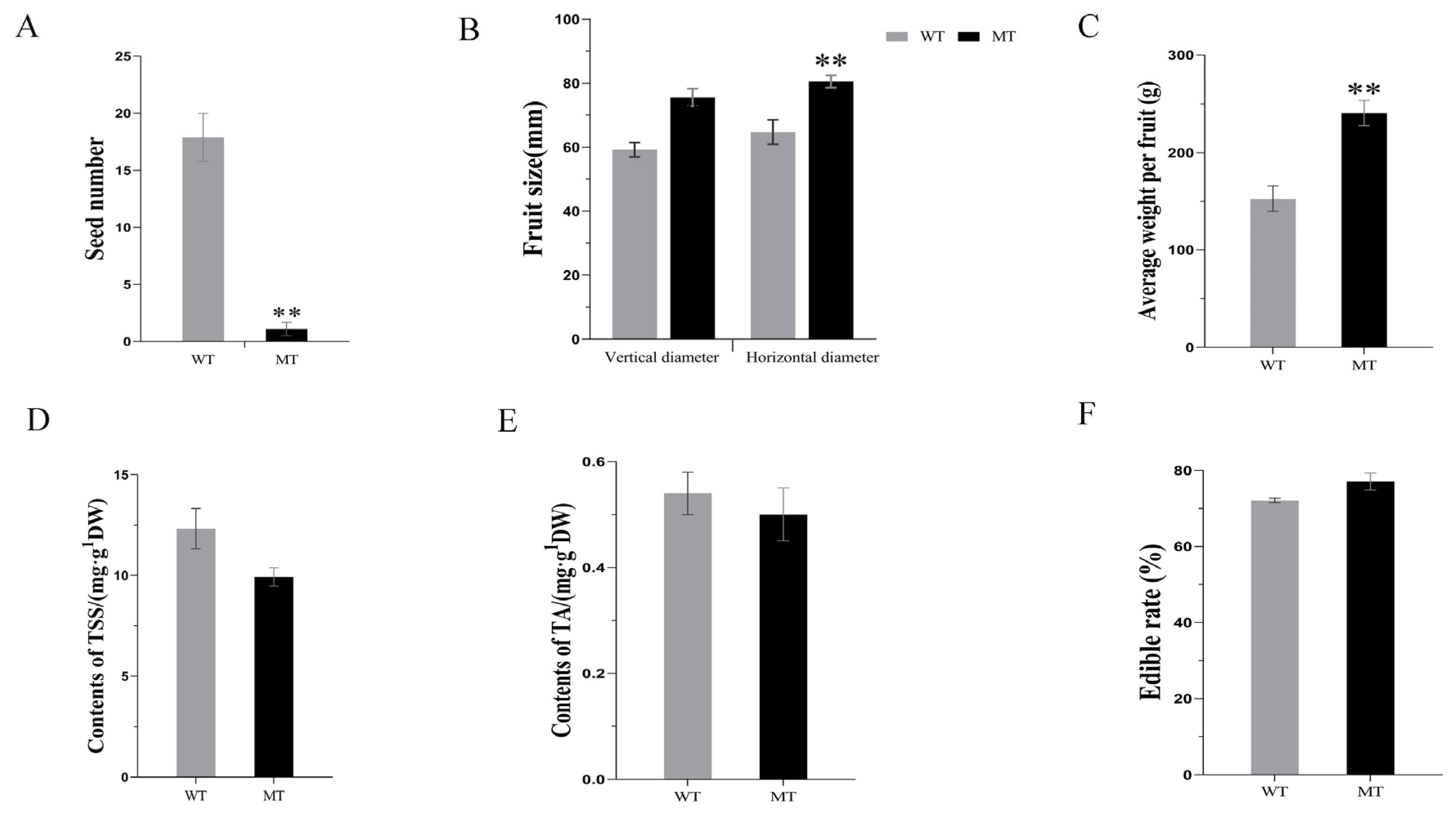
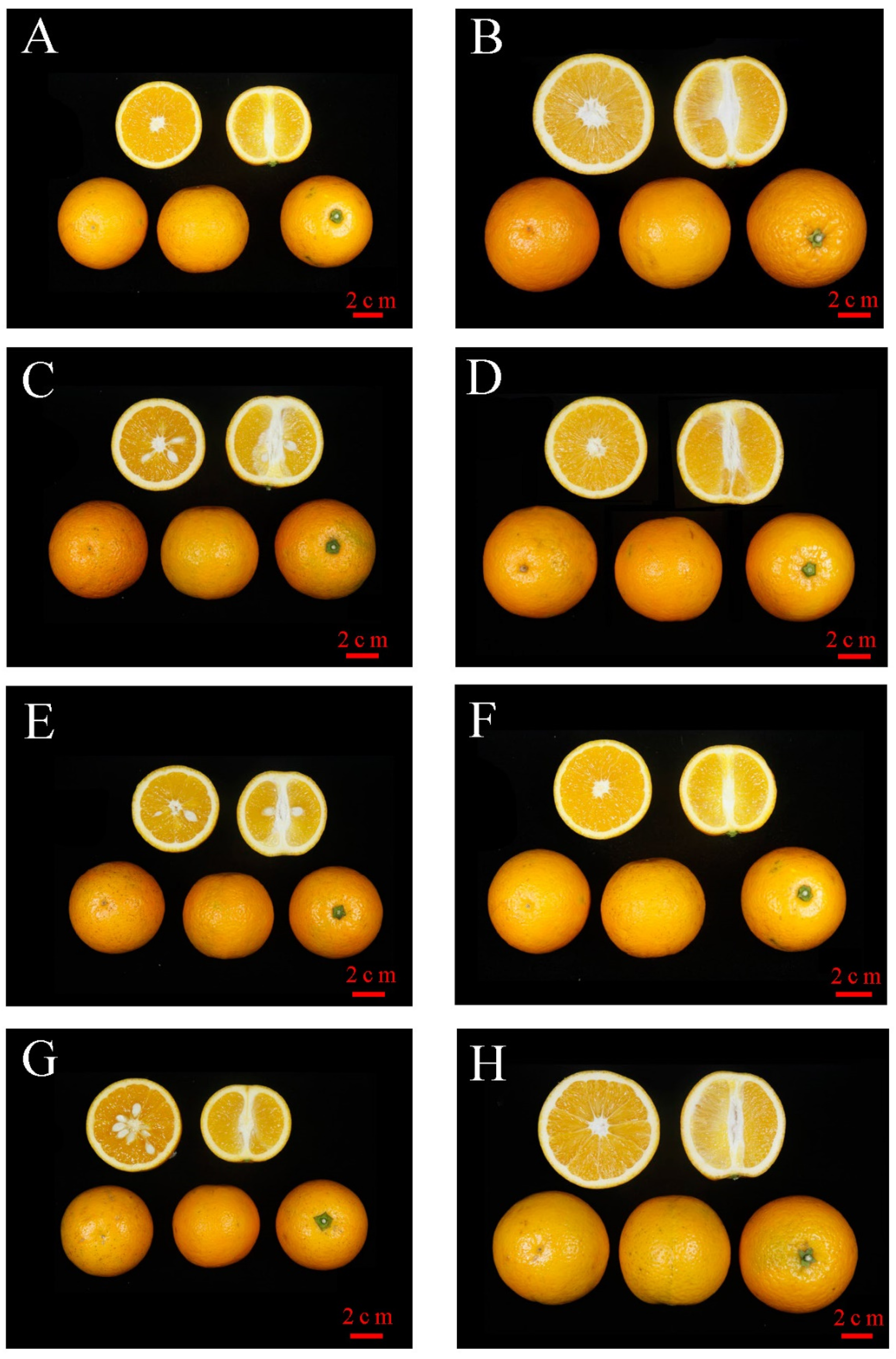
3.1. Fruit Characteristics of MT Compared to WT
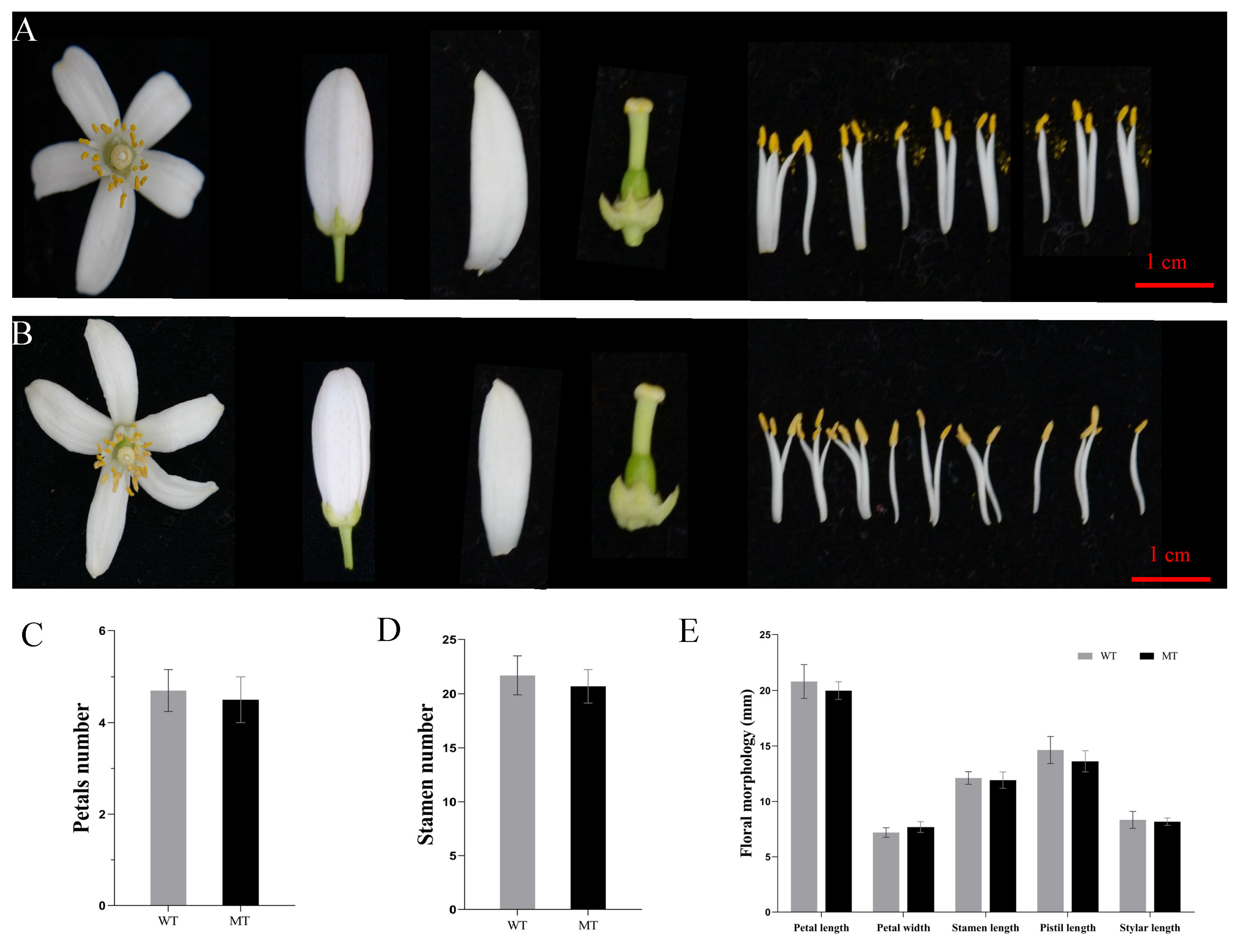
3.2. Floral Morphology and Quality of Two Cultivars
3.3. Pollen Morphology and Viability
3.4. Effects of Self- and Cross-Pollination on Seed Number in Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, J.M.; Liu, C.Y.; Feng, M.Q.; Liu, Y.; Guo, W.W.; Wu, X.M. miR156-SPL modules regulate induction of somatic embryogenesis in citrus callus. J. Exp. Bot. 2018, 69, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Cimen, B.; Yesiloglu, T.; Incesu, M.; Yilmaz, B.J.S.H. Studies on mutation breeding in citrus: Improving seedless types of ‘Kozan’ common orange by gamma irradiation. Sci. Hortic. 2021, 278, 109857. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, C.; Ye, Z.; Silva, J.A.T.D.; Hu, G. Seedless mechanism of a new citrus cultivar ‘Huami Wuhegonggan’ (Citrus sinensis × C. reticulata). Pak. J. Bot. 2015, 47, 2369–2378. [Google Scholar]
- Cheng, J.; Liao, L.; Zhou, H.; Gu, C.; Wang, L.; Han, Y. A small indel mutation in an anthocyanin transporter causes variegated colouration of peach flowers. J. Exp. Bot. 2015, 66, 7227–7239. [Google Scholar] [CrossRef]
- Kamatyanatt, M.; Singh, S.K.; Sekhon, B.S. Mutation Breeding In Citrus—A Review. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 1–8. [Google Scholar]
- Foster, T.M.; Aranzana, M.J. Attention sports fans! The far-reaching contributions of bud sport mutants to horticulture and plant biology. Hortic. Res. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, M.; Wen, J.; Yi, H.; Deng, X.; Xu, X. Abnormal Microspore Development Leads to Pollen Abortion in a Seedless Mutant of ‘Ougan’ Mandarin (Citrus suavissima Hort. ex Tanaka). Am. Soc. Hortic. Sci. 2007, 132, 777–782. [Google Scholar] [CrossRef]
- Ye, L.X.; Gan, Z.M.; Wang, W.F.; Ai, X.Y.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. Comparative analysis of the transcriptome, methylome, and metabolome during pollen abortion of a seedless citrus mutant. Plant Mol. Biol. 2020, 104, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.K.; Yun, S.H.; Yi, K.U.; Park, Y.C.; Lee, H.Y.; Kim, M.; Lee, Y.; Song, K.J.; Kim, H.B. Identification of Citrus Varieties Bred in Korea Using Microsatellite Markers. Hortic. Sci. Technol. 2020, 38, 374–384. [Google Scholar] [CrossRef]
- Deng, X. Advances in worldwide citrus breeding. Acta Hortic. Sin. 2005, 32, 1140–1146. [Google Scholar]
- Sudo, M.; Yasuda, K.; Yahata, M.; Sato, M.; Tominaga, A.; Mukai, H.; Ma, G.; Kato, M.; Kunitake, H. Morphological Characteristics, Fruit Qualities and Evaluation of Reproductive Functions in Autotetraploid Satsuma Mandarin (Citrus unshiu Marcow.). Agronomy 2021, 11, 2441. [Google Scholar] [CrossRef]
- Kumar, K.; Yu, Q.; Bhatia, D.; Honsho, C.; Gmitter, F.G., Jr. Construction of a high density genetic linkage map to define the locus conferring seedlessness from Mukaku Kishu mandarin. Hortic. J. 2023, 14, 1087023. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, L.; Peng, A.; Lei, T.; Li, Q.; Yao, L.; Jiang, G.; Chen, S.; Li, Z.; Zou, X. Production of marker-free transgenic plants from mature tissues of navel orange using a Cre/loxP site-recombination system. Hortic. Plant J. 2023, 9, 473–480. [Google Scholar] [CrossRef]
- Honsho, C. Self-incompatibility Related to Seedless Fruit Production in Citrus Plants. Hortic. J. 2023, 92, 1–12. [Google Scholar] [CrossRef]
- Vardi, A.; Levin, I.; Carmi, N. Induction of seedlessness in citrus: From classical techniques to emerging biotechnological approaches. J. Am. Soc. Hortic. Sci. 2008, 133, 117–126. [Google Scholar] [CrossRef]
- Bermejo, A.; Pardo, J.; Morales, J.; Cano, A. Comparative study of bioactive components and quality from juices of different mandarins: Discriminant multivariate analysis of their primary and secondary metabolites. Agric. Sci. 2016, 7, 341–351. [Google Scholar] [CrossRef]
- Goto, S.; Yoshioka, T.; Ohta, S.; Kita, M.; Hamada, H.; Shimizu, T. Segregation and heritability of male sterility in populations derived from progeny of Satsuma mandarin. PLoS ONE 2016, 11, e0162408. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M. Progress on studies for seedless breeding of citrus in Japan. Progress on studies for seedless breeding of citrus in Japan. Adv. Hortic. Sci. 2014, 28, 64–72. [Google Scholar]
- Tidy, A.C.; Ferjentsikova, I.; Vizcay-Barrena, G.; Liu, B.; Yin, W.; Higgins, J.D.; Xu, J.; Zhang, D.; Geelen, D.; Wilson, Z.A. Sporophytic control of pollen meiotic progression is mediated by tapetum expression of ABORTED MICROSPORES. J. Exp. Bot. 2022, 73, 5543–5558. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, D.; Ke, F.; Zhu, M.; Xu, J.; Zhang, M. Seedless mutant ‘Wuzi Ougan’ (Citrus suavissima Hort. ex Tanaka ‘seedless’) and the wild type were compared by iTRAQ-based quantitative proteomics and integratedly analyzed with transcriptome to improve understanding of male sterility. BMC Genet. 2018, 19, 106. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Zhu, Q.; Liu, Z.; Yan, F.; Wu, L.; Xu, X.; Zhou, X.Y.; Chen, X.; Amp, Z.A. Cytological observation of pollen development in ‘Ougan’ (Citrus suavissima Hort. ex Tanaka) and its seedless mutant. J. Fruit Sci. 2014, 31, 265–269. [Google Scholar]
- Catalano, C.; Las Casas, G.; Giuffrida, A.; Ferlito, F.; Di Guardo, M.; Continella, A.; Distefano, G. Reproductive biology factors hampering lemon [Citrus limon (L.) Burm. f.] genetic improvement. Agriculture 2022, 12, 2020. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Hu, C.Y.; Lu, Y.G.; Li, J.Q.; Liu, X.D. Abnormalities occurring during female gametophyte development result in the diversity of abnormal embryo sacs and leads to abnormal fertilization in indica/japonica hybrids in rice. J. Integr. Plant Biol. 2009, 51, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, L.; Fan, F.; Liu, X. Comparative transcript profiling reveals the mechanism of female sterility associated with seedless Ponkan mandarin (Citrus reticulata Blanco). Genome 2018, 61, 595–604. [Google Scholar] [CrossRef]
- Liang, M.; Cao, Z.; Zhu, A.; Liu, Y.; Tao, M.; Yang, H.; Xu, Q., Jr.; Wang, S.; Liu, J.; Li, Y.; et al. Evolution of self-compatibility by a mutant S(m)-RNase in citrus. Nat. Plants 2020, 6, 131–142. [Google Scholar] [CrossRef]
- Xue, M.N.; Chen, T.T.; Yang, X.H. Observations on self and cross-compatibility in Shatinyu. Acta Hortic. Sin. 1995, 22, 127–132. [Google Scholar]
- Qiao, L.; Cao, M.; Zheng, J.; Zhao, Y.; Zheng, Z.L. Gene coexpression network analysis of fruit transcriptomes uncovers a possible mechanistically distinct class of sugar/acid ratio-associated genes in sweet orange. BMC Plant Biol. 2017, 17, 186. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Huang, H.; Zhang, F.; Gentile, A.; Deng, Z.; Li, N. A new seedless orange ‘Juxiangyuan’ selected from ‘Succari Orange’ mutant. J. Fruit Sci. 2019, 36, 388–391. [Google Scholar]
- Bermejo, A.; Pardo, J.; Cano, A. Influence of gamma irradiation on seedless citrus production: Pollen germination and fruit quality. Food Nutr. Sci. 2011, 2, 169–180. [Google Scholar]
- Chai, L.; Ge, X.; Biswas, M.K.; Xu, Q.; Deng, X.X. Self-sterility in the mutant ‘Zigui shatian’ pummelo (Citrus grandis Osbeck) is due to abnormal post-zygotic embryo development and not self-incompatibility. Plant Cell Tissue Organ Cult. 2011, 104, 1–11. [Google Scholar] [CrossRef]
- Ateyyeh, A.F. Improving in vitro pollen germination of five species of fruit trees. Dirasat Agric. Sci. 2005, 32, 189–194. [Google Scholar]
- Li, F.; Gong, L.; Deng, Z.; Gentile, A.; Long, G.; Li, D.; Lu, X. Seed, sugar and acid characteristics of ‘Zaomi’ Ponkan (Citrus reticulata Blanco), a spontaneous mutant of ‘Xinnu’. Sci. Hortic. 2017, 225, 707–715. [Google Scholar] [CrossRef]
- Ye, W.; Qin, Y.; Ye, Z.; Silva, J.A.T.D.; Zhang, L.; Wu, X.; Lin, S.; Hu, G. Seedless mechanism of a new mandarin cultivar ‘Wuzishatangju’ (Citrus reticulata Blanco). Plant Sci. 2009, 177, 19–27. [Google Scholar] [CrossRef]
- Balaguera-López, H.E.; Fischer, G.; Magnitskiy, S. Seed-fruit relationships in fleshy fruit: Role of hormones. A review. Rev. Colomb. Cienc. Hortícolas 2020, 14, 90–103. [Google Scholar] [CrossRef]
- Bons, H.; Nirmaljit, K.; Rattanpal, H. Quality and Quantity Improvement of Citrus: Role of Plant Growth Regulators. Int. J. Agric. Environ. Biotechnol. 2015, 8, 433. [Google Scholar] [CrossRef]
- Mazzucato, A.; Cellini, F.; Bouzayen, M.; Zouine, M.; Mila, I.; Minoia, S.; Carriero, F. A TILLING allele of the tomato Aux/IAA9 gene offers new insights into fruit set mechanisms and perspectives for breeding seedless tomatoes. Mol. Breed. 2015, 35, 22. [Google Scholar] [CrossRef]
- Thanopoulos, C.; Bouranis, D.; Passam, H.J.S.H. Comparative development, maturation and ripening of seedless and seed-containing bell pepper fruits. Sci. Hortic. 2013, 164, 573–577. [Google Scholar] [CrossRef]
- Lovatt, C.J. Factors affecting fruit set/early fruit drop in avocado. Calif. Avocado Soc. Yearb. 1990, 74, 193–199. [Google Scholar]
- Yin, P.; Ding, W.; Zhang, H.; Liu, X.; Zhang, H.; Zeng, J.; Xu, J. Morphological, physiological and molecular characteristics of the seedless ‘Hongjiangcheng’ sweet orange. Hortic. Plant J. 2023, 9, 437–449. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Reig, C.; Martínez-Fuentes, A.; Iglesias, D.J.; Munoz-Fambuena, N. Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant Sci. 2016, 247, 13–24. [Google Scholar] [CrossRef]
- Garcia-Papi, M.A.; Garcia-Martinez, J.L. Endogenous plant growth substances content in young fruits of seeded and seedless Clementine mandarin as related to fruit set and development. Sci. Hortic. 1984, 22, 265–274. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsumoto, R.; Yamada, Y. Relationship between sterility and seedlessness in citrus. J. Jpn. Soc. Hortic. Sci. 1995, 64, 23–29. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. The effective pollination period in ‘Clemenules’ mandarin, ‘Owari’ Satsuma mandarin and ‘Valencia’ sweet orange. Plant Sci. 2007, 173, 223–230. [Google Scholar] [CrossRef]
- Atawia, A.; Abd EL-Latif, F.; EL-Badawy, H.; Abo-Aziz, A.; Abou Rayya, M.; Baiea, M. Effect of various pollination treatments on yield characteristics and fruit quality of shaddock fruits. Int. J. Sci. Eng. Res. 2016, 7, 1–9. [Google Scholar]
- Mesejo, C.; Yuste, R.; Martínez-Fuentes, A.; Reig, C.; Iglesias, D.J.; Primo-Millo, E. Self-pollination and parthenocarpic ability in developing ovaries of self-incompatible Clementine mandarins (Citrus clementina). Physiol. Plant. 2013, 148, 87–96. [Google Scholar] [CrossRef]
- Picarella, M.E.; Mazzucato, A. The Occurrence of Seedlessness in Higher Plants; Insights on Roles and Mechanisms of Parthenocarpy. Front. Plant Sci. 2019, 9, 1997. [Google Scholar] [CrossRef]
- Premachandran, A.; Dhayasree, K.; Kurien, S. Seedless fruits: Fruits of future. J. Pharmacogn. Phytochem. 2019, 8, 1053–1059. [Google Scholar]
- Chao, C.-C.T.; Fang, J.; Devanand, P.S. Long distance pollen flow in mandarin orchards determined by AFLP markers-implications for seedless mandarin production. J. Am. Soc. Hortic. Sci. 2005, 130, 374–380. [Google Scholar] [CrossRef]
- Hasegawa, Y. Programmed Citrus Breeding in Japan. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60, 609–613. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tominaga, S. Relationship between seedlessness of Keraji (Citrus keraji hort. ex Tanaka) and female sterility and self-incompatibility. J. Jpn. Soc. Hortic. Sci. 2002, 71, 183–186. [Google Scholar] [CrossRef]
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Raza, S.A.; Rehman, R.N.U. Innovative breeding methods to develop seedless citrus cultivars. Int. J. Biosci. 2013, 3, 191–201. [Google Scholar]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Distefano, G.; Gentile, A.; Hedhly, A.; La Malfa, S. Temperatures during flower bud development affect pollen germination, self-incompatibility reaction and early fruit development of clementine (Citrus clementina Hort. ex Tan.). Plant Biol. 2018, 20, 191–198. [Google Scholar] [CrossRef]
- Lu, L.; Yang, H.; Xu, Y.; Zhang, L.; Wu, J.; Yi, H. Laser capture microdissection-based spatiotemporal transcriptomes uncover regulatory networks during seed abortion in seedless Ponkan (Citrus reticulata). Plant J. Cell Mol. Biol. 2023, 115, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Gu, W.; Chen, J.; Song, N.; Gao, X.; Zhang, X. High Temporal-Resolution Transcriptome Landscape of Early Maize Seed Development. Plant Cell 2019, 31, 974–992. [Google Scholar] [CrossRef]
- Jiang, N.; Feng, M.Q.; Cheng, L.C.; Kuang, L.H.; Li, C.C.; Yin, Z.P.; Wang, R.; Xie, K.D.; Guo, W.W.; Wu, X.M. Spatiotemporal profiles of gene activity in stamen delineate nucleo-cytoplasmic interaction in a male-sterile somatic cybrid citrus. Hortic. Res. 2023, 10, uhad105. [Google Scholar] [CrossRef]



| Pollen Equatorial Axis Length (μm) | Pollen Polar Axis Length (μm) | Pollen Aberration Rate (%) | Pollen Viability (%) | Pollen Germination (%) | |
|---|---|---|---|---|---|
| WT | 36.2 ± 0.18 | 19.4 ± 0.38 | 3.57 ± 0.12 | 89.8 ± 1.32 | 87.49 ± 0.58 |
| MT | 28.41 ± 0.66 * | 16.17 ± 0.63 | 41.48 ± 0.46 ** | 9.59 ± 1.4 ** | 9.56 ± 0.02 ** |
| Female Parent | Male Parent | Average Seed Number |
|---|---|---|
| WT | - | 0 c |
| WT | WT | 11.47 ± 0.84 a |
| WT | MT | 3.51 ± 1.78 b |
| WT | ‘Dahong’ sweet orange | 2.4 ± 0.12 b |
| MT | - | 0 c |
| MT | WT | 1 ± 0.70 b |
| MT | MT | 1 ± 0.53 b |
| MT | ‘Dahong’ sweet orange | 0.67 ± 0.67 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wang, Q.; Luo, J.; Gentile, A.; Long, G.; Deng, Z.; Li, D.; Li, N. ‘Juxiangyuan’ Seedless Orange: A New Mutant with Male and Female Sterility. Horticulturae 2024, 10, 350. https://doi.org/10.3390/horticulturae10040350
Zhang F, Wang Q, Luo J, Gentile A, Long G, Deng Z, Li D, Li N. ‘Juxiangyuan’ Seedless Orange: A New Mutant with Male and Female Sterility. Horticulturae. 2024; 10(4):350. https://doi.org/10.3390/horticulturae10040350
Chicago/Turabian StyleZhang, Feng, Qinggang Wang, Jianming Luo, Alessandra Gentile, Guiyou Long, Ziniu Deng, Dazhi Li, and Na Li. 2024. "‘Juxiangyuan’ Seedless Orange: A New Mutant with Male and Female Sterility" Horticulturae 10, no. 4: 350. https://doi.org/10.3390/horticulturae10040350
APA StyleZhang, F., Wang, Q., Luo, J., Gentile, A., Long, G., Deng, Z., Li, D., & Li, N. (2024). ‘Juxiangyuan’ Seedless Orange: A New Mutant with Male and Female Sterility. Horticulturae, 10(4), 350. https://doi.org/10.3390/horticulturae10040350






