Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus ostreatus Grown on Different Commercial Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Set-Up
2.2. Substrate Chemical Characterization
2.3. Productive and Qualitative Traits
2.4. Statistical Analysis
3. Results
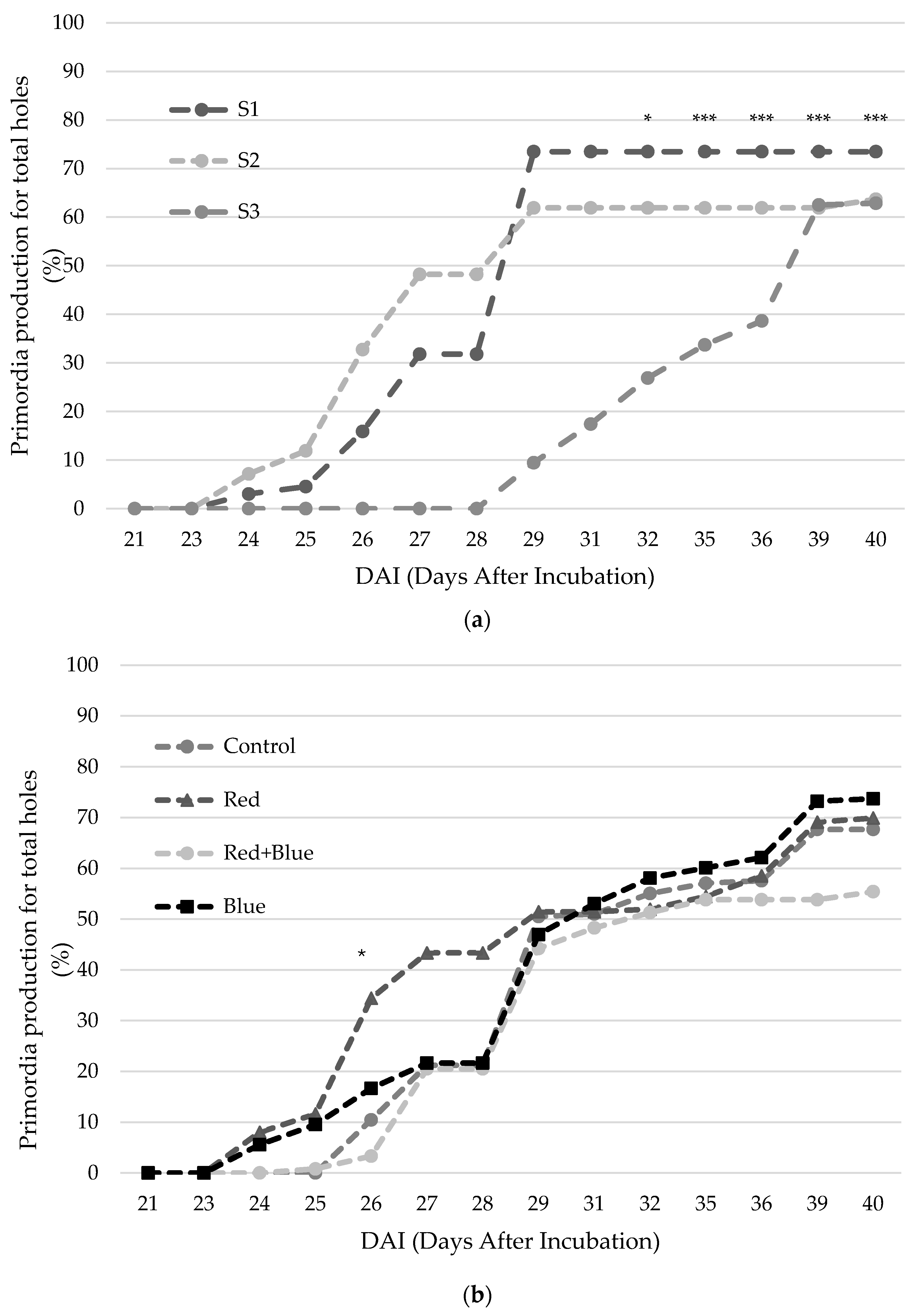
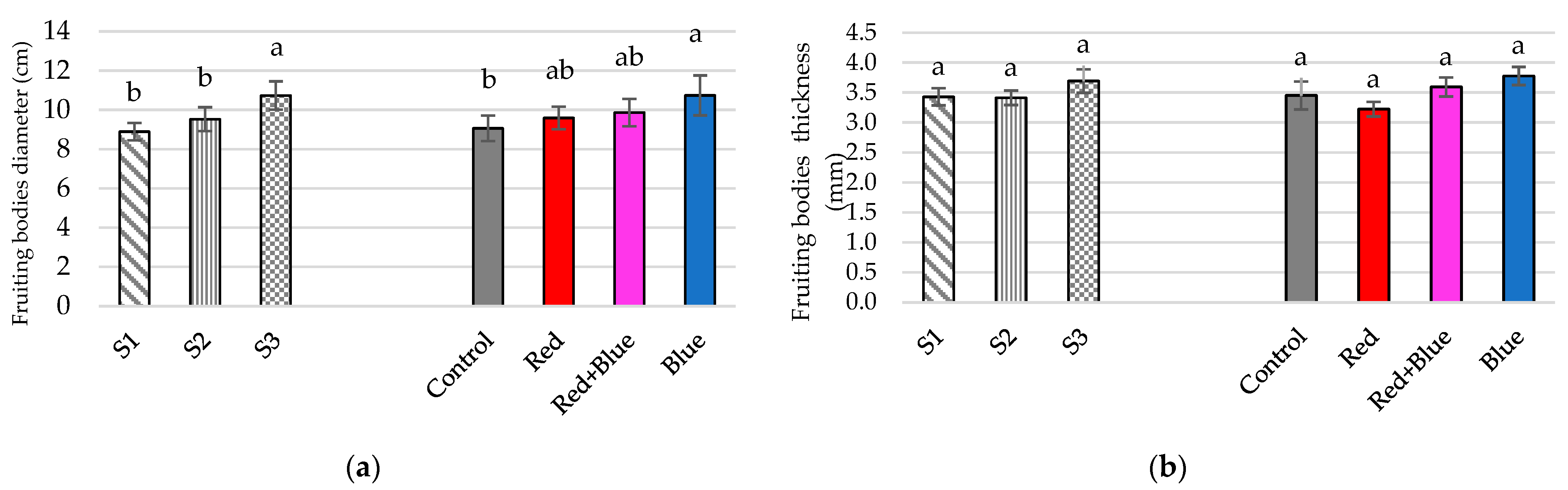
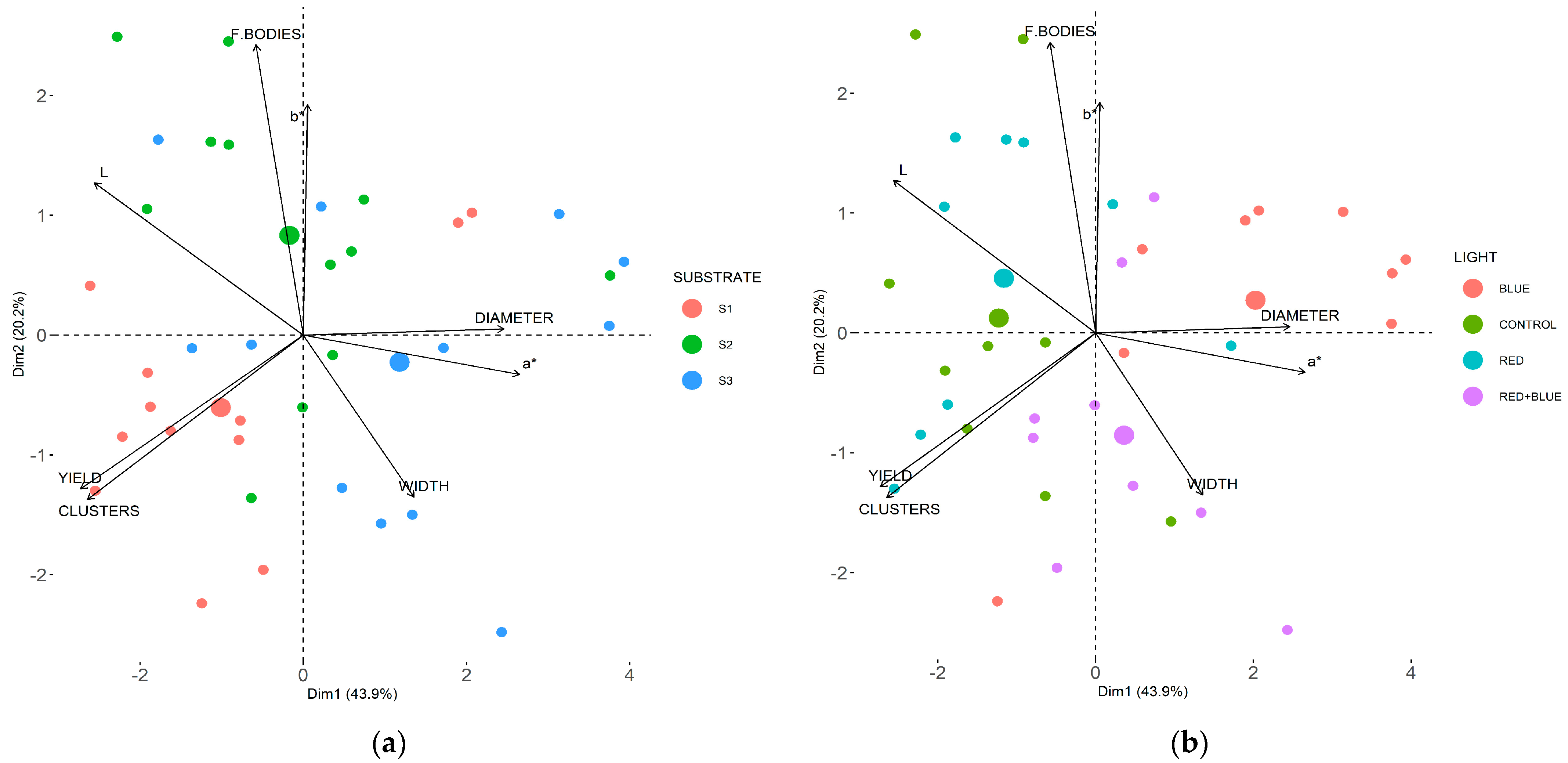
3.1. Productive Traits
3.2. Qualitative Traits
4. Discussion
4.1. Productive Traits
4.1.1. Lighting Treatments
4.1.2. Substrate
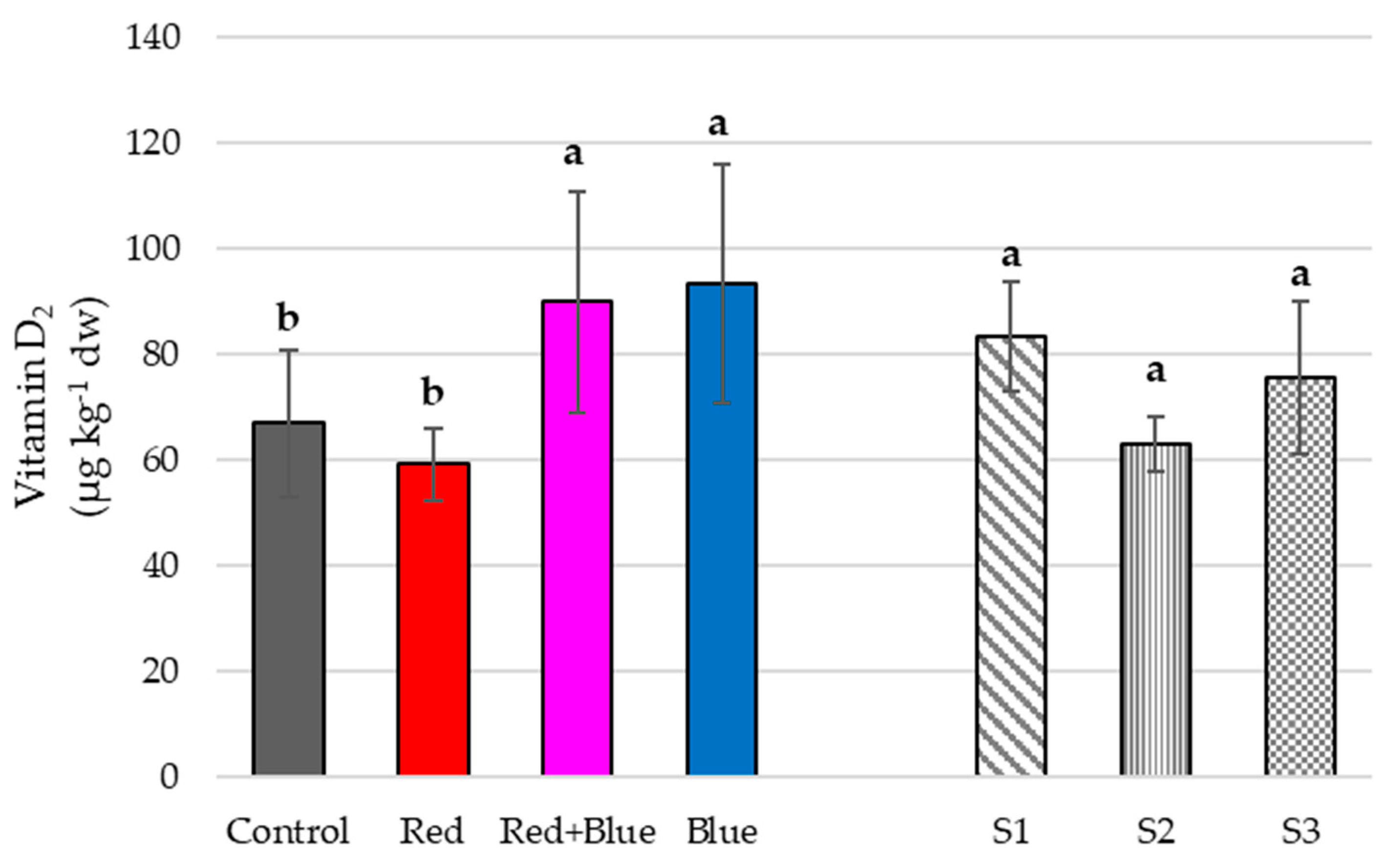
4.2. Qualitative Traits
Vitamin D2
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, M.P. Advances in Mushroom Production: Key to Food, Nutritional and Employment Security: A Review. Indian Phytopathol. 2020, 73, 377–395. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 5–13. ISBN 978-1-119-14941-5. [Google Scholar]
- Ferraro, V.; Venturella, G.; Pecoraro, L.; Gao, W.; Gargano, M.L. Cultivated Mushrooms: Importance of a Multipurpose Crop, with Special Focus on Italian Fungiculture. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2022, 156, 130–142. [Google Scholar] [CrossRef]
- Rawiniwati, W. Agribusiness Prospect of Banana Flowers and Oyster Mushrooms as Raw Materials of Meatballs Vegetarian. J. Trop. Biodivers. 2021, 1, 149–166. [Google Scholar] [CrossRef]
- Lesa, K.N.; Khandaker, M.U.; Mohammad Rashed Iqbal, F.; Sharma, R.; Islam, F.; Mitra, S.; Emran, T.B. Nutritional Value, Medicinal Importance, and Health-Promoting Effects of Dietary Mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Doroški, A.; Klaus, A.; Režek Jambrak, A.; Djekic, I. Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review. Sustainability 2022, 14, 12509. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom Cultivation in the Circular Economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Bonatti, M.; Karnopp, P.; Soares, H.M.; Furlan, S.A. Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju Nutritional Characteristics When Cultivated in Different Lignocellulosic Wastes. Food Chem. 2004, 88, 425–428. [Google Scholar] [CrossRef]
- Doroški, A.; Klaus, A.; Kozarski, M.; Cvetković, S.; Nikolić, B.; Jakovljević, D.; Tomasevic, I.; Vunduk, J.; Lazić, V.; Djekic, I. The Influence of Grape Pomace Substrate on Quality Characterization of Pleurotus ostreatus—Total Quality Index Approach. J. Food Process. Preserv. 2021, 45, e15096. [Google Scholar] [CrossRef]
- Mleczek, M.; Gąsecka, M.; Budka, A.; Niedzielski, P.; Siwulski, M.; Kalač, P.; Mleczek, P.; Rzymski, P. Changes in Mineral Composition of Six Strains of Pleurotus after Substrate Modifications with Different Share of Nitrogen Forms. Eur. Food Res. Technol. 2021, 247, 245–257. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C.; et al. A Review on Valorization of Oyster Mushroom and Waste Generated in the Mushroom Cultivation Industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef]
- Mandeel, Q.A.; Al-Laith, A.A.; Mohamed, S.A. Cultivation of Oyster Mushrooms (Pleurotus spp.) on Various Lignocellulosic Wastes. World J. Microbiol. Biotechnol. 2005, 21, 601–607. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Eger-Hummel, G. Blue-Light Photomorphogenesis in Mushrooms (Basidiomycetes). In The Blue Light Syndrome; Senger, H., Ed.; Proceedings in Life Sciences; Springer: Berlin/Heidelberg, Germany, 1980; pp. 555–562. ISBN 978-3-642-67650-5. [Google Scholar]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors Affecting Mushroom pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, H.; Sun, Y.; Xia, R.; Hou, Z.; Li, Y.; Wang, Y.; Pan, S.; Li, L.; Zhao, C.; et al. Effect of Light on Quality of Preharvest and Postharvest Edible Mushrooms and Its Action Mechanism: A Review. Trends Food Sci. Technol. 2023, 139, 104119. [Google Scholar] [CrossRef]
- Ko, J.A.; Lee, B.H.; Lee, J.S.; Park, H.J. Effect of UV-B Exposure on the Concentration of Vitamin D2 in Sliced Shiitake Mushroom (Lentinus Edodes) and White Button Mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, A.; Fornal, E.; Radzki, W.; Skrzypczak, K.; Zalewska-Korona, M.; Michalak-Majewska, M.; Parfieniuk, E.; Stachniuk, A. Study on Vitamin D2 Stability in Dried Mushrooms during Drying and Storage. Food Chem. 2016, 199, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.; James, A.; Black, L. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Fernandes, Â.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R. UV-Irradiated Mushrooms as a Source of Vitamin D 2: A Review. Trends Food Sci. Technol. 2017, 70, 82–94. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, W.; Liu, W.; Xu, J.; Liu, W.; Zhang, X. Effect of Different Light Qualities and Intensities on the Yield and Quality of Facility-Grown Pleurotus eryngii. J. Fungi 2022, 8, 1244. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Tian, F.; Jia, C.; Li, C.; Li, Y. Transcriptomic Profiling Sheds Light on the Blue-Light and Red-Light Response of Oyster Mushroom (Pleurotus ostreatus). AMB Express 2020, 10, 10. [Google Scholar] [CrossRef]
- Du, F.; Zou, Y.; Hu, Q.; Zhang, H.; Ye, D. Comparative Transcriptomic Analysis Reveals Molecular Processes Involved in Pileus Morphogenesis in Pleurotus eryngii under Different Light Conditions. Genomics 2020, 112, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Masuno, K.; Abe, M.; Nishizawa, H.; Matsumoto, T.; Kunitomo, S.; Sakata, H.; Nakamura, K.; Koyama, T.; Ito, M.; et al. Light-Stimulative Effects on The Cultivation of Edible Mushrooms by Using Blue Led. In Proceedings of the Mushroom Biology and Mushroom Products: 7th International Conference on Mushroom Biology and Mushroom Products, Arcachon, France, 4–7 October 2011; Volume 2. Poster session. [Google Scholar]
- Paucek, I.; Appolloni, E.; Pennisi, G.; Quaini, S.; Gianquinto, G.; Orsini, F. LED Lighting Systems for Horticulture: Business Growth and Global Distribution. Sustainability 2020, 12, 7516. [Google Scholar] [CrossRef]
- Nicoletto, C.; Tosini, F.; Sambo, P. Effect of Grafting on Biochemical and Nutritional Traits of ‘Cuore Di Bue’ Tomatoes Harvested at Different Ripening Stages. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2013, 63, 114–122. [Google Scholar] [CrossRef]
- Accredited Method for Quantification of Cholecalciferol: MP 1570 Rev 2/2017 (LC-MS/MS); Chelab—Mérieux NutriSciences Corporation: Resana, Italy, 2017.
- Roshita, I.; Goh, S.Y. Effect of Exposure to Different Colors Light Emitting Diode on the Yield and Physical Properties of Grey and White Oyster Mushrooms; AIP Publishing: Ho Chi Minh, Vietnam, 2018; p. 020110. [Google Scholar]
- Muswati, C.; Simango, K.; Tapfumaneyi, L.; Mutetwa, M.; Ngezimana, W. The Effects of Different Substrate Combinations on Growth and Yield of Oyster Mushroom (Pleurotus ostreatus). Int. J. Agron. 2021, 2021, 9962285. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.H.; Eira, A.F.D.; Kuramae, E.E.; Queiroz, E.C. Morphomolecular Characterization of Pleurotus ostreatus (Jacq. Fr.) Kummer Strains in Relation to Luminosity and Temperature of Frutification. Sci. Agric. 2003, 60, 531–535. [Google Scholar] [CrossRef]
- Villaescusa, R.; Gil, M.I. Quality Improvement of Pleurotus Mushrooms by Modified Atmosphere Packaging and Moisture Absorbers. Postharvest Biol. Technol. 2003, 28, 169–179. [Google Scholar] [CrossRef]
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.-C.; Bhuyan, D.J. Recovery of Ergosterol and Vitamin D2 from Mushroom Waste—Potential Valorization by Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Ložnjak, P.; Jakobsen, J. Stability of Vitamin D3 and Vitamin D2 in Oil, Fish and Mushrooms after Household Cooking. Food Chem. 2018, 254, 144–149. [Google Scholar] [CrossRef]
- Timpanaro, G.; Bellia, C.; Foti, V.T.; Scuderi, A. Consumer Behaviour of Purchasing Biofortified Food Products. Sustainability 2020, 12, 6297. [Google Scholar] [CrossRef]

| Substrate | Strain | C:N Ratio | Total Nitrogen | P | K | Cd | Cr | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| % | mg kg−1 dw | |||||||||
| S1 | P80 | 47.5 | 0.89 | 989 | 11,129 | <0.1 | 7.09 | 5.21 | 0.97 | 20.89 |
| S2 | 60.6 | 0.70 | 721 | 8798 | <0.1 | 11.47 | 6.37 | 1.42 | 19.08 | |
| S3 | 49.3 | 0.87 | 813 | 11,696 | <0.1 | 11.59 | 5.28 | 2.68 | 24.38 | |
| Yield kg kg−1 Substrate | Biological Efficiency (%) | Number Clusters kg−1 Substrate | Number Fruiting Bodies/Cluster | |
|---|---|---|---|---|
| Substrate | ||||
| S1 | 0.17 ± 0.01 | 50.1 ± 2.62 | 0.76 ± 0.10 a | 18.9 ± 0.96 |
| S2 | 0.21 ± 0.01 | 61.8 ± 3.03 | 0.47 ± 0.06 b | 19.8 ± 0.90 |
| S3 | 0.18 ± 0.02 | 53.1 ± 5.27 | 0.40 ± 0.06 b | 21.6 ± 1.49 |
| p-value | ns | ns | 0.0022 | ns |
| Lighting treatment | ||||
| Control | 0.19 ± 0.01 | 56.3 ± 3.44 | 0.68 ± 0.07 a | 21.9 ± 1.33 |
| Red | 0.19 ± 0.01 | 54.5 ± 2.68 | 0.66 ± 0.08 a | 23.2 ± 1.58 |
| Red + Blue | 0.20 ± 0.01 | 59.13 ± 3.18 | 0.56 ± 0.07 ab | 18.3 ± 0.82 |
| Blue | 0.17 ± 0.03 | 50.14 ± 7.48 | 0.28 ± 0.12 b | 20.0 ± 1.14 |
| p-value | ns | ns | 0.0043 | ns |
| L | a* | b* | Fruiting Bodies Color | |
|---|---|---|---|---|
| Substrate | ||||
| S1 | 61.0 ± 0.66 a | 2.71 ± 0.64 a | 9.2 ± 0.41 b | |
| S2 | 62.5 ± 0.92 a | 2.56 ± 0.70 a | 10.24 ± 0.25 a | |
| S3 | 56.0 ± 0.74 b | 3.29 ± 0.66 b | 8.76 ± 0.53 b | |
| p-value | 0.000 | 0.000 | 0.0001 | |
| Lighting treatment | ||||
| Control | 63.6 ± 0.94 a | 2.06 ± 0.15 c | 9.55 ± 0.41 | |
| Red | 63.8 ± 0.91 a | 2.27 ± 0.16 c | 9.40 ± 0.25 | |
| Red + Blue | 56.0 ± 0.89 b | 3.30± 0.16 b | 8.94 ± 0.26 | |
| Blue | 55.7 ± 1.08 b | 3.91 ± 0.17 a | 10.03 ± 0.30 | |
| p-value | 0.000 | 0.000 | ns | |
| pH | EC (mS cm−1) | Total Soluble Solids (°Brix) | Titratable Acidity (% Citric Acid eq.) | |
|---|---|---|---|---|
| Substrate | ||||
| S1 | 6.34 ± 0.04 | 4.34 ± 0.34 b | 2.91 ± 0.24 b | 0.72 ± 0.09 c |
| S2 | 6.26 ± 0.04 | 5.24 ± 0.13 a | 3.78 ± 0.22 a | 1.30 ± 0.07 a |
| S3 | 6.30 ± 0.05 | 4.78 ± 0.17 ab | 3.24 ± 0.19 ab | 1.00 ± 0.06 b |
| p-value | ns | 0.0345 | 0.0165 | 0.000 |
| Light treatments | ||||
| Control | 6.24 ± 0.04 | 4.44 ± 0.33 | 2.97 ± 0.22 | 1.01 ± 0.11 |
| Red | 6.28 ± 0.06 | 5.17 ± 0.16 | 3.71 ± 0.24 | 1.06 ± 0.15 |
| Red + Blue | 6.35 ± 0.03 | 4.95 ± 0.14 | 3.52 ± 0.25 | 1.03 ± 0.04 |
| Blue | 6.32 ± 0.06 | 4.63 ± 0.41 | 3.05 ± 0.34 | 0.96 ± 0.15 |
| p-value | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bonis, M.; Locatelli, S.; Sambo, P.; Zanin, G.; Pecchia, J.A.; Nicoletto, C. Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus ostreatus Grown on Different Commercial Substrates. Horticulturae 2024, 10, 349. https://doi.org/10.3390/horticulturae10040349
De Bonis M, Locatelli S, Sambo P, Zanin G, Pecchia JA, Nicoletto C. Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus ostreatus Grown on Different Commercial Substrates. Horticulturae. 2024; 10(4):349. https://doi.org/10.3390/horticulturae10040349
Chicago/Turabian StyleDe Bonis, Marina, Silvia Locatelli, Paolo Sambo, Giampaolo Zanin, John A. Pecchia, and Carlo Nicoletto. 2024. "Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus ostreatus Grown on Different Commercial Substrates" Horticulturae 10, no. 4: 349. https://doi.org/10.3390/horticulturae10040349
APA StyleDe Bonis, M., Locatelli, S., Sambo, P., Zanin, G., Pecchia, J. A., & Nicoletto, C. (2024). Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus ostreatus Grown on Different Commercial Substrates. Horticulturae, 10(4), 349. https://doi.org/10.3390/horticulturae10040349







