Postharvest Chemical Treatment of Physiologically Induced Stem End Blockage Improves Vase Life and Water Relation of Cut Flowers
Abstract
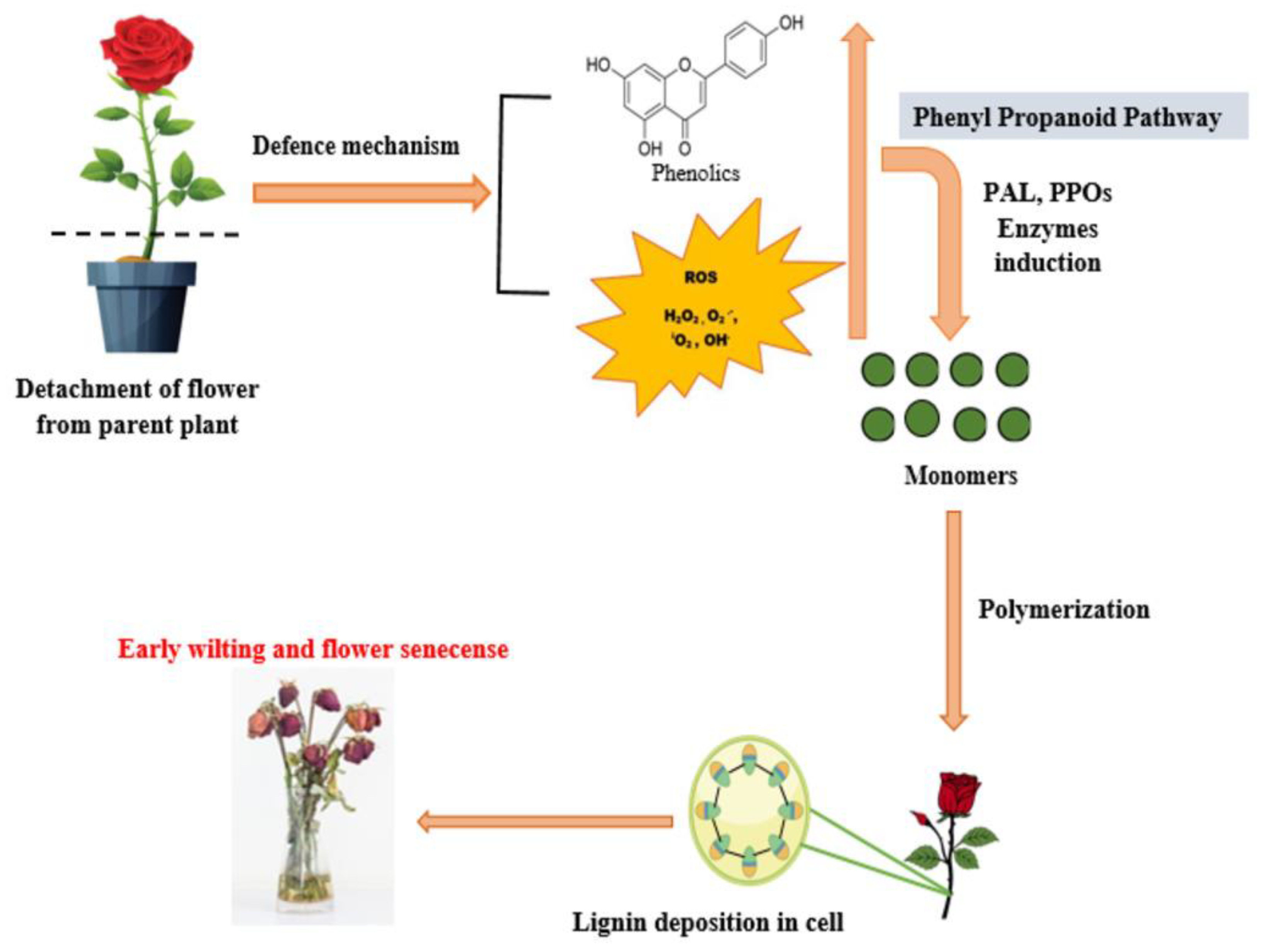
1. Introduction
2. Methodology
3. Wounding
4. Effect of Chemical Inhibitors on Enzyme Activity
4.1. Phenylalanine Ammonia Lyase (PAL)
4.2. Polyphenol Oxidases
4.2.1. Polyphenol Oxidase (PPOs)
4.2.2. Laccase (LACs)
4.2.3. Catechol Oxidase (COs)
4.2.4. Peroxidase Enzymes (PRXs)
4.3. pH
5. Effect of Chemical Inhibitors on Secondary Metabolite Deposition
5.1. Lignin
5.2. Suberin
5.3. Tyloses
5.4. Gel
5.5. Latex
5.6. Tannins
6. Ethylene Synthesis
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gerabeygi, K.; Roein, Z.; Rezvanipour, S. Control of stem bending in cut gerbera flowers through application of dithiothreitol and thioglycolic acid as wound reaction inhibitors. J. Hortic. Sci. Biotechnol. 2021, 96, 653–662. [Google Scholar] [CrossRef]
- Che Husin, N.M.; Joyce, D.C.; Irving, D.E. Gel xylem occlusions decrease hydraulic conductance of cut Acacia holosericea foliage stems. Postharvest Biol. Technol. 2018, 135, 27–37. [Google Scholar] [CrossRef]
- Gantait, S.S.; Ratnayake, K.; Joyce, D.C. Physiological Xylem Occlusion in Cut Flower Stems. In Proceedings of the National Symposium on Recent Advances in Floriculture and Urban Horticulture in Global Perspective, New Delhi, India, 4–5 January 2018. [Google Scholar]
- He, S.; Joyce, D.C.; Irving, D.E.; Faragher, J.D. Stem end blockage in cut Grevillea ‘Crimson Yul-lo’inflorescences. Postharvest Biol. Technol. 2006, 41, 78–84. [Google Scholar] [CrossRef]
- da Silva Vieira, M.R.; Santos, C.M.G.; de Souza, A.V.; de Alencar Paes, R.; da Silva, L.F. Physiological blockage in plants in response to postharvest stress. Afr. J. Biotechnol. 2013, 12, 1168–1170. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Qiao, A.; Li, H.; He, S.; Wang, W.; Sun, M.; Daryl, C.J. Two phenylalanine ammonia-lyase genes (DcPAL2 and DcPAL3) are involved in the cut-induced stem-end wound reaction of carnation (Dianthus caryophyllus L.). J. Hortic. Sci. Biotechnol. 2011, 86, 377–383. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; He, S.; Pang, Z.; Lin, X.; Lin, S.; Li, H. Transcriptomics profile reveals the temporal molecular events triggered by cut-wounding in stem-ends of cut ‘Tiber’lily flowers. Postharvest Biol. Technol. 2019, 156, 110950. [Google Scholar] [CrossRef]
- Ratnayake, K.; Joyce, D.C.; Webb, R.I. Cu2+ inhibition of gel secretion in the xylem and its potential implications for water uptake of cut Acacia holosericea stems. Physiol. Plant. 2013, 148, 538–548. [Google Scholar] [CrossRef]
- Williamson, V.G.; Faragher, J.; Parsons, S.; Franz, P. Inhibiting the Postharvest Wounding Response in Wildflowers; Rural Industries Research and Development Corporation: Barton, ACT, Australia, 2002; pp. 1–82. [Google Scholar]
- Hamidi, E.H.; Roein, Z.; Karimi, M. Extending the vase life of rose cut flower cv. Bakara using inhibitors of physiological vascular occlusion. J. Hortic. Postharvest Res. 2020, 3, 35–48. [Google Scholar]
- Van Doorn, W.G. Vascular occlusion in cut rose flowers: A survey. Acta Hortic. 1995, 405, 58–66. [Google Scholar] [CrossRef]
- Rahimian-Boogar, A.; Salehi, H.; Mir, N. Influence of citric acid and hydrogen peroxide on postharvest quality of tuberose (l.‘Pearl’) cut flowers. J. Hortic. Res. 2016, 24, 13–19. [Google Scholar] [CrossRef]
- Jedrzejuk, A.; Rochala, J.; Rabiza-Swider, J. Occurrence of blockage in cut stems of Clematis L. Acta Agrobot. 2013, 66, 3–8. [Google Scholar] [CrossRef][Green Version]
- Wang, R.; Zheng, X.; Xu, X. Evidence for physiological vascular occlusion in stems of cut gerbera cv. Hongyan. J. Agric. Sci. Technol. 2014, 16, 365–372. [Google Scholar]
- Eljodi, E.M.J.; Azizi, M. Postharvest assessment of lignifying enzymes activity, flower stem lignification and bending disorder of gerbera cut flower. Int. J. Hortic. Sci. Technol. 2015, 2, 87–95. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Leonardi, C.; Romano, D. PAL activities in asparagus spears during storage after ammonium sulfate treatments. Postharvest Biol. Technol. 2018, 140, 34–41. [Google Scholar] [CrossRef]
- Menino, T.V.R.; Joia, B.M.; Almeida, A.M.; Krzyzanowski, F.C.; Marchiosi, R.; Ferrarese-Filho, O. Lignin monomeric composition in soybean seed coats and resistance to mechanical damage. J. Seed Sci. 2023, 45, e202345035. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Song, Y.; Shang, W.; Wang, H.; Lei, X.; Ding, W.; He, D.; Jiang, L.; Shi, L.; et al. ClO2 treatment delays petal senescence and extends the vase life of Paeonia suffruticosa ‘Luoyang Hong’cut flowers. Sci. Hortic. 2024, 325, 112650. [Google Scholar] [CrossRef]
- Etalo, D.W.; Harbinson, J.; Van Meeteren, U. Could wound-induced xylem peroxide contribute to the postharvest loss of hydraulic conductivity in stems? Acta Hortic. 2009, 847, 287–294. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Cruz, P. Evidence for a wounding-induced xylem occlusion in stems of cut chrysanthemum flowers. Postharvest Biol. Technol. 2000, 19, 73–83. [Google Scholar] [CrossRef]
- Mohammadi, M.; Aelaei, M.; Saidi, M. Pre-harvest and pulse treatments of spermine, γ-and β-aminobutyric acid increased antioxidant activities and extended the vase life of gerbera cut flowers ‘Stanza’. Ornam. Hort. 2020, 26, 306–316. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Vaslier, N. Wounding-induced xylem occlusion in stems of cut chrysanthemum flowers: Roles of peroxidase and cathechol oxidase. Postharvest Biol. Technol. 2002, 26, 275–284. [Google Scholar] [CrossRef]
- Verdonk, J.C.; Van Ieperen, W.; Carvalho, D.R.; Van Geest, G.; Schouten, R.E. Effect of preharvest conditions on cut-flower quality. Front. Plant Sci. 2023, 14, 1281456. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Alberici, A.; Antonacci, S. Effect of promoter and inhibitors of phenylalanine ammonialyase enzyme on stem bending of cut gerbera flowers. Acta Hortic. 2007, 755, 471–476. [Google Scholar] [CrossRef]
- Damunupola, J.W.; Qian, T.; Muusers, R.; Joyce, D.C.; Irving, D.E.; Van Meeteren, U. Effect of S-carvone on vase life parameters of selected cut flower and foliage species. Postharvest Biol. Technol. 2010, 55, 66–69. [Google Scholar] [CrossRef]
- Kan, J.; Liu, Y.; Hui, Y.; Wan, B.; Liu, J.; Qian, C.; Jin, C. 2-aminoindan-2-phosphonic acid alleviates oxidative browning in fresh-cut lily bulbs. J. Food Process. Preserv. 2022, 46, e16449. [Google Scholar] [CrossRef]
- Imsabai, W.; Leethiti, P.; Netlak, P.; Van Doorn, W.G. Petal blackening and lack of bud opening in cut lotus flowers (Nelumbo nucifera): Role of adverse water relations. Postharvest Biol. Technol. 2013, 79, 32–38. [Google Scholar] [CrossRef]
- Holzwarth, M.; Wittig, J.; Carle, R.; Kammerer, D.R. Influence of putative polyphenoloxidase (PPO) inhibitors on strawberry (Fragaria × ananassa Duch.) PPO, anthocyanin and color stability of stored purées. LWT-Food Sci. Technol. 2013, 52, 116–122. [Google Scholar] [CrossRef]
- Celikel, F.G.; Joyce, D.C.; Faragher, J.D. Inhibitors of oxidative enzymes affect water uptake and vase life of cut Acacia holosericea and Chamelaucium uncinatum stems. Postharvest Biol. Technol. 2011, 60, 149–157. [Google Scholar] [CrossRef]
- Chaudhary, D.; Chong, F.; Neupane, T.; Choi, J.; Jee, J.G. New inhibitors of laccase and tyrosinase by examination of cross-inhibition between copper-containing enzymes. Int. J. Mol. Sci. 2021, 22, 13661. [Google Scholar] [CrossRef]
- Vaslier, N.; Van Doorn, W.G. Xylem occlusion in bouvardia flowers: Evidence for a role of peroxidase and cathechol oxidase. Postharvest Biol. Technol. 2003, 28, 231–237. [Google Scholar] [CrossRef]
- Loubaud, M.; Van Doorn, W.G. Wound-induced and bacteria-induced xylem blockage in roses, Astilbe, and Viburnum. Postharvest Biol. Technol. 2004, 32, 281–288. [Google Scholar] [CrossRef]
- van Meeteren, U.; Arévalo-Galarza, L.; Van Doorn, W.G. Inhibition of water uptake after dry storage of cut flowers: Role of aspired air and wound-induced processes in chrysanthemum. Postharvest Biol. Technol. 2006, 41, 70–77. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Stead, A.D. Abscission of flowers and floral parts. J. Exp. Bot. 1997, 48, 821–837. [Google Scholar] [CrossRef]
- Marques, A.E.; Silva, F.; Barbosa, J.G.; Finger, F.L. Ação de inibidores de enzimas oxidativas e crescimento bacteriano sobre a longevidade das flores de ave-do-paraíso (Strelitzia reginae Aiton). Ornam. Hortic. 2011, 17, 75–86. [Google Scholar] [CrossRef]
- Karsten, J.; Finger, F.L.; Barbosa, J.G.; Chaves, D.V. Characterisation of peroxidase activity in bird-of-paradise (Strelitzia reginae Ait.) stems. J. Hort. Sci. Biotechnol. 2012, 87, 668–672. [Google Scholar] [CrossRef]
- Sharifzadeh, K.; Asil, M.; Roein, Z.; Sharifzadeh, M. Effect of 8-hydroxyquinoline citrate, sucrose and peroxidase inhibitors on vase life of lisianthus (Eustoma grandiflorum L.) cut flowers. J. Hortic. Res. 2014, 22, 41–47. [Google Scholar] [CrossRef]
- Van Meeteren, U.; Arévalo-Galarza, L. Obstruction of water uptake in cut chrysanthemum stems after dry storage: Role of wound-induced increase in enzyme activities and air emboli. Acta Hortic. 2009, 847, 199–206. [Google Scholar] [CrossRef]
- Ghadimian, S.; Danaei, E. The effect of salicylic acid function on vase life and activity of the phenylalanine ammonia lyase enzyme of cut flowers (Rosa hybrid acv. Black Magic). Int. Conf. Res. Sci. Technol. 2016, 7, 20–25. [Google Scholar]
- Kanani, M.; Nazarideljou, M.J. Methyl jasmonate and α-aminooxi-β-phenyl propionic acid alter phenylalanine ammonia-lyase enzymatic activity to affect the longevity and floral scent of cut tuberose. Hortic. Environ. Biotechnol. 2017, 58, 136–143. [Google Scholar] [CrossRef]
- Blaschek, L.; Pesquet, E. Phenoloxidases in plants—How structural diversity enables functional specificity. Front. Plant Sci. 2021, 12, 754601. [Google Scholar] [CrossRef]
- Selvarajan, E.; Veena, R.; Manoj Kumar, N. Polyphenol oxidase, beyond enzyme browning. In Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 203–222. [Google Scholar] [CrossRef]
- Wold-McGimsey, F.; Krosch, C.; Alarcón-Reverte, R.; Ravet, K.; Katz, A.; Stromberger, J.; Mason, R.E.; Pearce, S. Multi-target genome editing reduces polyphenol oxidase activity in wheat (Triticum aestivum L.) grains. Front. Plant Sci. 2023, 14, 1247680. [Google Scholar] [CrossRef]
- Janick, J. (Ed.) . Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 49, pp. 144–146. [Google Scholar]
- Krush, G.H.; Rastegar, S. γ-Aminobutyric acid (GABA) inhibits the enzymatic browning of cut Narcissus tazetta cv.‘Shahla-e-Shiraz’ flowers during vase life. J. Plant Growth Regul. 2023, 42, 2602–2612. [Google Scholar] [CrossRef]
- Shabanian, S.; Nasr Esfahani, M.; Karamian, R.; Tran, L.S.P. Salicylic acid modulates cutting-induced physiological and biochemical responses to delay senescence in two gerbera cultivars. Plant Growth Regul. 2018, 87, 245–256. [Google Scholar] [CrossRef]
- Pourzarnegar, F.; Hashemabadi, D.; Kaviani, B. Cerium nitrate and salicylic acid on vase life, lipid peroxidation, and antioxidant enzymes activity in cut lisianthus flowers. Ornam. Hortic. 2020, 26, 658–669. [Google Scholar] [CrossRef]
- Kazemzadeh-Beneh, H.; Samsampour, D.; Zarbakhsh, S. Biochemical, physiological changes and antioxidant responses of cut gladiolus flower ‘White Prosperity’ induced by nitric oxide. Adv. Hortic. Sci. 2018, 32, 421–432. [Google Scholar] [CrossRef]
- Chan, J.C.; Paice, M.; Zhang, X. Enzymatic oxidation of lignin: Challenges and barriers toward practical applications. ChemCatChem 2020, 12, 401–425. [Google Scholar] [CrossRef]
- Madhavi, V.; Lele, S.S. Laccase: Properties and applications. BioResources 2009, 4, 1694–1717. [Google Scholar] [CrossRef]
- Luo, H.; Deng, S.; Fu, W.; Zhang, X.; Zhang, X.; Zhang, Z.; Pang, X. Characterization of active anthocyanin degradation in the petals of Rosa chinensis and Brunfelsia calycina reveals the effect of gallated catechins on pigment maintenance. Int. J. Mol. Sci. 2017, 18, 699. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Roein, Z.; Ghafouriyan, M.; Shiri, M.A. Improvement of vase life of cut gerbera flower by xylem blockage inhibitors. In Proceedings of the 1st International and 2nd National Ornamental Plants Congress, Mashhad, Iran, 23–25 August 2016; pp. 166–168. [Google Scholar]
- Liao, J.; Wei, X.; Tao, K.; Deng, G.; Shu, J.; Qiao, Q.; Chen, G.; Wei, Z.; Fan, M.; Saud, S.; et al. Phenoloxidases: Catechol oxidase-the temporary employer and laccase-the rising star of vascular plants. Hortic. Res. 2023, 10, uhad102. [Google Scholar] [CrossRef]
- Krebs, B.; Merkel, M.; Rompel, A. Catechol oxidase and biomimetic approaches. J. Argent. Chem. Soc. 2004, 92, 1–15. [Google Scholar]
- Knee, M. (Ed.) Fruit Quality and Its Biological Basis; CRC Press: Boca Raton, FL, USA, 2002; pp. 172–173. [Google Scholar]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Botany 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Kim, K.Y.; Jung, W.J.; Avice, J.C.; Ourry, A.; Kim, T.H. Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J. Experimental. Bot. 2007, 58, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, K.; Hassanpour Asil, M.; Roein, Z. Effect of copper sulphate on enzyme activity and vase life of lisianthus cut flower. Int. J. Hortic. Sci. Technol. 2013, 14, 219–228. [Google Scholar]
- Marandi, R.J.; Hassani, A.; Abdollahi, A.; Hanafi, S. Application of Carum copticum and Satureja hortensis essential oils and salicylic acid and silver thiosulfate in increasing the vase life of cut rose flower. J. Med. Plants Res. 2011, 5, 5034–5038. [Google Scholar]
- Van Doom, W.G.; Perik, R.R. Hydroxyquinoline citrate and low pH prevent vascular blockage in stems of cut rose flowers by reducing the number of bacteria. J. Am. Soc. Hortic. Sci. 1990, 115, 979–981. [Google Scholar] [CrossRef]
- Damunupola, J.W.; Joyce, D.C. When is a vase solution biocide not, or not only, antimicrobial? J. Jpn. Soc. Hortic. Sci. 2008, 77, 211–228. [Google Scholar] [CrossRef][Green Version]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef]
- Hamedan, H.J.; Sohani, M.M.; Aalami, A.; Nazarideljou, M.J. Genetic engineering of lignin biosynthesis pathway improved stem bending disorder in cut gerbera (Gerbera jamesonii) flowers. Sci. Hortic. 2019, 245, 274–279. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, D.; Meng, J.; Tao, J. EGTA reduces the inflorescence stem mechanical strength of herbaceous peony by modifying secondary wall biosynthesis. Hortic. Res. 2019, 6, 36. [Google Scholar] [CrossRef]
- Kashyap, A.; Planas-Marquès, M.; Capellades, M.; Valls, M.; Coll, N.S. Blocking intruders: Inducible physico-chemical barriers against plant vascular wilt pathogens. J. Exp. Bot. 2021, 72, 184–198. [Google Scholar] [CrossRef]
- Ramamurthy, M.S.; Ussuf, K.K.; Nair, P.M.; Thomas, P. Lignin biosynthesis during wound healing of potato tubers in response to gamma irradiation. Postharvest Biol. Technol. 2000, 18, 267–272. [Google Scholar] [CrossRef]
- Ros Barceló, A. Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 2005, 220, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ghafouriyan, M.; Roein, Z.; Shiri, M.A. Effect of inhibitors of lignin biosynthesis on vase life of gerbera cut flowers. Iran. J. Hortic. Sci. 2019, 49, 903–914. [Google Scholar] [CrossRef]
- Che Husin, N.M.; Joyce, D.C.; Irving, D.E. The limiting factor for commercial vase life of cut Acacia Holosericea foliage stem by dye tracking method. Plant Physiol. Addressing Green Econ. 2013, 21, 129–133. [Google Scholar]
- Wahrenburg, Z.; Benesch, E.; Lowe, C.; Jimenez, J.; Vulavala, V.K.; Lü, S.; Hammerschmidt, R.; Douches, D.; Yim, W.C.; Santos, P.; et al. Transcriptional regulation of wound suberin deposition in potato cultivars with differential wound healing capacity. Plant J. 2021, 107, 77–99. [Google Scholar] [CrossRef]
- Vehniwal, S.S.; Abbey, L. Cut flower vase life–influential factors, metabolism and organic formulation. Hortic. Int. J. 2019, 3, 275–281. [Google Scholar] [CrossRef]
- Jędrzejuk, A.; Zakrzewski, J. Xylem occlusions in the stems of common lilac during postharvest life. Acta Physiol. Plant 2009, 31, 1147–1153. [Google Scholar] [CrossRef]
- Jedrzejuk, A.; Rochala, J.; Zakrzewski, J.; Rabiza-Świder, J. Identification of xylem occlusions occurring in cut clematis (Clematis L., fam. Ranunculaceae Juss.) stems during their vase life. Sci. World J. 2012, 2012, 749281. [Google Scholar] [CrossRef]
- Schmitt, U.; Richter, H.G.; Muche, C. TEM study of wound-induced vessel occlusions in European ash (Fraxinus excelsior L.). IAWA J. 1997, 18, 401–404. [Google Scholar] [CrossRef]
- Rabiza-Świder, J.; Skutnik, E.; Jędrzejuk, A. The effect of preservatives on water balance in cut clematis flowers. J. Hortic. Sci. Biotechnol. 2017, 92, 270–278. [Google Scholar] [CrossRef]
- Rabiza-Świder, J.; Skutnik, E.; Jędrzejuk, A.; Rochala-Wojciechowska, J. Nanosilver and sucrose delay senescence of cut snapdragon flowers. Postharvest Biol. Technol. 2020, 165, 111165. [Google Scholar] [CrossRef]
- Jędrzejuk, A.; Rabiza-Świder, J.; Skutnik, E.; Łukaszewska, A. Some factors affecting longevity of cut lilacs. Postharvest Biol. Technol. 2016, 111, 247–255. [Google Scholar] [CrossRef]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): Tyloses in summer and gels in winter1. Am. J. Bot. 2008, 95, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, K.; Joyce, D.C.; Webb, R.I. Xylem plugging and postharvest longevity of cut Acacia holosericea. Acta Hortic. 2015, 1104, 287–294. [Google Scholar] [CrossRef]
- Che Husin, N.M.; Gupta, M.L.; George, D.L.; Joyce, D.C.; Irving, D.E. Gel occlusion in the xylem vessels of cut Acacia holosericea foliage stems. Acta Hortic. 2013, 1012, 369–373. [Google Scholar] [CrossRef]
- Ratnayake, K.; Bui, C.L.; Joyce, D.C. Copper distribution and ionic form effects for postharvest treatments of cut Acacia holosericea stems. Sci. Hortic. 2011, 130, 919–926. [Google Scholar] [CrossRef]
- Abarca, L.F.S.; Klinkhamer, P.G.; Choi, Y.H. Plant latex, from ecological interests to bioactive chemical resources. Planta Med. 2019, 85, 856–868. [Google Scholar] [CrossRef]
- Konno, K. Plant latex and other exudates as plant defense systems: Roles of various defense chemicals and proteins contained therein. Phytochemistry 2019, 72, 1510–1530. [Google Scholar] [CrossRef]
- Ramos, M.V.; Freitas, C.D.T.; Morais, F.S.; Prado, E.; Medina, M.C.; Demarco, D. Plant latex and latex-borne defense. Adv. Bot. Res. 2020, 93, 1–25. [Google Scholar] [CrossRef]
- Van Doorn, W.G. Water relation of cut flowers. Hortic. Rev. 2010, 18, 55–106. [Google Scholar] [CrossRef]
- Pinto, A.C.R.; Mello, S.C.; Jacomino, A.P.; Minami, K.; Barbosa, J.C. Postharvest characteristics and treatments to overcome latex exuded from cut flowers of lotus. Acta Hortic. 2009, 813, 671–678. [Google Scholar] [CrossRef]
- Ahmad, I.; Joyce, D.C.; Faragher, J.D. Physical stem-end treatment effects on cut rose and acacia vase life and water relations. Postharvest Biol. Technol. 2011, 59, 258–264. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A. Ornamental cut flowers: Physiology in practice. Floric. Ornam. Plant Biotechnol. 2006, 14, 124–140. [Google Scholar]
- Windell, N.E. Leaf Blackening and the Control Thereof in Selected Protea Species and Cultivars. Master’s Thesis, Stellanbosch Univeristy, Stellenbosch, South Africa, 2012; pp. 9–10. [Google Scholar]
- Peters, D.J.; Constabel, C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J. 2002, 32, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.W.; Vardien, W.; Jacobs, G.; Windell, N.E. Leaf blackening: A serious impediment to long-term cold storage, transport, and extended vase life in protea cut flowers. Hortic. Rev. 2018, 45, 73–104. [Google Scholar] [CrossRef]
- Che Husin, N.M.; Liu, J.; Joyce, D.C.; Irving, D.E. Cutting wound ethylene production does not limit the vase life of Acacia holosericea. Sci. Hortic. 2016, 212, 35–48. [Google Scholar] [CrossRef]
- Lakimova, E.T.; Woltering, E.J. Wound-induced ethylene synthesis in component flower parts of cut carnation flowers. Cyбmponuчecкoc Дeкopamuвнoe Caдoвoдcmвo 2013, 48, 191–199. [Google Scholar]
- Ferrante, A.; Serra, G. Lignin content and stem bending incidence on cut gerbera flowers. Acta Hortic. 2009, 847, 377–384. [Google Scholar] [CrossRef]
- Naing, A.H.; Soe, M.T.; Yeum, J.H.; Kim, C.K. Ethylene acts as a negative regulator of the stem-bending mechanism of different cut snapdragon cultivars. Front. Plant Sci. 2021, 12, 745038. [Google Scholar] [CrossRef]
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and gums: A review of structure, function and occurrence of vessel occlusions. IAWA J. 2016, 37, 186–205. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Harkema, H.; Otma, E. Is vascular blockage in stems of cut lilac flowers mediated by ethylene? Acta Hortic. 1991, 298, 177–182. [Google Scholar] [CrossRef]
| Enzyme | Chemical Inhibitor | Reference |
|---|---|---|
| Phenylalanine ammonia lyase (PAL) |
| [24] |
| S-carvone | [25] | |
| 2-aminoindan-2-phosphonic acid (AIP) | [26] | |
| Polyphenol oxidase (PPO) |
| [22] |
| Phenylhydrazine | [27] | |
| [28] | |
| [10] | |
| Laccase (LAC) | Cetyltrimethylammonium bromide (CTAB) | [29] |
| [1] | |
| Sodium azide | [30] | |
| Catechol oxidase (CO) | Calcium chloride | [22] |
| 2-Mercapto-ethanol | [31] | |
| 2,3-Dihydroxynaphthalene | [32] | |
| Tropolone | [33] | |
| Peroxidase (PRX) | Cycloheximide (CHI) | [34] |
| [20] | |
| 3-Amino-1,2,4, triazole (3-AT) | [22] | |
| Copper sulphate | [31] | |
| Hydroquinone | [32] | |
| Sodium metabisulphite | [35] | |
| [36] | |
| [37] |
| Crop | Chemical Inhibitor | Concentration | Type of Preservative | Experimental Conditions | Results | Reference |
|---|---|---|---|---|---|---|
| Gerbera (Gerbera jamesonii) | Thioglycolic acid (TGA) | 0.5 mM | 20 h Pulsing | 70 ± 5% Relative humidity 15 µmol m−2 s−1 Light intensity 20 ± 2 °C Temperature | Minimized lignin content in vessels | [1] |
| Sodium azide | 0.2 mM | 10 h Pulsing | 60 ± 5% Relative humidity 75 µmol m−2 s−1 Light intensity 10 °C Temperature | Improved healing of wounds and minimization of lignin deposition | [63] | |
| Catechol 8-HQC | 1.0 mM 0.45 mM | Holding solution | 35–55% Relative humidity 15 µmol m−2 s−1 Light intensity 18–22 °C Temperature | Decreased vascular occlusion by gums | [14] | |
| Leather flower (Clematis spp) | 8-HQC+ Sugar | 200 mg L−1 + 20 mg L−1 | Holding solution | 60% Relative humidity 35 µmol m−2 s−1 Light intensity 20 °C Temperature | Minimized vessel occlusion was achieved due to tylose deposition | [64] |
| Silver wattle (Acacia holosericea) | Cu2+ | 2.2 mM | 5 h Pulsing | 60 ± 5% Relative humidity 18 µmol m−2 s−1 Light intensity 19 ± 2 °C Temperature | Prevented gel secretion from parenchyma cells | [65] |
| S-carvone | 0.636 mM | Holding solution | 60 ± 10% Relative humidity 14 µmol m−2 s−1 Light intensity 20 ± 1 °C Temperature | Improved fresh weight and water uptake rate | [25] | |
| Lotus (Nelumbo nucifera) | Citric acid | 150 mg L−1 | Holding solution | 65–75% Relative humidity 15 µmol m−2 s−1 Light intensity 25 °C Temperature | Reduced latex flow from lactifiers | [27] |
| Geraldton waxflower (Chamelaucium uncinatum) | Phenyl hydrazine | 10 mM | 5 h Pulsing | 60 % Relative humidity 15 µmol m−2 s−1 Light intensity 20 °C Temperature | Prevented phenolic compound deposition and improved water uptake | [29] |
| Lilac (Syringa vulgaris L.) | Chrysal professional | Holding solution | 70 ± 5% Relative humidity 25 µmol m−2 s−1 Light intensity 18–20 °C Temperature | Reduced vessel blockage by tyloses by up to 9.6% | [66] | |
| Spider flower (Grevillea spp) | S-carvone 4-hexylresorcinol | 0.636 mM 2.5 mM | Holding solution | 60 ± 10% Relative humidity 12 µmol m−2 s−1 Light intensity 20 ± 1 °C Temperature | Prevented stem end blockage by inhibiting suberin deposition | [4] |
| Astilbe (Astilbe x arendsi) | Copper sulphate | 0.25 mM | Holding solution | 60 ± 5% Relative humidity 15 µmol m−2 s−1 Light intensity 20 °C Temperature | Improved water uptake by inhibiting phenolic compound secretion | [32] |
| Chrysanthemum (Chrysanthemum grandiflorum) | p-phenylene diamide | 10 mM | 5 h Pulsing | 60% Relative humidity 15 µmol m−2 s−1 Light intensity 20 °C Temperature | Delayed leaf wilting | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor, A.; Bashir, M.A.; Naveed, M.S.; Akhtar, M.T.; Saeed, S. Postharvest Chemical Treatment of Physiologically Induced Stem End Blockage Improves Vase Life and Water Relation of Cut Flowers. Horticulturae 2024, 10, 271. https://doi.org/10.3390/horticulturae10030271
Manzoor A, Bashir MA, Naveed MS, Akhtar MT, Saeed S. Postharvest Chemical Treatment of Physiologically Induced Stem End Blockage Improves Vase Life and Water Relation of Cut Flowers. Horticulturae. 2024; 10(3):271. https://doi.org/10.3390/horticulturae10030271
Chicago/Turabian StyleManzoor, Ayesha, Muhammad Ajmal Bashir, Muhammad Saqib Naveed, Muhammad Tanveer Akhtar, and Shaista Saeed. 2024. "Postharvest Chemical Treatment of Physiologically Induced Stem End Blockage Improves Vase Life and Water Relation of Cut Flowers" Horticulturae 10, no. 3: 271. https://doi.org/10.3390/horticulturae10030271
APA StyleManzoor, A., Bashir, M. A., Naveed, M. S., Akhtar, M. T., & Saeed, S. (2024). Postharvest Chemical Treatment of Physiologically Induced Stem End Blockage Improves Vase Life and Water Relation of Cut Flowers. Horticulturae, 10(3), 271. https://doi.org/10.3390/horticulturae10030271







