Abstract
The plant water status is crucial for growth and production, but the current climate change scenario makes it challenging to match the water plant demand. Blueberries are an economically important crop and play an acknowledged role in human health due to their antioxidant compounds. This research aimed to determine whether the exogenous application of methyl jasmonate (MeJA) improves the antioxidant defense mechanisms for protecting the photosynthetic performance in the blueberry plant cultivar ‘Brigitta’ under the stress condition of a water deficit (WD). A greenhouse experiment was carried out under a 16-h light period, at 20 °C, and 60–80% relative humidity for two weeks before the application of MeJA to the blueberry plants (Vaccinium corymbosum, cultivar ‘Brigitta’). The following treatments were maintained for 7-days: (i) 80% field capacity (NoWD), (ii) 80% field capacity plus MeJA application (NoWD + MeJA), (iii) 20% field capacity (WD), and (iv) 20% field capacity plus MeJA application (WD + MeJA). The MeJA was sprayed as an aqueous solution of 10 µM MeJA over the plant’s foliar system. At the end of the assay, the blueberry leaves were analyzed for their relative water content, specific leaf area, lipid peroxidation, total antioxidant activity, total phenols, total anthocyanins, anthocyanidin compounds, and photosynthetic performance. The ‘Brigitta’ cultivar showed a significant decrease in the oxidative stress at leaf levels, with an increase in antioxidant activity, phenolic compounds, total anthocyanins, delphinidin, petunidin, antheraxanthin, zeaxanthin, and an improvement in photosynthetic performance parameters. The ‘Brigitta’ blueberry cultivar was shown to be susceptible to WD, which mainly decreased photosynthesis. However, the application of MeJA to the leaves induced metabolic changes through an increase of the antioxidant strategy within the plant to counteract the negative effects of WD and protect the photosynthetic apparatus, which allowed the ‘Brigitta’ cultivar to withstand the period of a WD.
1. Introduction
The surface freshwater fraction is naturally unequally distributed worldwide, and anthropogenic activities, such as agriculture, have contributed to intensifying the limiting conditions in some regions [1,2]. Thus, the agriculture activity is the largest global consumer of water. About 40% of global crop production takes place on irrigated land, which accounts for approximately 20% of the global farmland [3,4]. The impact of climate change on the precipitation pattern has affected water availability. Some croplands are facing severe water restrictions, resulting in long-term water deficit (WD) episodes and decreasing crop production. Crops are highly water-demanding, being affected by WD due to climate change [5,6]. In this regard, it is reported that one of the most sensitive species to water shortages in soils is the blueberry (Vaccinium corymbosum) due to its superficial root system [7]. Even though blueberry plants are exposed for short periods to limited water (a few days without rain or irrigation), the first and quickest response of blueberry is to reduce its photosynthesis, decreasing growth and fruit production [8].
The WD plant stress condition affects biochemical and physiological processes, inducing plant morphological changes. Metabolic processes involved in electron transport and energy production are mainly affected by a WD, such as photosynthesis, dark respiration, and photorespiration [9]. Therefore, the production of highly antioxidant fruit species, like blueberries, can be seriously affected by a WD. These crops are attractive for securing food production. Under a WD condition, plant organelles are prone to undergo a sensitive imbalance, resulting in an exacerbated production and accumulation of reactive oxygen species (ROS) [10,11]. These partially reduced oxygen derivative components are highly reactive and toxic, causing irreversible oxidation of cellular organelles, and detrimental cell metabolic activities [11]. The consequence of the oxidative damage in the membrane of stomata cell guards produces an imbalance of the intra- and extra-cellular calcium, chloride, and potassium concentrations, extending the membrane depolarization and causing permanent stomata closure. In addition, ROS are reported as crucial participants in guard cell signaling, where H2O2 is involved in ABA-induced stomatal closure [12]. This stomata closure restricts the fixation of atmospheric CO2, down-regulates photosynthesis, limits the water absorption, promotes photorespiration, and stimulates even more production of ROS [13,14]. The impact of climate change on the precipitation pattern has affected water availability, and some croplands are facing severe water restrictions, resulting in long-term WD episodes and decreasing crop production and/or fruit quality [15]. Thus, a WD through ROS production is considered the major cause of crop productivity loss [16,17], including blueberry plant orchards.
On the other hand, under abiotic stress conditions such as a WD, the increased ROS formation can be linked to signals derived from changes in the regulation of phytohormones [18]. ROS are a common signal to down-regulate the release of phytohormones, such as salicylic acid (SA), abscisic acid (ABA), ethylene (ET), jasmonic acid (JA), and its methyl ester the methyl jasmonate (MeJA), involved in stress control [18,19,20]. MeJA is a phytohormone derived from the acetate pathway. It has been reported that its application alleviates aluminum stress in blueberry plants [21,22]; however, the effect of MeJA application to overcome a WD has not yet been reported in this species. On the other hand, the application of MeJA to wheat improved drought tolerance, increasing net photosynthesis [23]. Likewise, this phytohormone decreased the damage provoked by drought stress, improving antioxidant enzyme activities in rice seedlings [24]. Thus, this phytohormone might be a useful solution to avoid the plant stress through WD periods in blueberry cultivars, under the current uncertain climate change scenarios. Therefore, we hypothesized that the exogenous application of MeJA improves the antioxidant defense mechanisms, protecting the photosynthetic performance in the ‘Brigitta’ blueberry cultivar under the stress condition of a WD. Therefore, our aim was to determine whether the exogenous application of MeJA improves the antioxidant defense mechanisms for protecting the photosynthetic performance in the ‘Brigitta’ blueberry cultivar under a WD.
2. Materials and Methods
2.1. Plant Material
Twenty plants of the two-year-old Vaccinium corymbosum cultivar ‘Brigitta’ were selected based on the resilience of the plants to abiotic stresses [25,26]. Plants of the cultivar ‘Brigitta’ were provided by Berries San Luis (Quillém, Lautaro, Chile; 38°29′ S, 72°23′ W). Healthy plants with a similar size (60 cm) and without treatments were selected to set up the WD experiment.
2.2. Plant Water Deficit (WD) Condition Assay
The experiment was carried out in a greenhouse at the Instituto de Agroindustria, Universidad de La Frontera, Temuco, Chile (38°45′ S, 72°40′ W). The greenhouse growing conditions were established at a 16-h light period with mean of 500 µmol photons m−2 s−1, 20 ± 5 °C, and 60–80% relative humidity. The plants were placed into pots containing 1.5 kg of dried and sieved Andisol. The experimental design was completely randomized with five replicates per treatment. To minimize any positional effects, the pots with plants were daily re-randomized.
Water irrigation was applied periodically for plant conditioning, using distilled water over 14 days in order to maintain 80% field capacity (FC). After that, the plants were subjected to different treatments as follows: (i) 80% field capacity (NoWD), (ii) 80% field capacity plus MeJA (NoWD + MeJA), (iii) 20% field capacity (WD), and (iv) 20% field capacity plus MeJA (WD + MeJA). Plants were kept as control (NoWD) and MeJA treatments (NoWD + MeJA, WD, and WD + MeJA) for 7 days. In this period, an aqueous solution of 10 µM MeJA (Sigma-Aldrich, Darmstadt, Germany) was sprayed on the leaves at the start of the experiment on treatments NoWD and the WD, using the method described by Ulloa-Inostroza et al. [22]. To maintain the differential water content treatments, the pots were weighed, applying irrigation twice daily, using distilled water depending on the treatment. At the end of the experimental period, in vivo photosynthesis and fluorescence measurements were performed. The plant leaves and roots were harvested, and enough fresh material was separated for immediate lipid peroxidation (LP) analysis, and the remaining material was frozen at −80 °C for subsequent biochemical analyses (Revco Elite Series Ultra-Low Temperature, Thermo Scientific™, Waltham, MA, USA).
2.3. Physiological Analyses
The relative water content (RWC) was determined in 10 discs from the plants (replicate), which were weighed (FW), and quickly immersed in distilled water overnight to saturation. Then, the discs of leaves were weighed (turgor weight, SW) after removing the excess water. The discs of leaves were oven-dried until a constant weight at 70 °C and the dry weight (DW) was measured. RWC was calculated based on the equation described by Larcher [27] as follows:
where FW = fresh leaf weight, DW (g) = dry weight of leaves (g), and SW = saturation weight of the leaves (g). FW and DW were determined after drying at 60 °C and SW after soaking in water for 24 h at 20 °C.
RWC = [(FW − DW)/(SW − DW)] × 100
The specific leaf area (SLA) was determined by randomly selecting two leaves from each individual plant, which were placed on a white background and photographed with a reference point of 1 cm2. The SLA was calculated using ImageJ software, dividing each leaf area (LA) derived from the software by the dry weight (DW) [28].
2.4. Biochemical Analyses
The LP of the membranes was determined to be an indicator of oxidative stress. The measurement was carried out on fresh leaves and roots, using thiobarbituric acid-reacting substances (TBARS) according to the modified method described by Du and Bramlage [29]. In order to correct the interference produced by TBARS sugar complexes, the absorbance was measured spectrophotometrically at 440, 532, and 600 nm. The LP was expressed as nmol equivalents of malondialdehyde (MDA) concentration per gram of fresh weight (FW) in nmol MDA g–1 FW.
The total antioxidant activity (AA) in the leaves and roots was determined by using the 2.1-diphenyl1-1-picrylhydrazyl (DPPH) method according to Chinnici et al. [30]. The absorbance was measured at 515 nm using Trolox as standard. The RSA was expressed as microgram of Trolox equivalent per gram of fresh weight (µg TE g–1 FW).
The total phenol (TP) concentration was determined following the procedure described by Slinkard and Singleton [31] using a Folin–Ciocalteau reagent, measured spectrophotometrically at 765 nm, and expressed in chlorogenic acid equivalents (CAE) g−1 FW.
The total anthocyanins (TA) were estimated from the leaves via the pH differential method as described by Cheng and Breen [32], and measured spectrophotometrically at 530 nm and at 657 nm with a molar extinction coefficient for cyanidin-3-glucoside of 29,600 nm. The results were expressed as micrograms of cyanidin 3-O-glycoside equivalent per g of fresh weight (µg cyanidin 3-O-glycoside eq. g−1 FW).
The anthocyanin compound analyses were carried out through HPLC as described in Castrejón et al. [33]. The signals were detected at 530 nm and the data were expressed as milli- or micrograms per g of fresh weight (mg or μg g−1 FW). The mobile phase was performed using acidified water (A) and 100% methanol (B). The binary gradient was applied as follows: 0–39.9 min of 90% A–10% B, 40–41.9 min of 60% A–40% B, 42–48.9 min of 20% A–80% B, and 49–62 min of 90% A–10% B, according to Ribera-Fonseca et al. [34].
2.5. Photosynthetic Performance
The in vivo photochemical efficiency of the leaves was determined through the chlorophyll fluorescence, during the light period (08:00 to 10:00 h), with a modulated fluorometer (FMS 2; Hansatech Instruments, King’s Lynn, UK). Three completely expanded leaves of the plants were dark-adapted for 30 min (to obtain open centers) with leaf clips with a mobile shutter plate. The evaluated parameters were: the maximal photochemical efficiency of PSII [Fv/Fm = (Fm − Fo)/Fm], the effective photochemical efficiency of PSII (ΦPSII), the electron transport rate (ETR), and the non-photochemical quenching (NPQ). These conditions and parameters were measured according to Reyes-Díaz et al. [35], and the calculations were performed according to Maxwell and Johnson [36].
The in vivo measurements of the net photosynthesis (µmol CO2 m−2 s−1), transpiration (mmol H2O m−2s−1), and stomatal conductance (mol H2O m−2 s−1) were measured during the light period (08:00 to 10:00 h) with a portable infrared gas analyzer (Licor-6800, LI-COR Bioscience, Inc., Lincoln, NE, USA) following Reyes-Díaz et al. [25]. The measurements were made on attached leaves from the second to the fourth node of shoots with an equipped cuvette that controlled the light source (500 µmol photons m−2s−1), temperature (20 °C), and CO2 (400 ppm). Intrinsic water-use efficiency of photosynthesis (WUEPh) was calculated according to Locke and Ort [37] as follows:
WUE Ph= net photosynthesis/transpiration (µmol CO2 m−2s−1/mmol H2O m−2s−1).
The photosynthetic pigment concentrations in the leaves were carried out with acetone HPLC grade at 4 °C under a green safelight and centrifuged at 4 °C. The pigments were quantified using a high-performance liquid chromatography (HPLC) system (Agilent technologies 1200 series, column C-18 Waters spherisorb 5.0 μm ODS1 4.6 × 250 mm) according to Garcia-Plazaola and Becerril [38]. All standards for the pigments Vx, Ax, Zx, neoxanthin (Nx), chlorophyll (Chl) a, b, β-carotene (βCa), and lutein (Lt) were purchased from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO, USA).
2.6. Statistical Analyses
The data set was tested for normality applying equal variance and Kolmogorov–Smirnov normality tests. Then, a one-way ANOVA was carried out, and the differences between treatments were detected using the Tukey HSD test at a 0.05 level. The analyses were performed with statistical software SAS v. 8.01.
3. Results
3.1. Physiological Parameters
Significant differences for the relative leaf area (RLA) and relative water content (RWC) among the control treatment (NoWD) and the treatment at water deficit (WD) were observed. However, no significant differences in RLA and RWC between the control and treatment under a normal water regimen with the application of MeJA (NoWD + MeJA) were observed (Figure 1). A WD decreased the relative leaf area by about 33% compared to NoWD and NoWD + MeJA, increasing by 12% through the application of MeJA. A decrease of 22% in the relative water content (RWC) caused by the WD was observed, recovering by 12% with the application of MeJA (Figure 1).
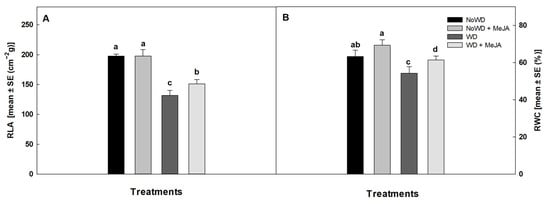
Figure 1.
Relative leaf area (RLA) and relative water content (RWC) in the blueberry ‘Brigitta’ cultivar under a WD and the application of MeJA to the leaves. (A): RLA of leaves and (B): RWC of leaves. Lowercase letters show the statistically significant differences among the control and treatments (p ≤ 0.05). Each value is the mean of five replicates with the standard deviation of the mean.
3.2. Biochemical Determinations
Regarding LP, this parameter increased directly according to the WD in the leaves (18.2%) and roots (2.4-fold) of each treatment compared with the control. However, the application of MeJA significantly decreased the LP in the leaves of the NoWD + MeJA treatment and the WD+ MeJA compared to the respective control treatment (Figure 2A). While in the roots, the LP slightly increased in all treatments compared to the control (Figure 2C).
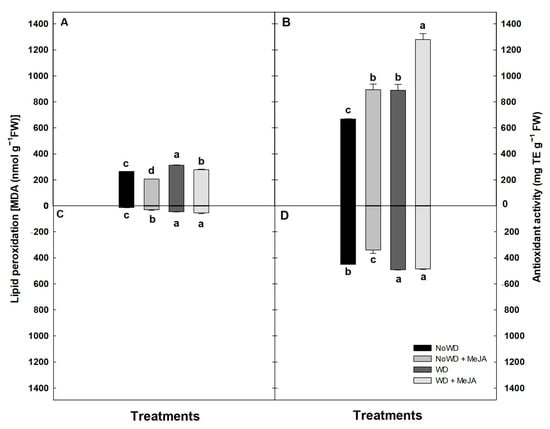
Figure 2.
The lipid peroxidation (LP) and antioxidant activity (AA) in the cultivar ‘Brigitta’ under a WD and the application of MeJA to the leaves. (A): LP of the leaves, (B): AA of the leaves, (C): LP of the roots and (D): AA of the roots. Lowercase letters show the statisticalyl significant differences among the control and treatments (p ≤ 0.05). Each value is the mean of five replicates with the standard deviation of the mean.
The highest values of the AA were obtained in the leaves and roots of the treatment WD + MeJA (50%) compared with the remaining treatments and NoWD (Figure 2B,D). Further, the AA from the roots of treatment NoWD + MeJA decreased by 25% with respect to the NoWD (Figure 2B,D). Non-statistically significant differences were observed in the leaves of treatments NoWD + MeJA and WD + MeJA, increasing by around 32% in this parameter with respect to the control (Figure 2B,D).
The highest decrease in TP (26.5%) was observed for the WD treatment compared with the NoWD (p ≤ 0.05). However, no significant differences among the NoWD + MeJA and WD + MeJA treatments with the control were observed (Figure 3).
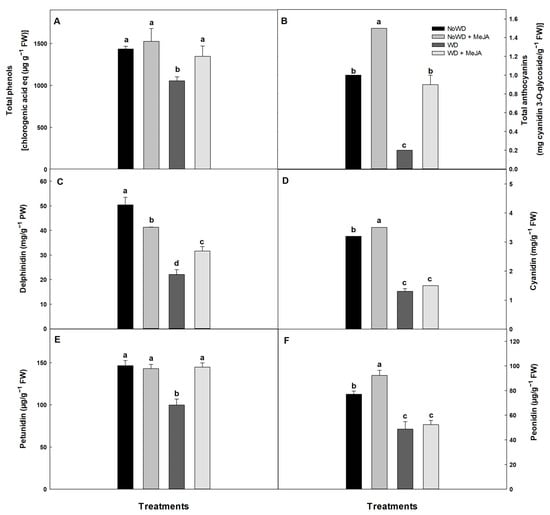
Figure 3.
Total phenols (TP), total anthocyanins (TA), delphinidin, cyanidin, petunidin, and peonidin in the ‘Brigitta’ cultivar under a WD and the application of MeJA to the leaves. (A): TP, (B): TA, (C): delphinidin, (D): cyanidin, (E): petunidin, and (F): peonidin. Lowercase letters show the statistically significant differences among the control and treatments (p ≤ 0.05). Each value is the mean of five replicates.
The lowest TA concentrations were observed for leaves of the WD treatment compared with the remaining treatments and NoWD (p ≤ 0.05). However, the application of MeJA increased the TA in the NoWD + MeJA treatment (50%) compared with the NoWD, respectively (Figure 3).
Regarding the anthocyanin profile in the leaves, it was observed that the delphinidin concentration decreased by 56% for the WD treatment compared with the NoWD. However, the application of MeJA in the NoWD + MeJA treatment seemed to improve the response via a lower decline in this parameter (38%) compared with the NoWD of this compound in the leaves. Likewise, the cyanidin and petunidin concentrations decreased significantly under a WD and the absence of MeJA (59.3%), being alleviated by applying MeJA in the NoWD + MeJA treatment (32%). On the other hand, for the peonidin concentration, the main affecting factor seemed to be a WD, because its lowest levels were found in the WD treatment compared with the remaining treatments and NoWD, which did not show significant statistical differences among themselves (Figure 3).
3.3. Photosynthetic Performance
The maximum quantum yield (Fv/Fm) for each WD and treatment (0.75–0.8) scored among the normal ranges (Figure 4). Thereby, the ΦPSII, ETR, and NPQ results did not show significant statistical differences between NoWD and the NoWD + MeJA treatment. In contrast, ΦPSII and ETR decreased by 28.5% and ~41% with a WD, respectively (Figure 4B,C), while an alleviation (around at 33%) was observed when MeJA [WD + MeJA] was applied (Figure 4B,C).
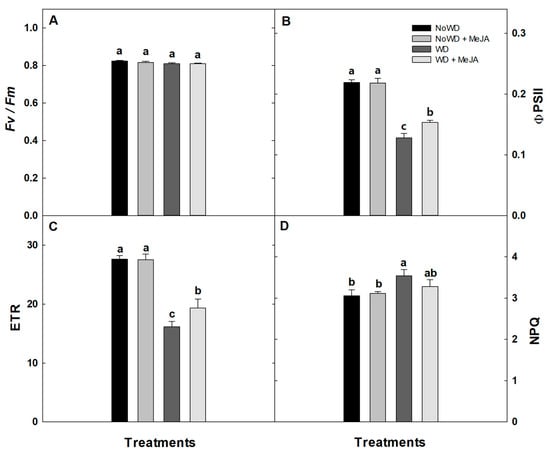
Figure 4.
Maximum quantum yield of PSII (Fv/Fm), effective quantum yield of PSII (ΦPSII), electron transport rate (ETR), and non-photochemical (NPQ) in the cultivar ‘Brigitta’ under a WD and the application of MeJA to the leaves. (A): Fv/Fm, (B): ΦPSII, (C): ETR, and (D): NPQ. Lowercase letters show the statistically significant differences among the control and treatments (p ≤ 0.05). Each value is the mean of five replicates with the standard deviation of the mean.
The photosynthetic parameters measured in the blueberry control plants (NoWD) were within the expected range for healthy plants. For the plant treatments with MeJA, i.e., (NoWD + MeJA) and (WD + MeJA), these parameters were improved (Figure 5). Specifically, the application of MeJA contrasted with the WD plants, causing an increase in the net photosynthesis by 4-fold, a 2-fold increase in the stomatal conductance and transpiration, and water use efficiency (Figure 5).
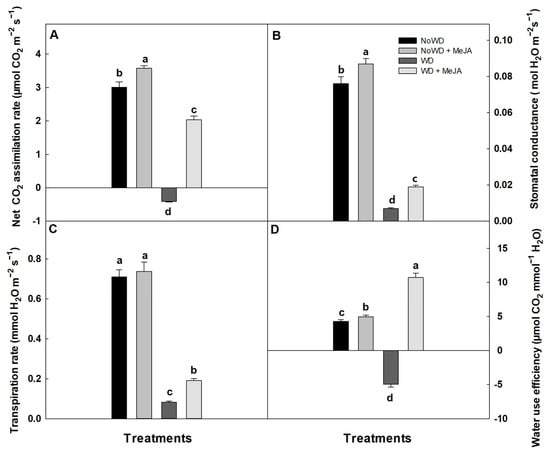
Figure 5.
Photosynthetic rate (A), stomatal conductance (B), transpiration rate (C) and water use efficiency (D) in the cultivar ‘Brigitta’ under a WD and the application of MeJA to the leaves. Lowercase letters show the statistically significant differences among the control and treatments (p ≤ 0.05). Each value is the mean of five replicates with the standard deviation of the mean.
The chlorophyll concentration decreased by 20% in the cultivar ‘Brigitta’ with WD treatment without significant differences with MeJA treatment (Figure 6). The β-carotene and lutein decreased in all treatments compared to the control plants, while zeaxanthin increased 1.8-fold with the application of MeJA and a WD compared to a WD (Figure 6).
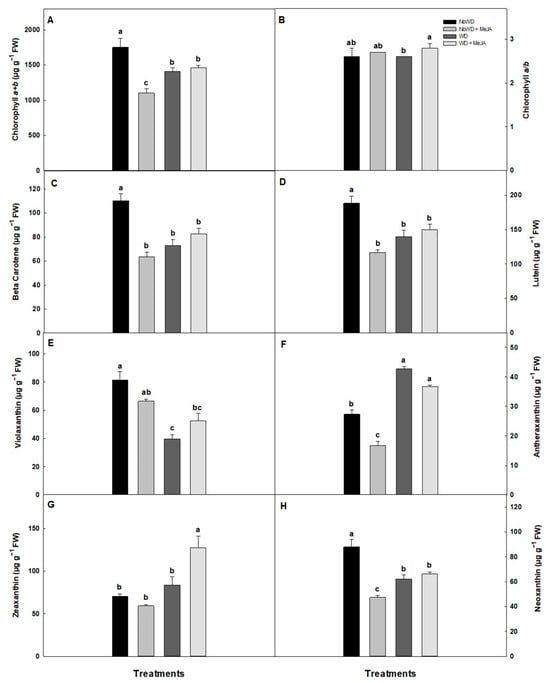
Figure 6.
The Chl a + b and Chl a/b concentration, and carotenoids concentration in the ‘Brigitta’ cultivar under WD and the application of MeJA to the leaves. (A): Chlorophyll a+b, (B): chlorophyll a/b, (C): beta-carotene, (D): lutein, (E): violaxanthin, (F): antheraxanthin, (G): zeaxanthin, and (H): neoxanthin. Lowercase letters show the statistically significant differences among the control and treatments (p ≤ 0.05). Each value is the mean of five replicates with the standard deviation of the mean.
4. Discussion
In the present study, blueberry plants improve their photosynthetic and antioxidant performance with the application of MeJA (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). Furthermore, these differences were observed for the leaves of plants without WD plus the MeJA, which significantly decreased its oxidative stress level and increased its antioxidant activity (Figure 2).
The application of MeJA significantly increased the TA, cyanidin, and peonidin in the ‘Brigitta’ cultivar’s leaves (Figure 3). Several studies report the increase of secondary metabolites and antioxidant compounds after the application of MeJA, but not on blueberry plants. Li et al. [39] reported a significantly increased concentration of phenolic compounds at the cuticular level on sunflower (Helianthus annuus), tomato (Solanum lycopersicum), and soybean (Glycine max) plants after treatment with 0.1, 0.5, 1.0, and 2.5 mM of MeJA. In turn, for blueberry plants, the effect of MeJA has been investigated on foliar tissue from the Legacy and Bluegold cultivars for other abiotic stress [22,40]. Ulloa-Inostroza et al. [22] reported an increase in the total antioxidant activity, the enzymatic activity of SOD and CAT, and the total phenols for the Legacy and Bluegold cultivars treated with 5 μM of exogenous MeJA under Al stress. Ribera-Fonseca et al. [41] showed that leaf extracts from the Legacy cultivars treated with 0.05 mM MeJA significantly increased their antioxidant properties, mainly attributed to anthocyanins. Besides this, the photosynthetic performance was concordant with typical values of MeJA-treated plants and similar to those reported by Ulloa-Inostroza et al. [22] in the Legacy and Bluegold cultivars. Thus, the results from the ‘Brigitta’ cultivar reinforce the capacity of this cultivar to respond to the application of MeJA, since the volatile nature of this compound efficiently permeates and enters the cell and makes itself available to participate in the biochemical secondary metabolic route. It seems that an intracellular concentration change scenario of MeJA is transcribed as an alert or a secondary message that activates an antioxidant mechanism triggered by a stress condition.
As a response after seven days of a WD, the ‘Brigitta’ cultivar presented significant shifts in the physiological and biochemical parameters, especially the increment of oxidative damage and antioxidant activity, and a reduction in the relative foliar area and photosynthesis (Figure 1, Figure 2, Figure 4 and Figure 5), which are indicators of a series of biochemical imbalances induced by an exacerbated production and accumulation of ROS. As a result, the oxidative damage to the membrane of stomatal cells increases, driving the permanent closure of stomas by differences in the intra- and extra-cellular levels of calcium (Ca2+), chloride (Cl-), and potassium (K+), and prolonged membrane depolarization. Consequently, the stomatal closure restricts photosynthesis, CO2 fixation, and water consumption, whereas photorespiration is promoted and ROS production is potentiated [10,11,12]. The strong reduction in photosynthesis in the ‘Brigitta’ cultivar under a WD could be indicative of severe stress due to a WD; it is known that under severe drought stress condition, many metabolic processes, like photosynthesis, are negatively affected, damaging the basic organizational structure and photosynthetic apparatus [42,43]. Likewise, our results also indicated that despite this strong decrease in net photosynthesis, becoming slightly negative under WD, the chlorophyll slightly decreased (15%), and the Chl a/b did not show changes, indicating that the plants did not show a state of leaf senescence. On the other hand, these results could suggest a negative balance in CO2 assimilation, indicating severe stress, as indicated above, which did not happen with the application of MeJA. Furthermore, the results showed that the ‘Brigitta’ cultivar promoted an increment in antioxidant activity in the leaves and roots as a response to the water stress to ameliorate the damage induced by the WD (Figure 2B–D), suggesting that the antioxidant machinery is working and the plant is not in a permanent wilting point due to a severe WD, supporting also the idea that the plants did not show a state of leaf senescence. Our results showed that the levels of the total phenolic compounds and anthocyanins decrease under a WD, and the total antioxidant activity could not be dependent on these compounds (Figure 3). On the other hand, we can also observe what was previously described by Inostroza-Blancheteau et al. [26] for aluminum toxicity. They demonstrated that the antioxidant response of the ‘Brigitta’ cultivar was mainly mediated by antioxidant enzymes such as SOD and CAT, which transform ROS into water. In this context, the strategy of ‘Brigitta’ could be to increase enzymatic antioxidants against a water deficit, since this cultivar is prone to decrease its physiological activity under these stressing conditions compared to other cultivars such as ‘Elliot’, ‘Bluegold’, ‘Biloxi’, and ‘Sharpblue’, which are more efficient at keeping their relative water content (RWC) under drought [44]. This evidence was supported by Améglio et al. [45], who determined that some cultivars, such as ‘Bluecrop’, can rapidly close their stomata and decrease evapotranspiration, which finally restricts water loss. Likewise, Molnar et al. [46] concluded that the blueberry cultivar ‘Goldtraube’ had the highest drought tolerance efficiency, followed by ‘Bluecrop’, ‘Hortblue Petite’, ‘Duke’, and ‘Brigitta Blue’.
The results from this research can support agronomic management decisions for the blueberry cultivar ‘Brigitta’, particularly under the climate change scenario where the availability of water for production is uncertain. This cultivar is sensitive to water stress, and critical production stages like transplanting, orchard settling, and under certain phenological periods, could benefit from the application of MeJA.
In recent years, MeJA has received particular attention due to its ability to increase the production of antioxidant compounds against the oxidative effect of ROS at photosyn-thetic levels. In this way, our results demonstrated a significant (p < 0.05) reduction in the oxidative stress (LP) in the ‘Brigitta’ cultivar treated with MeJA (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). This effect was significant at the leaf level, highlighting the increase in antioxidant activity, production of phenolic compounds, TA, delphinidin, petunidin, antheraxanthin, zeaxanthin, and photosynthetic performance parameters in the ‘Brigitta’ cultivar (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). The highest increase (50%) of zeaxanthin in ‘Brigitta’ under WD + MeJA could be involved in the protection of chloroplasts as reported by Brunetti et al. [47], where they highlighted that under severe drought, zeaxanthin and neoxanthin increased (75% and 25%, respectively), serving an important antioxidant function in protecting the chloroplast. Our results are in concordance with those shown by Wang [48], who observed that the increasing water retention by strawberry plants under a water deficit was improved after applying MeJA. Ma et al. [49] found that treating wheat plants with MeJA had a protective effect on their photosynthetic performance under a water deficit. This protecting effect was attributed to the decreasing effect on ROS damage by MeJA, which ameliorated the decreasing effect on the net photosynthetic ratio and stomatal conductance, while at the same time, the efficiency in water uptake was improved. Additionally, the improvement in the photo-dissipation of energy elements such as delphinidin, petunidin, antheraxanthin, and zeaxanthin, derived from applying MeJA, could protect the performance of the chloroplasts (Figure 3 and Figure 6). However, the activation of the antioxidant mechanism by MeJA is dependent on the WD degree [49]. Probably, the antioxidant response could be caused not only by MeJA but also by a summative effect of the stress condition. In this context, Su et al. [50] reported that ryegrass plants under heat stress treated with MeJA could activate the exact antioxidant mechanisms and elements for the photo-dissipation of energy than that observed for water deficit stress. This was at the same time associated with an increased expression of genes related to the biosynthetic pathway of jasmonate compounds (LpLOX2, LpAOC, LpOPR3, and LpJMT).
5. Conclusions
This study has demonstrated that the application of MeJA with a concentration of 10 µM ameliorates the negative effect of a WD in blueberry cultivar ‘Brigitta’. The stress condition caused by a WD triggered plant metabolic changes, increasing antioxidants (up to 50%), such as anthocyanins, antheraxanthin, zeaxanthin, among others, associated with a strategy for decreasing the harmful effects of drought by protecting the photosynthetic apparatus and its functionality in highbush blueberries, allowing the ‘Brigitta’ cultivar to endure a WD. On the other hand, our study has great relevance to the crops with economic importance since our results demonstrated that the application of MeJA could be an interesting tool to improve the resistance of crops subjected to changing climatic conditions. For a better understanding, future research should be addressed for a more in-depth study of the molecular mechanisms that may be acting under stress due to a water deficit and the application of MeJA.
Author Contributions
E.M.U.-I. and M.R.-D.; methodology, formal analysis, investigation, resources, and writing the original draft, E.M.U.-I., M.R.-D., C.C. and M.C.; review and editing, E.M.U.-I. and M.R.-D.; supervision, M.R.-D. and E.M.U.-I.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was supported by funds from the FONDECYT Postdoctoral 3180754, and ANID/FONDECYT 1211856 projects from ANID (Chile), FONDEQUIP-UAysén Ulloa-2018 project, URY 20993 “Centro de Innovación e Incubadora de Ideas U. Aysén (3i U. Aysén)”, Chilean Ministry of Education, and ANID/FONDAP/15130015 and ANID/FONDAP/1523A0001.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Centro de Innovación e Incubadora de Ideas U. Aysén (3i U. Aysén, Universidad de Aysén) and the Center of Plant, Soil Interaction and Natural Resources Biotechnology, Scientific and Technological Bioresource Nucleus (BIOREN-UFRO), Universidad de La Frontera.
Conflicts of Interest
Author Carolin Córdova was employed by the company Terra Sustineri Limitada. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Westmacott, J.R.; Burn, D.H. Climate change effects on the hydrologic regime within the Churchill-Nelson River Basin. J. Hydrol. 1997, 202, 263–279. [Google Scholar] [CrossRef]
- Wolf, A.T. Criteria for equitable allocations: The heart of international water conflict. Nat. Resour. Forum. 1999, 23, 3–30. [Google Scholar] [CrossRef]
- Meier, J.; Zabel, F.; Mauser, W. A global approach to estimate irrigated areas—A comparison between different data and statistics. Hydrol. Earth Syst. Sci. 2018, 22, 1119–1133. [Google Scholar] [CrossRef]
- Nagaraj, D.; Proust, E.; Todeschini, A.; Rulli, M.C.; D’Odorico, P. A new dataset of global irrigation areas from 2001 to 2015. Adv. Water Resour. 2021, 152, 103910. [Google Scholar] [CrossRef]
- Boyer, J. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global agricultural economic water scarcity. Sci. Adv. 2020, 6, eaaz6031. [Google Scholar] [CrossRef]
- Bryla, D.R.; Strik, B.C. Effects of cultivar and plant spacing on the seasonal water requirements of highbush blueberry. J. Am. Soc. Hortic. Sci. 2007, 132, 270–277. [Google Scholar] [CrossRef]
- Bryla, D.R. Crop evapotranspiration and irrigation scheduling in blueberry. In Evapotranspiration—From Measurements to Agricultural and Environmental Applications; Gerosa, G., Ed.; Intech: Rijeka, Croatia, 2011; Chapter 9; pp. 167–186. [Google Scholar]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.d.S.; Zulfiqar, F.; Alam, M.d.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Singh, R.; Parihara, P.; Singha, S.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Reactive oxygen species signaling and stomatal movement: Current updates and future perspectives. Redox Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Keles, Y.; Oncel, I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2005, 56, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.; Fucile, M.; Manzi, D.; Peruzzi, E.; Mattii, G.B. Effects of Zeowine and compost on leaf functionality and berry composition in Sangiovese grapevines. J. Agric. Sci. 2023, 161, 412–427. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Díaz, G.A.; Abbas, A.; Parveen, A.; Atiq, M.N.; Alshaya, H.; et al. Phytohormones trigger drought tolerance in crop plants: Outlook and future perspectives. Front. Plant Sci. 2022, 12, 799318. [Google Scholar] [CrossRef]
- Kwak, J.M.; Nguyen, V.; Schroeder, J.I. The role of reactive oxygen species in hormonal responses1. Plant Physiol. 2006, 141, 323–329. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Ulloa-Inostroza, E.M.; Gonzalez-Villagra, J.; Ivanov, A.G.; Kurepin, L.V. Phytohormonal responses to soil acidity in plants. In Plant Hormones under Challenging Environmental Factors; Ahammed, G.J., Yu, J.-Q., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2016; pp. 133–155. [Google Scholar]
- Ulloa-Inostroza, E.M.; Alberdi, M.; Meriño-Gergichevich, C.; Reyes-Díaz, M. Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum. Plant Soil 2017, 412, 81–96. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.Q.; Zhang, L.T.; Sun, M.M.; Lin, T.B. Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica 2014, 52, 377–385. [Google Scholar] [CrossRef]
- Dong, T.X.; Cai, K.Z.; Zhang, J.X.; Rong, H.; Xie, G.Z.; Zeng, R.S. The physiological roles of methyl jasmonate (MeJA) in drought resistance of rice seedlings. Ecol. Environ. 2007, 16, 1261–1265. [Google Scholar]
- Reyes-Díaz, M.; Meriño-Gergichevich, C.; Alarcón, E.; Alberdi, M.; Horst, W.J. Calcium sulfate ameliorates the effect of aluminum toxicity differentially in genotypes of highbush blueberry (Vaccinium corymbosum L.). J. Soil Sci. Plant Nutr. 2011, 11, 59–78. [Google Scholar] [CrossRef]
- Inostroza-Blancheteau, C.; Reyes-Diaz, M.; Aquea, F.; Nunes-Nesi, A.; Alberdi, M.; Arce-Johnson, P. Biochemical and molecular changes in response to aluminium-stress in highbush blueberry (Vaccinium corymbosum L). Plant Physiol. Biochem. 2011, 49, 1005–1012. [Google Scholar] [CrossRef]
- Larcher, W. Physiological plant ecology. In Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003; 504p. [Google Scholar]
- Maloof, J.N.; Nozue, K.; Mumbach, M.R.; Palmer, C.M. LeafJ: An imageJ Plugin for semi-automated leaf shape measurement. J. Vis. Exp. 2013, 71, e50028. [Google Scholar]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipidoxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1556–1570. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, Y.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.A. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 29–55. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hort. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Díaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Alberdi, M.; Mora, M.L. Short-term aluminum stress differentially affects the photochemical efficiency of photosystem II in highbush blueberry genotypes. J. Am. Soc. Hortic. Sci. 2009, 134, 14–21. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G. Chlorophyll fluorescence. A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Locke, A.M.; Ort, D.R. Leaf hydraulic conductance declines in coordination with photosynthesis, transpiration and leaf water status as soybean leaves age regardless of soil moisture. J. Exp. Bot. 2014, 65, 6617–6627. [Google Scholar] [CrossRef]
- Garcia-Plazaola, J.I.; Becerril, J.M. A rapid HPLC method to measure liphophilic antioxidant in stressed plants: Simultaneous determination of carotenoids and tocopherols. Phytochem. Anal. 1999, 10, 307–313. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of methyl jasmonate on plant growth and leaf properties. J. Plant Nutr. Soil Sci. 2018, 181, 409–418. [Google Scholar] [CrossRef]
- González-Villagra, J.; Pino, R.; Inostroza-Blancheteau, C.; Cartes, P.; Ribera-Fonseca, A.; Reyes-Díaz, M. Pre-Harvest MeJA application counteracts the deleterious impact of Al and Mn toxicity in highbush blueberry grown in acid soils. Plants 2021, 10, 2730. [Google Scholar] [CrossRef] [PubMed]
- Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The anti-proliferative and anti-invasive effect of leaf extracts of blueberry plants treated with methyl jasmonate on human gastric cancer in vitro is related to their antioxidant properties. Antioxidants 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Golldack, D.; Lüking, I.; Yang, O. Plant tolerance to drought and salinity: Stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011, 30, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Balboa, K.; Ballesteros, G.I.; Molina-Montenegro, M.A. Integration of physiological and molecular traits would help to improve the insights of drought resistance in highbush blueberry cultivars. Plants 2020, 9, 1457. [Google Scholar] [CrossRef] [PubMed]
- Améglio, T.; Roux, L.; Perrier, M.C. Water relations of highbush blueberry under drought conditions. Acta Hortic. 2000, 537, 273–278. [Google Scholar] [CrossRef]
- Molnar, S.; Clapa, D.; Viorel, M. Response of the five highbush blueberry cultivars to in vitro induced drought stress by polyethylene glycol. Agronomy 2022, 12, 732. [Google Scholar] [CrossRef]
- Brunetti, C.; Tattini, M.; Guidi, L.; Velikova, V.; Ferrini, F.; Fini, A. An integrated overview of physiological and biochemical responses of Celtis australis to drought stress. Urban For. Urban Green. 2019, 46, 126480. [Google Scholar] [CrossRef]
- Wang, S.Y. Methyl jasmonate reduces water stress in strawberry. Plant Growth Regul. 1999, 18, 127–134. [Google Scholar] [CrossRef]
- Ðurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Milošević, S. Alterations in physiological, biochemical, and molecular responses of Impatiens walleriana to drought by methyl jasmonate foliar application. Genes 2023, 14, 1072. [Google Scholar]
- Su, Y.; Huang, Y.; Dong, X.; Wang, R.; Tang, M.; Cai, J.; Chen, J.; Zhang, X.; Nie, G. Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front. Plant Sci. 2021, 12, 664519. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).