Comparison of Methods to Determine Nutrient Uptake of Tomato Grown in Free-Draining Perlite Substrate—Key Information for Optimal Fertigation Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Crop Details
2.2. Experimental Design
2.3. Measurements
2.3.1. Climate
2.3.2. Determination of Nutrients Uptake in Dry Matter
2.3.3. Determination of Nutrient Uptake with the Mass Balance Method
2.3.4. Determination of Nutrient Retention in Perlite Substrate
2.3.5. Estimation of Nutrient Uptake by Roots
3. Results
3.1. Climate
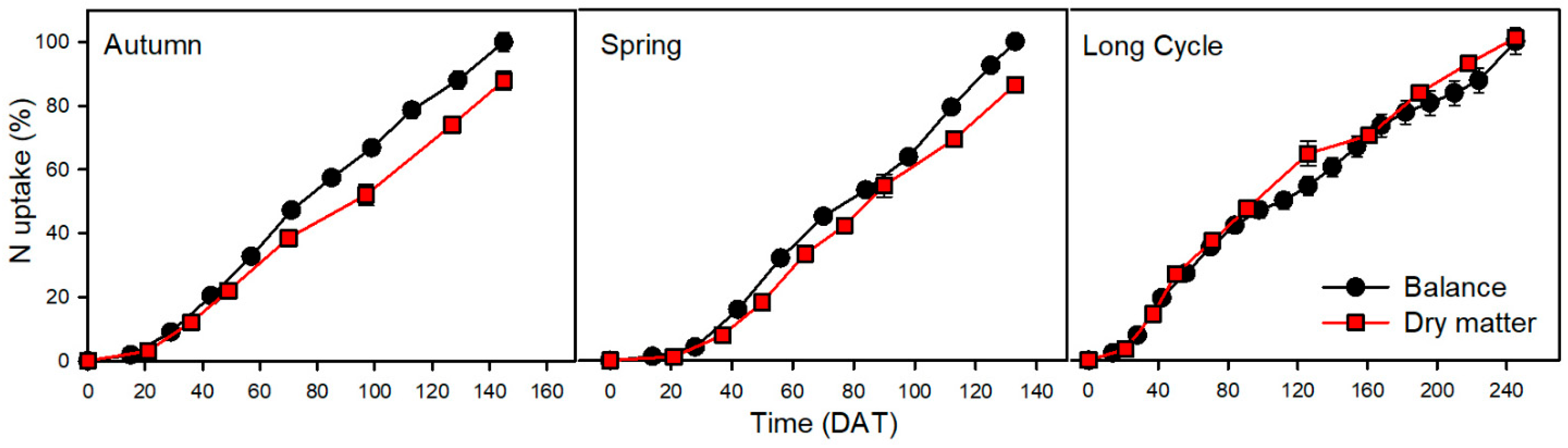
3.2. Nitrogen
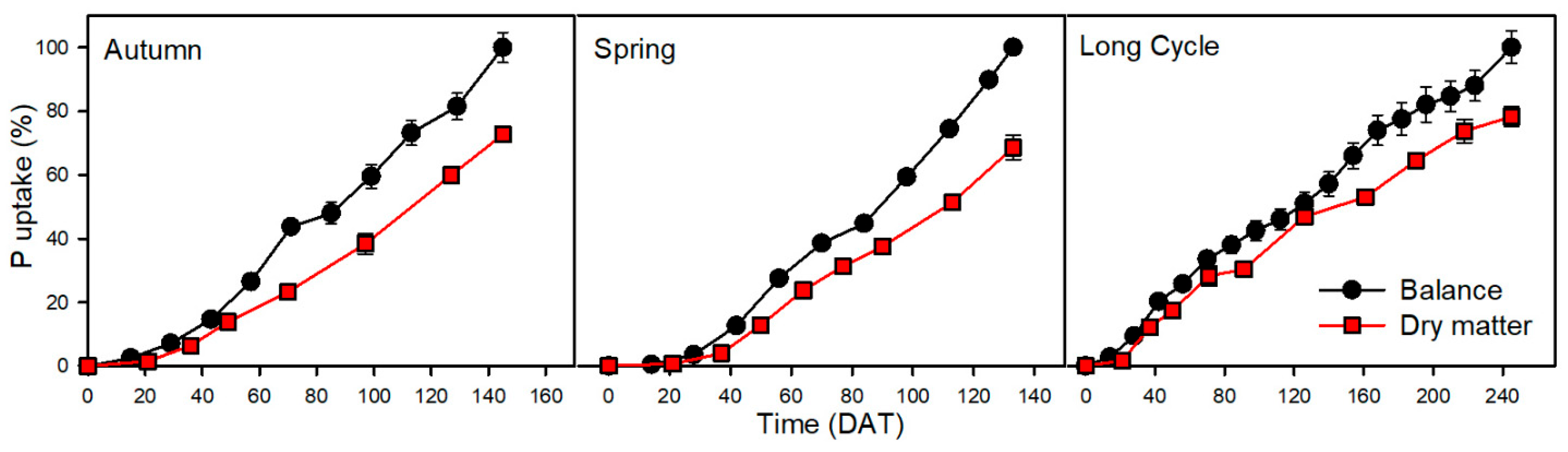
3.3. Phosphorus
3.4. Potassium
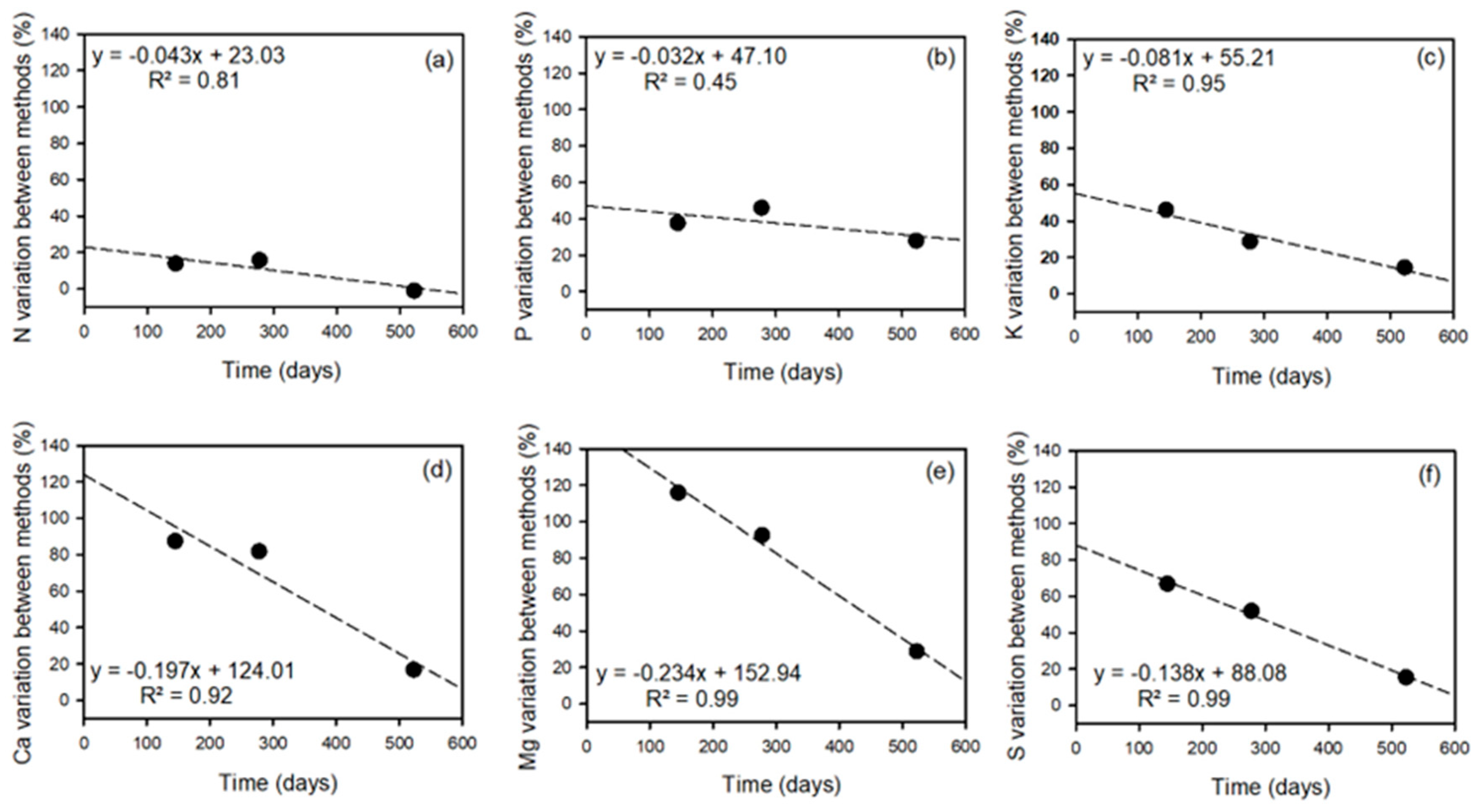
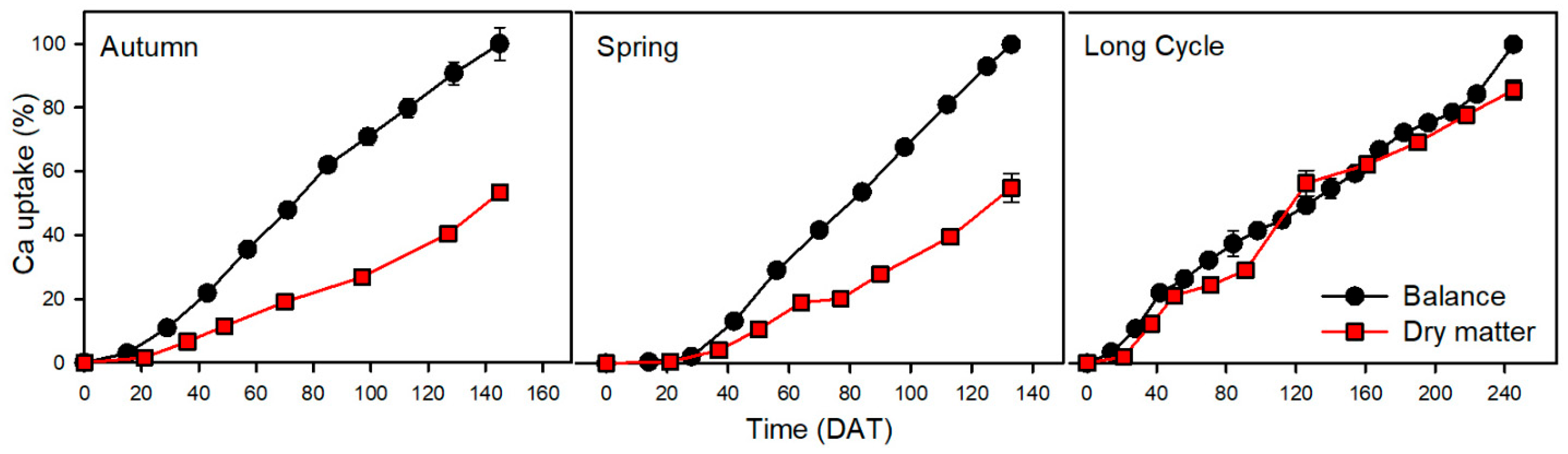
3.5. Calcium
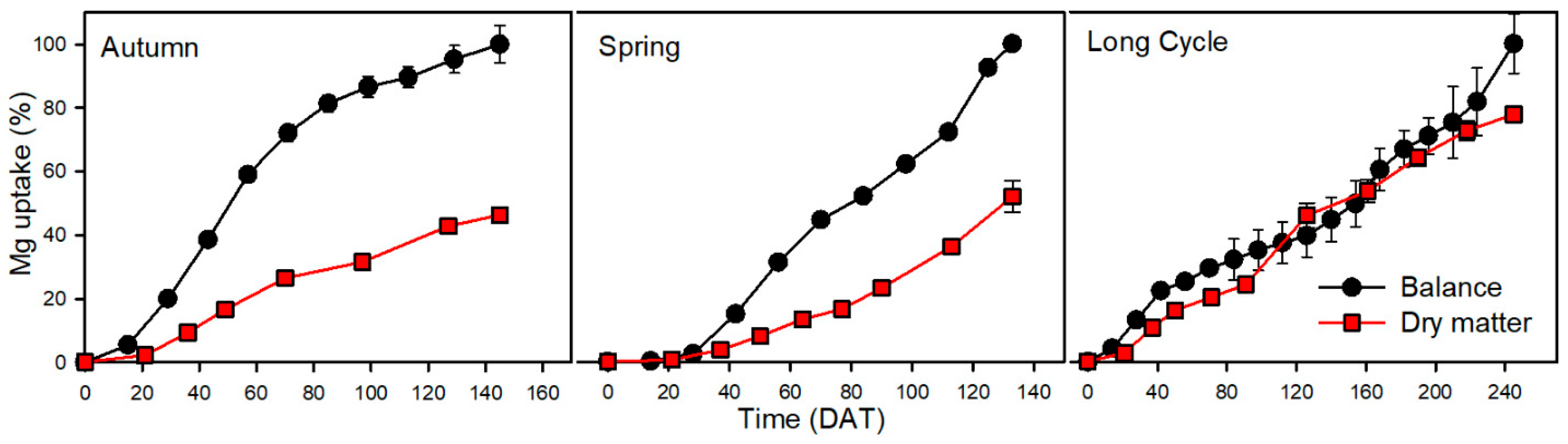
3.6. Magnesium
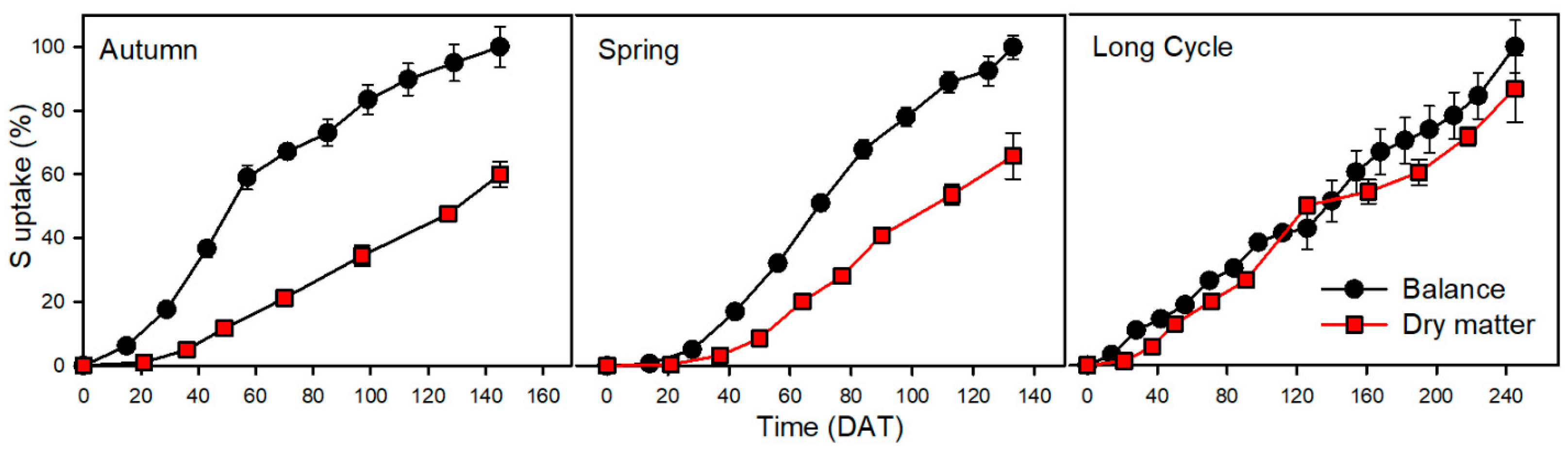
3.7. Sulphur
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardossi, A.; Tognoni, F.; Incrocci, L. Mediterranean Greenhouse Technology. Chronica Horticulturae. Chron. Horticult. 2004, 44, 28–34. [Google Scholar]
- Incrocci, L.; Thompson, R.B.; Fernandez, M.D.; De Pascale, S.; Pardossi, A.; Stanghellini, C.; Rouphael, Y.; Gallardo, M. Irrigation Management of European Greenhouse Vegetable Crops. Agric. Water Manag. 2020, 242, 106393. [Google Scholar] [CrossRef]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing Water and Nutrient Losses from Soilless Cropping in Southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Perilla, G.A.; Mas, J.-F. High-Resolution Mapping of Protected Agriculture in Mexico, through Remote Sensing Data Cloud Geoprocessing. Eur. J. Remote Sens. 2019, 52, 532–541. [Google Scholar] [CrossRef]
- Acharki, S.; Kozhikkodan Veettil, B. Mapping Plastic-Covered Greenhouse Farming Areas Using High-Resolution PlanetScope and RapidEye Imagery: Studies from Loukkos Perimeter (Morocco) and Dalat City (Vietnam). Environ. Sci. Pollut. Res. 2023, 30, 23012–23022. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Zhang, Z.; Yang, L.; Pan, X.; Jia, Z. Mapping Agricultural Plastic Greenhouses Using Google Earth Images and Deep Learning. Comput. Electron. Agric. 2021, 191, 106552. [Google Scholar] [CrossRef]
- Bartosik, M.-L.; Salonen, K.; Jokinen, R.; Hukkanen, K.R. Comparison of Open and Closed Growing Methods on Peat and Rockwool and the Leaching of Nutrients. Acta Hortic. 1993, 342, 303–306. [Google Scholar] [CrossRef]
- Le Bot, J.; Jeannequin, B.; Fabre, R. Impacts of N-Deprivation on the Yield and Nitrogen Budget of Rockwool Grown Tomatoes. Agronomie 2001, 21, 341–350. [Google Scholar] [CrossRef]
- Thompson, R.B.; Gallardo, M.; Rodríguez, J.S.; Sánchez, J.A.; Magán, J.J. Effect of N Uptake Concentration on Nitrate Leaching from Tomato Grown in Free-Draining Soilless Culture under Mediterranean Conditions. Sci. Hortic. 2013, 150, 387–398. [Google Scholar] [CrossRef]
- Peña-Fleitas, M.T.; Thompson, R.; Gallardo, M.; Fernández-Fernández, M.D. Regional Model of Nitrate Leaching for an Intensive Vegetable Production System. In Proceedings of the International Workshop of Nitrogen, Environment, and Vegetables, Torino, Italy, 15–17 April 2013, Book of Abstracts/NEV2013; Fontana, E., Grignani, C., Nicola, S., Eds.; Department of Agricultural, Forest and Food Sciences-University of Turin: Turin, Italy, 2013; pp. 73–74. [Google Scholar]
- Incrocci, L.; Massa, D.; Pardossi, A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Pardossi, A.; Malorgio, F.; Incrocci, L.; Carmassi, G.; Maggini, R.; Massa, D.; Tognoni, F. Simplified Models for the Water Relations of Soilless Cultures: What They Do or Suggest for Sustainable Water Use in Intensive Horticulture. Acta Hortic. 2006, 718, 425–434. [Google Scholar] [CrossRef]
- Min, J.; Zhao, X.; Shi, W.-M.; Xing, G.-X.; Zhu, Z.-L. Nitrogen Balance and Loss in a Greenhouse Vegetable System in Southeastern China. Pedosphere 2011, 21, 464–472. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Zhang, F.S.; Christie, P. Nitrogen Balance and Groundwater Nitrate Contamination: Comparison among Three Intensive Cropping Systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef]
- Qasim, W.; Xia, L.; Lin, S.; Wan, L.; Zhao, Y.; Butterbach-Bahl, K. Global Greenhouse Vegetable Production Systems Are Hotspots of Soil N2O Emissions and Nitrogen Leaching: A Meta-Analysis. Environ. Pollut. 2021, 272, 116372. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, Y.; Shi, X.; Chen, X. Nitrate Leaching from Open-Field and Greenhouse Vegetable Systems in China: A Meta-Analysis. Environ. Sci. Pollut. Res. 2018, 25, 31007–31016. [Google Scholar] [CrossRef]
- Anonymous. Council Directive 91/676/EEC Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources; Official Journal of the European Communities: Luxembourg, 1991; pp. 1–8. [Google Scholar]
- Anonymous. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; Official Journal of the European Communities: Luxembourg, 2000; pp. 1–73. [Google Scholar]
- Nikolaou, G.; Neocleous, D.; Katsoulas, N.; Kittas, C. Irrigation of Greenhouse Crops. Horticulturae 2019, 5, 7. [Google Scholar] [CrossRef]
- Van der Salm, C.; Voogt, W.; Beerling, E.; van Ruijven, J.; van Os, E. Minimising Emissions to Water Bodies from NW European Greenhouses; with Focus on Dutch Vegetable Cultivation. Agric. Water Manag. 2020, 242, 106398. [Google Scholar] [CrossRef]
- Cameira, M.d.R.; Mota, M. Nitrogen Related Diffuse Pollution from Horticulture Production—Mitigation Practices and Assessment Strategies. Horticulturae 2017, 3, 25. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A Targeted Management of the Nutrient Solution in a Soilless Tomato Crop According to Plant Needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-90-481-2531-9. [Google Scholar]
- Savvas, D.; Gruda, N. Application of Soilless Culture Technologies in the Modern Greenhouse Industry—A Review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Christou, A.; Polycarpou, P.; Kitta, E.; Katsoulas, N. Energy and Water Related Parameters in Tomato and Cucumber Greenhouse Crops in Semiarid Mediterranean Regions. A Review, Part Ii: Irrigation and Fertigation. Horticulturae 2021, 7, 548. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Chemical Analysis in Substrate Systems and Hydroponics-Use and Interpretation. Acta Hortic. 2001, 548, 247–260. [Google Scholar] [CrossRef]
- Sonneveld, C. Composition of Nutrient Solutions. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H., Eds.; Embryo Publications: Athens, Greece, 2002; pp. 179–210. [Google Scholar]
- Sonneveld, C. Effects of Salinity on Substrate Grown Vegetables and Ornamentals in Greenhouse Horticulture. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2000. [Google Scholar]
- Van Noordwijk, M. Synchronisation of Supply and Demand Is Necessary to Increase Efficiency of Nutrient Use in Soilless Horticulture. In Plant Nutrition-Physiology and Applications; Van Beusichem, M.L., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1990; pp. 525–531. [Google Scholar]
- Cedeño, J.; Magán, J.J.; Thompson, R.B.; Fernández, M.D.; Gallardo, M. Reducing Nutrient Loss in Drainage from Tomato Grown in Free-Draining Substrate in Greenhouses Using Dynamic Nutrient Management. Agric. Water Manag. 2023, 287, 108418. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Bibbiani, C.; Carmassi, G.; Malorgio, F.; Pardossi, A. Simulation of Crop Water and Mineral Relations in Greenhouse Soilless Culture. Environ. Model. Softw. 2011, 26, 711–722. [Google Scholar] [CrossRef]
- Gallardo, M.; Elia, A.; Thompson, R.B. Decision Support Systems and Models for Aiding Irrigation and Nutrient Management of Vegetable Crops. Agric. Water Manag. 2020, 240, 106209. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Carmassi, G.; Campiotti, C.A.; Pardossi, A. Strategies to Decrease Water Drainage and Nitrate Emission from Soilless Cultures of Greenhouse Tomato. Agric. Water Manag. 2010, 97, 971–980. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. Validating a Smart Nutrient Solution Replenishment Strategy to Save Water and Nutrients in Hydroponic Crops. Front. Environ. Sci. 2022, 10, 965964. [Google Scholar] [CrossRef]
- Voogt, W. Nutrient Uptake of Year Round Tomato Crops. Acta Hortic. 1993, 339, 99–112. [Google Scholar] [CrossRef]
- Heinen, M.; Sonneveld, C.; Voogt, W.; Baas, R.; Keltjens, W.G.; Veen, B.W. Mineral Balance of Young Tomato Plants Grown on Nutrient Solution; DLO Research Institute for Agrobiology and Soil Fertility: Haren, The Netherlands, 1996; Available online: https://library.wur.nl/WebQuery/wurpubs/302591 (accessed on 28 January 2024).
- Gallardo, M.; Fernández, M.D.; Giménez, C.; Padilla, F.M.; Thompson, R.B. Revised VegSyst Model to Calculate Dry Matter Production, Critical N Uptake and ETc of Several Vegetable Species Grown in Mediterranean Greenhouses. Agric. Syst. 2016, 146, 30–43. [Google Scholar] [CrossRef]
- Pedersen, A.; Zhang, K.; Thorup-Kristensen, K.; Jensen, L.S. Modelling Diverse Root Density Dynamics and Deep Nitrogen Uptake—A Simple Approach. Plant Soil 2010, 326, 493–510. [Google Scholar] [CrossRef]
- Sanjuan-Delmás, D.; Josa, A.; Muñoz, P.; Gassó, S.; Rieradevall, J.; Gabarrell, X. Applying Nutrient Dynamics to Adjust the Nutrient-Water Balance in Hydroponic Crops. A Case Study with Open Hydroponic Tomato Crops from Barcelona. Sci. Hortic. 2020, 261, 108908. [Google Scholar] [CrossRef]
- Xaxiri, E.; Darivakis, E.; Karavidas, I.; Ntatsi, G.; Savvas, D. Comparing the Nutritional Needs of Two Solanaceae and One Cucurbitaceae Species Grown Hydroponically under the Same Cropping Conditions. Plants 2023, 12, 3642. [Google Scholar] [CrossRef]
- Savvas, D.; Öztekin, G.B.; Tepecik, M.; Ropokis, A.; Tüzel, Y.; Ntatsi, G.; Schwarz, D. Impact of Grafting and Rootstock on Nutrient-to-Water Uptake Ratios during the First Month after Planting of Hydroponically Grown Tomato. J. Hortic. Sci. Biotechnol. 2017, 92, 294–302. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. Effect of Different Macronutrient Cation Ratios on Macronutrient and Water Uptake by Melon (Cucumis melo) Grown in Recirculating Nutrient Solution. J. Plant Nutr. Soil Sci. 2015, 178, 320–332. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G.; Rodopoulou, M.; Goumenaki, F. Nutrient Uptake Concentrations in a Cucumber Crop Grown in a Closed Hydroponic System under Mediterranean Climatic Conditions as Influenced by Irrigation Schedule. Acta Hortic. 2014, 1034, 545–552. [Google Scholar] [CrossRef]
- Gertsson, U.E. Nutrient Uptake by Tomatoes Grown in Hydroponics. Acta Hortic. 1995, 401, 351–356. [Google Scholar] [CrossRef]
- Xiong, J.; Tian, Y.; Wang, J.; Liu, W.; Chen, Q. Comparison of Coconut Coir, Rockwool, and Peat Cultivations for Tomato Production: Nutrient Balance, Plant Growth and Fruit Quality. Front. Plant Sci. 2017, 8, 280598. [Google Scholar] [CrossRef]
- Rivera-del Rio, R.; Pineda-Pineda, J.; Avitia-Garcia, E.; Castillo-González, A.M.; Vargas-Hernandez, M. Alteration of Physical Properties of Substrates and Accumulation of Nutrients in Strawberry Hydroponic Systems (Fragaria x Ananassa Duch.). Acta Hortic. 2017, 1170, 679–686. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Similon, L.; Moelants, J.; Gage, E. End-of-Life of Organic Growing Media: Assessing the Residual Nutrient Concentrations in Mineral and Organic Growbags of Tomato. Acta Hortic. 2023, 1377, 545–552. [Google Scholar] [CrossRef]

| Crop | EC | pH | Macronutrients (mmol L−1) | Micronutrients (mg L−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO3− | PO43− | SO42− | NH4+ | K+ | Ca2+ | Mg2+ | Fe | Mn | Zn | B | Cu | ||||
| Supply | Autumn | 3.4 | 6.0 | 9.0 | 1.7 | 3.0 | 1.2 | 7.0 | 4.8 | 2.6 | 2.1 | 1.8 | 0.6 | 0.4 | 0.1 |
| Spring | 3.3 | 6.2 | 9.9 | 1.5 | 3.1 | 0.9 | 6.1 | 4.6 | 2.7 | 1.7 | 1.6 | 0.4 | 0.3 | 0.1 | |
| Long Cycle | 3.5 | 6.3 | 9.2 | 1.4 | 3.3 | 0.8 | 6.1 | 4.9 | 2.8 | 1.4 | 1.3 | 0.3 | 0.1 | 0.1 | |
| Drainage | Autumn | 5.2 | 6.2 | 8.5 | 1.9 | 5.8 | 0.1 | 8.0 | 7.2 | 5.0 | 3.2 | 2.0 | 0.8 | 0.6 | 0.2 |
| Spring | 4.9 | 6.1 | 11.0 | 1.6 | 5.5 | 0.2 | 7.0 | 6.2 | 4.9 | 2.6 | 1.7 | 0.6 | 0.4 | 0.2 | |
| Long Cycle | 5.3 | 6.2 | 10.2 | 1.7 | 5.7 | 0.1 | 6.6 | 7.3 | 5.2 | 2.4 | 1.3 | 0.4 | 0.2 | 0.1 | |
| Crop | Mean of Daily Climatic Values for Duration of Each Crop | ||||||
|---|---|---|---|---|---|---|---|
| Air Temperature (°C) | VPD (kPa) | Solar Radiation (MJ m−2 d−1) | |||||
| Average | Maximum | Minimum | Average | Maximum | Minimum | Integral | |
| Autumn | 16.9 | 24.8 | 12.3 | 0.73 | 1.99 | 0.08 | 6.4 |
| Spring | 20.1 | 27.2 | 14.2 | 0.90 | 1.71 | 0.01 | 10.1 |
| Long Cycle | 16.2 | 24.6 | 12.2 | 0.76 | 1.95 | 0.23 | 6.1 |
| Crop | Nutrient | Uptake by Balance Method (kg ha−1) | Uptake by Dry Matter Method (kg ha−1) | Statistical Significance of Difference between Methods | Difference—Balance Minus Dry Matter Method (kg ha−1) | Relative Difference (Relative to Balance Method) (%) | Relative Difference (Relative to Dry Matter Method) (%) |
|---|---|---|---|---|---|---|---|
| Autumn | N | 278 | 245 | * | 33 | 12 | 13 |
| P | 83 | 60 | ** | 23 | 28 | 38 | |
| K | 448 | 307 | *** | 141 | 31 | 46 | |
| Ca | 247 | 132 | ** | 115 | 47 | 87 | |
| Mg | 57 | 27 | *** | 30 | 53 | 111 | |
| S | 81 | 49 | ** | 32 | 40 | 65 | |
| Spring | N | 377 | 326 | *** | 51 | 14 | 16 |
| P | 112 | 77 | *** | 35 | 31 | 45 | |
| K | 555 | 431 | ** | 124 | 22 | 29 | |
| Ca | 367 | 202 | *** | 165 | 45 | 82 | |
| Mg | 78 | 40 | *** | 38 | 49 | 95 | |
| S | 120 | 79 | ** | 41 | 34 | 52 | |
| Long Cycle | N | 491 | 497 | ns | −6 | −1 | −1 |
| P | 135 | 106 | * | 29 | 21 | 27 | |
| K | 809 | 708 | ** | 101 | 12 | 14 | |
| Ca | 428 | 367 | ns | 61 | 14 | 17 | |
| Mg | 86 | 67 | ns | 19 | 22 | 28 | |
| S | 162 | 141 | ns | 21 | 13 | 15 |
| Crop | Nutrient | Amount Applied (kg ha−1) | Difference in Uptake between Methods (kg ha−1) | Measured Retention in Perlite (kg ha−1) | Percentage of Applied Retained in Perlite (%) | Estimated Root Uptake (kg ha−1) | Residual Difference (kg ha−1) | Relative Residual Difference in Relation to Balance Method (%) |
|---|---|---|---|---|---|---|---|---|
| Autumn | N | 405 | 33 | 32 | 8 | 10 | −9 | −3 |
| P | 146 | 23 | 19 | 13 | 3 | 1 | 2 | |
| K | 776 | 141 | 77 | 10 | 10 | 54 | 12 | |
| Ca | 546 | 115 | 11 | 2 | 14 | 90 | 36 | |
| Mg | 182 | 30 | 18 | 10 | 2 | 10 | 18 | |
| S | 273 | 32 | 20 | 8 | 3 | 9 | 11 | |
| Long Cycle | N | 908 | −6 | 26 | 3 | 19 | −51 | −10 |
| P | 286 | 29 | 22 | 8 | 6 | 1 | 0 | |
| K | 1554 | 101 | 44 | 3 | 20 | 37 | 5 | |
| Ca | 1266 | 61 | 29 | 2 | 28 | 4 | 1 | |
| Mg | 443 | 19 | −2 | 0 | 3 | 18 | 21 | |
| S | 683 | 21 | 2 | 0 | 8 | 11 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedeño, J.M.; Magán, J.-J.; Thompson, R.B.; Fernández, M.-D.; Gallardo, M. Comparison of Methods to Determine Nutrient Uptake of Tomato Grown in Free-Draining Perlite Substrate—Key Information for Optimal Fertigation Management. Horticulturae 2024, 10, 232. https://doi.org/10.3390/horticulturae10030232
Cedeño JM, Magán J-J, Thompson RB, Fernández M-D, Gallardo M. Comparison of Methods to Determine Nutrient Uptake of Tomato Grown in Free-Draining Perlite Substrate—Key Information for Optimal Fertigation Management. Horticulturae. 2024; 10(3):232. https://doi.org/10.3390/horticulturae10030232
Chicago/Turabian StyleCedeño, Juan M., Juan-José Magán, Rodney Bruce Thompson, María-Dolores Fernández, and Marisa Gallardo. 2024. "Comparison of Methods to Determine Nutrient Uptake of Tomato Grown in Free-Draining Perlite Substrate—Key Information for Optimal Fertigation Management" Horticulturae 10, no. 3: 232. https://doi.org/10.3390/horticulturae10030232
APA StyleCedeño, J. M., Magán, J.-J., Thompson, R. B., Fernández, M.-D., & Gallardo, M. (2024). Comparison of Methods to Determine Nutrient Uptake of Tomato Grown in Free-Draining Perlite Substrate—Key Information for Optimal Fertigation Management. Horticulturae, 10(3), 232. https://doi.org/10.3390/horticulturae10030232









