Study of the Phenolic Compounds and Biological Activities of the Wild Fruits of Vaccinium leucanthum Schltdl.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Quality
2.2. Extract Preparation
2.3. Total Phenol Quantification
2.4. Total Flavonoid Quantification
2.5. Total Anthocyanin Quantification
2.6. Total Tannins Quantification
2.7. Detection and Quantification of Chlorogenic Acid, Hyperoside and Anthocyanins by HPTLC
2.8. Antioxidant Activity
2.8.1. Antioxidant Activity Using ABTS Assay
2.8.2. Antioxidant Activity Using DPPH Assay
2.8.3. Antioxidant Activity Using HPTLC-DPPH●
2.9. Antimicrobial Activity
2.10. Xanthine Oxidase (XO) Inhibitory Assay
2.11. Angiotensin I-Converting Enzyme (ACE) Inhibition Assay
2.12. Statistical Analysis
3. Results and Discussion
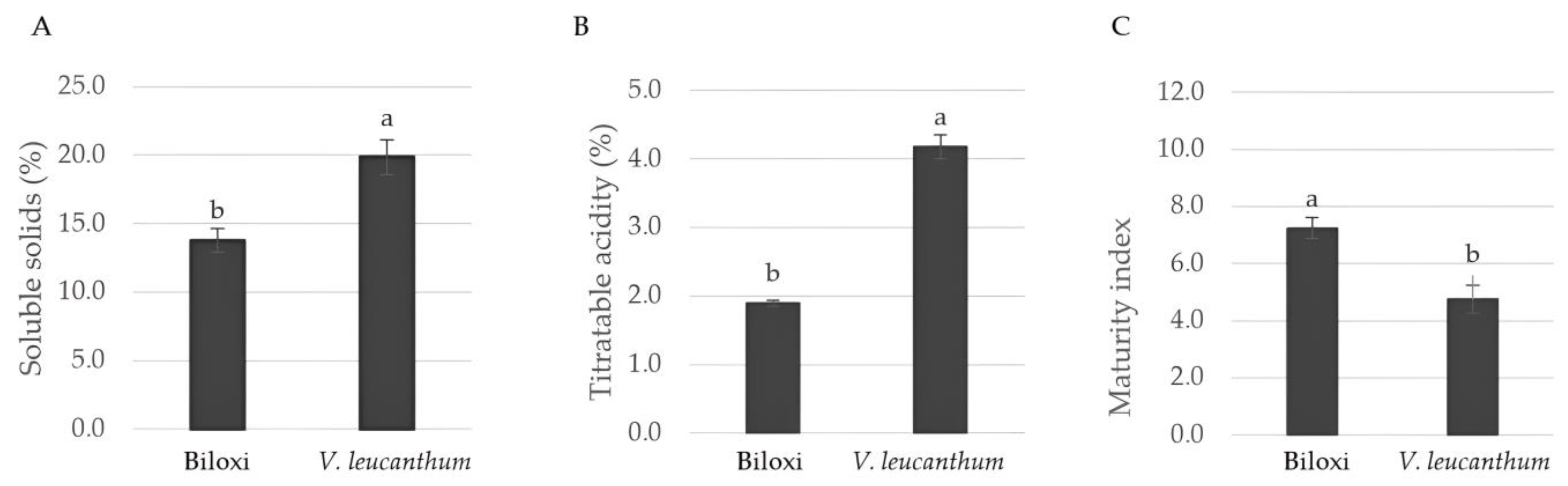
3.1. Quality Attributes
3.2. Total Phenols
3.3. Total Flavonoids
3.4. Total Anthocyanins
3.5. Total Tannins
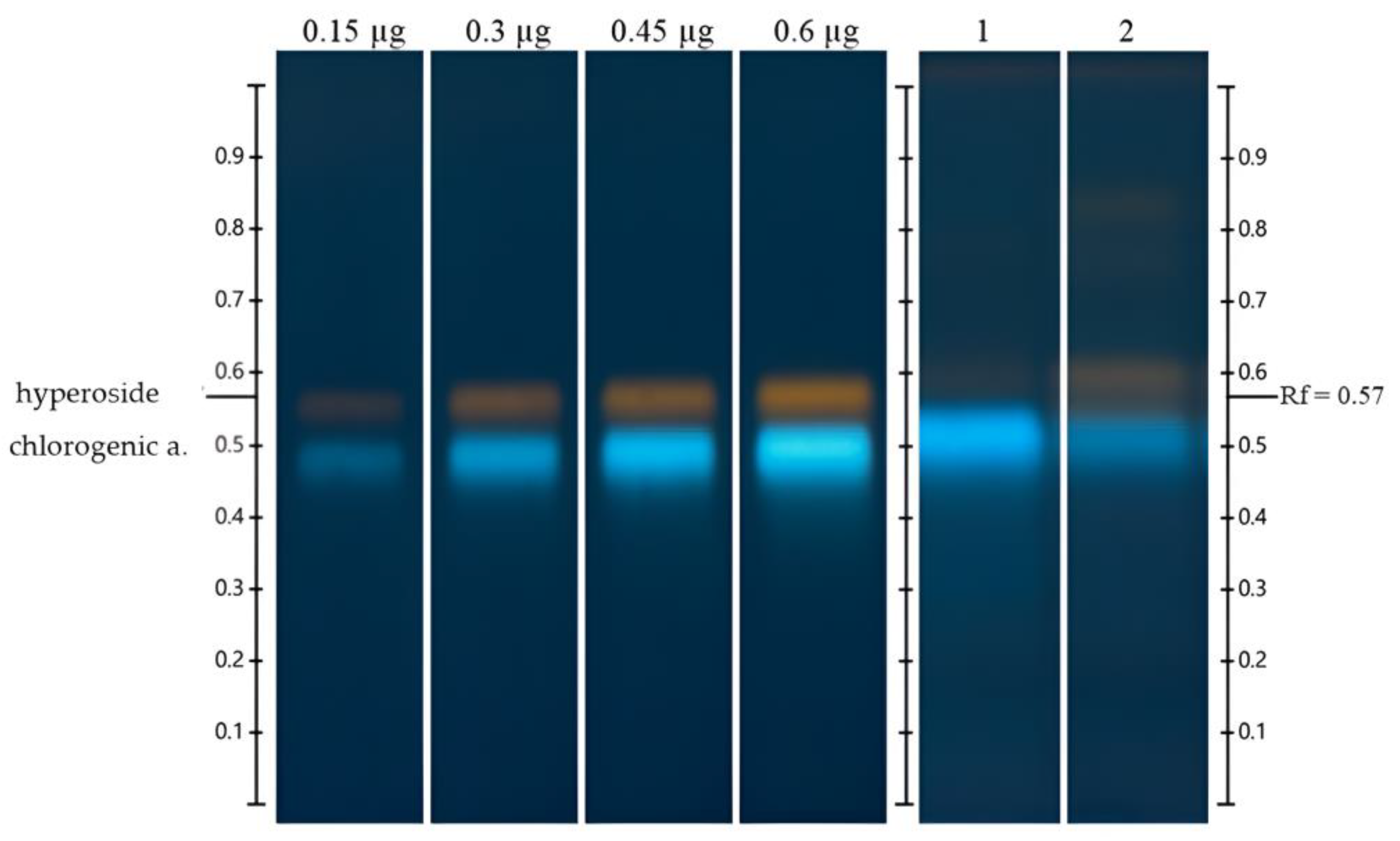
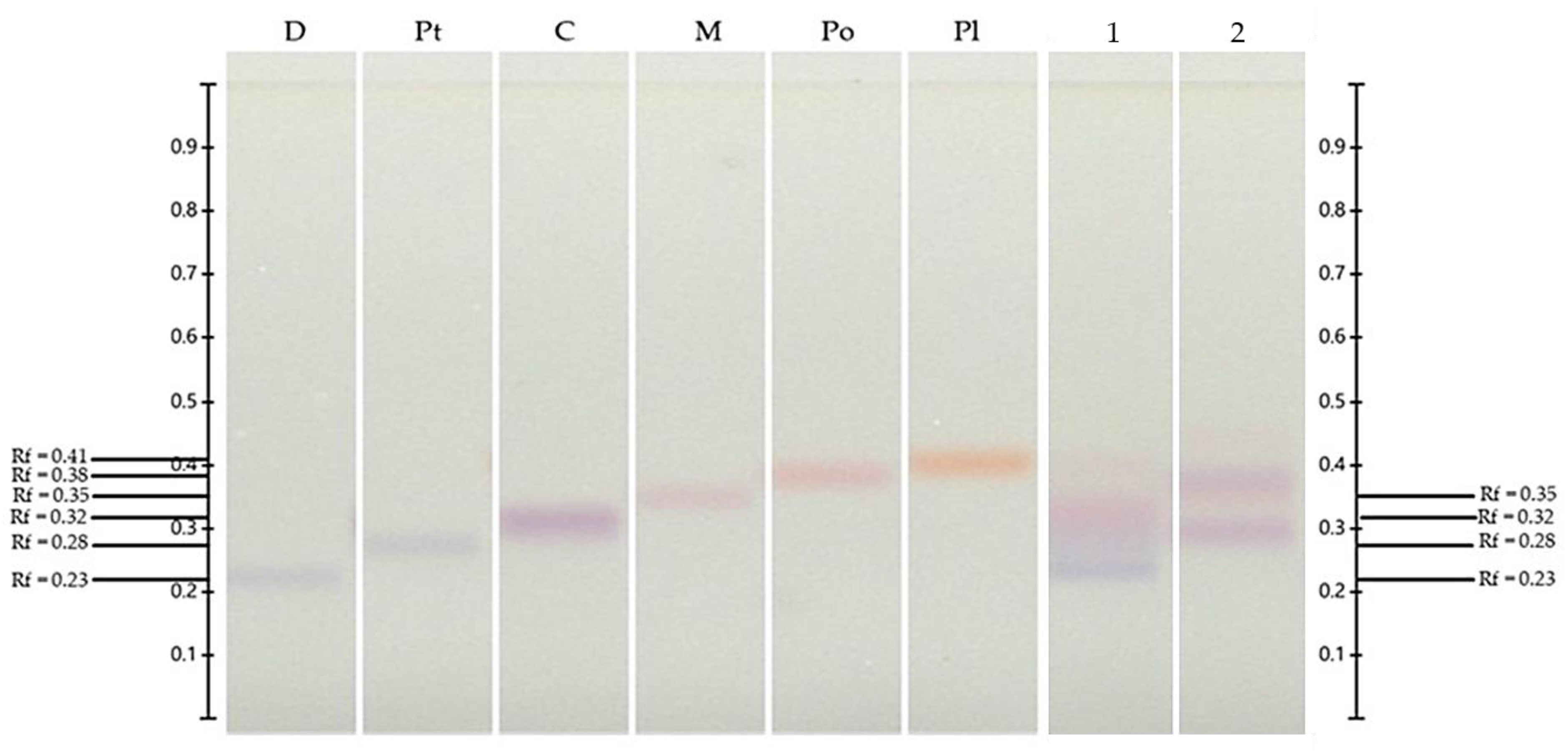
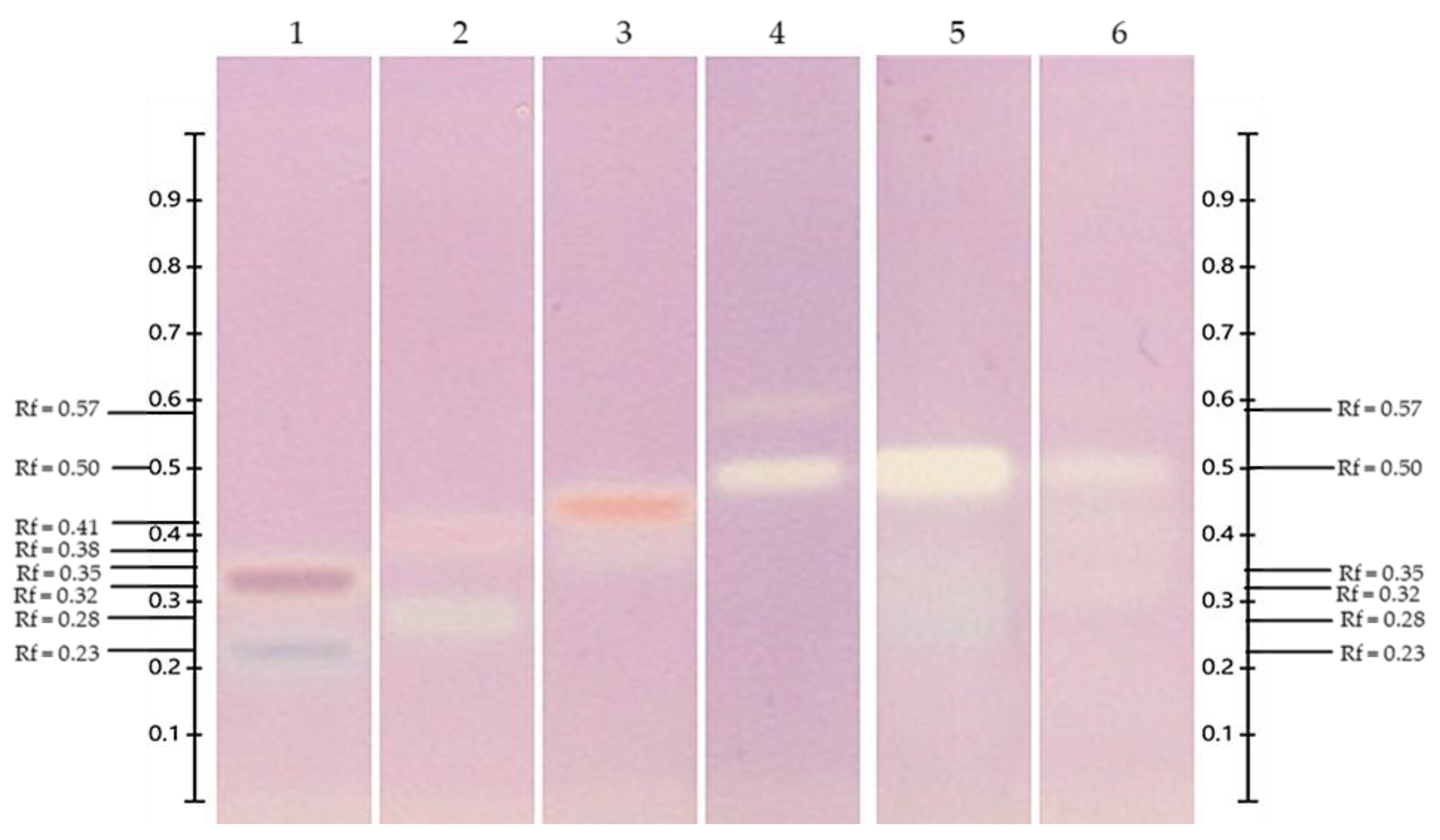
3.6. HPTLC
3.6.1. Detection and Quantification of Chlorogenic Acid and Hyperoside
3.6.2. Detection and Quantification of Anthocyanins
3.7. Antioxidant Activity
3.7.1. DPPH● and ABTS●+ Assay
3.7.2. HPTLC-DPPH● Antioxidant Assay
3.8. MIC and MBC of Methanolic Extracts of Fruits of V. leucanthum Schltdl. and ‘Biloxi’
3.9. Xanthine Oxidase Inhibitory Assay
3.10. Angiotensin I-Converting Enzyme Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albert, N.W.; Iorizzo, M.; Mengist, M.F.; Montanari, S.; Zalapa, J.; Maule, A.; Edger, P.P.; Yocca, A.E.; Platts, A.E.; Pucker, B.; et al. Vaccinium as a Comparative System for Understanding of Complex Flavonoid Accumulation Profiles and Regulation in Fruit. Plant Physiol. 2023, 192, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef] [PubMed]
- FAO. Bases de Datos Estadísticos. 2023. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 20 May 2024).
- SIAP. Base de Datos Estadísticos. México. 2022. Available online: http://infosiap.siap.gob.mx/gobmx/datosAbiertos.php (accessed on 10 May 2024).
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd, ed.; CABI: East Lansing, MI, USA, 2018; Volume 1, ISBN 9781780647272. [Google Scholar]
- Huang, H.; Luo, Y.; Wang, Q.; Zhang, Y.; Li, Z.; He, R.; Chen, X.; Dong, Z. Vaccinium as Potential Therapy for Diabetes and Microvascular Complications. Nutrients 2023, 15, 2031. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive Compounds, Antioxidant Activity, and Biological Effects of European Cranberry (Vaccinium oxycoccos). Molecules 2019, 24, 24. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.J.; Shin, Y. Assessment of Physicochemical Quality, Antioxidant Content and Activity, and Inhibition of Cholinesterase between Unripe and Ripe Blueberry Fruit. Foods 2020, 9, 690. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.C.; Tanase, C.; Popa, M.E. Phenolic Antioxidants in Aerial Parts of Wild Vaccinium Species: Towards Pharmaceutical and Biological Properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health. Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Wang, Y.; Fong, S.K.; Singh, A.P.; Vorsa, N.; Johnson-Cicalese, J. Variation of Anthocyanins, Proanthocyanidins, Flavonols, and Organic Acids in Cultivated and Wild Diploid Blueberry Species. HortScience 2019, 54, 576–585. [Google Scholar] [CrossRef]
- Ştefănescu, B.E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crişan, G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Herniter, I.A.; Kim, Y.; Wang, Y.; Havill, J.S.; Johnson-Cicalese, J.; Muehlbauer, G.J.; Iorizzo, M.; Vorsa, N. Trait Mapping of Phenolic Acids in an Interspecific (Vaccinium corymbosum var. caesariense × V. darrowii) Diploid Blueberry Population. Plants 2023, 12, 1346. [Google Scholar] [CrossRef]
- Hicks, J.M.; Muhammad, A.; Ferrier, J.; Saleem, A.; Cuerrier, A.; Arnason, J.T.; Colson, K.L. Quantification of Chlorogenic Acid and Hyperoside Directly from Crude Blueberry (Vaccinium angustifolium) Leaf Extract by NMR Spectroscopy Analysis: Single-Laboratory Validation. J. AOAC Int. 2012, 95, 1406–1411. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Nasution, S.L.; Suhartina, S.; Munshy, U.Z.; Rizal, R.; Widowati, W. Antioxidant and Anti-Inflammatory Activity of Chlorogenic Acid on Lead-Induced Fibroblast Cells. J. Phys. Conf. Ser. 2019, 1374, 012006. [Google Scholar] [CrossRef]
- Xu, P.; Xu, X.; Fotina, H.; Fotina, T. Anti-Inflammatory Effects of Chlorogenic Acid from Taraxacum Officinale on LTA-Stimulated Bovine Mammary Epithelial Cells via the TLR2/ NF-ΚB Pathway. PLoS ONE 2023, 18, e0282343. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Wang, X.S.; Peng, M.J.; He, C.T. The Antihypertensive Effects of Eucommia Ulmoides Leaf Water/Ethanol Extracts Are Chlorogenic Acid Dependent. J. Funct. Foods 2022, 94, 105129. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Liu, H.; Wu, H.; Wang, Y.; Wang, F.; Liu, X.; Zhou, J. Enhancement on Antioxidant and Antibacterial Activities of Brightwell Blueberry by Extraction and Purification. Appl. Biol. Chem. 2021, 64, 78. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Meirinho, S.; Ayuso-Calles, M.; Roca-Couso, R.; Rivas, R.; Falcão, A.; Alves, G.; Silva, L.R.; Flores-Félix, J.D. Exploring the Antioxidant, Antidiabetic, and Antimicrobial Capacity of Phenolics from Blueberries and Sweet Cherries. Appl. Sci. 2023, 13, 6348. [Google Scholar] [CrossRef]
- Qiao, Y.; Shen, Y.; Jiang, H.; Li, D.; Li, B. Structural Characterization, Antioxidant and Antibacterial Activity of Three Pectin Polysaccharides from Blueberry. Int. J. Biol. Macromol. 2024, 262, 129707. [Google Scholar] [CrossRef] [PubMed]
- Colak, A.M.; Kupe, M.; Bozhuyuk, M.R.; Ercisli, S.; Gundogdu, M. Identifizierung Einiger Fruchtmerkmale von Akzessionen Der Wildheidelbeere (Vaccinium myrtillus L.) Aus Ostanatolien. Gesunde Pflanz. 2018, 70, 31–38. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin Composition of Different Wild and Cultivated Berry Species. LWT 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium myrtillus L.) Growing at Different Locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the Developmental and Environmental Regulation of Secondary Metabolism in Vaccinium Spp. Berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef]
- Correia, S.; Matos, M. Advances in Blueberry (Vaccinium spp.) In Vitro Culture: A Review. Molecules 2024, 10, 533. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, Andecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Bernal-Gallardo, J.O.; Molina-Torres, J.; Angoa-Pérez, V.; Cárdenas-Valdovinos, J.G.; García-Ruíz, I.; Ceja-Díaz, J.A.; Mena-Violante, H.G. Phenolic Compound Content and the Antioxidant and Antimicrobial Activity of Wild Blueberries (Vaccinium stenophyllum Steud.) Fruits Extracts during Ripening. Horticulturae 2022, 8, 15. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, J.; Oh, D.R.; Kim, D.W.; Lee, H.S.; Choi, C. Complete Chloroplast Genome Sequences of Vaccinium bracteatum Thunb., V. vitis-idaea L., and V. uliginosum L. (Ericaceae). Mitochondrial DNA Part B Resour. 2020, 5, 1843–1844. [Google Scholar] [CrossRef]
- Edger, P.P.; Iorizzo, M.; Bassil, N.V.; Benevenuto, J.; Ferrão, L.F.V.; Giongo, L.; Hummer, K.; Lawas, L.M.F.; Leisner, C.P.; Li, C.; et al. There and Back Again; Historical Perspective and Future Directions for Vaccinium Breeding and Research Studies. Hortic. Res. 2022, 9, uhac083. [Google Scholar] [CrossRef] [PubMed]
- CONABIO Catálogo de Autoridades Taxonómicas de Especies de Flora y Fauna Con Distribución En México. Base datos SNIB-CONABIO 2024. Available online: https://www.snib.mx/taxonomia/descarga/ (accessed on 20 May 2024).
- Sánchez-Franco, J.A.; Ayala-Niño, A.; Cariño-Cortés, R.; Hernández-Fuentes, A.D.; Castañeda-Ovando, A.; Campos-Montiel, R.G.; Román-Guerrero, A.; Jiménez-Alvarado, R. Vaccinium leucanthum SCHLECHTENDAHL FRUIT, A NEW SOURCE OF DIETARYFIBER AND ANTIOXIDANT COMPOUNDS. Rev. Mex. Ing. Química 2019, 18, 901–911. [Google Scholar] [CrossRef]
- González-Elizondo, M.S.; González-Elizondo, M. Flora Del Bajío y de Regiones Adyacentes. Fascículo 183, Familia Ericaceae; Instituto de Ecología A.C.: Pátzcuaro, México, 2014. [Google Scholar]
- Santos, R.O.; Trindade, S.C.; Maurer, L.H.; Bersch, A.M.; Sautter, C.K.; Penna, N.G. Physicochemical, Antioxidant and Sensory Quality of Brazilian Blueberry Wine. An. Acad. Bras. Cienc. 2016, 88, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Kryvtsova, M.V.; Salamon, I.; Koscova, J.; Spivak, M.Y. Antibiofilm Forming, Antimicrobial Activity and Some Biochemical Properties of Vaccinium Vitis Idaea Leaf and Berry Extracts on Staphylococcus Aureus. Biosyst. Divers. 2020, 28, 238–242. [Google Scholar] [CrossRef]
- Creţu, G.; Morlock, G.; Miron, A.R.; Nechifor, A.C. A High-Performance Thin-Layer Chromatographic Method for Chlorogenic Acid and Hyperoside Determination from Berry Extracts. Rom. Biotechnol. Lett. 2013, 18, 8657–8665. [Google Scholar]
- Hosu, A.; Cimpoiu, C.; David, L.; Moldovan, B. Study of the Antioxidant Property Variation of Cornelian Cherry Fruits during Storage Using HPTLC and Spectrophotometric Assays. J. Anal. Methods Chem. 2016, 2016, 5. [Google Scholar] [CrossRef]
- UNTEA, A.; LUPU, A.; SARACILA, M.; PANAITE, T. Comparison of ABTS, DPPH, Phosphomolybdenum Assays for Estimating Antioxidant Activity and Phenolic Compounds in Five Different Plant Extracts. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2018, 75, 110. [Google Scholar] [CrossRef]
- Orsini, F.; Vovk, I.; Glavnik, V.; Jug, U.; Corradini, D. HPTLC, HPTLC-MS/MS and HPTLC-DPPH Methods for Analyses of Flavonoids and Their Antioxidant Activity in Cyclanthera Pedata Leaves, Fruits and Dietary Supplement. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 290–301. [Google Scholar] [CrossRef]
- Sun, X.H.; Hao, L.R.; Xie, Q.C.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Wu, V.C.H. Antimicrobial Effects and Membrane Damage Mechanism of Blueberry (Vaccinium corymbosum L.) Extract against Vibrio Parahaemolyticus. Food Control 2020, 111, 107020. [Google Scholar] [CrossRef]
- Wee, S.P.; Loh, K.E.; Lam, K.W.; Ismail, I.S. A Study of the Interaction between Xanthine Oxidase and Its Inhibitors from Chrysanthemum Morifolium Using Computational Simulation and Multispectroscopic Methods. Metabolites 2023, 13, 113. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Ye, R.; Wua, Y.; Xia, W. Comparison of Analytical Methods to Assay Inhibitors of Angiotensin I-Converting Enzyme. Food Chem. 2013, 141, 3329–3334. [Google Scholar] [CrossRef]
- Franco-Tobón, Y.N.; Rojano, B.; Alzate-Arbeláez, A.F.; Restrepo-Florez, C.E.; Rivero-Barrios, D.M.; Maldonado-Celis, M.E. Propiedades Fisicoquímicas Y Antioxidantes De Productos Derivados Del Fruto Agraz. Vitae 2016, 23, 184–193. [Google Scholar] [CrossRef]
- Hera, O.; Sturzeanu, M.; Vîjan, L.E.; Tudor, V.; Teodorescu, R. Biochemical Evaluation of Some Fruit Characteristics of Blueberry Progenies Obtained from ‘Simultan × Duke’. ACS Omega 2023, 8, 18603–18616. [Google Scholar] [CrossRef]
- Stajčić, S.M.; Tepić, A.N.; Djilas, S.M.; Šumić, Z.M.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Vulić, J.J.; Tumbas, V.T. Chemical Composition and Antioxidant Activity of Berry Fruits. Acta Period. Technol. 2012, 43, 93–105. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudonis, R.; Motiekaityte, V.; Vainoriene, R.; Burdulis, D.; Viskelis, J.; Raudone, L. Composition of Sugars in Wild and Cultivated Lingonberries (Vaccinium vitis-idaea L.). Molecules 2019, 24, 4225. [Google Scholar] [CrossRef]
- Gibson, L.; Rupasinghe, H.P.V.; Forney, C.F.; Eaton, L. Characterization of Changes in Polyphenols, Antioxidant Capacity and Physico-Chemical Parameters during Lowbush Blueberry Fruit Ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef]
- Aliman, J.; Michalak, I.; Bušatlić, E.; Aliman, L.; Kulina, M.; Radović, M.; Hasanbegović, J. Study of the Physicochemical Properties of Highbush Blueberry and Wild Bilberry Fruit in Central Bosnia. Turkish J. Agric. For. 2020, 44, 156–168. [Google Scholar] [CrossRef]
- Ross, K.A.; Ehret, D.; Godfrey, D.; Fukumoto, L.; Diarra, M. Characterization of Pilot Scale Processed Canadian Organic Cranberry (Vaccinium macrocarpon) and Blueberry (Vaccinium angustifolium) Juice Pressing Residues and Phenolic-Enriched Extractives. Int. J. Fruit Sci. 2017, 17, 202–232. [Google Scholar] [CrossRef]
- Kalt, W.; Lawand, C.; Ryan, D.A.J.; McDonald, J.E.; Donner, H.; Forney, C.F. Oxygen Radical Absorbing Capacity, Anthocyanin and Phenolic Content of Highbush Blueberries (Vaccinium corymbosum L.) during Ripening and Storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of Antioxidant Capacity and Phenolic Composition of Blueberry, Blackberry, and Strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.A.; Wu, V.C.H. Antimicrobial Effect of Blueberry (Vaccinium corymbosum L.) Extracts against the Growth of Listeria Monocytogenes and Salmonella Enteritidis. Food Control 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Okan, O.T.; Deniz, I.; Yayli, N.; Şat, I.G.; Öz, M.; Serdar, G.H. Antioxidant Activity, Sugar Content and Phenolic Profiling of Blueberries Cultivars: A Comprehensive Comparison. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 639–652. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Savić, Z.; Brala, A.; Levaj, B.; Kovaćević, D.B.; Biško, A. Evaluation of Phenolic Content and Antioxidant Capacity of Blueberry Cultivars (Vaccinium corymbosum L.) Grown in the Northwest Croatia. Food Technol. Biotechnol. 2010, 48, 214–221. [Google Scholar]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative Analysis of Phenolic Content and Profile, Antioxidant Capacity and Anti-Inflammatory Bioactivity in Wild Alaskan and Commercial Vaccinium Berries Plants for Human Health Institute, Food Bioprocessing and Nutrition Sciences Department. Agric. food Chem. 2013, 62, 4007–4017. [Google Scholar] [CrossRef]
- Narváez, S.C.; Carrión, M.H. Evaluation of Antioxidant Activity, Phenolic Content, Anthocyanins, and Flavonoids of Fresh and Dried ‘Biloxi’ Blueberries. Vitae 2022, 29, 1–9. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry Anthocyanins: An Updated Review on Approaches to Enhancing Their Bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and Function of Blueberry Anthocyanins: A Review of Recent Advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, H.; Huang, Z.; Zhang, C.; Lyu, L.; Li, W.; Wu, W. Metabolite Profiling and Classification of Highbush Blueberry Leaves under Different Shade Treatments. Metabolites 2022, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, Y.; Li, B.; Tan, H.; Li, D.; Li, L.; Liu, X.; Han, J.; Meng, X. Comparative Transcriptome Analysis of Genes Involved in Anthocyanin Synthesis in Blueberry. Plant Physiol. Biochem. 2018, 127, 561–572. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Lingonberry (Vaccinium vitis-idaea L.) Grown in the Pacific Northwest of North America: Anthocyanin and Free Amino Acid Composition. J. Funct. Foods 2012, 4, 213–218. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European Cranberry (Vaccinium microcarpon) Proanthocyanidins: Isolation, Identification, and Bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef]
- Suvanto, J.; Karppinen, K.; Riihinen, K.; Jaakola, L.; Salminen, J.P. Changes in the Proanthocyanidin Composition and Related Gene Expression in Bilberry (Vaccinium myrtillus L.) Tissues. J. Agric. Food Chem. 2020, 68, 7378–7386. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Soviguidi, D.R.J.; Pan, R.; Liu, Y.; Rao, L.; Zhang, W.; Yang, X. Chlorogenic Acid Metabolism: The Evolution and Roles in Plant Response to Abiotic Stress. Phyton-International J. Exp. Bot. 2022, 91, 239–255. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef] [PubMed]
- Prada-Muñoz, J.; Coy-Barrera, E. Targeted Anthocyanin Profiling of Fruits from Three Southern Highbush Blueberry Cultivars Propagated in Colombia. Molecules 2024, 29, 691. [Google Scholar] [CrossRef] [PubMed]
- González-Cruz, E.M.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Ragazzo-Sánchez, J.A. Evaluation of Biopolymers in the Encapsulation by Electrospraying of Polyphenolic Compounds Extracted from Blueberry (Vaccinium corymbosum L.) Variety Biloxi. Polym. Bull. 2021, 78, 3561–3576. [Google Scholar] [CrossRef]
- Müller, D.; Schantz, M.; Richling, E. High Performance Liquid Chromatography Analysis of Anthocyanins in Bilberries (Vaccinium myrtillus L.), Blueberries (Vaccinium corymbosum L.), and Corresponding Juices. J. Food Sci. 2012, 77, C340–C345. [Google Scholar] [CrossRef]
- Su, Z. Anthocyanins and Flavonoids of Vaccinium L. Pharm. Crops 2012, 3, 7–37. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Cardeñosa, V.; Girones-Vilaplana, A.; Muriel, J.L.; Moreno, D.A.; Moreno-Rojas, J.M. Influence of Genotype, Cultivation System and Irrigation Regime on Antioxidant Capacity and Selected Phenolics of Blueberries (Vaccinium corymbosum L.). Food Chem. 2016, 202, 276–283. [Google Scholar] [CrossRef]
- Namiesnik, J.; Vearasilp, K.; Kupska, M. Antioxidant Activities and Bioactive Components in Some Berries. Eur. Food Res. Technol. 2013, 237, 819–829. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are Antioxidants Helpful for Disease Prevention? Res. Pharm. Sci. 2010, 5, 5–12. [Google Scholar]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Cano, A.; Maestre, A.B.; Hernández-Ruiz, J.; Arnao, M.B. ABTS/TAC Methodology: Main Milestones and Recent Applications. Processes 2023, 11, 185. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic Profile and Antioxidant Activity of Highbush Blueberry (Vaccinium corymbosum L.) during Fruit Maturation and Ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Salinas, C.G.; Estrada, B.C.; Vidal Martínez, V.A. Variabilidad En Contenido y Tipos de Antocianinas En Granos de Color Azul/Morado de Poblaciones Mexicanas de Maíz. Rev. Fitotec. Mex. 2013, 36, 285–294. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Radovanović, B.C.; Andelković, A.S.M.; Radovanović, A.B.; Andelković, M.Z. Antioxidant and Antimicrobial Activity of Polyphenol Extracts from Wild Berry Fruits Grown in Southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 813–819. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Carmen Garcia-Parrilla, M.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef]
- Silva, A.C.O.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.A.; Pisciottano, M.N.C.; Amorim, E.L.C.; Albuquerque, U.P. Which Approach Is More Effective in the Selection of Plants with Antimicrobial Activity? Evid. -Based Complement. Altern. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef]
- Kim, S.H.; Adeyemi, D.E.; Park, M.K. Characterization of a New and Efficient Polyvalent Phage Infecting e. Coli O157:H7, Salmonella Spp., and Shigella Sonnei. Microorganisms 2021, 9, 2105. [Google Scholar] [CrossRef]
- Yang, C.; Xiang, Y.; Qiu, S. Resistance in Enteric Shigella and Nontyphoidal Salmonella: Emerging Concepts. Curr. Opin. Infect. Dis. 2023, 36, 360–365. [Google Scholar] [CrossRef]
- Salamon, I.; Şimşek Sezer, E.N.; Kryvtsova, M.; Labun, P. Antiproliferative and Antimicrobial Activity of Anthocyanins from Berry Fruits after Their Isolation and Freeze-Drying. Appl. Sci. 2021, 11, 2096. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The Effect of Chlorogenic Acid on Bacillus Subtilis Based on Metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Yen Nguyen, T.K.; Kase, E.T.; Tadesse, M.; Barsett, H.; Wangensteen, H. Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts. Molecules 2017, 22, 1806. [Google Scholar] [CrossRef]
- Kostić, D.A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Dordević, A.S.; Ickovski, J.D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef]
- Hille, R. Xanthine Oxidase—A Personal History. Molecules 2023, 28, 1921. [Google Scholar] [CrossRef]
- Li, X.; Jin, W.; Zhang, W.; Zheng, G. The Inhibitory Kinetics and Mechanism of Quercetin-3-O-Rhamnoside and Chlorogenic Acid Derived from Smilax China L. EtOAc Fraction on Xanthine Oxidase. Int. J. Biol. Macromol. 2022, 213, 447–455. [Google Scholar] [CrossRef]
- Xue, H.; Xu, M.; Gong, D.; Zhang, G. Mechanism of Flavonoids Inhibiting Xanthine Oxidase and Alleviating Hyperuricemia from Structure–Activity Relationship and Animal Experiments: A Review. Food Front. 2023, 4, 1643–1665. [Google Scholar] [CrossRef]
- Bieber, E.D.; Edelsohn, G.A.; McGee, M.E.; Shekunov, J.; Romanowicz, M.; Vande Voort, J.L.; McKean, A.J.S. The Role of Parental Capacity for Medical Decision-Making in Medical Ethics and the Care of Psychiatrically Ill Youth: Case Report. Front. Psychiatry 2020, 11, 559263. [Google Scholar] [CrossRef]
- Sarkar, D.; Agustinah, W.; Woods, F.; Coneva, E.; Vinson, E.; Shetty, K. In Vitro Screening and Evaluation of Phenolic Antioxidant-Linked Anti-Hyperglycemic Functions of Rabbit-Eye Blueberry (Vaccinium ashei) Cultivars. J. Berry Res. 2017, 7, 163–177. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Fu, L.; Li, C.-Y.; Xu, L.-P.; Zhang, L.-X.; Zhang, W.-M. Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
| Standard | Expression | Calibration Curve |
|---|---|---|
| chlorogenic acid | milligrams chlorogenic acid equivalent per gram of dry weight (mg CAE/g DW) | y = 0.9723x − 0.0823, R2 = 0.9821 |
| hyperoside | milligrams of hyperoside equivalent per gram of dry weight (mg HE/g DW) | y = 0.239x + 0.0182, R2 = 0.9878 |
| cyanidin-3-glucoside | milligrams cyanidin-3-glucoside equivalent per gram of dry weight (mg CGE/g DW) | y = 0.9138x + 0.0203, R2 = 0.9742 |
| petunidin-3-glucoside | milligrams of petunidin-3-glucoside equivalent per gram of dry weight (mg PGE/g DW) | y = 0.7244x − 0.0112, R2 = 0.9896 |
| malvidin-3-glucoside | milligrams of malvidin-3-glucoside equivalent per gram of dry weight (mg MGE/g DW) | y = 0.3203x + 0.0172, R2 = 0.9955 |
| delphinidin-3-glucoside | milligrams of delphinidin-3-glucoside equivalents per gram of dry weight (mg DGE/g DW) | y = 0.9331x + 0.0002, R2 = 0.9804 |
| Extracts | Total Phenols (mg GAE/g DW) | Total Flavonoids (mg QE/g DW) | Total Anthocyanins (mg CGE/g DW) | Total Tannins (mg TAE/g DW) |
|---|---|---|---|---|
| ‘Biloxi’ | 19.23 ± 0.08 a | 3.12 ± 0.15 b | 0.221 ± 0.016 b | 9.32 ± 0.12 a |
| V. leucanthum | 16.75 ± 0.09 b | 4.85 ± 0.34 a | 0.303 ± 0.008 a | 8.81 ± 0.53 a |
| Extracts | Chlorogenic Acid (mg CAE/g DW) | Hyperoside (mg HE/g DW) |
|---|---|---|
| ‘Biloxi’ | 16.87 ± 0.33 a | 3.52 ± 0.36 b |
| V. leucanthum | 7.25 ± 0.11 b | 5.14 ± 0.10 a |
| Extracts | mg MGE/g DW | mg PGE/g DW | mg DGE/g DW | mg CGE/g DW |
|---|---|---|---|---|
| ‘Biloxi’ | n.d. | n.d. | 6.06 ± 0.23 | 3.47 ± 0.30 |
| V. leucanthum | 11.80 ± 0.10 | 6.92 ± 0.12 | n.d. | n.d. |
| Extracts | ABTS●+ (µM TE/g DW) | DPPH● (µM TE/g DW) |
|---|---|---|
| ‘Biloxi’ | 259.93 ± 15.2 a | 214.08 ± 15.9 a |
| V. leucanthum | 127.92 ± 4.9 b | 121.83 ± 5.9 b |
| Extracts | E. coli ATCC 12792 | S. flexneri ATCC 10708 | S. choleraesuis ATCC 12022 | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| ‘Biloxi’ | 8.89 | 17.78 | 8.89 | 17.78 | 8.89 | 17.78 |
| V. leucanthum | 8.89 | 17.78 | 8.89 | 17.78 | 8.89 | 17.78 |
| Ceftriaxone | 0.015 | 0.031 | 0.015 | 0.031 | 0.015 | 0.031 |
| Extracts | Inhibition (%) |
|---|---|
| ‘Biloxi’ | 67.66 ± 1.194 a |
| V. leucanthum | 56.97 ± 0.782 b |
| Extracts | Inhibition (%) |
|---|---|
| ‘Biloxi’ | 43.03 ± 4.0 a |
| V. leucanthum | 42.28 ± 2.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Gallardo, J.O.; Mena-Violante, H.G.; Luna-Suárez, S. Study of the Phenolic Compounds and Biological Activities of the Wild Fruits of Vaccinium leucanthum Schltdl. Horticulturae 2024, 10, 1091. https://doi.org/10.3390/horticulturae10101091
Bernal-Gallardo JO, Mena-Violante HG, Luna-Suárez S. Study of the Phenolic Compounds and Biological Activities of the Wild Fruits of Vaccinium leucanthum Schltdl. Horticulturae. 2024; 10(10):1091. https://doi.org/10.3390/horticulturae10101091
Chicago/Turabian StyleBernal-Gallardo, José Osvaldo, Hortencia Gabriela Mena-Violante, and Silvia Luna-Suárez. 2024. "Study of the Phenolic Compounds and Biological Activities of the Wild Fruits of Vaccinium leucanthum Schltdl." Horticulturae 10, no. 10: 1091. https://doi.org/10.3390/horticulturae10101091
APA StyleBernal-Gallardo, J. O., Mena-Violante, H. G., & Luna-Suárez, S. (2024). Study of the Phenolic Compounds and Biological Activities of the Wild Fruits of Vaccinium leucanthum Schltdl. Horticulturae, 10(10), 1091. https://doi.org/10.3390/horticulturae10101091






