Abstract
Litchi (Litchi chinensis Sonn.) is considered one of the most important sub-tropical fruits of the world. In the western part of Odisha, India, litchi growers are facing problems of unstable and lower marketable yield and inferior quality due to a higher incidence of fruit cracking, fruit drop, low sugar content, and higher fruit acidity. Keeping in mind the positive effects of nutrients and bioregulators, the current study was conducted to elucidate their impact on fruit yield and quality in the farmers’ field of Jamankira block in Sambalpur district of Odisha, which is under the care of Odisha University of Agriculture and Technology, India. For this study, eight-year-old litchi trees were selected. With 12 treatments, the experiment was set up in a Randomized Block Design replicated thrice, as follows: T1: spray treatment with Borax—0.5%; T2: spray treatment with Borax—0.3%; T3: spray treatment with ZnSO4—0.75%; T4: spray treatment with ZnSO4—0.5%; T5: spray treatment with CaCl2—0.5%; T6: spray treatment with CaCl2—0.1%; T7: spray treatment with humic acid—1.5%; T8: spray treatment with humic acid—1%; T9: spray treatment with seaweed extract—0.5%; T10: spray treatment with seaweed extract—0.1%; T11: foliar spray with NAA—20 ppm; and T12: control (Water Spray). The current study compared foliar feeding treatments comprising different nutrient and bioregulators, which were applied during the first week of December, just after the completed formation of new leaves and the untreated control. The highest total number of fruits per plant was recorded in plants sprayed with 0.5% ZnSO4 (T4) followed by those treated with 1% humic acid (T8). The highest total fruit yield was recorded in plants subjected to foliar feeding with 0.3% Borax (T2) which was found to be statistically similar to plants treated with 0.1% seaweed extract (T10) and 0.5% seaweed extract (T9). Among the treatments, a better response, i.e., a higher number of marketable fruits and marketable yield, was recorded in litchi plants treated with 0.3% Borax (T2) followed by 0.5% zinc sulphate (T4), 1% humic acid (T8), and 0.1% CaCl2 (T6). The application of 1% humic acid (T8) followed by 1.5% humic acid (T9) enhanced fruit setting (%) and fruit retention rates (%) and reduced the fruit drop rate (%). The enhanced fruit size (fruit length and fruit width) and higher fruit weight was obtained in litchi plants treated with 0.3% Borax. The foliar application of 0.3% Borax (T2) also resulted in a higher TSS, total sugars, reducing sugar content, lower acidity, the highest aril weight, and lower seed weight in litchi cv. Bombai. In this research, among the five principal components, only PC1 demonstrated approximately 45.14% variability within the influential axes. PC1 contributed the highest proportion (48.9%) to the overall variability, followed by PC2 with 29.1%, PC3 with 11.9%, PC4 with 0.59%, and PC5 with 0.20%. Consequently, the outcomes of the principal component analysis indicate the presence of extensive variability among treatments.
1. Introduction
Litchi is a member of the subfamily Nepheleae of the family Sapindaceae, which comprises 2000 species and 150 genera [1], and is native to Southern China. It is regarded as the queen of fruits because of its superb quality, juicy fruit, superb balance of sugar and acid, unique and pleasant flavor, appealing color, and high nutrient content [2]. Its delightful flavor, superb taste, nice aroma, appealing look, and high nutritional content have made it popular throughout the world, creating new opportunities for faster export growth [3]. Litchi fruits primarily consist of organic acids, carbohydrates, protein, fat, pigments, vitamins, and organic acids [4,5,6]. This fruit contains 83.6 g of moisture, 0.7 g of protein, 0.1 g of fat, 15.0 g of carbohydrates, 4.0 mg of calcium, 32.0 mg of phosphorus, 0.7 mg of iron, 0.02 mg of thiamine, 0.07 mg of riboflavin, 1.1 mg of niacin, and 15.0 mg of ascorbic acid [4]. The fruit is highly appreciated as a table fruit as well as when consumed both in dried and canned forms. Jam, jelly, squash, and cordial can also be prepared from this fruit. In general, the reddening of the epicarp and the flattening of the tubercles are regarded as the signs of fruit maturity [7].
The yield and quality of plants are enhanced by the foliar application of nutrients and bioregulators during key developmental and critical stages [8,9]. The application of nutrients and plant growth regulators has been proven to have a beneficial impact on litchi fruit yield and quality [9]. Zinc and boron deficiencies result in decreased flower induction and fruit setting, which diminishes fruit quality [10]. Calcium primarily affects fruit quality through the synthesis of calcium pectate, which is linked to an increase in the strength of the middle lamella and cell wall [11]. When micronutrients are given in the right quantities, plants develop more quickly, which improves flowering and increases fruit setting, both of which increase yield [12]. Zinc and boron play an important role in a number of enzymatic processes and aid in the buildup of sugar in fruit obtained from source organs to sinks [13]. Since the foliar application of micronutrients is an established means of completing as well as improving plant nutrition, it is a frequently used method [12,13]. When a plant is subjected to stress or unfavorable soil conditions, the absorption of nutrients from the soil by the plant is restricted. Under this circumstance, a foliar spray can be used to meet nutritional needs of the plant [8]. Plant bioregulators encourage increased fruit retention, flowering, and fruiting. Boron is unique among the micronutrients in that it is linked to the reproductive system and carbohydrate chemistry of plants, in addition to being directly related to photosynthesis and enzyme performance.
Bioregulators are naturally occurring organic products that encourage plants to reach their full growth and yield potentials [7]. Humic acid is extremely useful in releasing nutrients from the soil, making them available to the plant when needed. Humic acids have multiple significant functions, including improving the physical and biochemical activities of soil by enhancing its structure, texture, water-holding capacity, and microbial population [14]. They also increase the availability of soil nutrients, particularly micronutrients, by chelating and co-transporting them to plants [15].
Seaweeds have been shown to be beneficial when employed as biofertilizers not only because they have a biological impact but also owing to their biocompatibility, contributing biological molecules that are common with plants. Seaweeds are multicellular, macroscopic organisms that inhabit coastal and marine environments. They are abundant in polysaccharides, polyunsaturated fatty acids, and enzymes [16,17]. Seaweed extracts are considered bio-stimulants because they include a variety of macronutrients (Ca, P, and K), micronutrients (Fe, Cu, Zn, B, Mn, Co, and Mo), and growth regulators (cytokinin, auxins, and gibberellins) that are essential for plant growth and development [18,19]. The most common method for boosting yields of a number of commercial crops is by the foliar spraying of seaweed extract [19]. Seaweed extracts have been found to contain a variety of growth regulators, including gibberellins, auxins, and cytokinins [20]. Because seaweed extracts can promote the rapid development and yield of horticultural plants like vegetable, fruit, and cereals, they have recently acquired a great deal of attention [21]. Despite its distinct and appealing qualities, poor fruit setting [22], excessive fruit drop [9,23], fruit cracking [23], and poor fruit quality [24] are the main obstacles in litchi production, which result in poor yield and profitability. Fruit drop in litchi has been linked to endogenous hormonal disruption [18,25], internal factors such as high temperatures, low humidity and strong winds [26], as well as, embryo abortion, internal nutrition, and hormonal imbalance [22,23,27]. Fruit cracking in growing fruits is a global issue in the cultivation of litchi. Because of the aforementioned limitations, there is a huge discrepancy between the supply and demand for litchi, raising its price on the market. Many nutrients and bioregulators are applied in addition to the recommended irrigation practices in order to reduce this gap. Many research investigations have documented the various advantages of seaweed extracts on the growth, development and higher yield of many crops [28,29]. Growers are using nutrients and bioregulators to improve yield-related attributes in litchi, thereby boosting the yield. It is established that nutrients increase both the quantity and quality of litchi fruit, but the beneficial effects of combining nutrients with bioregulators have not yet been thoroughly investigated. After China, India is the world’s second-largest producer of litchi. It is mostly cultivated in Eastern Indian states like Bihar, West Bengal, Odisha, etc. During 2022–2023, according to the data released from the Directorate of Horticulture, Krushi Bhawan, Bhubaneswar, the total area of land used for litchi cultivation in Odisha was 4380 ha with a production of 24,220 metric tonnes of fruit. Of this, more than half of the total area of land used for litchi cultivation is found in the western region of Odisha, which includes districts like Sundergarh, Sambalpur, Angual, Deogarh, Kandhmal, and Kalahandi. Due to lower fruit setting, fruit drop, and sugar content, and increased fruit cracking, acidity, and other factors, the growers of litchi in the western region of Odisha, India, have been facing problems with the inconsistent of the yield, the marketable of the fruit, and their inferior quality. Due to the abovementioned constraints, the actual productivity realized by the farmers of the western part of Odisha (6.6 t/ha) is comparatively less than that of major litchi-producing states in India like Bihar (8.0 t/ha) and West Bengal (10.5 t/ha). The other factors that may contribute towards the low yield of poor-quality litchi fruits include the low organic matter content in the soil, low soil pH, and improper management techniques employed by the farmers. However, according to many researchers, using nutrients and bioregulators like humic acids and seaweed extracts improved the quantity and quality of litchi.
A set of possibly correlated variables can be reduced to a smaller set of uncorrelated variables known as principal components using a mathematical technique called principal component analysis (PCA). This statistical technique is important for plant breeding programs because it makes it possible to identify important polygenic features. By lowering the dimensionality of a complicated dataset, PCA provides a way to simplify it and reveal hidden structures inside it. The degree of trait variation explained by a particular main component is indicated by the eigenvalue linked to that component. The breeding program’s later stages can be effectively guided by this knowledge. Studies on the impact of nutrients and bioregulators on litchi have not yet been carried out in western part of Odisha in India. Hence, an experiment was carried out in this region based on this background to evaluate the impact of nutrients and bioregulators on the yield and fruit quality of Litchi cv. Bombai.
2. Materials and Methods
2.1. Experimental Material
Eight-year-old Litchi trees (Litchi chinensis Sonn.) cv. Bombai were used as experimental material.The plants were produced by layering and planted in July 2012. The plants were planted in a square system with a spacing of 8 m × 8 m.
2.2. Experimental Site & Design
The present investigation was conducted in a farmer’s field at Jamankira block (21°54′ N latitude, 84°39′ longitude, and altitude 171 m above the mean sea level), Sambalpur district, Odisha, India, during the years of 2020–2022 in a Randomized Block Design (RBD) with 12 treatments (Table 1) replicated thrice.

Table 1.
Treatment details.
In the first week of December, just after the completed production of new leaves, the nutrients and bioregulators were applied. The climate of the experimental site was warm and semi-dry, with hot, dry summers and mild winters. The meteorological information gathered between 2020 and 2022 at the Regional Research and Technology Transfer Station (RRTTS), Chiplima, during the meteorological observational period is shown in Figure 1, Figure 2, Figure 3 and Figure 4.
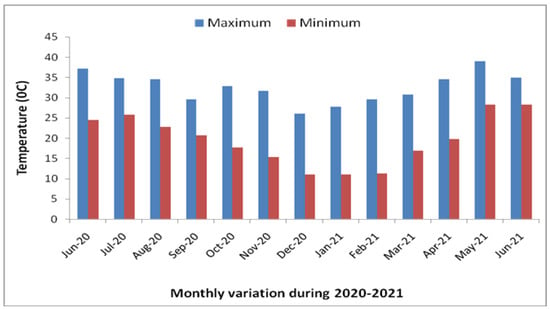
Figure 1.
Monthly variation in temperature during 2020–2021.
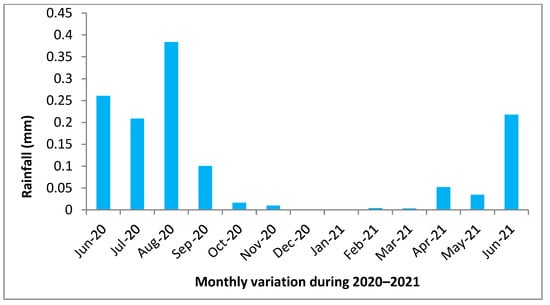
Figure 2.
Monthly variation in rainfall (mm) during 2020–2021.
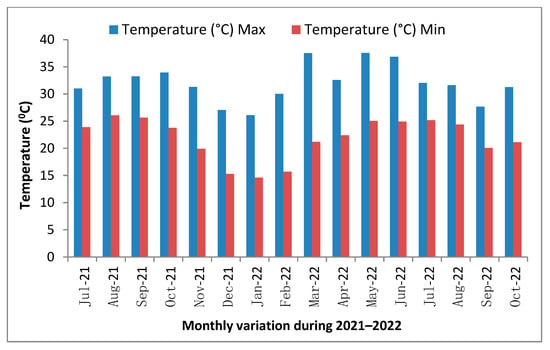
Figure 3.
Monthly variation in temperature during 2021–2022.
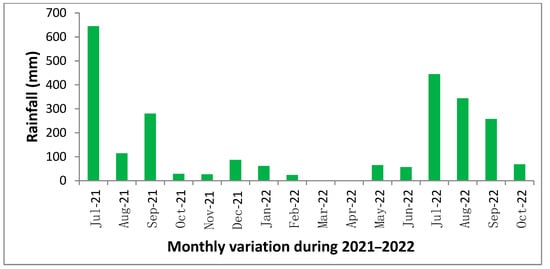
Figure 4.
Monthly variation in rainfall (mm) during 2021–2022.
2.3. Trait Measurement
The fruits were harvested when their color turned red. They were analyzed for yield parameters, which included total number of fruits, total number of marketable fruits, total yield, and total marketable yield. Physico-chemical parameters included fruit weight, aril weight, seed weight, pericarp weight, fruit length and width, and nut length and width, while biochemical parameters included total sugars, titratable acidity, total sugars, reducing sugar content, non-reducing sugar content, TSS/acid ratio, and ascorbic acid content.
2.3.1. Determination of Fruit and Aril Weight
The average fruit weight, aril weight, seed weight, and pericarp weight of 10 samples from each replication were analyzed by using an electronic weighing balance, and their average weight was determined and expressed in grams (g).
2.3.2. Determination of Fruit and Nut Size
The length and breadth of fruit and nut were measured by using a digital slide caliper, and the mean was expressed in centimeter (cm).
2.3.3. Biochemical Parameters
Determination of Total Soluble Solids (0Brix)
TSS is generally measured using digital refractometer (0–320Brix). TSS stands for total soluble solids which include sugar, vitamins, amino acids, acids, and other soluble solids present in the juice. To measure the TSS of litchi fruit, two to three drops of juice were placed on the prism of the refractometer, and observations were recorded. Before and after taking the reading, the refractometer was properly washed. The reading of refractometer is expressed as a percentage or in 0Brix.
Determination of Titratable Acidity (%)
A known volume of aqueous extract was titrated against an alkali solution with known normality to determine the total acidity of the fruit. It is measured against an equivalent volume of any organic acid, such as citric or malic acid [30]. The titratable acidity is calculated in terms of malic acid, as shown in Equation (1), and expressed in percentage [31].
where, TA = Total titratable acidity (%); T = Titrated value; N = Normality of NAOH; V = Volume made up; WE = Equivalent weight of acidity (66); VA = Volume of aliquote taken; Vs = Volume of sample taken
Total acidity (%) = TA = [(T × N × V × WE)/(VA × Vs × 1000)] × 100
Determination of Total Sugar (%)
By utilizing Fehling’s A and Fehling’s B solutions and the method outlined by [32], the total sugar content of the fruit aril was calculated. It was titrated against the sample in a burette until a bright red color appeared, which indicates the endpoint. The total sugar content was calculated using the following formula shown in Equation (2):
Total Sugar (%) = [(Dilution factor (0.05) × Dilution (100))/(Titrate value × volume of sample taken)] × 100
Determination of Reducing Sugar Content (%)
The method described by [33] was used to calculate the reducing sugar content, which was computed using Equation (3):
Reducing Sugar (%) = [(Dilution factor (0.05) × Dilution (100))/(Titrate value × volume of sample taken)] × 100
Determination of Non-Reducing Sugar Content (%)
By subtracting the reducing sugar content from the total carbohydrates and multiplying the outcome by 0.95, the amount of non-reducing sugar was determined.
Determination of TSS/Acid Ratio
By dividing the TSS value by the titratable acidity, the TSS/Acid ratio was calculated computationally, and the outcome of that calculation was expressed as the TSS/acid ratio.
TSS/acid ratio = TSS/titratable acidity
Determination of Ascorbic Acid Content (mg/100 g)
The 2,6-dichlorophenol indophenol visual titration method described by [32] was used to determine the ascorbic content of the litchi fruit. This was expressed in terms of mg ascorbic acid per 100 g of fruit aril and was calculated by using following formula shown in Equation (4).
Ascorbic acid (mg/100g) = (titrate reading × dye factor × dilution × 100)/(Aliquat of extract × weight of sample taken)
2.3.4. Statistical Analysis
The data were analyzed according to the method of analysis suggested by [33] for a Randomized Block Design. The significant variation among the treatments was observed by applying the “F” test and Critical Difference (CD) at a 5% level of significance which was calculated to compare the mean values of the treatments for all the characters. Duncan’s Multiple Range (DMR) test was performed to compare the treatment means.
2.3.5. Principal Component Analysis (PCA)
The principal component analysis decreases the dimensions of multivariate data to a few principal axes, generates an eigenvector for each axis, and produces component scores for the characteristics [34,35]. A PCA analysis is employed to condense the dimensions of multivariate data into a small set of principal axes, each associated with an eigenvector. Component scores are then generated for each individual characteristics. In this study, a graph was plotted based on PC1 and PC2. The statistical analysis and graphical representations were conducted using Minitab 19, a statistical software package.
3. Results
3.1. Fruit Setting
The results regarding the percentage of fruit setting in litchi influenced by different nutrients and bioregulators applications are given in Table 2. The obtained data revealed that the highest fruit setting rate (59.76%) was recorded with T7, i.e.,1.5%-humic-acid-treated trees which was statistically similar to that of plants treated with 1% humic acid, i.e., T8, (58.17%) while the minimum fruit setting rate (46.49%) was observed in T12 (control) which was found to be statistically inferior compared to the rest of treatments and was followed by T9 (53.53%), T2 (53.11%), and T1 (53.07%).

Table 2.
Influence of foliar feeding with nutrients and bioregulators on fruit setting, fruit drop, fruit retention, and fruit cracking percentage in Litchi cv. Bombai.
3.2. Fruit Drop
It is evident from the pooled data presented in Table 2 and Table S10, that the fruit drop rate varied from 65.94 to 80.91 percent. The minimum fruit drop (65.94%) was recorded in plants in the T8 treatment (humic acid—1%) which was followed by the statistically similar T7 treatment (70.19%), while the maximum fruit drop rate (80.91%) was recorded in the T12 treatment (control) which was followed by the statistically similar T3 (79.59%) and T4 (80.01%) treatments. The pooled data further indicated that the mean value of all the concentrations of humic acid had the lowest value (65.94% in T8 and 70.19% in T7) followed by the seaweed extracts (73.72% in T9 and 74.18% in T10).
3.3. Fruit Retention
The T8 treatment group (humic acid—1%) showed the highest fruit retention rate (34.07%) and was statistically superior to all other treatments; it was followed (29.81%) byT7 (humic acid—1.5%). The control plants (T12) had the lowest fruit retention rate (19.10%), which was found to be statistically different and less than that of the other treatments (Table 2 and Table S10). When compared to the control, all other treatments significantly improved fruit retention in litchi.
3.4. Fruit Cracking
From the combined data shown in Table 2 and Table S10, it becomes apparent that foliar feeding with different nutrients and bioregulators significantly reduced the fruit cracking percentage in litchi cv. Bombai, and that the values ranged from 8.46% in T2 to 30.36% in the control (T12). The pooled data clearly show that the T2 treatment (Borax—0.3%) produced the lowest percentage of fruit cracking (8.46%), which was found to be statistically equivalent to T1 (8.73%), and that all other treatments recorded more cracking than T2, while the highest fruit cracking rate (30.36%) was recorded in the untreated control trees (T12).
3.5. Total Number of Fruits per Plant
The number of fruits per plant varied significantly due to different treatments, as shown in Table 3 and Figure 5. The highest total number of fruits per plant (1972.08) was obtained in T4 (ZnSO4—0.5%) and was found to be statistically equal to T8 (humic acid—1%), T2 (Borax—0.3%), and T10 (seaweed extract—0.1%), recording 1864.91, 1858.03, and 1836.79 fruits per plant, respectively. The control plants (T12) produced the lowest number of fruits per plant (1627.77).

Table 3.
Influence of foliar feeding with nutrients and bioregulators on number of fruits per plant and yield of Litchi cv. Bombai.
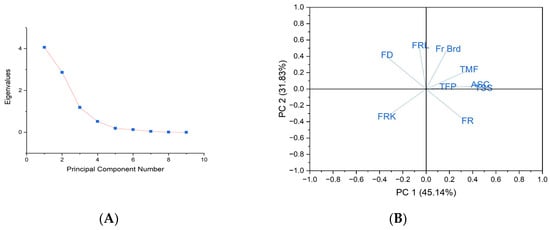
Figure 5.
(A) Slope declining of the treatments from first treatment to last treatment; (B) Scattered graph of Principal Component Analysis for treatments (where, TFP—Total number of fruits per plat; TMF—Total number of marketable fruits; FD—Fruit drop; FRK—Fruit cracking; FR—Fruit retention; TSS—Total soluble solids; FRL—Fruit length; FB—Fruit width, ASC—Ascorbic acid.
3.6. Total Number of Marketable Fruits per Plant
The pooled data presented in Table 3 and Table S11, show that the maximum number of marketable fruits (1698.46) was observed in plants under the T2 treatment (Borax—0.3%), which has been shown to be superior to all other treatments. It was statistically equivalent (1632.43) to T4, while all other treatments recorded higher number of marketable fruits than T12 (control). Out of all the treatments, the control plants (T12) had the lowest marketable fruits per plant (144.18), and they were found to be significantly less effective than the other treatments.
3.7. Total Yield per Plant
The litchi plants under the T2 treatment (Borax—0.3%) produced the highest total fruit yield (38.69 kg per plant), which was found to be statistically equal to T10 (36.68 kg/plant), T9 (36.41 kg/plant), and T4 (35.85 kg/plant) and was also observed to be superior to all other treatments, whereas the lowest fruit yield (29.65 kg/plant) was found in T12 (control). The influence of the treatments (T2, T10, T9, and T4) on the total fruit yield per plant was found to be statistically equivalent between treatments. The pooled data further shows that the mean fruit yield obtained from T2 (38.69 kg/plant) is 23.3% higher than the control and that there is a 19.16% higher yield in the T10 treatment compared to the control (Table 3 and Table S11).
3.8. Total Marketable Yield
The maximum marketable yield (35.34 kg/plant) was observed in litchi plants under T2 (i.e., Borax—0.3%), which was found to be statistically superior to all other treatments, pursuant to the pooled data shown in Table 3 and Table S11. There was statistical similarity observed among the T4 (29.56 kg/plant), T5 (28.29 kg/plant), T6 (28.11 kg/plant), and T8 (28.39 kg/plant) treatments. The T12 treatment (control) showed the lowest marketable fruit yield (20.88 kg/plant), and it appeared to be significantly less effective than any other treatment. The pooled mean value of the marketable yield obtained from all the treatments revealed that T2, T4, T5, and T8 showed a 41.03%, 29.76%, 26.19%, and 25.72% higher marketable fruit yield than the control.
3.9. Physico-Chemical Parameters
3.9.1. Fruit Length
All the plant bioregulators and nutrients significantly increased the fruit length in comparison to the control. The plants under the T2 treatment (foliar feeding with 0.3% Borax) showed the longest fruit (3.66 cm) based on the pooled data shown in Table 4 and Table S12. The following treatments were found to be statistically similar with each other with respect to fruit length in litchi: T3, i.e., foliar feeding with 0.75% ZnSO4 (3.53 cm); T5, i.e., foliar feeding with 0.5% CaCl2 (3.56 cm); T9, i.e., foliar feeding with 0.3% seaweed extract (3.43 cm); and T10, i.e., foliar feeding with 0.1% seaweed extract (3.45 cm). The fruit with the minimum length (2.83 cm) was observed in the T7 treatment (1% humic acid).

Table 4.
Influence of foliar feeding with nutrients and bioregulators on fruit and nut size of Litchi. cv. Bombai.
3.9.2. Fruit Width
It is apparent from the data shown in Table 4 and Table S12 that the fruit width of litchi was not significantly influenced by the various foliar treatments. From the pooled data, it can be seen that the foliar application of 0.3% Borax (T2) resulted in the highest fruit width (3.12 cm) followed by plants in T5 (3.08 cm) and T10 (3.02 cm).The plants in T8 produced the lowest fruits width (2.61 cm).
3.9.3. Nut Length
3.9.4. Nut Width
3.9.5. Fruit Weight
During the study period, there was a significant variation in the average fruit weight of litchi fruit due to influence of different treatments. The pooled data presented in Table 5 and Table S13, clearly show that the plants treated with 0.3% Borax (T2) had the highest fruit weight (20.85 g), which was statistically found to be equal to the T3 (20.39 g), T10 (20.02 g), and T5 (19.81 g) treatment. The lowest fruit weight (18.26 g) was obtained in the control plants (T12) which was found to be statistically similar with the T4 (18.30 g), T6 (18.59 g), T7 (18.63 g), T8 (18.84 g), and T1 (18.91 g) treatments.

Table 5.
Influence of foliar feeding with nutrients and bioregulators on fruit weight, aril weight, seed weight, and pericarp weight of Litchi cv. Bombai.
3.9.6. Aril Weight, Seed Weight, Pericarp Weight, Aril/Seed Ratio, and Aril/Pericarp Ratio
The data recorded with respect to the aril weight in litchi and the effects of foliar feeding with nutrient and bioregulator treatments are presented in Table 5 and Table S13. The pooled data exhibited a significant variation among the treatments with respect to aril content in litchi cv. Bombai. The highest was recorded in T2 (16.01 g) followed by T5 (15.70 g), which were found to be statistically similar to each other. The lowest aril content (12.20 g) was recorded in T12 which was found to be statistically different from rest of the treatments (Table 4).
The plants treated with 0.3% Borax (T2) had the lowest seed weight (2.19 g) and were statistically different from the other treatments, according to the data presented in Table 5. The untreated control plants (T12) had the highest recorded seed weight (3.09 g) among all the treatments. There was a non-significant effect of various treatments on the pericarp weight of litchi cv. Bombai (Table 5 and Table S13), yet T2 showed the lowest pericarp weight, and the control plants had recorded the highest pericarp weight. From the pooled data, the minimum value was obtained (2.23 g) in T2, and the maximum value (2.54 g) was obtained in T12 (control). Assessing the data set displayed in Table 5 and Table S13, it was found that plants subjected to foliar feeding with 0.3% Borax (T2) had the highest aril/seed ratio (7.31) compared to the other treatments. On the other hand, the lowest aril/seed ratio (3.95) was found in T12 (control) which was observed to be statistically similar to the T4 (4.47), T7 (4.82), T9 (4.71), and T11 (4.49) treatments. It is evident from the pooled data that maximum aril/pericarp ratio (7.18) was found in T2, whereas the lowest was recorded in the control (T12) plants (4.81) (Table 5 and Table S13). The aril/pericarp ratio was found to be significantly superior in all the treatments in reference to the control.
3.9.7. Chemical Analysis
Total Soluble Solids
The treatments had significant effects on the total soluble solids (TSS) content of the litchi fruit (Table 6 and Table S14). Based on the pooled data, it was determined that the highest TSS was recorded in T2 (14.070Brix) followed a significantly similar value in T1 (14.020Brix), T8 (13.730Brix), T7 (13.420Brix), and T10 (13.220Brix). However, the lowest TSS was observed in the control, i.e., T12, (9.820Brix) which was statistically different than the values obtained from the treatments; it was followed by the statistically significant T3 (11.470Brix), T11 (11.780Brix), T4 (11.980Brix), and T6 (12.550Brix) treatments.

Table 6.
Influence of foliar feeding with nutrients and bioregulators on total soluble solids, acidity, total sugar content, and reducing and non-reducing sugar content of Litchi cv. Bombai.
Titratable Acidity
A significant effect for titratable acidity (%) was observed in the fruits treated with various nutrients and bioregulators (Table 6 and Table S14). The lowest titratable acidity was obtained in T2 (0.72%), which was followed by significantly similar T1 (0.78%), T8 (0.79%), and T7 (0.80%) treatments. The control plants, however, had the highest calculated titratable acidity, i.e., T12, (1.00%) followed by statistically equivalent T10 (0.91%) and statistically different T9 (0.88%), T4 (0.85%), and T3 (0.85%) treatments.
Total Sugar Content
The treatments had a notable effect on the total sugar content (%) of the litchi fruit (Table 6 and Table S14). The pooled data showed that the T2 treatment had the highest total sugar percentage (10.40%), followed by the statistically similar T1 treatment (10.12%). These were followed by the statistically different T8 (9.98%) and T7 (9.88%) treatments. However, the lowest total sugar content was observed in the control, i.e., T12, (7.85) which was followed by the statistically different T11 (8.63%) and T3 (8.88%) treatments. However, T11 and T3 were statistically similar to each other.
Reducing Sugar Content
The T2 treatment showed the highest reducing sugar content (8.15%) followed by the statistically identical T1 (7.93%) treatment, which is evident from the pooled data for the two years studied (Table 6 and Table S14). These were followed by the statistically different T8 (7.88%) and T7 (7.82%) treatments. However, the lowest reducing sugar content was observed in the control (6.08%), which was followed by the statistically similar T4 (6.65%) treatment and statistically different T11 (6.78%) and T7 (7.01%) treatments.
Non-Reducing Sugar Content
There was non-significant effect of the treatments on the non-reducing sugar content (%) of the litchi fruit (Table 6 and Table S14). In the pooled data of the two years studied, the highest non-reducing sugar content was observed in T2 (2.14%), which was followed by the statistically similar T1 (2.07%), T8 (2.00%), and T7 (1.96%) treatments. However, the lowest non-reducing sugar was observed in the control (1.69%), which was followed by the statistically similar T4 (1.75%), T11 (1.76%), and T3 (1.78%) treatments.
TSS/Acidity Ratio
The TSS/acid ratio varied significantly due to different treatments and reflected in Table 7 and Table S15. The pooled results showed the highest TSS/acidity ratio. in the T2 (20.33), which was followed by statistically significant T1 (18.76) and statistically different T8 (17.96), T7 (17.25), and T5 (16.35). However, the lowest TSS/acidity ratio was observed in the control, i.e., T12, (9.99) which was statistically different than all other treatments. It was followed by T11 (12.74), T3 (14.39), T4 (14.74), and T10 (14.98).

Table 7.
Influence of foliar feeding with nutrients and bioregulators on TSS/acid ratio and ascorbic acid content of Litchi cv. Bombai.
Ascorbic Acid
There was a significant effect of the treatments on the ascorbic content (mg/100 g) of the litchi fruit (Table 7 and Table S15).The highest ascorbic acid content was observed in the T2 treatment (32.22 mg/100 g), which was followed by the statistically similar T1 treatment (32.06 mg/100 g) as indicated from the pooled data presented in Table 7. This was followed by the statistically different T7 (30.67 mg per 100 g) and T8 (30.56 mg per 100 g) treatments. The lowest ascorbic content was observed in the control, i.e., T12, (27.06 mg/100 g) which was followed by the statistically different T11 (27.83 mg per 100 g), T3 (28.11 mg per 100 g), and T4 (28.50 mg per 100 g) treatments.
Principal Component Analysis
Due to the many characteristics studied, including the total number of fruits per plant, total number of marketable fruits, fruit drop, fruit retention, total soluble solids, ascorbic acid, fruit cracking, fruit length, and fruit width, principal component analysis was performed.
The eigen values slowly declined starting from the total number of fruits followed by the total number of marketable fruits and fruit drop, and the remaining characteristics showed negligible responses for the treatments effect. The eigen values corresponding to the variables measuring the total number of fruits per plant (4.06255) suggest a pronounced response to the applied treatments in this investigation, with the fruit drop (1.1867) and fruit retention (0.51802) following in significance. The eigen value is notably low for fruit width and also for fruit length. The overall variance is distributed across ten components, elucidating the impact of treatments on various measured traits in the current study. The principal component analysis (PCA) reveals a transformation in the dimensionality of variables such as fruit cracking and ascorbic acid content, with varying degrees of variance. Notably, PCA1 (45.14%) and PCA2 (31.83%) collectively account for the total variance in this study (Tables S7–S9 in the Supplementary Materials).
4. Discussion
Compared to earlier research, this study delivers more precise information since it supported our hypothesis that applying nutrients and bioregulators topically could enhance the yield and physico-chemical parameters of litchi fruit.
Plants sprayed with 0.5% ZnSO4 had the highest total number of fruits per plant, followed by those treated with 1% humic acid. This result corroborates the findings of the authors of [36], who obtained a considerably high number of fruits per tree by spraying litchi plants with zinc sulphate (0.6%). Similar results were also reported by [37]. Since humic acids can adhere to ionized nutrients and prevent them from leaching away, they could act as a medium for transporting nutrients from the soil to the plant. They provide water and nutrients to plants when they reach the roots. This would have facilitated greater nutrient availability and utilization [38]. In addition to producing plant hormones and enzymes, it has also been demonstrated that the application of humic acid increases the weight of the roots and shoots, chlorophyll content, and the photosynthetic rate [39].
The litchi plants treated with 0.3% Borax by foliar feeding had the highest total number of fruits per plant, which was found to be statistically identical to plants treated with 0.1% and 0.5% seaweed extracts. This result was close to the findings of the authors of [40], who recorded an enhanced total fruit yield over their control due to the application of seaweed extract in kiwi fruit. The essential nutrients contained in seaweed extracts, viz. nitrogen, potassium, phosphorous, calcium, magnesium, sulfur, iron, sodium, zinc, and copper [41], might reduce the production losses caused by cracking without compromising either the quality or the yield of crops. The findings also corroborate the claims of a higher yield per plant when humic acid is applied to peaches [42], apples [43], and pears [44]. The favorable effects of boron, namely boosting the rates of carbohydrate and RNA metabolism [45] and accelerating the passage of photosynthates from the leaves to the developing fruits [46], may contribute to the yield enhancement that results from treatment with boron.
Among the treatments, plants treated with 0.3% Borax and 0.5% zinc sulphate demonstrated the best response in terms of a higher number of marketable fruits and marketable output. The application of 1% humic acid and 0.1% CaCl2 also enhanced the marketable yield of litchi. This result is in line with that of the authors of [47], who found that applying boron to plants increased the yield of marketable fruits per plant. The right dose of boron may contribute significantly to the mechanisms related to flowering and fruiting, nitrogen metabolism, hormone synthesis, and cell division, among other positive functions [48]. It might also facilitate the mobilization of nutrient to the fruits, augmenting the yield of nutritious fruit. Additionally, the results concur with those reported in [49]. Boron is also relevant in the biosynthesis of auxin in the meristem of plants. A boron deficiency leads to decreased levels of bound auxin and a reduction in IAA oxidase activity [50]. The marketable fruits represent the number of fruits excluding cracked and pest- and disease-affected fruit. The results of the authors of [51], who found that applying boron greatly decreased the amount of fruit cracking, provided complete support for the data we gave on fruit cracking in litchi in our experiment. The application of Boron has also been shown to reduce fruit cracking in litchi, according to [52,53]. The presence of boron, zinc, and other micronutrients influences the uptake of water and solutes. In the case of enhanced water uptake, solutes accumulate in the fruits and minimize the pressure on the skin, resulting in less cracking. Auxin stimulation brought about by the use of bioregulators may be the cause of the quicker accumulation of building blocks and the improved source–sink relationship, which results in higher fruit setting, retention, and less cracking.
The application of 1% humic acid followed by 1.5% humic acid improved the percentage of fruit setting and fruit retention in litchi and decreased the fruit drop percentage. Humic acid in the soil acts as a chelating agent, making already present nutrients in the soil available to plants. The findings of this investigation are parallel with those of [54], in which the influence of humic acid on pomegranates was investigated, and it was found that higher amounts of humic acid contributed to higher percentages of fruit setting and fruit retention. Additionally, they observed that with the increased application of humic acid, the percentage of fruit drop decreased. Humic acid has also been shown to have a significant beneficial effect on grapes [55] and pears [56,57].
The litchi plants treated with 0.3% Borax yielded fruits with increased fruit weight and size (both length and width). The application of boron directly accelerates the processes of cell division and elongation, which may be the cause of enhanced fruit size and fruit weight in litchi. Fruits grew larger because of their faster development and increased food material mobilization from the production site to the storage organs as a result of the applied nutrients. A similar result was observed in litchi after boron treatment and has already been reported [58]. The current results further support the findings of the authors of [59], who found no discernible variations in the fruits’ vertical diameter, transverse diameter, lateral diameter, and fruit shape index among fruits of all treatments and their control. These results are in conformity with those reported by [60] in guava and by [61,62] in litchi. The increase in fruit weight might be due to the rapid increase in the size of cells, or it may also due the fact that the foliar application of boron eventually increased the fruit weight by maintaining lower levels of auxins in various parts of the fruit which helped in increasing the growth rate of the fruit [62].
The highest aril weight and lowest seed weight was observed in plants treated with 0.3% Borax. The application of boron increased the weight of the aril and decreased the weight of the seed, resulting in a high aril/seed ratio. These results are consistent with those of [61] in litchi and those of [32] in apricot. The aril weight also depends on the fruit and seed size [63] but is also affected by plant nutrition [64]. Boron treatments resulted in the production of fruits with smaller seed. This may be due to involvement of boron in the metabolism of IAA which reduces seed size. The decrease in seed weight may be due to the fact that auxin induced a parthenocarpic effect to some extent, thereby resulting in a reduced seed weight [65].
The ratio between the weight of the aril to the weight of the seed is known as the aril/seed ratio. Fruits collected from boron-treated plants had the highest aril/seed ratio, while fruits that were not treated had the lowest ratio. This is related to the fact that the application of boron enhanced the pulp weight and reduced the stone weight which, as a consequence, resulted in a high pulp/stone ratio [61]. Similar findings were obtained by [25,66] in litchi.
The foliar application of Borax resulted in a higher TSS and lower acidity in litchi cv. Bombai. The application of Borax also improved the total sugar and reducing sugar contents. This is in accordance with the results of the authors of [67], who found that applying boric acid prior to harvest increased the TSS, total sugar content, and reducing sugar content, and decreased the titratable acidity. The data presented on the acidity of litchi was fully supported with the findings of the authors of [68] who observed that foliar spraying with boric acid reduced acid levels in the fruits of litchi. Lower acidity in fruits might be due to increased sugar buildup, improved sugar release into fruit tissues, and the conversion of organic acids to sugars [69]. Another possible cause for limiting the titratable acidity might also be due to fast acid consumption of organic acids during respiration. The higher TSS/acid ratio and ascorbic acid content were obtained in litchi due to the foliar application of 0.3% Borax followed by1.5% humic acid and 1% humic acid to litchi. Similar results were also reported by the authors of [70], who found that spraying litchi fruits with 0.5% or 1% Borax enhanced the TSS and lowered the acidity. It was also discovered that while the acidity was lowest, a 0.4% Borax spray increased the TSS, sugar, and ascorbic acid levels in litchi cv. Purvi [68].
The highest total fruit yield and marketable fruit yield were recorded in plants treated with 0.3% Borax (T2) among the other nutrient treatments applied to litchi. From a quality point of view, the same treatment, i.e., 0.3% Borax (T2), was found to be the best, resulting in a higher TSS, lower acidity, higher ascorbic acid content, etc. Regarding the bioregulator treatments, the plants treated with0.1% seaweed extract (T10) and 0.5% seaweed extract (T9) produced the highest total fruit yield. However, the highest marketable yield was recorded in plants treated with 1% humic acid (T8).
5. Conclusions
Determining the effectiveness of applying nutrients and bioregulators to enhance fruit yield and quality in litchi was the primary objective of this study. During both the two years studied, all the treatments increased the fruit yield and yield-attributing parameters such as fruit weight, fruit width, and fruit length in litchi when compared to the control. The highest total fruit yield was recorded (38.44 kg/plant) in plants treated with 0.3% Borax (T2) followed by plants treated with0.1% seaweed extract (T10) with 36.68 kg per plant and0.5% seaweed extract (T9) with 36.41 kg per plant. However, the highest marketable yield (35.34 kg per plant) was recorded in plants treated with 0.3% Borax (T2) followed by 0.5% zinc sulphate (T4) (with 29.56 kg per plan) and 1% humic acid (T8) with 28.39 kg per plant. The increase in fruit weight and the number of fruits per plant, along with other fruit-related physical attributes, might be responsible for the yield improvement. Additionally, it was discovered that foliar spraying with 0.3% Borax (T2) was superior in improving certain quality parameters such as total soluble solids (14.070Brix), total sugar content (10.40%), and ascorbic acid (32.06 mg per 100 g edible portion). The improvement of fruit quality may be attributed to better growth of plants with different treatments of humic acid which might have favored the production of better-quality fruit. The increase in the fruit yield might also be due to the accumulation of sugars and other soluble solids in the fruits.
On the basis of the above findings, it may be concluded that among the nutrients studied, 0.3% Borax (T2), 0.1% seaweed extract (T10), and 1% humic acid (T8) were found to be superior treatments among the bioregulators that can be recommended for improving the yield and quality parameters of litchi cv. Bombai. However, for further studies, combinations of these nutrients and bioregulators should be studied to obtain the best result.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10020188/s1.
Author Contributions
Conceptualization, S.N.; data curation, S.K.B.; formal analysis, S.N. and P.R.; investigation, S.N. and B.K.S.; methodology, R.K.T., D.M.V. and P.R.; software, S.K.B.; supervision, R.K.T., S.P. and P.M.; validation, S.C.S., S.S. (Sunil Samal) and P.M.; writing—original draft, S.N.; writing—review and editing, R.K.T., S.P. and S.S. (Senthamizh Selvi). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by contribution from individual authors.
Data Availability Statement
Available data are provided in the publication, and any other information will be provided upon request.
Acknowledgments
The authors are grateful to the Department of Fruit Science and Horticulture Technology, Odisha University of Agriculture and Technology, Bhubaneswar, Odisha, India, and the farmers of Jamankira block of Sambalpur, Odisha, for aiding in the research trial.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menzel, C.M. Plant Resources of South East Asia. No. 2; Edible Fruit and Nuts; Verheij, E.W.M., Coronel, R.E., Eds.; Pudoe: Wageningen, The Netherlands, 1991; 191p. [Google Scholar]
- Raghavan, M.; Hazarika, B.N.; Susmita, D.; Ramjan, M.; Langstieh, L.B. Integrated nutrient management in litchi (Litchi chinensis Sonn.) cv. Muzaffarpur for yield and fruit quality at foothills of Arunachal Pradesh. Int. J. Chem. Stud. 2018, 6, 2809–2812. [Google Scholar]
- Mozumder, S.N.; Faisal, S.M.; Sultana, D.; Firoz, Z.A. Effect of Growth Regulator and Irrigation on Fruit Cracking and Yield of Litchi in the Hilly Area. Int. J. Adv. Innov. Res. 2016, 5, 104–108. [Google Scholar]
- Deng, X.P.; Han, Z.H.; Li, S.H. Fruit Tree Biology; Higher Education Press: Beijing, China, 1999. [Google Scholar]
- Kumar, D.; Mishra, D.S.; Chakraborty, B.; Kumar, P. Pericarp browning and quality management of litchi fruit by antioxidants and salicylic acid during ambient storage. J. Food Sci. Technol. 2011, 50, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Hajare, S.N.; More, V.; Kumar, S.; Wadhawan, S.; Parte, M.N.P. Antioxidant and radio protective properties of commercially grown litchi (Litchi chinensis) from India. Food Chem. 2011, 126, 39–45. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, P.; Karuna, K.; Aftab, A.; Kumar, A.; Ahmad, F. Effect of Pre-harvest Salicylic Acid Spray on Shelf Life and Biochemical Changes of Litchi during Storage. Curr. J. Appl. Sci. Technol. 2023, 42, 59–65. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press Limited Harcourt Brace and Company: London, UK, 2012; pp. 347–364. [Google Scholar]
- Singh, O.P.; Phogat, K.P.S. Effect of plant growth regulators on fruit drop, size and quality of litchi cv. Calcuttia. Punjab Hortic. J. 1984, 24, 83–88. [Google Scholar]
- Menzel, C.M.; Simpson, D.R. Lychee nutrition: A review. Sci. Hortic. 1987, 31, 195–224. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; Mason, B.; Critchley, A.T. Seaweeds as a source of proteins for use in pharmaceuticals and high-value applications. In Novel Proteins for Food, Pharmaceuticals, and Agriculture: Sources, Applications, and Advances; Hayes, M., Ed.; Wiley: Hoboken, NJ, USA, 2018; 217p. [Google Scholar]
- Davarpanaha, S.; Tehranifara, A.; Abadíab, J.; Valb, J.; Davarynejada, G.; Aranc, M.; Khorassanid, R. Foliar calcium fertilization reduces fruit cracking in pomegranate (Punica granatum cv. Ardestani). Sci. Hortic. 2018, 230, 86–91. [Google Scholar] [CrossRef]
- Ram, R.A.; Bose, T.K. Effect of foliar application of of magnesium and micronutrients on growth, yield and fruit quality mandarin orange (Citrus reticulata Blanco.). Indian J. Hortic. 2000, 57, 215–220. [Google Scholar]
- Priyadarshi, V.; Hota, D.; Karna, A.K. Effect of Growth Regulators and Micronutrients Spray on Chemical Parameters of Litchi (Litchi chinensis Sonn.) cv. Calcuttia. Int. J. Econ. Plants 2018, 5, 99–103. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Bordoloi, B.C.; Singha, D.D.; Ojha, N.J. Role of seaweed extract on growth, yield and quality of some agricultural crops: A review. Agric. Rev. 2018, 39, 321–326. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physico-chemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Mosa, W.F.; Sas-Paszt, L.; Górnik, K.; Ali, H.M.; Salem, M.Z. Vegetative growth, yield, and fruit quality of guava (Psidium guajava L.) cv. maamoura as affected by some biostimulants. Bioresources 2021, 16, 7379–7399. [Google Scholar] [CrossRef]
- Ashour, M.; El-Shafei, A.A.; Khairy, H.M.; Abd-Elkader, D.Y.; Mattar, M.M.; Alataway, A.; Hassan, S.M. Effect of Pterocladia capillacea seaweed extracts on growth parameters and biochemical constituents of Jew’s Mallow. Agronomy 2020, 10, 420. [Google Scholar] [CrossRef]
- Sarkar, G.K.; Sinha, M.M.; Misra, R.S. Effect of NAA on fruit set, fruit drop, cracking, fruit size and quality in litchi cv. Rose Scented. Progress. Hortic. 1984, 16, 301–304. [Google Scholar]
- Bhat, S.K.; Raina, B.L.; Chogtu, S.K.; Muthoo, A.K. Effect of exogenous auxin application on fruit drop and cracking of litchi (Litchi chinensis Sonn.) cv. Dehradun. Adv. Plant Sci. 1997, 10, 83–86. [Google Scholar]
- Brahmachari, V.S.; Rani, R. Effect of growth substances on fruit drop, yield and physico-chemical composition of litchi fruits. Progress. Hortic. 2001, 32, 50–55. [Google Scholar]
- Awasthi, R.P.; Tripathi, B.R.; Singh, A. Effect of foliar sprays of zinc on the fruit drop and quality of the litchi. Punjab Hortic. J. 1975, 15, 14–16. [Google Scholar]
- Menzel, C.M.; Simpson, D.R. Lychee cultivars: Description and performance of major litchi cultivars in sub-tropical Queensland. Qld. Agric. J. 1986, 112, 126–136. [Google Scholar]
- Kanwar, J.S.; Njjar, G.S. Litchi cultivation in the Punjab problem and prospect. Punjab Hortic. J. 1975, 15, 9–13. [Google Scholar]
- Ahmed, Y.M.; Shalaby, E.A. Effect of different seaweed extracts and compost on vegetative growth, yield and fruit quality of cucumber. J. Hortic. Sci. Ornam. Plants 2012, 4, 235–240. [Google Scholar]
- Hamed, S.M.; Abd El-Rhman, A.A.; Abdel-Raouf, N.; Ibraheem, I.B.M. Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni Suef Univ. J. Basic Appl. Sci. 2018, 7, 104–110. [Google Scholar]
- AOAC International. A.O.A.C. Official methods of analysis. In Association of Official Analytical Chemists, 20th ed.; Benjamin Franklin Station: Washington, DC, USA, 2016. [Google Scholar]
- Priyadarshi, V.; Mehta, K.; Hota, D.; Mishra, G.; Jogur, A. Effect of growth regulators and micronutrients spray on vegetative growth of litchi (Litchi chinensis Sonn.) cv.Calcuttia. Agric. Update 2018, 12, 707–712. [Google Scholar] [CrossRef]
- Rangana, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products, II ed.; Tata McGraw-Hill Publ. Co.: New Delhi, India, 1986. [Google Scholar]
- Panse, V.G.; Sukhatme, P.V. Statistical Methods for Agricultural Workers; Indian Council of Agricultural Research: New Delhi, India, 1989; pp. 152–165. [Google Scholar]
- Massay, W.F. Principal components regression in exploratory statistical research. J. Am. Stat. Assoc. 1965, 60, 234–246. [Google Scholar] [CrossRef]
- Jolliffie, I.T. Principal Component Analysis; Springer: New York, NY, USA, 1986. [Google Scholar]
- Brijpal, B.; Tiwari, J.P.; Mishra, K.K.; Lal, S. Effect of Zinc Sulphate on Vegetative Growth, Yield and Leaf Nutrient Status of Guava (Psidium guajava L.). Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1265–1273. [Google Scholar]
- Sharma, R.K.; Thakur, S.; Kumar, R. A note on the effect of foliar feeding of zinc and GA on fruiting of guava trees (Psidium guajava L.). Haryana J. Hort. Sci. 1993, 22, 207–208. [Google Scholar]
- Nurbhanje, K.H.; Varu, D.K. Effect of biostimulant and biofertilizers on flowering and fruiting of pomegranate (Punica granatum L.) cv.Bhagwa. Int. J. Chem. Stud. 2019, 7, 378–382. [Google Scholar]
- Bybordi, A.; Ebrahimian, E. Growth, yield and quality components of canola fertilized with urea and zeolite. Commun. Soil Sci. Plant Anal. 2013, 44, 2896–2915. [Google Scholar] [CrossRef]
- Rana, V.S.; Sharma, V.; Sharma, S.; Rana, N.; Kumar, V.; Sharma, U.; Almutairi, K.R.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Gudeta, K. Seaweed Extract as a biostimulant agent to enhance the fruit Growth, yield, and quality of Kiwifruit. Horticulturae 2023, 9, 432. [Google Scholar] [CrossRef]
- Margal, P.B.; Thakare, R.S.; Kamble, B.M.; Patil, V.S.; Patil, K.B.; Titirmare, N.S. Effect of Seaweed Extracts on Crop Growth and Soil: A Review. J. Exp. Agric. Int. 2023, 45, 9–19. [Google Scholar] [CrossRef]
- El-Khawaga, A.S. Partial replacement of mineral N fertilizers by Using humic acid and Spirulina platensis algae biofertilizer in Florida Prince peach orchards. Middle East J. Appl. Sci. 2011, 1, 5–10. [Google Scholar]
- Fathi, M.A.; Eissa, F.M.; Yehia, M.M. Improving growth, yield and fruit quality of Desert Red peach and Anna apple by using some biostimulants. Minia J. Agric. Res. Develop. 2002, 22, 519–534. [Google Scholar]
- Kabeel, H.; Abd El-Atif, F.M.; Baza, M.S.M. Growth, fruiting and nutritional status of “Le-Conte”pear trees in response to mineral and humate fertilizers. Ann. Agric. Sci. Moshtohor. 2008, 46, 139–156. [Google Scholar]
- Parr, A.J.; Loughman, B.C. Boron and membrane functions in plants. In Metals and Micronutrients Uptake and Utilization by Plants; Annual Proceedings of the Phytochemical Society of Europe No. 21; Roff, D.A., Pirepoint, W.S., Eds.; Academic Press: London, UK, 1983; pp. 87–107. [Google Scholar]
- Rajput, C.B.S.; Chand, S. Significance of boron and zinc in guava (Psidium guajava L.). Bangladesh Hort. 1975, 3, 22–27. [Google Scholar]
- Singh, S.; Singh, J.P. Effect of foliar sprays of NAA and boron on flowering, fruiting, fruit retention and yield of litchi (Litchi chinensis Sonn.). Int. J. Chem. Stud. 2019, 7, 1995–1999. [Google Scholar]
- Russel, D.A. Boron and Soil fertility. In The Year Book of Agriculture; U.S.D.A.: Washington, DC, USA, 1957. [Google Scholar]
- Banyal, A.K.; Rangra, A.K. Response of yield and quality attributes of litchi cv. Dehradun to soil and foliar application of boron. J. Hill Agric. 2011, 2, 33–37. [Google Scholar]
- Dugger, W.M. Boron in plant metabolism. In Encyclopedia of Plant Physiology; Lalichli, A., Bieleski, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1968; Volume 5B, pp. 628–650. [Google Scholar]
- Sharma, N.; Belsare, C. Effect of Plant Bio-Regulators and Nutrients on Fruit Cracking and Quality in Pomegranate (Punica granatum L.) ‘G-137′ in Himachal Pradesh. Acta Hortic. 2011, 890, 347–352. [Google Scholar] [CrossRef]
- Brahmachari, V.S.; Kumar, R. Effect of foliar sprays of mineral nutrients on fruit set retention and cracking in litchi (Litchi chinensis Sonn.) fruits. Haryana J. Hortic. Sci. 1997, 26, 177–180. [Google Scholar]
- Babu, N.; Singh, A.R.; Babu, N. Effect of micronutrients spray on fruit cracking and fruit maturity in litchi (Litchi chinensis Sonn.) fruits. Indian Agric. 2002, 46, 203–204. [Google Scholar]
- Khattab, M.M.; Shaban, A.E.; El-Shrief, A.; Mohamed, A. Effect of humic acid and amino acids on pomegranate trees under deficit irrigation. I: Growth, flowering and fruiting. J. Hortic. Sci. Ornam. Plants 2012, 4, 253–259. [Google Scholar]
- Omar, A.H.; Abdelall, A.H. Influence of sulphuric acid, humic acid, sulphur and irrigation water on growth and productivity of Superior seedless vines grown under saline condition. J. Agric. Sci. 2005, 30, 6951–6961. [Google Scholar] [CrossRef]
- Ismail, M.; Wanden, M.T.; EI-Sheikh, M. Response of ‘Thompson seedless’ and ‘Roomy Red’ grape cultivars to foliar sprays with yeast extract and GA. J. Agric. Sci. 2003, 28, 6321–6334. [Google Scholar]
- Sanchez-Sanchez, A.; Sanchez-Andreu, J.; Jorda, J.; Bermudez, D. Humic substances and amino acids improve effectiveness of chelate Fe EDDHA in lemon trees. J. Plant Nutr. 2002, 25, 2433–2442. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Singh, R.P. Effect of micronutrients and plant growth regulators on fruiting of litchi. Int. J. Agric. Sci. 2009, 5, 521–524. [Google Scholar]
- Xu, W.P.; Wang, L.; Yang, Q.; Wei, Y.H.; Zhang, C.X.; Wang, S.P. Effect of Calcium and Boron on the Quality of Kiwifruit. Acta Hortic. 2015, 1096, 317–320. [Google Scholar] [CrossRef]
- Gaur, B.; Beer, K.; Hada, T.S.; Kanth, N.; Syamal, M.M. Studies on the effect of foliar application of nutrients and GA3 on fruit yield and quality of winter Season Guava. Ecoscan 2014, 6, 479–483. [Google Scholar]
- Singh, N.; Kaur, A.; Gill, B.S. Effect of foliar application of zinc and boron on yield and fruit quality of litchi cv. Dehradun. Int. J. Dev. Res. 2016, 6, 8686–8688. [Google Scholar]
- Haq, I.; Rab, A.; Sajid, M. Foliar application of calcium chloride and borax enhance the fruit quality of litchi cultivars. Anim. Plant Sci. 2013, 23, 1385–1390. [Google Scholar]
- Li, J.G.; Huang, H.B.; Gao, F.F.; Huang, X.M.; Wang, H.C. An overview of litchi fruit cracking. Acta Hortic. 2008, 558, 205–208. [Google Scholar] [CrossRef]
- Kazuhiro, I.; Masashi, M.; Hiroyuki, F. The effect of spraying of calcium to the fruit quality the quality keeping period and the tree vigour of Kousui in green house. Bull. Saga Prefect. Fruit Tree Exp. Stn. 2004, 15, 8–14. [Google Scholar]
- Singh, R.; Godara, N.R.; Singh, R.; Dahiya, S.S. Responses of foliar application of growth regulators and nutrients on ber cv. Umran. Haryana J. Hort. Sci. 2001, 30, 161–164. [Google Scholar]
- Brahmachari, V.S.; Yadav, G.S.; Kumar, N. Effect of foliar feeding of calcium, zinc and boron on field and quality attributes of litchi (Litchi chinensis Sonn.). Orissa J. Hortic. 1997, 25, 49–52. [Google Scholar]
- Alila, P.; Achumi, I. Pre-harvest chemical treatments affect post-harvest quality of litchi fruit. Acta Hortic. 2012, 934, 755–762. [Google Scholar] [CrossRef]
- Misra, A.R.; Khan, I. Trichloro phenoxy acetic acid and micronutrient on fruit size, cracking, maturity quality of litchi cv. Rose Scented. Progress. Hortic. 1981, 13, 87–90. [Google Scholar]
- Soppelsa, S.; Kelserer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production. Effects on tree growth, yield and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Kumar, M.; Ojha, O.K.; Jha, K.K. Yield and physico-chemical properties of litchi fruits as affected by different rates of pruning and chemical spray. Progress. Hortic. 2012, 44, 166–169. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).