The Postharvest Safety and Quality of Fresh Basil as Affected by the Use of Cypriot Oregano (Origanum dubium) Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Media
2.2. Plant Material
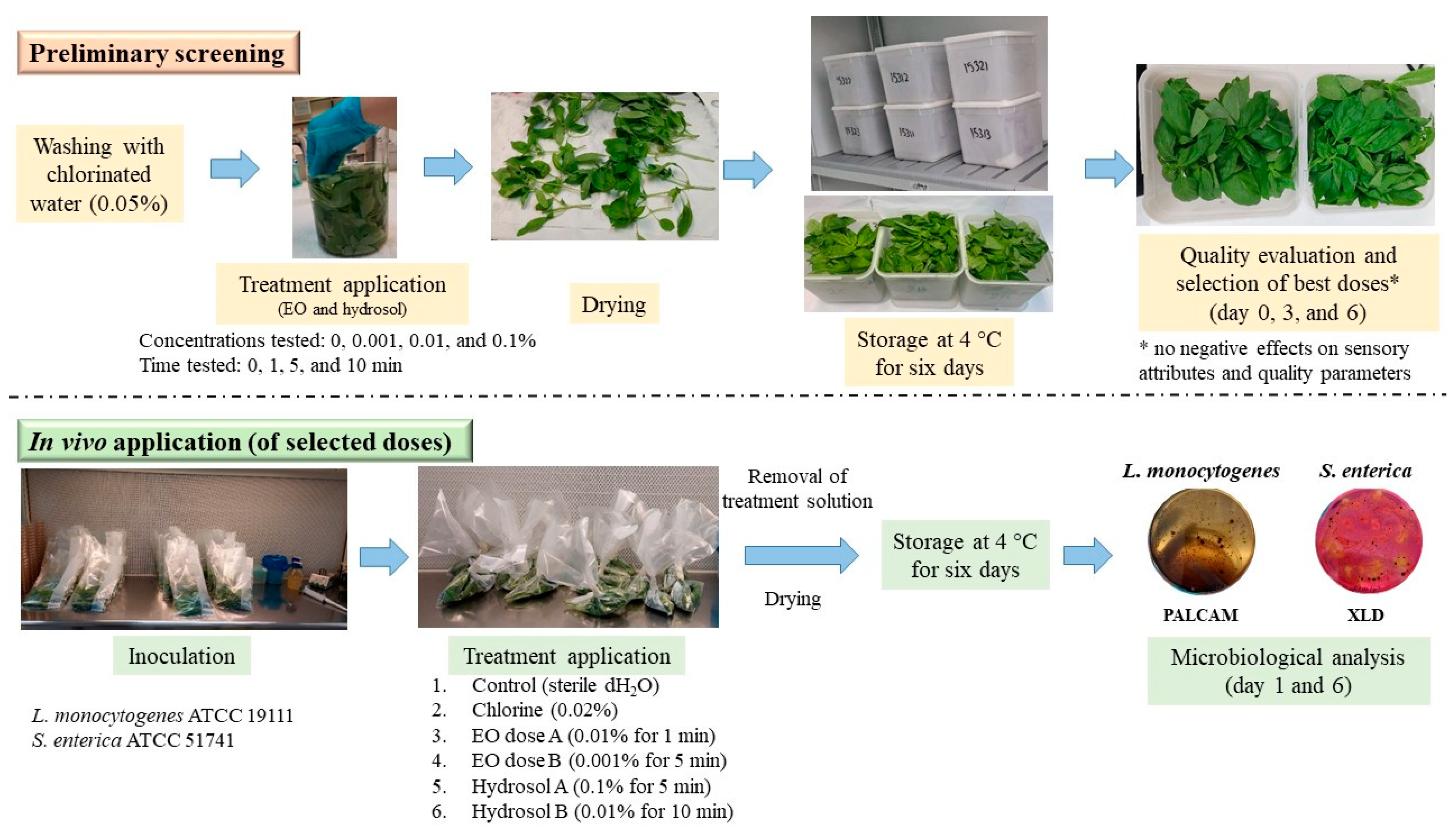
2.3. Preliminary Screening
2.3.1. The Effects on Basil’s Weight Loss and Respiration Rate
2.3.2. The Effects on Basil’s Sensory Characteristics
2.3.3. The Effects on Basil’s Quality Parameters
Color
Leaf Pigments
Polyphenols, Antioxidant Activity, Total Flavonoids and Ascorbic Acid Content
2.3.4. The Determination of Damage Indexes
2.4. The In Vivo Application of a Selected Sanitation Mean
2.4.1. The Preparation of the Bacterial Inoculum
2.4.2. The Procedure for Treatment Application
2.4.3. Microbiological Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Screening
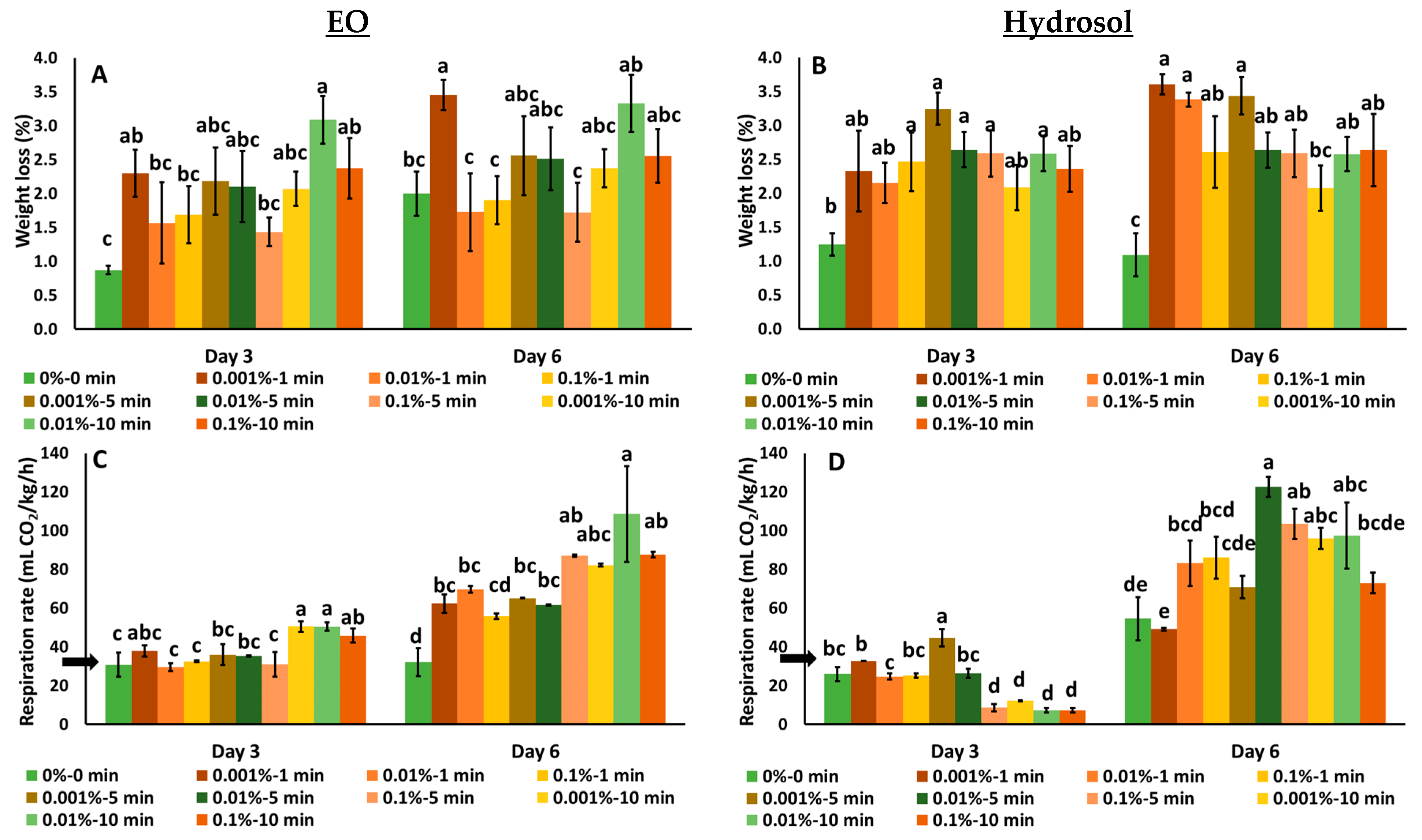
3.1.1. The Effects on Weight Loss and Respiration Rate
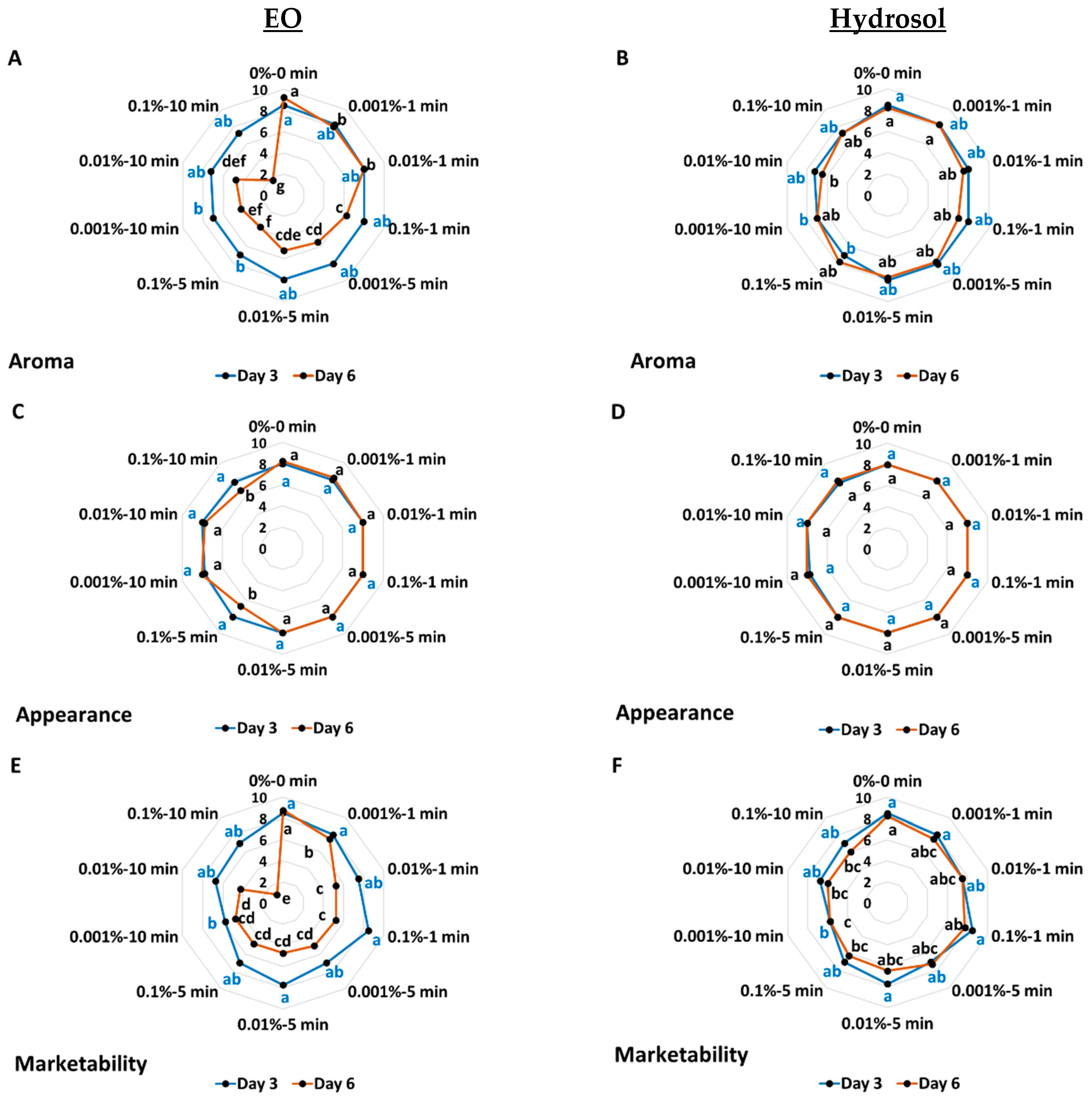
3.1.2. The Effects on Sensory Attributes
3.1.3. The Effects on Quality Parameters
Effects on Color
The Effects on Pigments, Phenols, Antioxidants, Flavonoids and Ascorbic Acid Content
The Effects on Damage Indexes
3.2. In Vivo Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, F.A.S.; Ali, E.F.; Mostafa, N.Y.; Mazrou, R. Shelf-life extension of sweet basil leaves by edible coating with thyme volatile oil encapsulated chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 177, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, R.; Pace, B.; Cefola, M.; Martignetti, A.; Stocchero, M.; Fratianni, F.; Nazzaro, F.; De Giulio, B. Assessment of volatile profile as potential marker of chilling injury of basil leaves during postharvest storage. Food Chem. 2016, 213, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, A.; Dellacassa, E.; Ringuelet, J.A.; Chaves, A.R.; Viña, S.Z. Nutritional and sensory quality during refrigerated storage of fresh-cut mints (Mentha × piperita and M. spicata). Food Chem. 2014, 143, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Cefola, M.; Pace, B.; Cozzolino, R.; De Giulio, B.; Cozzolino, A.; d’Acierno, A.; Coppola, R.; Logrieco, A.F.; Nazzaro, F. Changes in visual quality, physiological and biochemical parameters assessed during the postharvest storage at chilling or non-chilling temperatures of three sweet basil (Ocimum basilicum L.) cultivars. Food Chem. 2017, 229, 752–760. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Gil, M.I.; Marín, A.; Andujar, S.; Allende, A. Should chlorate residues be of concern in fresh-cut salads? Food Control 2016, 60, 416–421. [Google Scholar] [CrossRef]
- Chen, X.; Hung, Y.C. Development of a Chlorine Dosing Strategy for Fresh Produce Washing Process to Maintain Microbial Food Safety and Minimize Residual Chlorine. J. Food Sci. 2018, 83, 1701–1706. [Google Scholar] [CrossRef]
- Pizzo, J.S.; Visentainer, J.V.; da Silva, A.L.B.R.; Rodrigues, C. Application of essential oils as sanitizer alternatives on the postharvest washing of fresh produce. Food Chem. 2023, 407, 135101. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant hydrolates—Antioxidant properties, chemical composition and potential applications. Biomed. Pharmacother. 2021, 142, 112033. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The combined and single effect of marjoram essential oil, ascorbic acid, and chitosan on fresh-cut lettuce preservation. Foods 2021, 10, 575. [Google Scholar] [CrossRef]
- Poimenidou, S.V.; Bikouli, V.C.; Gardeli, C.; Mitsi, C.; Tarantilis, P.A.; Nychas, G.J.; Skandamis, P.N. Effect of single or combined chemical and natural antimicrobial interventions on Escherichia coli O157: H7, total microbiota and color of packaged spinach and lettuce. Int. J. Food Microbiol. 2016, 220, 6–18. [Google Scholar] [CrossRef]
- Ozturk, I.; Tornuk, F.; Caliskan-Aydogan, O.; Durak, M.Z.; Sagdic, O. Decontamination of iceberg lettuce by some plant hydrosols. LWT 2016, 74, 48–54. [Google Scholar] [CrossRef]
- Gerber, C.; Patel, J.; Friedman, M.; Ravishankar, S. Antimicrobial activity of lemongrass oil against Salmonella enterica on organic leafy greens. J. Appl. Microbiol. 2011, 112, 485–492. [Google Scholar]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Xylia, P.; Fasko, K.G.; Chrysargyris, A.; Tzortzakis, N. Heat treatment, sodium carbonate, ascorbic acid and rosemary essential oil application for the preservation of fresh Rosmarinus officinalis quality. Postharvest Biol. Technol. 2022, 187, 111868. [Google Scholar] [CrossRef]
- Bolin, H.R.; Huxsoll, C.C. Effect of preparation procedures and storage parameters on quality retension of salad-cut lettuce. J. Food Sci. 1991, 56, 60–62. [Google Scholar] [CrossRef]
- Goyeneche, R.; Agüero, M.V.; Roura, S.; Di Scala, K. Application of citric acid and mild heat shock to minimally processed sliced radish: Color evaluation. Postharvest Biol. Technol. 2014, 93, 106–113. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Možina, S.S.; Di Mattia, C.; Scuota, S.; Luzzi, I.; Jenič, T.; Paparella, A.; Serio, A. Salmonella enterica adhesion: Effect of Cinnamomum zeylanicum essential oil on lettuce. LWT 2019, 111, 16–22. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of Chlorophylls a and b, as well as total carotenids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 489. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Singh, P.; Hung, Y.C.; Qi, H. Efficacy of Peracetic Acid in Inactivating Foodborne Pathogens on Fresh Produce Surface. J. Food Sci. 2018, 83, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.P.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Changsawake, K.; Krusong, W.; Laosinwattana, C.; Teerarak, M. Retarding changes of postharvest qualities of sweet basil (Ocimum basilicum Linn.) by vapor-phase vinegar. J. Herbs Spices Med. Plants 2017, 23, 284–298. [Google Scholar] [CrossRef]
- Sánchez-García, F.; Hernandez, I.; Palacios, V.M.; Roldan, A.M. Freshness quality and shelf life evaluation of the seaweed Ulva rigida through physical, chemical, microbiological, and sensory methods. Foods 2021, 10, 181. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Miltiadous, P.; Tzortzakis, N. Origanum dubium (Cypriot oregano) as a Promising Sanitizing Agent against Salmonella enterica and Listeria monocytogenes on Tomato and Cucumber Fruits. Biology 2022, 11, 1772. [Google Scholar] [CrossRef]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Ripoll, J.; Charles, F.; Vidal, V.; Laurent, S.; Klopp, C.; Lauri, F.; Sallanon, H.; Roux, D. Postharvest Biology and Technology Transcriptomic view of detached lettuce leaves during storage: A crosstalk between wounding, dehydration and senescence. Postharvest Biol. Technol. 2019, 152, 73–88. [Google Scholar] [CrossRef]
- Mahmoud, E.; Starowicz, M.; Ciska, E.; Topolska, J.; Farouk, A. Determination of volatiles, antioxidant activity, and polyphenol content in the postharvest waste of Ocimum basilicum L. Food Chem. 2022, 375, 131692. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Basim, H.; Turgut, K.; Kaplan, B.; Basim, E.; Turgut, A. The Potential application of Origanum dubium Boiss. Essential oil as a seed protectant against bean and tomato seed-borne bacterial pathogens. Acta Sci. Pol. Hortorum Cultus 2019, 18, 79–86. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- Karioti, A.; Vrahimi-Hadjilouca, T.; Droushiotis, D.; Rancic, A.; Hadjipavlou-Litina, D.; Skaltsa, H. Analysis of the essential oil of Origanum dubium growing wild in Cyprus. Investigation of its antioxidant capacity and antimicrobial activity. Planta Med. 2006, 72, 1330–1334. [Google Scholar] [CrossRef]
- Kenigsbuch, D.; Chalupowicz, D.; Aharon, Z.; Maurer, D.; Aharoni, N. The effect of CO2 and 1-methylcyclopropene on the regulation of postharvest senescence of mint, Mentha longifolia L. Postharvest Biol. Technol. 2007, 43, 165–173. [Google Scholar] [CrossRef]
- Pace, B.; Cardinali, A.; Antuono, I.D.; Serio, F.; Cefola, M. Relationship between quality parameters and the overall appearance in lettuce during storage. Int. J. Food Process. Technol. 2014, 1, 18–26. [Google Scholar]
- Viacava, G.E.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Ansorena, M.R. Effect of free and microencapsulated thyme essential oil on quality attributes of minimally processed lettuce. Postharvest Biol. Technol. 2018, 145, 125–133. [Google Scholar] [CrossRef]
- Ghoname, A.A.; Abou-Hussei, S.D.; El-Tohamy, W.A. Eustress (Positive stress) Salinity as an enhancement tool for bioactive ingredients and quality characteristics of vegetables. A review. Sciences 2019, 9, 456–463. [Google Scholar]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of some citrus essential oils on post-harvest shelf life and physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Kesraoui, S.; Andrés, M.F.; Berrocal-Lobo, M.; Soudani, S.; Gonzalez-Coloma, A. Direct and indirect effects of essential oils for sustainable crop protection. Plants 2022, 11, 2144. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Gu, Y.; Shi, J.; Yan, J.; Wang, X.; Li, B.; Wang, B.; Zhong, W.; Cao, H.; et al. Dietary flavonoids—Microbiota crosstalk in intestinal inflammation and carcinogenesis. J. Nutr. Biochem. 2023, 125, 109494. [Google Scholar]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Htwe, N.M.P.S.; Rawangpai, M.; Ruangrak, E. Effect of post-harvesting with different photoperiods under artificial light sources on nitrate and vitamin C contents in hydroponic green oak lettuce. ASEAN J. Sci. Technol. Rep. 2023, 26, 10–19. [Google Scholar] [CrossRef]
- Koukounaras, A. Senescence and quality of green leafy vegetables. Stewart Postharvest Rev. 2009, 5, 1–5. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in plants–maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Bardsley, C.A.; Boyer, R.R.; Rideout, S.L.; Strawn, L.K. Survival of Listeria monocytogenes on the surface of basil, cilantro, dill, and parsley plants. Food Control 2019, 95, 90–94. [Google Scholar] [CrossRef]
- Faour-Klingbeil, D.; Kuri, V.; Todd, E.C.D. The influence of pre-wash chopping and storage conditions of parsley on the efficacy of disinfection against S. Typhimurium. Food Control 2016, 65, 121–131. [Google Scholar] [CrossRef]
- Pizzo, J.S.; Pelvine, R.A.; da Silva, A.L.B.R.; Mikcha, J.M.G.; Visentainer, J.V.; Rodrigues, C. Use of essential oil emulsions to control Escherichia coli O157:H7 in the postharvest washing of lettuce. Foods 2023, 12, 2571. [Google Scholar] [CrossRef]
- Tornuk, F.; Cankurt, H.; Ozturk, I.; Sagdic, O.; Bayram, O.; Yetim, H. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella Typhimurium on fresh cut carrots and apples. Int. J. Food Microbiol. 2011, 148, 30–35. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros Barbosa, I.; da Costa Medeiros, J.A.; de Oliveira, K.Á.R.; Gomes-Neto, N.J.; Tavares, J.F.; Magnani, M.; de Souza, E.L. Efficacy of the combined application of oregano and rosemary essential oils for the control of Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis in leafy vegetables. Food Control 2016, 59, 468–477. [Google Scholar] [CrossRef]
- Sharifi, A.; Mohammadzadeh, A.; Zahraei Salehi, T.; Mahmoodi, P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 2018, 124, 379–388. [Google Scholar] [CrossRef] [PubMed]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Hajibonabi, A.; Yekani, M.; Sharifi, S.; Nahad, J.S.; Dizaj, S.M.; Memar, M.Y. Antimicrobial activity of nanoformulations of carvacrol and thymol: New trend and applications. OpenNano 2023, 13, 100170. [Google Scholar] [CrossRef]
- Marik, C.M.; Zuchel, J.; Schaffner, D.W.; Strawn, L.K. Growth and survival of listeria monocytogenes on intact fruit and vegetable surfaces during postharvest handling: A systematic literature review. J. Food Prot. 2020, 83, 108–128. [Google Scholar] [CrossRef]
- Lund, P.; Tramonti, A.; De Biase, D. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef]
- Donsì, F.; Cuomo, A.; Marchese, E.; Ferrari, G. Infusion of essential oils for food stabilization: Unraveling the role of nanoemulsion-based delivery systems on mass transfer and antimicrobial activity. Innov. Food Sci. Emerg. Technol. 2014, 22, 212–220. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Martino, L. De Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Mavis, M.; Ali, M.S.B.; Hanoglu, A.; Ozalp, Y.; Yavuz, D.O.; Baser, K.H.C.; Serakinci, N. Evaluation of Therapeutic role of Thymus capitatus (L.) Hoffm. & Link, Origanum dubium Boiss. Essential Oils and Their Major Constituents as Enhancers in Cancer Therapy. Rec. Nat. Prod. 2023, 17, 715–720. [Google Scholar]
- Chrysargyris, A.; Petrovic, J.D.; Tomou, E.; Kyriakou, K.; Xylia, P.; Kotsoni, A.; Gkretsi, V.; Miltiadous, P.; Skaltsa, H.; Sokovi, M.D.; et al. Phytochemical Profiles and Biological Activities of Plant Extracts from Aromatic Plants Cultivated in Cyprus. Biology 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Concentration | Time |
|---|---|---|
| Control | 0.00% | 1 min |
| EO | 0.001%, 0.01%, 0.1% | 5 min |
| Hydrosol | 0.001%, 0.01%, 0.01% | 10 min |
| Time (min) | Concentration | L* | a* | b* | h | C | CI | |
|---|---|---|---|---|---|---|---|---|
| Day 0 | 0 | 0.00% | 45.75 ± 0.51 | −18.15 ± 0.30 | 25.77 ± 0.39 | 125.16 ± 0.23 | 31.52 ± 0.48 | −15.41 ± 0.28 |
| EO | 0 | 0.00% | 44.48 ± 1.10 b | −18.23 ± 0.95 | 25.47 ± 1.54 | 125.67 ± 0.36 a | 31.32 ± 1.80 | −16.21 ± 0.55 b |
| 1 | 0.001% | 45.83 ± 0.84 ab | −17.42 ± 0.62 | 25.39 ± 0.60 | 124.42 ± 0.60 ab | 30.80 ± 0.80 | −14.96 ± 0.09 ab | |
| 0.01% | 48.38 ± 0.53 a | −18.58 ± 0.18 | 27.32 ± 0.39 | 124.24 ± 0.32 ab | 33.04 ± 0.39 | −14.08 ± 0.31 a | ||
| 0.10% | 44.89 ± 1.24 b | −17.65 ± 0.89 | 26.16 ± 1.50 | 124.06 ± 0.53 b | 31.56 ± 1.72 | −15.12 ± 0.44 ab | ||
| 5 | 0.001% | 46.17 ± 0.81 ab | −17.73 ± 0.34 | 26.17 ± 0.80 | 124.16 ± 0.47 b | 31.62 ± 0.83 | −14.74 ± 0.49 a | |
| 0.01% | 46.20 ± 0.93 ab | −16.99 ± 0.33 | 24.64 ± 0.39 | 124.59 ± 0.45 ab | 29.93 ± 0.45 | −14.98 ± 0.52 ab | ||
| 0.10% | 46.68 ± 0.69 ab | −17.05 ± 0.89 | 24.70 ± 1.49 | 124.68 ± 0.39 ab | 30.02 ± 1.72 | −14.85 ± 0.35 a | ||
| 10 | 0.001% | 46.34 ± 1.25 ab | −17.47 ± 0.54 | 26.02 ± 0.99 | 123.93 ± 0.54 b | 31.35 ± 1.09 | −14.60 ± 0.61 a | |
| 0.01% | 47.59 ± 0.75 ab | −18.71 ± 0.39 | 27.08 ± 0.80 | 124.69 ± 0.36 ab | 32.92 ± 0.86 | −14.58 ± 0.42 a | ||
| 0.10% | 46.27 ± 1.04 ab | −17.43 ± 0.54 | 27.57 ± 0.45 | 122.26 ± 0.40 c | 32.62 ± 0.66 | −13.72 ± 0.24 a | ||
| Hydrosol | 0 | 0.00% | 47.57 ± 1.38 | −18.58 ± 0.73 | 27.53 ± 1.34 ab | 124.07 ± 0.46 ab | 33.22 ± 1.50 ab | −14.30 ± 0.58 b |
| 1 | 0.001% | 47.52 ± 0.62 | −17.73 ± 0.51 | 26.23 ± 1.05 b | 124.011 ± 0.54 ab | 31.67 ± 1.13 b | −14.28 ± 0.41 b | |
| 0.01% | 47.73 ± 0.68 | −18.04 ± 0.26 | 27.17 ± 0.54 ab | 123.59 ± 0.34 ab | 32.62 ± 0.57 ab | −13.94 ± 0.29 ab | ||
| 0.10% | 48.18 ± 1.37 | −18.96 ± 0.78 | 29.29 ± 2.02 ab | 123.12 ± 0.69 ab | 34.91 ± 2.12 ab | −13.65 ± 0.66 ab | ||
| 5 | 0.001% | 47.67 ± 0.71 | −18.80 ± 0.55 | 28.03 ± 0.90 ab | 123.86 ± 0.40 ab | 33.76 ± 1.03 ab | −14.10 ± 0.31 ab | |
| 0.01% | 47.16 ± 0.78 | −18.38 ± 0.24 | 26.83 ± 0.77 ab | 124.48 ± 0.55 a | 32.53 ± 0.74 ab | −14.61 ± 0.50 b | ||
| 0.10% | 48.42 ± 0.89 | −18.65 ± 0.63 | 28.30 ± 1.16 ab | 123.43 ± 0.51 ab | 33.90 ± 1.29 ab | −13.68 ± 0.47 ab | ||
| 10 | 0.001% | 50.40 ± 0.72 | −19.45 ± 0.48 | 30.40 ± 0.82 a | 122.61 ± 0.32 b | 36.09 ± 0.93 a | −12.71 ± 0.26 a | |
| 0.01% | 47.98 ± 1.00 | −19.26 ± 0.49 | 27.96 ± 0.87 ab | 124.58 ± 0.25 a | 33.95 ± 0.99 ab | −14.41 ± 0.42 b | ||
| 0.10% | 49.02 ± 1.19 | −19.05 ± 0.91 | 28.59 ± 1.58 ab | 123.74 ± 0.27 ab | 34.36 ± 1.81 ab | −13.68 ± 0.45 ab |
| Time (min) | Concentration | Chl a (mg/g) | Chl b (mg/g) | Tot Chl (mg/g) | Tot Car (mg/g) | |
|---|---|---|---|---|---|---|
| Day 0 | 0 | 0.00% | 0.38 ± 0.06 | 0.14 ± 0.02 | 0.51 ± 0.08 | 0.09 ± 0.01 |
| EO | 0 | 0.00% | 0.72 ± 0.08 ab* | 0.31 ± 0.07 ab | 1.03 ± 0.14 ab* | 0.15 ± 0.01 ab* |
| 1 | 0.001% | 0.70 ± 0.03 ab | 0.27 ± 0.02 ab | 0.98 ± 0.06 ab | 0.15 ± 0.01 ab | |
| 0.01% | 0.61 ± 0.08 ab | 0.22 ± 0.04 ab | 0.83 ± 0.12 ab | 0.13 ± 0.02 ab | ||
| 0.10% | 0.66 ± 0.11 ab | 0.26 ± 0.07 ab | 0.92 ± 0.18 ab | 0.14 ± 0.02 ab | ||
| 5 | 0.001% | 0.77 ± 0.04 ab | 0.32 ± 0.03 ab | 1.09 ± 0.07 ab | 0.15 ± 0.00 ab | |
| 0.01% | 0.81 ± 0.03 a | 0.34 ± 0.02 a | 1.15 ± 0.05 a | 0.17 ± 0.01 a | ||
| 0.10% | 0.62 ± 0.02 ab | 0.22 ± 0.01 ab | 0.84 ± 0.03 ab | 0.14 ± 0.00 ab | ||
| 10 | 0.001% | 0.54 ± 0.06 b | 0.19 ± 0.03 b | 0.73 ± 0.09 b | 0.12 ± 0.01 b | |
| 0.01% | 0.71 ± 0.06 ab | 0.26 ± 0.03 ab | 0.97 ± 0.09 ab | 0.15 ± 0.01 ab | ||
| 0.10% | 0.64 ± 0.12 ab | 0.24 ± 0.05 ab | 0.87 ± 0.017 ab | 0.14 ± 0.02 ab | ||
| Hydrosol | 0 | 0.00% | 0.59 ± 0.08 abc | 0.22 ± 0.05 abc | 0.81 ± 0.12 abc | 0.13 ± 0.01 abc |
| 1 | 0.001% | 0.37 ± 0.10 c | 0.13 ± 0.03 c | 0.50 ± 0.13 c | 0.09 ± 0.02 c | |
| 0.01% | 0.73 ± 0.09 ab | 0.28 ± 0.06 ab | 1.01 ± 0.14 ab | 0.16 ± 0.02 a | ||
| 0.10% | 0.53 ± 0.06 abc | 0.18 ± 0.02 abc | 0.71 ± 0.08 abc | 0.12 ± 0.01 abc | ||
| 5 | 0.001% | 0.77 ± 0.03 a | 0.31 ± 0.03 a | 1.07 ± 0.06 a | 0.17 ± 0.01 a | |
| 0.01% | 0.51 ± 0.05 bc | 0.16 ± 0.01 bc | 0.67 ± 0.06 bc | 0.12 ± 0.01 bc | ||
| 0.10% | 0.54 ± 0.04 abc | 0.19 ± 0.02 abc | 0.73 ± 0.06 abc | 0.11 ± 0.01 bc | ||
| 10 | 0.001% | 0.72 ± 0.10 ab | 0.29 ± 0.06 ab | 1.01 ± 0.16 ab | 0.15 ± 0.02 ab | |
| 0.01% | 0.76 ± 0.06 a | 0.30 ± 0.05 a | 1.06 ± 0.11 a | 0.16 ± 0.01 a | ||
| 0.10% | 0.59 ± 0.09 abc | 0.21 ± 0.04 abc | 0.80 ± 0.13 abc | 0.13 ± 0.02 abc |
| Time (min) | Concentration | Phenols (mg GEA/g) | DPPH (mg trolox/g) | FRAP (mg trolox/g) | Flavonoids (mg rutin/g) | AA (mg AA/100 g) | |
|---|---|---|---|---|---|---|---|
| Day 0 | 0 | 0.00% | 3.92 ± 0.23 | 8.42 ± 0.24 | 11.26 ± 0.51 | 13.21 ± 0.59 | 5.52 ± 0.20 |
| EO | 0 | 0.00% | 5.67 ± 0.32 bc* | 7.64 ± 0.24 bcd | 10.32 ± 0.31 bcde | 11.17 ± 1.00 bc | 4.89 ± 0.18 cde |
| 1 | 0.001% | 5.65 ± 0.23 bc | 8.30 ± 0.05 abc | 11.39 ± 0.42 abcd | 11.97 ± 0.06 bc | 5.75 ± 0.21 abc | |
| 0.01% | 6.60 ± 0.23 b | 8.97 ± 0.21 a | 11.62 ± 0.86 abcd | 13.01 ± 1.06 bc | 5.39 ± 0.11 bcd | ||
| 0.10% | 4.53 ± 0.21 d | 6.70 ± 0.20 de | 8.40 ± 0.10 e | 9.40 ± 0.28 c | 4.27 ± 0.19 e | ||
| 5 | 0.001% | 5.96 ± 0.22 cd | 6.38 ± 0.37 e | 12.17 ± 1.71 abc | 15.10 ± 2.46 b | 5.47 ± 0.39 abcd | |
| 0.01% | 5.52 ± 0.20 bcd | 7.36 ± 0.26 cd | 9.73 ± 0.02 de | 13.39 ± 2.07 bc | 5.04 ± 0.22 bcde | ||
| 0.10% | 6.20 ± 0.66 b | 9.29 ± 0.34 a | 12.26 ± 0.73 ab | 15.20 ± 1.04 b | 4.24 ± 0.16 e | ||
| 10 | 0.001% | 6.09 ± 0.12 b | 8.63 ± 0.43 ab | 12.10 ± 0.41 abc | 12.46 ± 1.58 bc | 4.81 ± 0.35 de | |
| 0.01% | 8.51 ± 0.57 a | 9.18 ± 0.52 a | 12.85 ± 0.29 a | 20.46 ± 3.16 a | 6.27 ± 0.43 a | ||
| 0.10% | 5.85 ± 0.16 bc | 7.65 ± 0.24 bcd | 9.87 ± 0.38 cde | 10.90 ± 0.76 bc | 5.82 ± 0.27 ab | ||
| Hydrosol | 0 | 0.00% | 5.83 ± 0.61 ab | 7.92 ± 1.07 a | 10.75 ± 1.24 abc | 13.78 ± 1.03 a | 5.11 ± 0.09 c |
| 1 | 0.001% | 3.73 ± 0.45 c | 4.64 ± 0.38 c | 8.18 ± 0.32 d | 9.85 ± 1.03 ab | 6.59 ± 0.31 ab | |
| 0.01% | 6.60 ± 0.63 a | 8.51 ± 0.13 a | 11.85 ± 0.72 a | 13.48 ± 0.99 a | 6.40 ± 0.45 abc | ||
| 0.10% | 6.36 ± 0.26 a | 8.46 ± 0.60 a | 11.60 ± 1.00 a | 12.91 ± 1.89 a | 7.10 ± 0.39 ab | ||
| 5 | 0.001% | 5.38 ± 0.61 abc | 6.91 ± 1.04 ab | 9.14 ± 1.40 abcd | 11.68 ± 0.86 ab | 5.83 ± 0.33 bc | |
| 0.01% | 5.24 ± 0.18 abc | 6.90 ± 0.37 ab | 9.14 ± 0.62 abcd | 10.22 ± 0.77 ab | 5.85 ± 0.26 bc | ||
| 0.10% | 4.47 ± 0.76 bc | 4.91 ± 0.19 bc | 8.11 ± 1.07 bcd | 8.01 ± 0.70 b | 7.41 ± 0.53 a | ||
| 10 | 0.001% | 4.27 ± 0.49 bc | 5.45 ± 0.68 bc | 7.68 ± 1.01 cd | 8.18 ± 1.49 b | 7.16 ± 0.47 ab | |
| 0.01% | 5.90 ± 0.49 ab | 7.92 ± 0.52 a | 10.40 ± 0.87 abc | 11.57 ± 1.28 ab | 7.58 ± 0.73 a | ||
| 0.10% | 5.28 ± 0.71 abc | 7.83 ± 1.02 a | 11.50 ± 1.70 a | 13.44 ± 2.04 a | 6.52 ± 0.38 ab |
| Time (min) | Concentration | H2O2 (μmol/g) | MDA (nmol/g) | |
|---|---|---|---|---|
| Day 0 | 0 | 0.00% | 0.26 ± 0.02 | 4.00 ± 0.10 |
| EO | 0 | 0.00% | 0.37 ± 0.04 b | 7.04 ± 0.24 ab* |
| 1 | 0.001% | 0.39 ± 0.01 b | 6.73 ± 0.49 abc | |
| 0.01% | 0.40 ± 0.03 b | 7.68 ± 0.44 a | ||
| 0.10% | 0.39 ± 0.02 b | 6.69 ± 0.34 abc | ||
| 5 | 0.001% | 0.43 ± 0.01 ab | 6.84 ± 0.40 ab | |
| 0.01% | 0.50 ± 0.05 a | 6.41 ± 0.37 abc | ||
| 0.10% | 0.37 ± 0.01 b | 5.86 ± 0.33 bc | ||
| 10 | 0.001% | 0.39 ± 0.03 b | 5.88 ± 0.36 bc | |
| 0.01% | 0.39 ± 0.01 b | 5.75 ± 0.42 bc | ||
| 0.10% | 0.37 ± 0.02 b | 5.39 ± 0.64 c | ||
| Hydrosol | 0 | 0.00% | 0.42 ± 0.01 a* | 6.65 ± 0.15 a* |
| 1 | 0.001% | 0.35 ± 0.01 ab | 4.19 ± 0.55 e | |
| 0.01% | 0.38 ± 0.05 ab | 4.77 ± 0.35 de | ||
| 0.10% | 0.32 ± 0.01 b | 4.81 ± 0.21 de | ||
| 5 | 0.001% | 0.38 ± 0.02 ab | 5.75 ± 0.07 bc | |
| 0.01% | 0.38 ± 0.01 ab | 6.73 ± 0.13 a | ||
| 0.10% | 0.33 ± 0.01 b | 6.09 ± 0.10 ab | ||
| 10 | 0.001% | 0.33 ± 0.01 b | 6.05 ± 0.31 ab | |
| 0.01% | 0.38 ± 0.05 ab | 5.05 ± 0.12 cd | ||
| 0.10% | 0.32 ± 0.01 b | 4.49 ± 0.08 de |
| Concentration | S. enterica (log cfu/mL) | L. monocytogenes (log cfu/mL) | |
|---|---|---|---|
| Day 1 | Control | 6.29 ± 0.09 a | 6.09 ± 0.06 a |
| Chlorine | 4.37 ± 0.12 c | 5.11 ± 0.10 bc | |
| EO dose A | 4.53 ± 0.11 c | 5.35 ± 0.08 b | |
| EO dose B | 4.72 ± 0.33 bc | 4.88 ± 0.12 c | |
| Hydrosol dose A | 5.24 ± 0.15 b | 5.28 ± 0.05 b | |
| Hydrosol dose B | 4.68 ± 0.31 bc | 5.01 ± 0.03 c | |
| Day 6 | Control | 6.13 ± 0.02 a | 6.04 ± 0.05 a |
| Chlorine | 4.94 ± 0.23 bc | 4.36 ± 0.09 d | |
| EO dose A | 4.45 ± 0.16 c | 4.35 ± 0.06 d | |
| EO dose B | 5.18 ± 0.30 b | 4.46 ± 0.11 d | |
| Hydrosol dose A | 5.77 ± 0.04 a | 4.88 ± 0.05 c | |
| Hydrosol dose B | 5.72 ± 0.15 a | 5.39 ± 0.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The Postharvest Safety and Quality of Fresh Basil as Affected by the Use of Cypriot Oregano (Origanum dubium) Extracts. Horticulturae 2024, 10, 159. https://doi.org/10.3390/horticulturae10020159
Xylia P, Chrysargyris A, Tzortzakis N. The Postharvest Safety and Quality of Fresh Basil as Affected by the Use of Cypriot Oregano (Origanum dubium) Extracts. Horticulturae. 2024; 10(2):159. https://doi.org/10.3390/horticulturae10020159
Chicago/Turabian StyleXylia, Panayiota, Antonios Chrysargyris, and Nikolaos Tzortzakis. 2024. "The Postharvest Safety and Quality of Fresh Basil as Affected by the Use of Cypriot Oregano (Origanum dubium) Extracts" Horticulturae 10, no. 2: 159. https://doi.org/10.3390/horticulturae10020159
APA StyleXylia, P., Chrysargyris, A., & Tzortzakis, N. (2024). The Postharvest Safety and Quality of Fresh Basil as Affected by the Use of Cypriot Oregano (Origanum dubium) Extracts. Horticulturae, 10(2), 159. https://doi.org/10.3390/horticulturae10020159








