Exogenous Melatonin as Pre- and Postharvest Application on Quality Attributes, Antioxidant Capacity, and Extension of Shelf Life of Papaya
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Treatment
2.2. Determination of Weight Loss and Fruit Firmness
2.3. Determination of Ripening Enzymes (Pectin Methylesterase, Polygalacturonase, Amylase and Cellulase)
2.4. Determination of Antioxidant Enzymes (POD, CAT, SOD)
2.5. Determination of Ethylene, CO2 Evolution Rate and Textural Characteristics
2.6. Statistics
3. Results
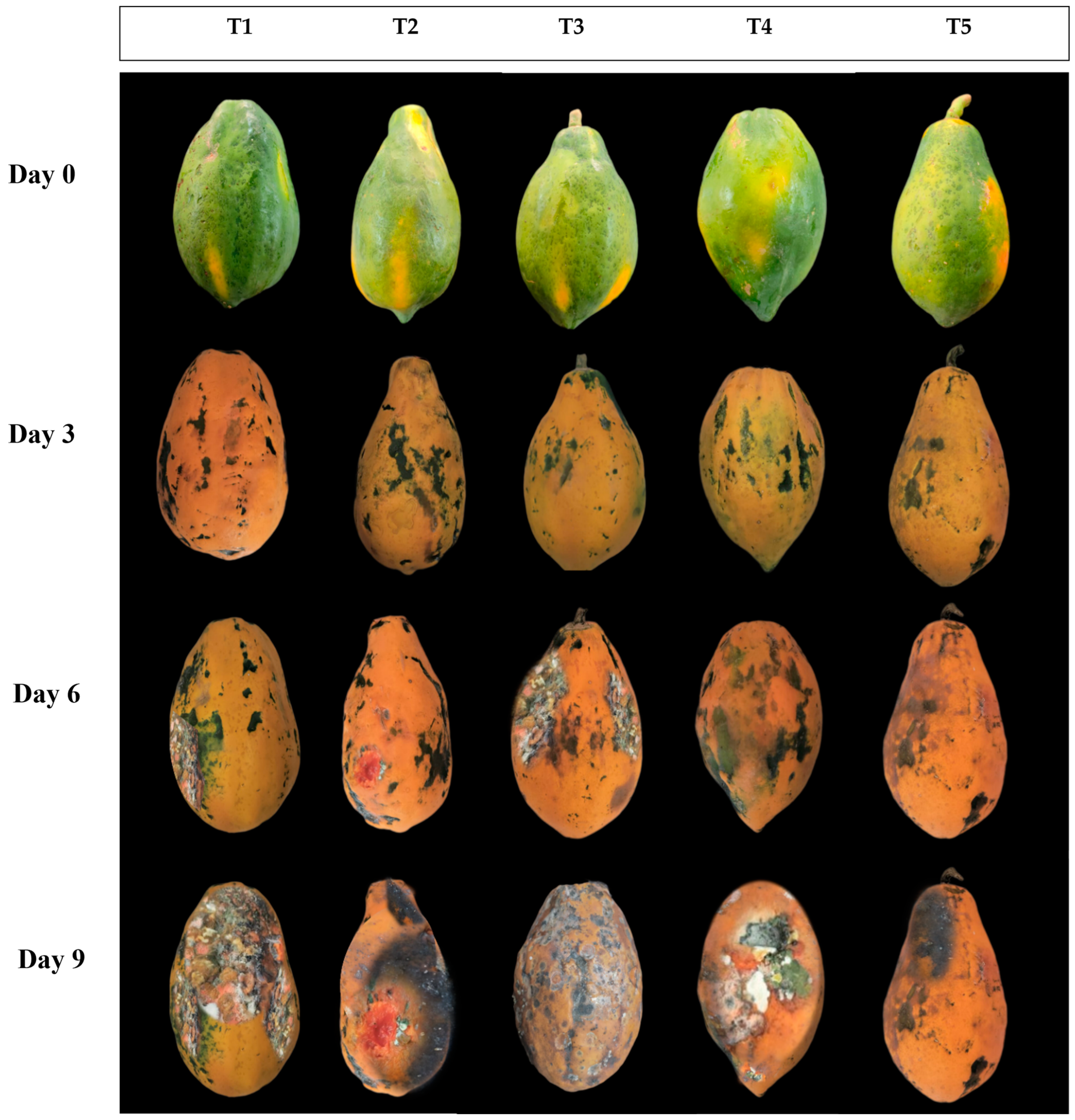
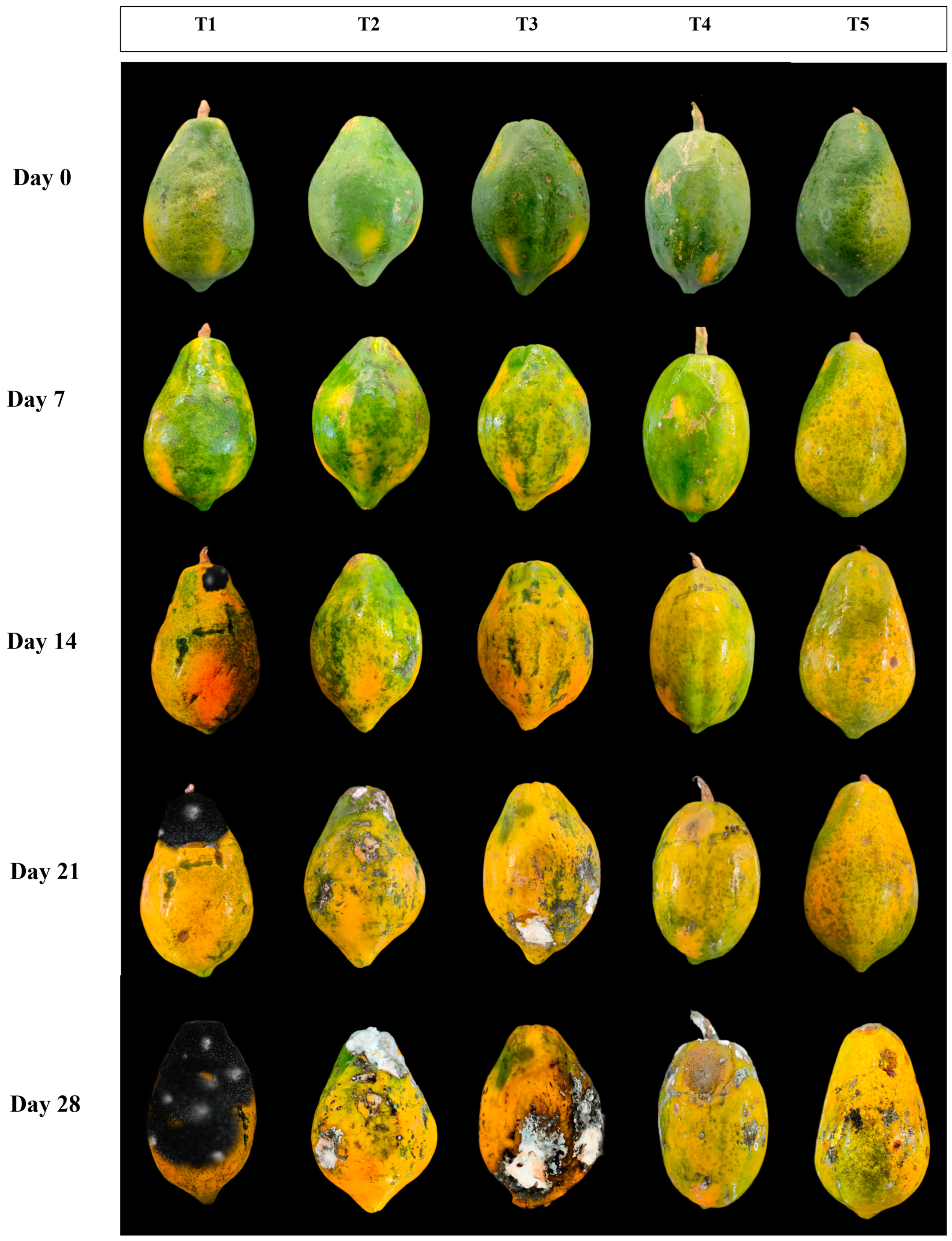
3.1. Influence of EMT Treatment on Physiological Loss in Weight and Fruit Firmness
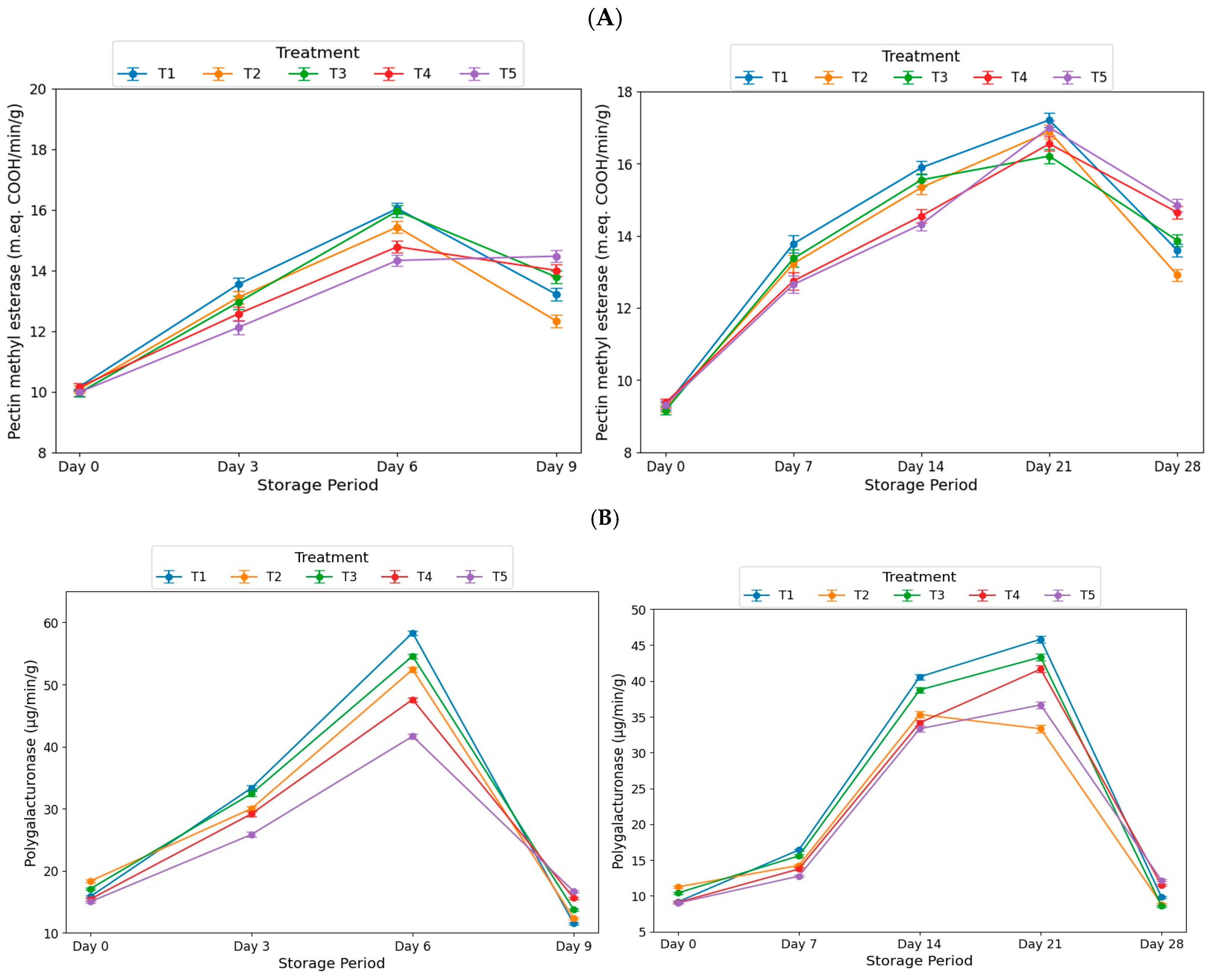
3.2. Effect of EMT Treatment on Ripening Enzymes
3.3. Influence of EMT Treatment on Antioxidant Enzymes
3.4. Effect of EMT Treatment on Ethylene, CO2 Evolution Rate and Textural Characteristics
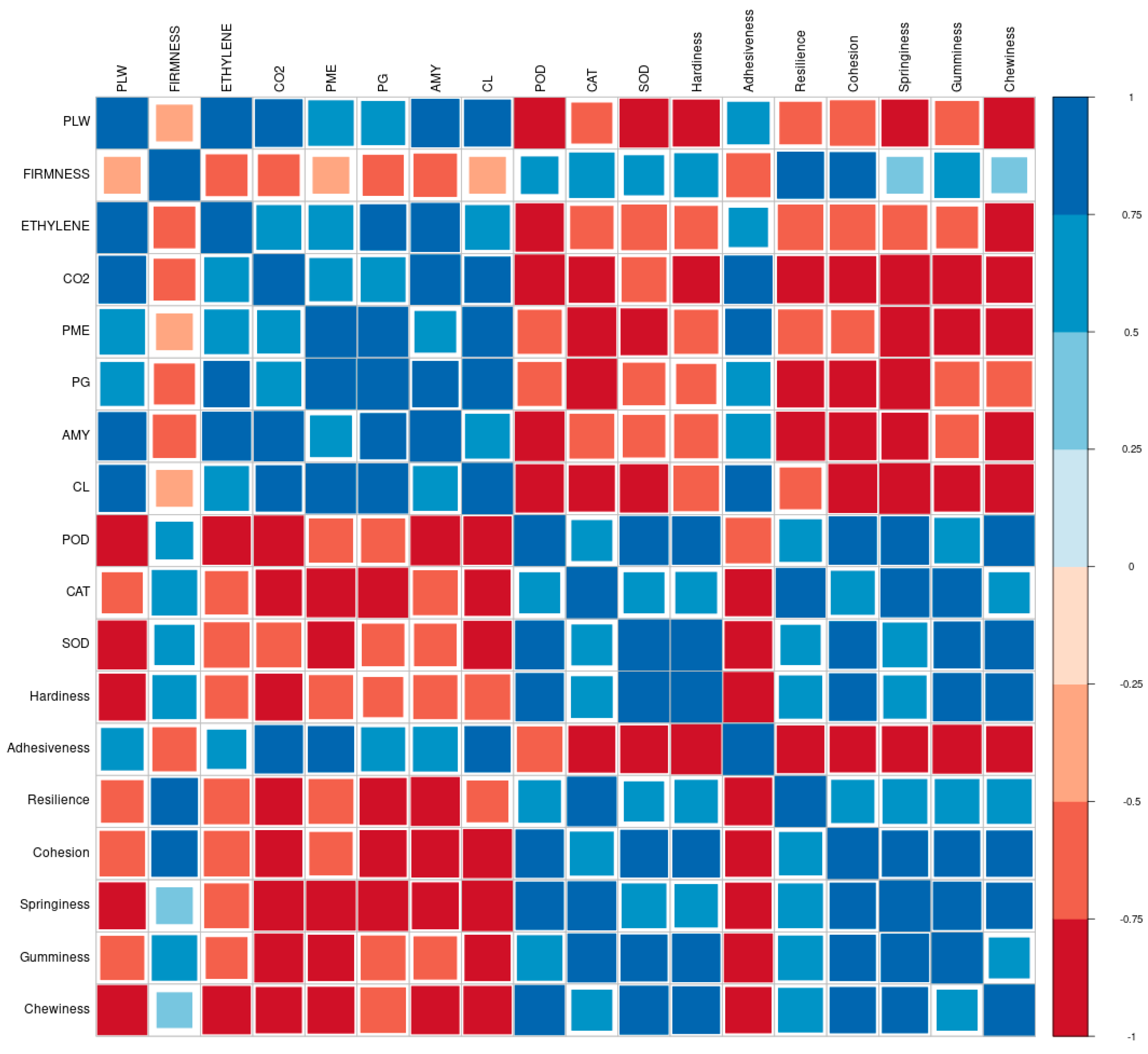
3.5. Correlation Matrix
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. 2022. Available online: https://www.statista.com/statistics/578039/world-papaya-production (accessed on 4 September 2024).
- de Vasconcellos Santos Batista, D.; Reis, R.C.; Almeida, J.M.; Rezende, B.; Bragança, C.A.D.; da Silva, F. Edible coatings in post-harvest papaya: Impact on physical–chemical and sensory characteristics. J. Food Sci. Technol. 2020, 57, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Palma, J.M.; Corpas, F.J. NADPH as a quality footprinting in horticultural crops marketability. Trends Food Sci. Technol. 2020, 103, 152–161. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Barrett, D.M.; Lloyd, B. Advanced preservation methods and nutrient retention in fruits and vegetables. J. Sci. Food Agric. 2012, 92, 7–22. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Z.; Chen, X.; Yun, Z.; Li, T.; Jiang, Y. Comparative metabolites profiling of harvested papaya (Carica papaya L.) peel in response to chilling stress. J. Sci. Food Agric. 2019, 99, 6868–6881. [Google Scholar] [CrossRef]
- Wang, D.; Randhawa, M.S.; Azam, M.; Liu, H.; Ejaz, S.; Ilahy, R.; Qadri, R.; Khan, M.I.; Umer, M.A.; Khan, M.A. Exogenous melatonin treatment reduces postharvest senescence and maintains the quality of papaya fruit during cold storage. Front. Plant Sci. 2022, 13, 1039373. [Google Scholar] [CrossRef]
- Hanif, A.; Ahmad, S.; Shahzad, S.; Liaquat, M.; Anwar, R. Postharvest application of salicylic acid reduced decay and enhanced storage life of papaya fruit during cold storage. J. Food Meas. Charact. 2020, 14, 3078–3088. [Google Scholar] [CrossRef]
- Ong, M.K.; Kazi, F.K.; Forney, C.F.; Ali, A. Effect of gaseous ozone on papaya anthracnose. Food Bioprocess Technol. 2013, 6, 2996–3005. [Google Scholar] [CrossRef]
- Albertini, S.; Reyes, A.E.L.; Trigo, J.M.; Sarriés, G.A.; Spoto, M.H.F. Effects of chemical treatments on fresh-cut papaya. Food Chem. 2016, 190, 1182–1189. [Google Scholar] [CrossRef]
- Vilaplana, R.; Chicaiza, G.; Vaca, C.; Valencia-Chamorro, S. Combination of hot water treatment and chitosan coating to control anthracnose in papaya (Carica papaya L.) during the postharvest period. Crop Prot. 2020, 128, 105007. [Google Scholar] [CrossRef]
- Cia, P.; Pascholati, S.F.; Benato, E.A.; Camili, E.C.; Santos, C.A. Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biol. Technol. 2007, 43, 366–373. [Google Scholar] [CrossRef]
- dos Passos Braga, S.; Magnani, M.; Madruga, M.S.; de Souza Galvão, M.; de Medeiros, L.L.; Batista, A.U.D.; Dias, R.T.A.; Fernandes, L.R.; de Medeiros, E.S.; de Souza, E.L. Characterization of edible coatings formulated with chitosan and Mentha essential oils and their use to preserve papaya (Carica papaya L.). Innov. Food Sci. Emerg. Technol. 2020, 65, 102472. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Vanyo, M. Trends in the airglow temperatures in the MLT region—Part 1: Model simulations. Atmosphere 2020, 11, 468. [Google Scholar] [CrossRef]
- Fan, Y.; Li, C.; Li, Y.; Huang, R.; Guo, M.; Liu, J.; Sun, T.; Ge, Y. Postharvest melatonin dipping maintains quality of apples by mediating sucrose metabolism. Plant Physiol. Biochem. 2022, 174, 43–50. [Google Scholar] [CrossRef]
- Annadurai, M.K.K.; Alagarsamy, S.; Karuppasami, K.M.; Ramakrishnan, S.; Subramanian, M.; Venugopal, P.R.; Muthurajan, R.; Vellingiri, G.; Dhashnamurthi, V.; Veerasamy, R. Melatonin decreases negative effects of combined drought and high temperature stresses through enhanced antioxidant defense system in tomato leaves. Horticulturae 2023, 9, 673. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria× anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.-X. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.; Zheng, X.; Tian, Y.; Shi, L.; Dong, P.; Li, Z. Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Sharafi, Y.; Jannatizadeh, A.; Fard, J.R.; Aghdam, M.S. Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci. Hortic. 2021, 288, 110304. [Google Scholar] [CrossRef]
- Wang, Z.; Pu, H.; Shan, S.; Zhang, P.; Li, J.; Song, H.; Xu, X. Melatonin enhanced chilling tolerance and alleviated peel browning of banana fruit under low temperature storage. Postharvest Biol. Technol. 2021, 179, 111571. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Austin, P.J. Continuous spectrophotometric assay for plant pectin methyl esterase. J. Agric. Food Chem. 1986, 34, 440–444. [Google Scholar] [CrossRef]
- Gayathri, T.; Nair, A.S. Biochemical analysis and activity profiling of fruit ripening enzymes in banana cultivars from Kerala. J. Food Meas. Charact. 2017, 11, 1274–1283. [Google Scholar] [CrossRef]
- Bernfeld, B. Amylases α and α. Methods Enzym. 1955, 1, 149–158. [Google Scholar]
- Li, R.; Wang, Y.; Li, W.; Shao, Y. Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage. Horticulturae 2023, 9, 126. [Google Scholar] [CrossRef]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; Volume 2, pp. 685–690. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Zhao, N.; Fu, D.; Li, J.; Chen, W.; Chen, W. Effects of hot water treatment on anthracnose disease in papaya fruit and its possible mechanism. Postharvest Biol. Technol. 2013, 86, 437–446. [Google Scholar] [CrossRef]
- Zhu, X.; Ye, L.; Ding, X.; Gao, Q.; Xiao, S.; Tan, Q.; Huang, J.; Chen, W.; Li, X. Transcriptomic analysis reveals key factors in fruit ripening and rubbery texture caused by 1-MCP in papaya. BMC Plant Biol. 2019, 19, 1–22. [Google Scholar] [CrossRef]
- Fabi, J.P.; Cordenunsi, B.R.; de Mattos Barreto, G.P.; Mercadante, A.Z.; Lajolo, F.M.; Nascimento, J.R.O.D. Papaya fruit ripening: Response to ethylene and 1-methylcyclopropene (1-MCP). J. Agric. Food Chem. 2007, 55, 6118–6123. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, S.; Wang, Y.; Xu, G.; Zhao, D. Evaluation of the Effect of Preharvest Melatonin Spraying on Fruit Quality of ‘Yuluxiang’ Pear Based on Principal Component Analysis. Foods 2023, 12, 3507. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.-F.; Xu, T.-F.; Song, C.-Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z.-W.; Xi, Z.-M. Melatonin treatment of pre-veraison grape berries to increase size and synchronicity of berries and modify wine aroma components. Food Chem. 2015, 185, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tijero, V.; Munoz, P.; Munne-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef]
- El-Naby, A.; Mohamed, M.A.A.; El-Naggar, Y.I.M. Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘Canino’apricot fruits. Acta Sci. Polonorum. Hortorum Cultus 2019, 18, 167–174. [Google Scholar]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Kucuker, E.; Aglar, E.; Sakaldaş, M.; Şen, F.; Gundogdu, M. Impact of Postharvest Putrescine Treatments on Phenolic Compounds, Antioxidant Capacity, Organic Acid Contents and Some Quality Characteristics of Fresh Fig Fruits during Cold Storage. Plants 2023, 12, 1291. [Google Scholar] [CrossRef]
- Anchana, K.; Kavitha, C.; Shanmugasundaram, K.; Djanaguiraman, M.; Johnson, I. Role of Exogenous Melatonin in Enhancing Shelf Life of Traditional Banana Varieties. Int. J. Environ. Clim. Chang. 2023, 13, 992–998. [Google Scholar] [CrossRef]
- Rasouli, M.; Saba, M.K.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin treatment improves postharvest preservation and resistance of guava fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid treatment alleviates chilling injury in peach fruit by promoting sugar and ethylene metabolism. Food Chem. 2021, 338, 128005. [Google Scholar] [CrossRef]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Sati, H.; Bhardwaj, R.; Fawole, O.A.; Pareek, S. Postharvest Melatonin Application Preserves Quality and Imparts Chilling Tolerance in Peaches. J. Food Biochem. 2023, 1, 8126640. [Google Scholar] [CrossRef]
- Badiche-El Hilali, F.; Valverde, J.M.; García-Pastor, M.E.; Serrano, M.; Castillo, S.; Valero, D. Melatonin postharvest treatment in leafy ‘Fino’ lemon maintains quality and bioactive compounds. Foods 2023, 12, 2979. [Google Scholar] [CrossRef]
- Dogan, A. Effects of different oxygen levels with high-carbon dioxide atmosphere on postharvest quality of fresh fig under palliflex storage systems. Horticulturae 2022, 8, 353. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-Lopez, A. Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.E.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M. Postharvest exogenous melatonin treatment of table grape berry enhances quality and maintains bioactive compounds during refrigerated storage. Horticulturae 2022, 8, 860. [Google Scholar] [CrossRef]
- Li, C.; Xin, M.; Li, L.; He, X.; Liu, G.; Li, J.; Sheng, J.; Sun, J. Transcriptome profiling reveals the mechanism of ripening and epidermal senescence in passion (Passiflora edulia Sims) fruit. bioRxiv 2020. [Google Scholar] [CrossRef]
- Khattak, S.H.; Kaleem, I.; Farrukh, A.; Noor, S.; Jamil, K.; Kamal, T.; Siddiqui, N.R.; Ali, G.M. Fruit Ripening Characterization and Amylase Mystery in Bananas. Glob. J. Nutr. Food Sci. 2022, 4. [Google Scholar] [CrossRef]
- Goyal, H.; Gill, M.S.; Gill, P.S.; Jawandha, S.K.; Singh, N. Changes in physicochemical and enzymatic activities of mango hybrids during fruit ripening. Erwerbs-Obstbau 2023, 65, 355–362. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, D.T.; Ali, M.; Liu, Y.; Zhuang, Q.G.; Wadood, S.A.; Liao, Q.H.; Liu, H.Y.; Gan, R.Y. Innovative postharvest strategies for maintaining the quality of kiwifruit during storage: An updated review. Food Front. 2024, 5, 1933–1950. [Google Scholar] [CrossRef]
- Liu, B.; Xin, Q.; Zhang, M.; Chen, J.; Lu, Q.; Zhou, X.; Li, X.; Zhang, W.; Feng, W.; Pei, H. Research progress on mango post-harvest ripening physiology and the regulatory technologies. Foods 2022, 12, 173. [Google Scholar] [CrossRef]
- Njie, A.; Zhang, W.e.; Dong, X.; Lu, C.; Pan, X.; Liu, Q. Effect of Melatonin on Fruit Quality via Decay Inhibition and Enhancement of Antioxidative Enzyme Activities and Genes Expression of Two Mango Cultivars during Cold Storage. Foods 2022, 11, 3209. [Google Scholar] [CrossRef]
- Edema, H.; Ashraf, M.F.; Samkumar, A.; Jaakola, L.; Karppinen, K. Characterization of cellulases from softening fruit for enzymatic depolymerization of cellulose. Carbohydr. Polym. 2024, 343, 122493. [Google Scholar] [CrossRef]
- Gülsoy, E.; Kaya, T.; Pehluvan, M.; Çokran, B.D. Textural and physico-chemical characteristics of Șalak (Apricose) apricot cultivar. In Proceedings of the VII International Scientific Agriculture Symposium, Jahorina, Bosnia and Herzegovina, 6–9 October 2016. [Google Scholar]
- Wang, H.; Wang, J.; Mujumdar, A.; Jin, X.; Liu, Z.-L.; Zhang, Y.; Xiao, H.-W. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 118, 106808. [Google Scholar] [CrossRef]
- Ramasamy, K.; Karuppasami, K.M.; Alagarswamy, S.; Shanmugam, K.P.; Rathinavelu, S.; Vellingiri, G.; Muniyappan, U.; Kanthan, T.; Kuppusamy, A.; Rajendran, M. Role of melatonin in directing plant physiology. Agronomy 2023, 13, 2405. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Ngoc, T.T.B.; Thu, V.M.; Hien, N.T.P.; Tra, H.T.T.; Anh, H.D.; Hue, T.T. Effect of exogenous melatonin on antioxidant enzyme activities and membrane lipid peroxidation in avocado fruit during ripening. Vietnam. J. Biotechnol. 2022, 20, 495–504. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Madebo, M.P.; Hu, S.; Zheng, Y.; Jin, P. Mechanisms of chilling tolerance in melatonin treated postharvest fruits and vegetables: A review. J. Future Foods 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Shang, F.; Liu, R.; Wu, W.; Han, Y.; Fang, X.; Chen, H.; Gao, H. Effects of melatonin on the components, quality and antioxidant activities of blueberry fruits. LWT 2021, 147, 111582. [Google Scholar] [CrossRef]
- Pan, Y.-G.; Yuan, M.-Q.; Zhang, W.-M.; Zhang, Z.-K. Effect of low temperatures on chilling injury in relation to energy status in papaya fruit during storage. Postharvest Biol. Technol. 2017, 125, 181–187. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Yahia, E.M.; Golding, J.B.; Vand, S.H. Postharvest melatonin treatment reduces chilling injury in sapota fruit. J. Sci. Food Agric. 2020, 100, 1897–1903. [Google Scholar] [CrossRef]



| Storage Period Treatment | Ambient | Cold | |||||
|---|---|---|---|---|---|---|---|
| 3 Days | 6 Days | 9 Days | 7 Days | 14 Days | 21 Days | 28 Days | |
| T1 | 1.73 b | 4.67 a | 7.42 b | 1.77 c | 5.05 a | 6.84 a | 10.09 a |
| T2 | 1.78 a | 3.99 c | 6.07 c | 2.12 a | 4.48 b | 6.72 a | 9.90 ab |
| T3 | 1.82 a | 4.48 b | 6.85 a | 2.43 b | 3.99 c | 6.06 b | 9.70 bc |
| T4 | 1.57 c | 3.14 d | 5.94 c | 1.63 d | 3.70 d | 5.48 c | 9.62 c |
| T5 | 1.04 d | 2.61 e | 5.74 d | 1.62 d | 3.59 e | 5.32 c | 9.06 d |
| SE (d) | 0.02 | 0.07 | 0.07 | 0.03 | 0.04 | 0.09 | 0.09 |
| CD (p ≤ 0.05) | 0.04 | 0.14 | 0.16 | 0.06 | 0.08 | 0.19 | 0.21 |
| Storage Period Treatment | Ambient | Cold | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 3 Days | 6 Days | 9 Days | 0 Day | 7 Days | 14 Days | 21 Days | 28 Days | |
| T1 | 8.00 ab | 7.54 ab | 6.89 b | 6.21 b | 7.90 a | 7.44 ab | 7.27 a | 6.91 bc | 6.00 b |
| T2 | 8.20 a | 7.38 bc | 6.55 c | 5.83 c | 7.80 a | 7.29 bc | 6.95 b | 6.77 cd | 5.54 d |
| T3 | 7.83 b | 7.23 c | 6.71 bc | 6.00 c | 7.72 a | 7.16 c | 6.81 b | 6.59 d | 5.76 c |
| T4 | 7.90 ab | 7.60 ab | 7.00 ab | 6.77 a | 8.00 a | 7.55 a | 7.33 a | 7.17 ab | 6.21 a |
| T5 | 7.95 ab | 7.71 a | 7.20 a | 6.91 a | 7.86 a | 7.60 a | 7.42 a | 7.26 a | 6.37 a |
| SE (d) | 0.14 | 0.11 | 0.14 | 0.08 | 0.14 | 0.08 | 0.07 | 0.13 | 0.09 |
| CD (p ≤ 0.05) | 0.30 | 0.24 | 0.29 | 0.18 | 0.30 | 0.17 | 0.17 | 0.28 | 0.20 |
| Parameters | Storage Period Treatment | Day 3 | Day 6 | Day 9 |
|---|---|---|---|---|
| Hardness | T1 | 2768.78 a | 1523.07 b | 362.11 b |
| T2 | 2608.05 b | 2008.04 a | 1906.22 a | |
| SE (d) | 33.83 | 31.10 | 9.47 | |
| CD | 82.79 | 76.12 | 23.18 | |
| Adhesiveness | T1 | −73.29 a | −149.35 a | −210.95 a |
| T2 | −167.62 b | −201.25 b | −438.14 b | |
| SE (d) | 1.52 | 3.36 | 4.34 | |
| CD | 3.73 | 8.23 | 10.64 | |
| Resilience | T1 | 3.08 b | 1.84 b | 0.66 b |
| T2 | 3.25 a | 2.51 a | 1.98 a | |
| SE (d) | 0.06 | 0.03 | 0.02 | |
| CD | 0.16 | 0.07 | 0.06 | |
| Cohesion | T1 | 0.27 b | 0.25 b | 0.15 b |
| T2 | 0.43 a | 0.37 a | 0.29 a | |
| SE (d) | 0.003 | 0.006 | 0.001 | |
| CD | 0.008 | 0.016 | 0.002 | |
| Springiness | T1 | 90.37 b | 84.20 b | 83.70 b |
| T2 | 94.32 a | 92.84 a | 90.37 a | |
| SE (d) | 1.27 | 0.95 | 0.54 | |
| CD | 3.12 | 2.34 | 1.33 | |
| Gumminess | T1 | 680.14 b | 346.01 b | 134.80 b |
| T2 | 1113.78 a | 579.77 a | 279.37 a | |
| SE (d) | 10.98 | 7.12 | 2.06 | |
| CD | 26.86 | 17.43 | 5.05 | |
| Chewiness | T1 | 614.65 b | 306.09 b | 127.14 b |
| T2 | 1034.02 a | 523.94 a | 233.84 a | |
| SE (d) | 10.43 | 5.02 | 1.99 | |
| CD | 25.53 | 12.30 | 4.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borthakur, P.; Chinnasamy, K.; Paramasivam, S.K.; Venkatachalam, S.; Alagarswamy, S.; Iruthayasamy, J.; Thiyagarajan, E.; Muthusamy, S. Exogenous Melatonin as Pre- and Postharvest Application on Quality Attributes, Antioxidant Capacity, and Extension of Shelf Life of Papaya. Horticulturae 2024, 10, 1099. https://doi.org/10.3390/horticulturae10101099
Borthakur P, Chinnasamy K, Paramasivam SK, Venkatachalam S, Alagarswamy S, Iruthayasamy J, Thiyagarajan E, Muthusamy S. Exogenous Melatonin as Pre- and Postharvest Application on Quality Attributes, Antioxidant Capacity, and Extension of Shelf Life of Papaya. Horticulturae. 2024; 10(10):1099. https://doi.org/10.3390/horticulturae10101099
Chicago/Turabian StyleBorthakur, Priyaxee, Kavitha Chinnasamy, Suresh Kumar Paramasivam, Sivakumar Venkatachalam, Senthil Alagarswamy, Johnson Iruthayasamy, Elaiyabarathi Thiyagarajan, and Saraladevi Muthusamy. 2024. "Exogenous Melatonin as Pre- and Postharvest Application on Quality Attributes, Antioxidant Capacity, and Extension of Shelf Life of Papaya" Horticulturae 10, no. 10: 1099. https://doi.org/10.3390/horticulturae10101099
APA StyleBorthakur, P., Chinnasamy, K., Paramasivam, S. K., Venkatachalam, S., Alagarswamy, S., Iruthayasamy, J., Thiyagarajan, E., & Muthusamy, S. (2024). Exogenous Melatonin as Pre- and Postharvest Application on Quality Attributes, Antioxidant Capacity, and Extension of Shelf Life of Papaya. Horticulturae, 10(10), 1099. https://doi.org/10.3390/horticulturae10101099







