Effects of Different Solvents on the Extraction of Phenolic and Flavonoid Compounds, and Antioxidant Activities, in Scutellaria baicalensis Hairy Roots
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of the Sample Extract
2.2.1. Hot Water Extraction
2.2.2. Alcoholic Solvent Extraction
2.3. Analysis of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Analysis of Individual Phenolic and Flavonoid Contents by High-Performance Liquid Chromatography (HPLC)
2.5. Antioxidant Activities
2.5.1. 2,2-Di Phenyl-1-Picryl Hydrazyl (DPPH) Free Radical-Scavenging Activity
2.5.2. 2,2′-Azino-bis 3-Ethylbenzo Thiazoline-6-Sulfonic Acid (ABTS) Free Radical-Scavenging Activity
2.5.3. Super Oxide Dismutase (SOD)-like-Scavenging Activity
2.5.4. Reducing Power Assay
2.6. Statistical Analysis
3. Results
3.1. Analysis of Yield (%), TPC, and TFC
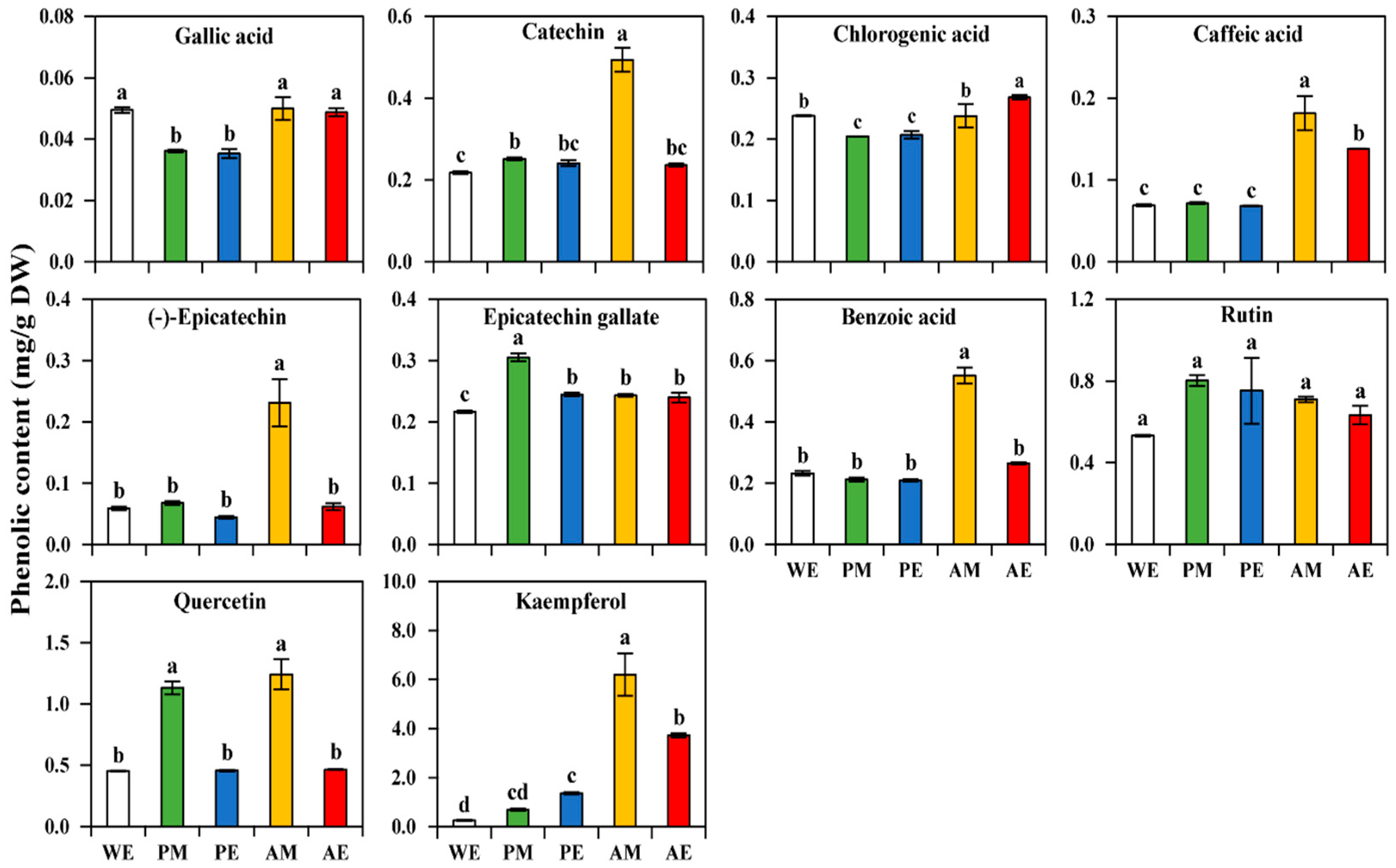
3.2. Analysis of Individual Phenolics (Including Flavanols and Flavonols) Using HPLC
3.3. Analysis of Individual Flavonoids Using HPLC
3.4. In Vitro Antioxidant Activities of S. baicalensis HR Extracts
3.5. Pearson Correlation Analysis between the Phenolic and Flavonoid Compounds and Antioxidant Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Choe, S.; Yang, K. Toxicological studies of antioxidants, butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA). Korean J. Food Sci. Technol. 1982, 14, 283–288. [Google Scholar]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-R.; Lee, J.-S.; Lim, H.-S. Changes in physiological activities of Scutellariae baicalensis by heating. J. Life Sci. 2007, 17, 1381–1386. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Xu, M.; Sun, J.; Wang, B.; Guo, D. Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. B 2007, 848, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.O.; Park, C.H.; Lee, G.-H.; Yokozawa, T.; Roh, S.-S.; Rhee, M.H. Heat-processed Scutellariae radix enhances anti-inflammatory effect against lipopolysaccharide-induced acute lung injury in mice via NF-κB signaling. Evid. Based Complement. Altern. Med. 2015, 2015, 456846. [Google Scholar] [CrossRef]
- Horvath, C.R.; Martos, P.A.; Saxena, P.K. Identification and quantification of eight flavones in root and shoot tissues of the medicinal plant Huang-qin (Scutellaria baicalensis Georgi) using high-performance liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A 2005, 1062, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Kwon, H.-S.; No, R.H.; Choi, G.P.; Lee, H.Y. Enhancement of anti-inflammatory activities of fermented Scutellaria baicalensis extracts using Lactobacillus rhamnosus. J. Soc. Cosmet. Sci. Korea 2013, 39, 303–311. [Google Scholar]
- Zhang, D.Y.; Wu, J.; Ye, F.; Xue, L.; Jiang, S.; Yi, J.; Zhang, W.; Wei, H.; Sung, M.; Wang, W. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003, 63, 4037–4043. [Google Scholar]
- Park, J.-H.; Kim, R.-Y.; Park, E. Antioxidant and α-glucosidase inhibitory activities of different solvent extracts of skullcap (Scutellaria baicalensis). Food Sci. Biotechnol. 2011, 20, 1107–1112. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta Gen. Subj. 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy root culture: A potent method for improved secondary metabolite production of Solanaceous plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy root cultures—A versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Rawat, J.M.; Bhandari, A.; Raturi, M.; Rawat, B. Agrobacterium rhizogenes Mediated Hairy Root Cultures: A Promising Approach for Production of Useful Metabolites. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–118. [Google Scholar]
- Huang, W.-H.; Lee, A.-R.; Yang, C.-H. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci. Biotechnol. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef]
- Kim, Y. Antibiofilm Activity of Scutellaria baicalensis through the inhibition of synthesis of the cell wall (1,3)-β-D-Glucan Polymer. MBL 2013, 41, 88–95. [Google Scholar]
- Lee, H.-H.; Kim, J.-S.; Jeong, J.-H.; Park, S.M.; Sathasivam, R.; Lee, S.Y.; Kim, C.S. Effect of different solvents on the extraction of compounds from different parts of Undaria pinnatifida (Harvey) Suringar. J. Mar. Sci. Eng. 2022, 10, 1193. [Google Scholar] [CrossRef]
- Park, C.H.; Xu, H.; Yeo, H.J.; Park, Y.E.; Hwang, G.-S.; Park, N.I.; Park, S.U. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metab. Eng. 2021, 64, 64–73. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Impact of different extraction solvents on phenolic content and antioxidant potential of Pinus densiflora bark extract. Biomed. Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef]
- El Mannoubi, I. Impact of different solvents on extraction yield, phenolic composition, in vitro antioxidant and antibacterial activities of deseeded Opuntia stricta fruit. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 176–184. [Google Scholar] [CrossRef]
- Park, C.H.; Baskar, T.B.; Park, S.-Y.; Kim, S.-J.; Valan Arasu, M.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus). Molecules 2016, 21, 157. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Park, W.T.; Yeo, S.K.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Influence of light-emitting diodes on phenylpropanoid biosynthetic gene expression and phenylpropanoid accumulation in Agastache rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- De Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; Da Rosa, M.B. A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV–Vis spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Oktay, M.; Küfrevioğlu, Ö.İ.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica L. Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.; Gu, Y.R.; Hong, J.-H. Extraction solvent-dependent antioxidant activities and cancer cell growth inhibitory effects of Scutellaria baicalensis extracts. Korean J. Food Preserv. 2019, 26, 566–575. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Solov’eva, A.I.; Stepanova, A.Y.; Panov, Y.M.; Gladkov, E.A. Metabolic characteristics of hairy root clones of Scutellaria pycnoclada and Scutellaria baicalensis. Processes 2023, 11, 2102. [Google Scholar] [CrossRef]
- Solov’eva, A.; Stepanova, A.; Ivanova, T.; Voronkov, A.; Panov, Y.M. Dose-dependent effect of H2O2 on hairy roots of Scutellaria baicalensis: Growth, composition of fatty acids, and flavones. Plant Cell Tissue Organ Cult. 2023, 155, 893–905. [Google Scholar] [CrossRef]
- Kovács, G.; Kuzovkina, I.; Szoke, E.; Kursinszki, L. HPLC determination of flavonoids in hairy-root cultures of Scutellaria baicalensis Georgi. Chromatographia 2004, 60, S81–S85. [Google Scholar] [CrossRef]
- Wilczańska-Barska, A.; Królicka, A.; Głód, D.; Majdan, M.; Kawiak, A.; Krauze-Baranowska, M. Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol. Lett. 2012, 34, 1757–1763. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Solov’eva, A.I.; Malunova, M.V.; Salamaikina, S.A.; Panov, Y.M.; Lelishentsev, A.A. Hairy roots of Scutellaria spp. (Lamiaceae) as promising producers of antiviral flavones. Molecules 2021, 26, 3927. [Google Scholar] [CrossRef]
- Pei, T.; Yan, M.; Li, T.; Li, X.; Yin, Y.; Cui, M.; Fang, Y.; Liu, J.; Kong, Y.; Xu, P. Characterization of UDP-glycosyltransferase family members reveals how major flavonoid glycoside accumulates in the roots of Scutellaria baicalensis. BMC Genom. 2022, 23, 169. [Google Scholar] [CrossRef]
- Korkina, L. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar]
- Pei, T.; Yan, M.; Huang, Y.; Wei, Y.; Martin, C.; Zhao, Q. Specific flavonoids and their biosynthetic pathway in Scutellaria baicalensis. Front. Plant Sci. 2022, 13, 866282. [Google Scholar] [CrossRef]
- Kumar, M.; Kasala, E.R.; Bodduluru, L.N.; Dahiya, V.; Lahkar, M. Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm. Res. 2016, 65, 613–622. [Google Scholar] [CrossRef]
- Halim, M.A.; Kanan, K.A.; Nahar, T.; Rahman, M.J.; Ahmed, K.S.; Hossain, H.; Mozumder, N.R.; Ahmed, M. Metabolic profiling of phenolics of the extracts from the various parts of blackberry plant (Syzygium cumini L.) and their antioxidant activities. LWT 2022, 167, 113813. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]




| Yield (%) | Total Phenolic Content (mg GAE/g DW) | Total Flavonoid Content (mg QE/g DW) | |
|---|---|---|---|
| WE | 16.7 ± 0.2 a | 17.37 ± 0.29 e | 8.71 ± 0.36 e |
| PM | 8.3 ± 0.1 c | 24.48 ± 0.1 d | 19.91 ± 0.26 d |
| PE | 7.35 ± 0.05 d | 32.81 ± 0.25 c | 25.41 ± 0.5 c |
| AM | 15.6 ± 0.1 b | 57.64 ± 0.25 b | 35.14 ± 1.34 b |
| AE | 16.85 ± 0.15 a | 66.03 ± 0.44 a | 40.11 ± 1.31 a |
| AIC50 of DPPH | AIC50 of ABTS | AIC50 of SOD | |
|---|---|---|---|
| WE | 3.53 ± 0.53 c | 1.1 ± 0.07 e | 0.66 ± 0.07 bc |
| PM | 1.04 ± 0.01 ab | 0.8 ± 0.01 d | 0.84 ± 0.11 c |
| PE | 1.2 ± 0.21 b | 0.62 ± 0.01 c | 1.09 ± 0.16 d |
| AM | 0.73 ± 0.09 ab | 0.38 ± 0.02 b | 0.54 ± 0.12 b |
| AE | 0.49 ± 0.04 a | 0.29 ± 0.01 a | 0.29 ± 0.04 a |
| GA | CAT | CA | CFA | EPC | EG | BA | RUT | QUE | KAE | Baicalin | Baicalein | WOG | TPC | TFC | DPPH | ABTS | SOD | RPA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | 1 | 0.319 | 0.848 | 0.630 | 0.473 | −0.629 | 0.541 | −0.525 | −0.014 | 0.523 | 0.311 | 0.243 | 0.165 | 0.432 | 0.190 | 0.231 | −0.162 | −0.808 | 0.442 |
| CAT | 1 | 0.005 | 0.793 | 0.945 | −0.021 | 0.955 | 0.153 | 0.730 | 0.823 | 0.982 | 0.285 | 0.576 | 0.486 | 0.455 | −0.371 | −0.467 | −0.200 | 0.425 | |
| CA | 1 | 0.557 | 0.164 | −0.529 | 0.262 | −0.430 | −0.272 | 0.463 | −0.008 | 0.556 | 0.342 | 0.621 | 0.431 | −0.035 | −0.396 | −0.889 | 0.658 | ||
| CFA | 1 | 0.844 | −0.181 | 0.891 | −0.014 | 0.483 | 0.984 | 0.780 | 0.691 | 0.810 | 0.850 | 0.758 | −0.517 | −0.756 | −0.649 | 0.824 | |||
| EPC | 1 | −0.051 | 0.973 | 0.095 | 0.752 | 0.845 | 0.967 | 0.249 | 0.531 | 0.465 | 0.396 | −0.290 | −0.415 | −0.326 | 0.413 | ||||
| EG | 1 | −0.176 | 0.649 | 0.584 | −0.163 | 0.091 | −0.073 | 0.181 | −0.159 | 0.027 | −0.460 | −0.027 | 0.291 | −0.141 | |||||
| BA | 1 | 0.029 | 0.640 | 0.896 | 0.949 | 0.359 | 0.590 | 0.573 | 0.486 | −0.316 | −0.499 | −0.408 | 0.521 | ||||||
| RUT | 1 | 0.446 | 0.062 | 0.214 | 0.086 | 0.287 | 0.018 | 0.200 | −0.498 | −0.236 | 0.247 | 0.010 | |||||||
| QUE | 1 | 0.477 | 0.828 | −0.001 | 0.407 | 0.135 | 0.178 | −0.382 | −0.195 | −0.036 | 0.105 | ||||||||
| KAE | 1 | 0.796 | 0.720 | 0.847 | 0.861 | 0.802 | −0.585 | −0.809 | −0.544 | 0.826 | |||||||||
| Baicalin | 1 | 0.227 | 0.555 | 0.429 | 0.399 | −0.366 | −0.417 | −0.220 | 0.373 | ||||||||||
| Baicalein | 1 | 0.909 | 0.962 | 0.971 | −0.784 | −0.959 | −0.540 | 0.967 | |||||||||||
| WOG | 1 | 0.925 | 0.965 | −0.890 | −0.961 | −0.456 | 0.913 | ||||||||||||
| TPC | 1 | 0.965 | −0.719 | −0.947 | −0.643 | 0.996 | |||||||||||||
| TFC | 1 | −0.856 | −0.989 | −0.469 | 0.957 | ||||||||||||||
| DPPH | 1 | 0.863 | 0.170 | −0.712 | |||||||||||||||
| ABTS | 1 | 0.433 | −0.935 | ||||||||||||||||
| SOD | 1 | −0.680 | |||||||||||||||||
| RPA | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Kim, K.; Kwon, D.Y.; Kim, J.K.; Sathasivam, R.; Park, S.U. Effects of Different Solvents on the Extraction of Phenolic and Flavonoid Compounds, and Antioxidant Activities, in Scutellaria baicalensis Hairy Roots. Horticulturae 2024, 10, 160. https://doi.org/10.3390/horticulturae10020160
Lim J, Kim K, Kwon DY, Kim JK, Sathasivam R, Park SU. Effects of Different Solvents on the Extraction of Phenolic and Flavonoid Compounds, and Antioxidant Activities, in Scutellaria baicalensis Hairy Roots. Horticulturae. 2024; 10(2):160. https://doi.org/10.3390/horticulturae10020160
Chicago/Turabian StyleLim, Jinsu, Kihyun Kim, Do Yeon Kwon, Jae Kwang Kim, Ramaraj Sathasivam, and Sang Un Park. 2024. "Effects of Different Solvents on the Extraction of Phenolic and Flavonoid Compounds, and Antioxidant Activities, in Scutellaria baicalensis Hairy Roots" Horticulturae 10, no. 2: 160. https://doi.org/10.3390/horticulturae10020160
APA StyleLim, J., Kim, K., Kwon, D. Y., Kim, J. K., Sathasivam, R., & Park, S. U. (2024). Effects of Different Solvents on the Extraction of Phenolic and Flavonoid Compounds, and Antioxidant Activities, in Scutellaria baicalensis Hairy Roots. Horticulturae, 10(2), 160. https://doi.org/10.3390/horticulturae10020160






