Assessment of Phytotoxicity in Untreated and Electrochemically Treated Leachates through the Analysis of Early Seed Growth and Inductively Coupled Plasma-Optical Emission Spectroscopy Characterization
Abstract
1. Introduction
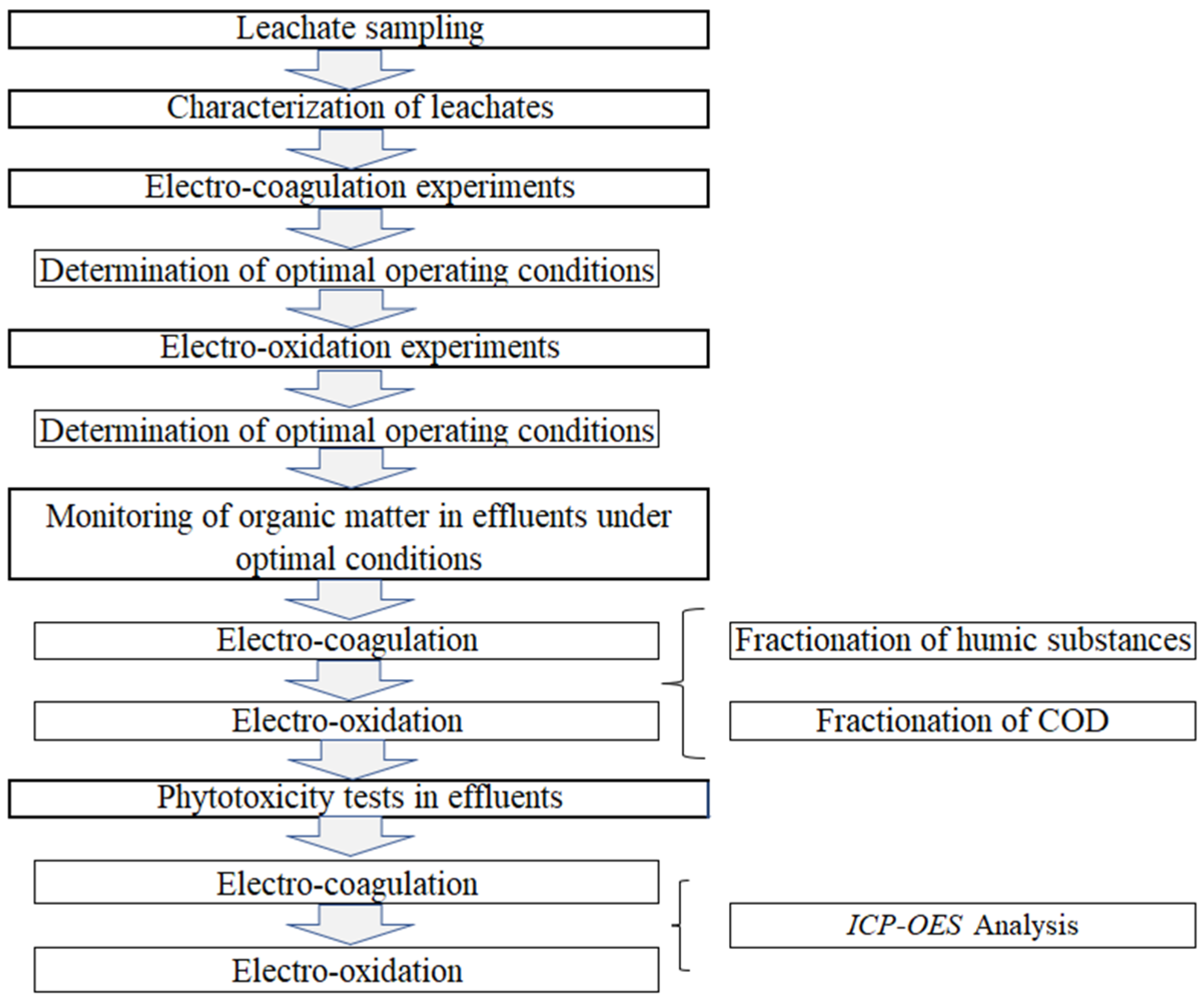
2. Materials and Methods
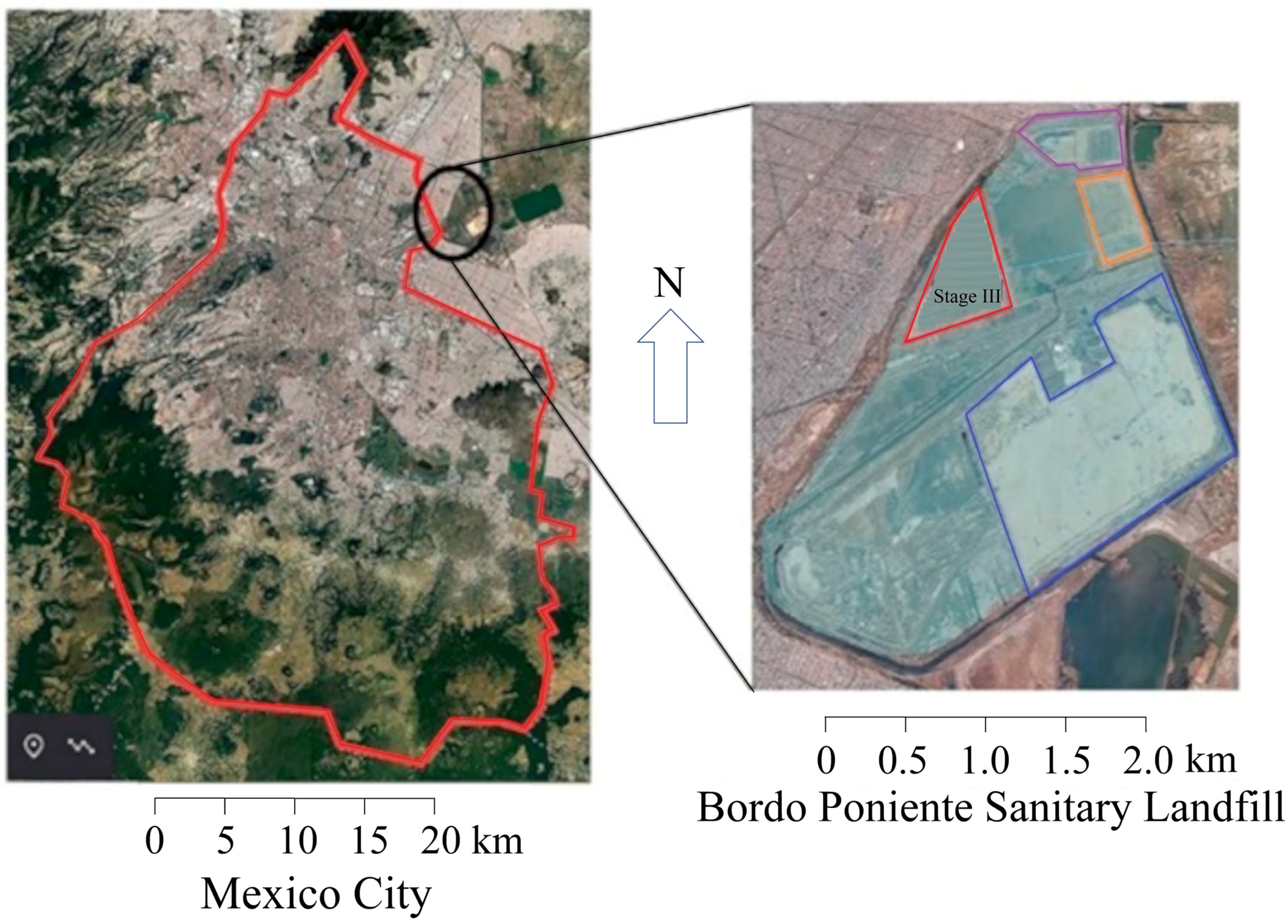
2.1. Landfill Leachate Collection and Characterization of Leachates and Effluents
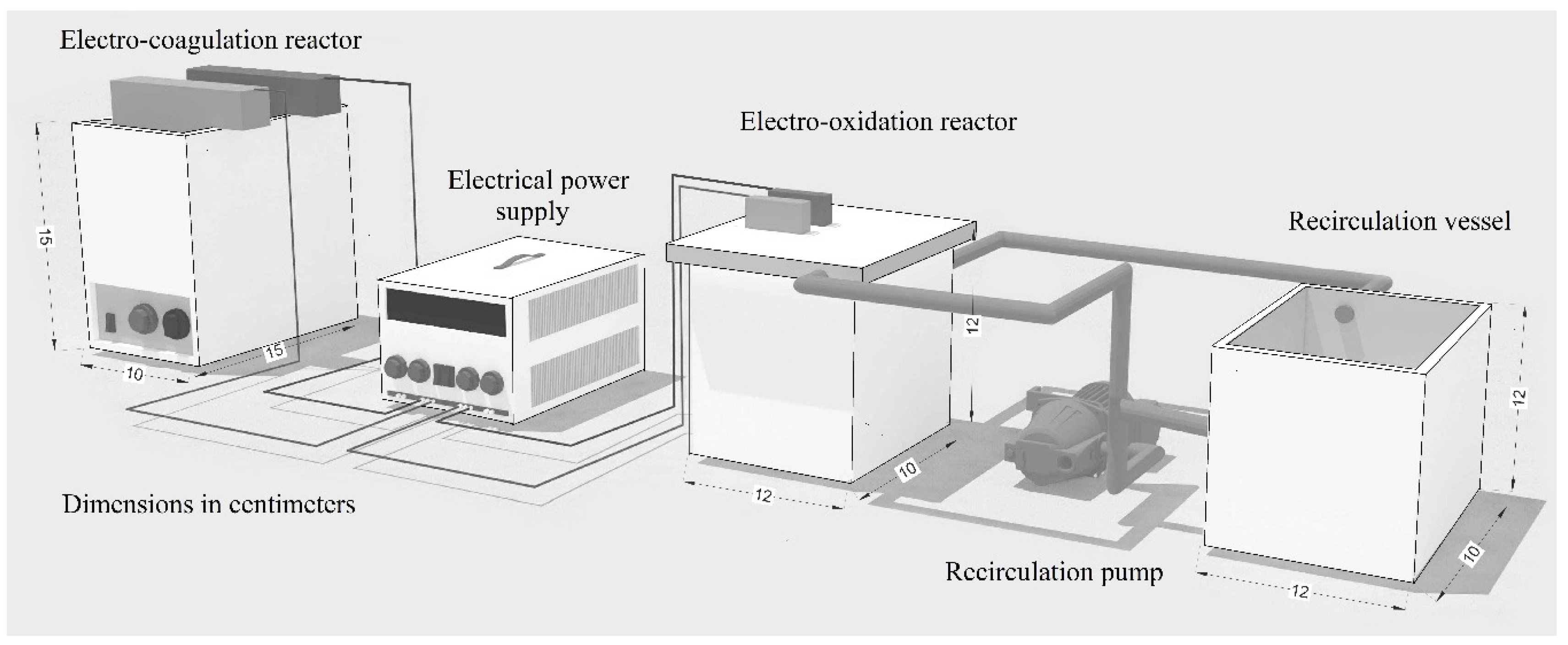
2.2. Leachate Treatment System
2.2.1. Electro-Coagulation Process
2.2.2. Electro-Oxidation Process
2.3. Evaluation of Phytotoxicity
2.3.1. Experiment Design
2.3.2. Transfer Coefficient and Enrichment Coefficient
2.4. Analysis Using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
2.4.1. Chemical Reagents and Materials and Preparation of Samples
2.4.2. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) Equipment
3. Results and Discussion
3.1. Treatment System under Conditions of Increased Removal of Organic Matter and Characterization of Leachates and Effluents
Mechanisms Involved in the Processes
- In the initial stage of direct EO, organic pollutants diffuse from the electrolyte to the anode surface where they are adsorbed. Subsequently, the organic compounds were oxidized at the anode surface through electron transfer, as demonstrated in Equation (8), where “R” represents the organic pollutant and “P” represents the oxidized organic pollutant [85]. Direct EO leads to the formation of •OH radicals adsorbed on the anode surface, which further oxidize the organic compounds through indirect electrolysis [85].R → P + e−
- Indirect EO is a process that takes place at a potential higher than the “water stability” potential, resulting in the generation of hydroxyl radicals (•OH). These radicals adsorb onto the anode surface and prove to be efficient in the oxidation of organic compounds, including the degradation of recalcitrant aromatic substances such as humic substances present in stabilized leachates. A model has been proposed to elucidate the degradation of organic compounds using BDD as an anode, which is described by Equation (9) [88].BDD(•OH) + R→ BDD + m CO2 + n H2O + H+ + e−
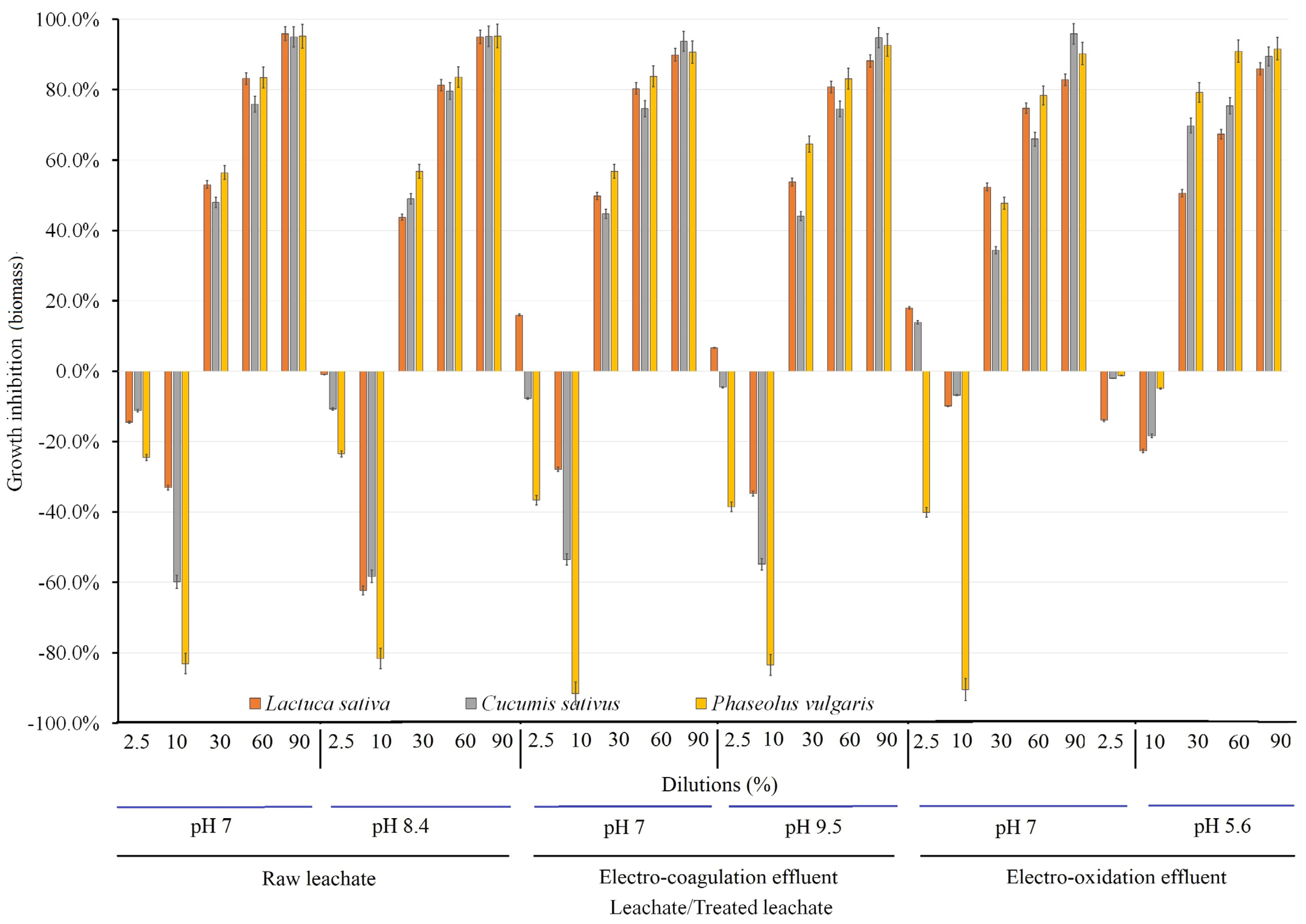
3.2. Evaluation of Phytotoxicity
3.2.1. Research on Seed Germination
3.2.2. Effects of Radicle Length and Acquired Biomass on Growth Inhibition
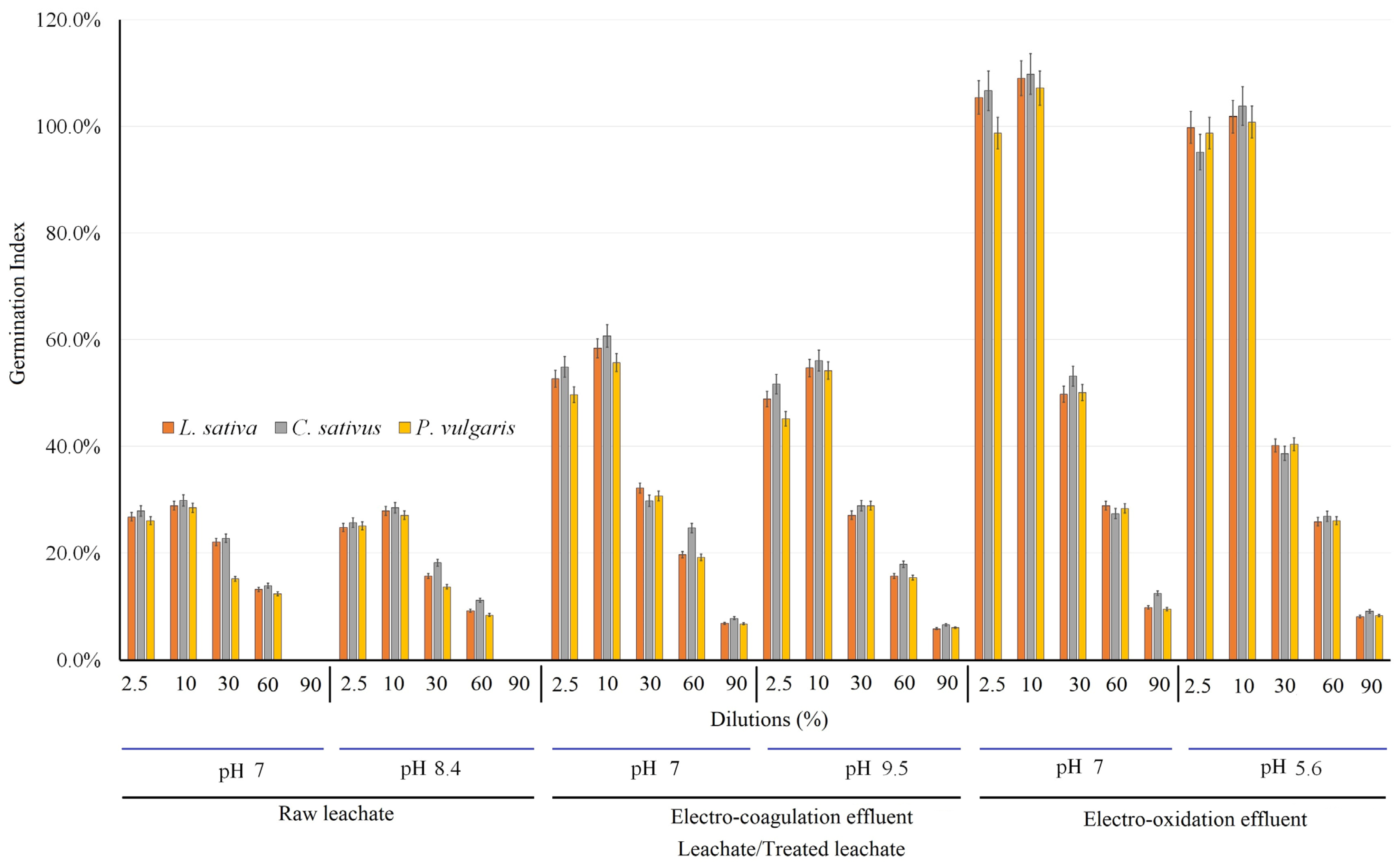
3.2.3. Results of Germination Index
3.2.4. Half Maximal Effective Concentration
3.2.5. Transfer Coefficient and Enrichment Coefficient
3.3. Analysis Using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
3.3.1. Samples of Used Garden Soil
3.3.2. Samples of Roots
- Although aluminum (Al) is not considered an essential nutrient, it has agronomic significance because of its toxic effects on plants [109]. Al toxicity was first observed in the root systems of plants, which are particularly vulnerable to the toxic effects of Al. Al inhibits root elongation and cell division, resulting in poor growth and reduced plant development [110].
- Barium (Ba) can be considered to have a slightly detrimental effect on plant growth as it competes with calcium, which is necessary for plant growth. However, this effect would only occur if the Ba levels in the soil exceeded the recommended maximum [105].
- Copper (Cu) is an essential micronutrient for plants in small amounts. However, excessive Cu in the growing medium can have a detrimental impact on root development by burning its tips, leading to excessive lateral growth, reduced branching, and ultimately plant decline [111]. Excessive Cu can also cause chlorosis, a condition that negatively affects plant growth and development [112].
- Iron (Fe) plays a fundamental role in plant growth and is involved in various biochemical processes, including respiration, chlorophyll synthesis, pathogen defense, the generation and elimination of reactive oxygen species, and photosynthesis. Both Fe deficiency and excess result in harmful effects on plant development such as chlorosis [112].
- Nickel (Ni), another essential micronutrient for plant growth, plays a crucial role in enzymatic catalysis as a component of various compounds. However, excessive Ni can negatively affect plant growth by affecting enzyme function [113].
- Zinc (Zn) is frequently present in insoluble forms in the soil and serves as an essential micronutrient for plants. Nevertheless, excessive levels of Zn can have deleterious effects on plant growth, as revealed by recent research [114]. It plays a critical role in the synthesis of carbohydrates during photosynthesis and in the metabolism of hormones by regulating the levels of auxins (a plant hormone that promotes plant growth and development).
- Calcium (Ca) is a structural element in plants that is present in the cell wall and membrane and plays a fundamental role in cell division and elongation [115]. Ca deficiency symptoms are commonly observed in growing organs, including apical meristems, which promote growth when plants germinate [116].
- Magnesium (Mg) plays a critical role in plant metabolism and its mobility within plants is highly beneficial for growth. As a fundamental component of chlorophyll, Mg enables plants to effectively perform photosynthesis, making it essential for crop health and productivity [117].
- Potassium (K), a crucial plant nutrient, plays an indispensable role in plant health and development. As a significant macronutrient, it comprises the majority of inorganic cations in plants and accounts for 10% of plant dry weight. This essential nutrient is primarily sourced from the soil [118].
- Manganese (Mn) is an essential micronutrient crucial for the proper functioning of various plant processes, including root cell elongation. Plants can actively absorb Mn in the form of Mn2+, but excessive levels of Mn can have detrimental effects on plant growth and development by replacing Mg in enzymatic reactions [119].
4. Conclusions
- The characterization of the leachates employed in this research project indicates that they possess a biodegradability index of 0.094, a chemical oxygen demand of 3.4 ± 0.1 g L−1, a dissolved organic carbon of 1.2 ± 0.05 gL−1, a color of 3200 ± 100 Pt-Co U, and a NH3-N content of 0.66 ± 0.03 gL−1. Consequently, it can be asserted that the leachates in question are mature.
- The parameters for the enhanced elimination of organic matter, as measured by COD, were established for both EC and EO processes. For EC, the optimal current density was found to be 23.3 mA cm−2, with a stirring rate of 120 revolutions per minute and a pH of 7. For EO, the conditions were determined to be a NaCl concentration of 1.0 g L−1, an electrode distance of 0.75 cm, a current density of 33.3 mA cm−2, and a pH of 7.
- Under conditions of greater removal of organic matter, measured as COD, removal values were reached in the chemical demand of oxygen, dissolved organic carbon, color, and NH3-N in the EC process of 63%, 69%, 94%, and 50%, respectively. For the EO process, these values were 82, 86, 99, and 81%, respectively.
- The proposed treatment system resulted in a significant enhancement of biodegradable organic matter. The concentration of biodegradable COD increased from 26% in the raw leachate to 39% following the EC process and further increased to 58% in the effluent of the EO process. Additionally, the biodegradability index, which was initially 0.094 in the crude leachate, improved to 0.26 with the EC process and attained a value of 0.46, following the EO process.
- The concentration of particulate COD in the EC effluent decreased from 48% to 23%. The EC process effectively removed colloidal species that could have impeded the subsequent EO process, demonstrating its suitability as an initial treatment stage.
- The conversion of a portion of the recalcitrant organic matter present in raw leachates into biodegradable materials and CO2 was achieved through both EC and EO processes. These processes resulted in a significant alteration in the chemical structure of the recalcitrant organic matter.
- By analyzing the organic matter content in an EC and EO system used to treat mature leachates, the structural changes that enhance the biodegradability of the resulting wastewater were uncovered.
- Based on the data collected in this study, it can be concluded that the parameters that significantly contributed to the toxicity in the leachates examined were aluminum, copper, iron, and zinc.
- The findings of the phytotoxicity assessments indicated that the proposed treatment approach led to a diminution of the phytotoxicity of the effluents produced. This outcome can be ascribed to alterations in the molecular composition of the organic matter.
5. Recommendations
- In this investigation, it was found that garden soil was a consistent factor in all of the experiments conducted. Therefore, future phytotoxicity trials should assess the effects of different soil types, such as sandy and clayey soil, to better understand the relationship between plants and the chemical composition of leachates in the soil.
- A valuable area of inquiry is to evaluate the influence of emerging pollutants on phytotoxicity tests. Although these pollutants have received considerable attention in recent times, the cessation of operations at the Bordo landfill in 2012 restricts the applicability of this variable. Therefore, it is proposed that phytotoxicity tests incorporate leachates from landfills with nearer closure dates, or even those that continue to function.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, N.; Palani, S.G. A Comprehensive Investigation of Toxicity and Pollution Potential of Municipal Solid Waste Landfill Leachate. Sci. Total Environ. 2022, 838, 155891. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Available online: https://openknowledge.worldbank.org/entities/publication/d3f9d45e-115f-559b-b14f-28552410e90a (accessed on 1 December 2023).
- Kamaruddin, M.A.; Yusoff, M.S.; Rui, L.M.; Isa, A.M.; Zawawi, M.H.; Alrozi, R. An Overview of Municipal Solid Waste Management and Landfill Leachate Treatment: Malaysia and Asian Perspectives. Environ. Sci. Pollut. Res. 2017, 24, 26988–27020. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Thakur, I.S.; Kaushik, A. Bioassays for Toxicological Risk Assessment of Landfill Leachate: A Review. Ecotoxicol. Environ. Saf. 2017, 141, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Kow, S.; Fahmi, M.R.; Abidin, C.Z.A.; Soon-An, O. Advanced Oxidation Processes: Process Mechanisms, Affecting Parameters and Landfill Leachate Treatment. Water Environ. Res. 2016, 88, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Dhanke, P.; Wagh, S. Treatment of Vegetable Oil Refinery Wastewater with Biodegradability Index Improvement. Mater. Today Proc. 2020, 27, 181–187. [Google Scholar] [CrossRef]
- Martínez-Cruz, A.; Rojas-Valencia, M.N. Evaluation of the Different Fractions of Organic Matter in an Electrochemical Treatment System Applied to Stabilized Leachates from the Bordo Poniente Landfill in Mexico City. Appl. Sci. 2023, 13, 5605. [Google Scholar] [CrossRef]
- Martínez-Cruz, A.; Rojas Valencia, M.N.; Araiza-Aguilar, J.A.; Nájera-Aguilar, H.A.; Gutiérrez-Hernández, R.F. Leachate Treatment: Comparison of a Bio-Coagulant (Opuntia ficus Mucilage) and Conventional Coagulants Using Multi-Criteria Decision Analysis. Heliyon 2021, 7, e07510. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Murshid, A.; Chanikya, P. Combination of Electrochemically Activated Persulfate Process and Electro-Coagulation for the Treatment of Municipal Landfill Leachate with Low Biodegradability. Chemosphere 2023, 338, 139449. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and Treatment of Landfill Leachate: A Review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, X.; Chen, N.; Feng, C.; Wang, H.; Kuang, P.; Hu, W. Review on Electrochemical System for Landfill Leachate Treatment: Performance, Mechanism, Application, Shortcoming, and Improvement Scheme. Sci. Total Environ. 2020, 745, 140768. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An Overview on the Removal of Synthetic Dyes from Water by Electrochemical Advanced Oxidation Processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical Advanced Oxidation Processes: Today and Tomorrow. A Review. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Oturan, M.A. Electrochemistry: As Cause and Cure in Water Pollution—An Overview. Environ. Chem. Lett. 2014, 12, 97–108. [Google Scholar] [CrossRef]
- Baiju, A.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Combined Heterogeneous Electro-Fenton, and Biological Process for the Treatment of Stabilized Landfill Leachate. J. Environ. Manag. 2018, 210, 328–337. [Google Scholar] [CrossRef]
- Mahmud, K.; Hossain, M.D.; Shams, S. Different Treatment Strategies for Highly Polluted Landfill Leachate in Developing Countries. Waste Manag. 2012, 32, 2096–2105. [Google Scholar] [CrossRef]
- Moradi, M.; Ghanbari, F. Application of Response Surface Method for Coagulation Process in Leachate Treatment as Pretreatment for Fenton Process: Biodegradability Improvement. J. Water Process. Eng. 2014, 4, 67–73. [Google Scholar] [CrossRef]
- Gandhimathi, R.; Babu, A.; Nidheesh, P.V.; Ramesh, S.T.; Anantha Singh, T.S. Laboratory Study on Leachate Treatment by Electrocoagulation Using Fly Ash, and Bottom Ash as Supporting Electrolytes. J. Hazard. Toxic. Radioact. Waste 2015, 19, 3. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent Advances in Municipal Landfill Leachate: A Review Focusing on Its Characteristics, Treatment, and Toxicity Assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar]
- Kulikowska, D. Usability of Powdered Activated Carbon for Landfill Leachate Treatment—Continued Research. Desalination Water Treat. 2016, 57, 28560–28569. [Google Scholar] [CrossRef]
- Verma, M.; Naresh Kumar, R. Can Coagulation-Flocculation Be an Effective Pre-Treatment Option for Landfill Leachate and Municipal Wastewater Co-Treatment? Perspect. Sci. 2016, 8, 492–494. [Google Scholar] [CrossRef]
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on Landfill Leachate Treatments. Am. J. Appl. Sci. 2009, 6, 672–684. [Google Scholar] [CrossRef]
- Gomes, A.I.; Santos, S.G.S.; Silva, T.F.C.V.; Boaventura, R.A.R.; Vilar, V.J.P. Treatment Train for Mature Landfill Leachates: Optimization Studies. Sci. Total Environ. 2019, 673, 470–479. [Google Scholar] [CrossRef]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and Conventional Technologies for Landfill Leachates Treatment: A Review. Sustainability 2017, 9, 9. [Google Scholar] [CrossRef]
- Alver, A.; Altaş, L. Characterization and Electrocoagulative Treatment of Landfill Leachates: A Statistical Approach. Process Saf. Environ. Prot. 2017, 111, 102–111. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill Leachate Treatment: Review and Opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Seibert, D.; Quesada, H.; Bergamasco, R.; Borba, F.H.; Pellenz, L. Presence of Endocrine Disrupting Chemicals in Sanitary Landfill Leachate, Its Treatment and Degradation by Fenton Based Processes: A Review. Process Saf. Environ. Prot. 2019, 131, 255–267. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.H.; Chan, G.Y.S. Physico-Chemical Treatments for Removal of Recalcitrant Contaminants from Landfill Leachate. J. Hazard. Mater. 2006, 129, 80–100. [Google Scholar] [CrossRef]
- Chaudhari, L.B.; Murthy, Z.V.P. Treatment of Landfill Leachates by Nanofiltration. J. Environ. Manag. 2010, 91, 1209–1217. [Google Scholar] [CrossRef]
- Álvarez-Vázquez, H.; Jefferson, B.; Judd, S.J. Membrane Bioreactors vs Conventional Biological Treatment of Landfill Leachate: A Brief Review. J. Chem. Technol. Biotechnol. 2004, 79, 1043–1049. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Karlis, P.K.; Mahnken, G. Influence of Various Parameters on the Electrochemical Treatment of Landfill Leachates. J. Appl. Electrochem. 2003, 33, 155–159. [Google Scholar] [CrossRef]
- Ozturk, I.; Altinbas, M.; Koyuncu, I.; Arikan, O.; Gomec-Yangin, C. Advanced Physico-Chemical Treatment Experiences on Young Municipal Landfill Leachates. Waste Manag. 2003, 23, 441–446. [Google Scholar] [CrossRef]
- Pastore, C.; Barca, E.; Del Moro, G.; Di Iaconi, C.; Loos, M.; Singer, H.P.; Mascolo, G. Comparison of Different Types of Landfill Leachate Treatments by Employment of Nontarget Screening to Identify Residual Refractory Organics and Principal Component Analysis. Sci. Total Environ. 2018, 635, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Nájera-Aguilar, H.A.; Gutiérrez-Hernández, R.F.; Bautista-Ramírez, J.; Martínez-Salinas, R.I.; Escobar-Castillejos, D.; Borraz-Garzón, R.; Rojas-Valencia, M.N.; Giácoman-Vallejos, G. Treatment of Low Biodegradability Leachates in a Serial System of Aged Refuse-Filled Bioreactors. Sustainability 2019, 11, 3193. [Google Scholar] [CrossRef]
- Oulego, P.; Collado, S.; Laca, A.; Díaz, M. Tertiary Treatment of Biologically Pre-Treated Landfill Leachates by Non-Catalytic Wet Oxidation. Chem. Eng. J. 2015, 273, 647–655. [Google Scholar] [CrossRef]
- Bulska, E.; Wagner, B. Quantitative Aspects of Inductively Coupled Plasma Mass Spectrometry. Philos. Trans. R. Soc. A Math. Phil. Trans. 2016, 374, 20150369. [Google Scholar] [CrossRef] [PubMed]
- Douvris, C.; Vaughan, T.; Bussan, D.; Bartzas, G.; Thomas, R. How ICP-OES Changed the Face of Trace Element Analysis: Review of the Global Application Landscape. Sci. Total Environ. 2023, 905, 167242. [Google Scholar] [CrossRef]
- Derdera, S.E.; Ogato, G.S. Towards Integrated, and Sustainable Municipal Solid Waste Management System in Shashemane City Administration, Ethiopia. Heliyon 2023, 9, e21865. [Google Scholar] [CrossRef]
- Zambezi, F.M.; Muisa-Zikali, N.; Utete, B. Effectiveness of Community Participation as Anti-Litter Monitors in Solid Waste Management in Metropolitan Areas in a Developing Country. Environ. Dev. Sustain. 2021, 23, 747–764. [Google Scholar] [CrossRef]
- Asefi, H.; Shahparvari, S.; Chhetri, P. Advances in Sustainable Integrated Solid Waste Management Systems: Lessons Learned over the Decade 2007–2018. J. Environ. Plan 2020, 63, 2287–2312. [Google Scholar] [CrossRef]
- Pacurariu, R.L.; Vatca, S.D.; Lakatos, E.S.; Bacali, L.; Vlad, M. A Critical Review of EU Key Indicators for the Transition to the Circular Economy. Int. J. Environ. Res. Public Health 2021, 18, 8840. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.Y.; Chang, C.-K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.-S.; Show, P.L. Waste Biorefinery towards a Sustainable Circular Bioeconomy: A Solution to Global Issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Pap, S.; Boyd, K.G.; Taggart, M.A.; Turk Sekulic, M. Circular Economy Based Landfill Leachate Treatment with Sulphur-Doped Microporous Biochar. Waste Manag. 2021, 124, 160–171. [Google Scholar] [CrossRef]
- Ilmasari, D.; Kamyab, H.; Yuzir, A.; Riyadi, F.A.; Khademi, T.; Al-Qaim, F.F.; Kirpichnikova, I.; Krishnan, S. A Review of the Biological Treatment of Leachate: Available Technologies and Future Requirements for the Circular Economy Implementation. Biochem. Eng. J. 2022, 187, 108605. [Google Scholar] [CrossRef]
- D1209-05 19; ASTM Standard Test Method for Color of Clear Liquids (Platinum-Cobalt Scale). ASTM International: West Conshohocken, PA, USA, 2019. Available online: https://www.astm.org/d1209-05r19.html (accessed on 2 December 2023).
- D7573-09; ASTM Standard Test Method for Total Carbon and Organic Carbon in Water by High Temperature Catalytic Combustion and Infrared Detection. ASTM International: West Conshohocken, PA, USA, 2019. Available online: https://www.astm.org/d7573-18ae01.html (accessed on 8 December 2023).
- Lipps, W.C.; Baxter, T.E.; Braun-Howland, E. (Eds.) APHA Standard Methods for the Examination of Water and Wastewater, 24th ed.; APHA Press: Washington, DC, USA, 2022; pp. 50–120. ISBN 9780875532998. [Google Scholar]
- Bashir, M.J.K.; Aziz, H.A.; Aziz, S.Q.; Abu Amr, S.S. An Overview of Electro-Oxidation Processes Performance in Stabilized Landfill Leachate Treatment. Desalin. Water Treat. 2013, 51, 2170–2184. [Google Scholar] [CrossRef]
- Abu Amr, S.S.; Aziz, H.A.; Adlan, M.N.; Alkasseh, J.M.A. Effect of Ozone and Ozone/Persulfate Processes on Biodegradable and Soluble Characteristics of Semiaerobic Stabilized Leachate. Environ. Prog. Sustain. Energy 2014, 33, 184–191. [Google Scholar] [CrossRef]
- Dia, O.; Drogui, P.; Buelna, G.; Dubé, R. Hybrid Process, Electrocoagulation-Biofiltration for Landfill Leachate Treatment. Waste Manag. 2018, 75, 391–399. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Arizon State University: Hoboken, NJ, USA, 2017; pp. 395–405, 433. ISBN 9781118146927. [Google Scholar]
- Taguchi, G.; Chowdhury, S.; Wu, Y. Taguchi’s Quality Engineering Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 423–424, 503–506, 584–588. ISBN 9780470258354. [Google Scholar]
- Deng, Y.; Feng, C.; Chen, N.; Hu, W.; Kuang, P.; Liu, H.; Hu, Z.; Li, R. Research on the Treatment of Biologically Treated Landfill Leachate by Joint Electrochemical System. Waste Manag. 2018, 82, 177–187. [Google Scholar] [CrossRef]
- Ghanbari, F.; Wu, J.; Khatebasreh, M.; Ding, D.; Lin, K.Y.A. Efficient Treatment for Landfill Leachate through Sequential Electrocoagulation, Electrooxidation and PMS/UV/CuFe2O4 Process. Sep. Purif. Technol. 2020, 242, 116828. [Google Scholar] [CrossRef]
- Tanyol, M.; Ogedey, A.; Oguz, E. COD Removal from Leachate by Electrocoagulation Process: Treatment with Monopolar Electrodes in Parallel Connection. Water Sci. Technol. 2018, 77, 177–186. [Google Scholar] [CrossRef]
- Guvenc, S.Y.; Dincer, K.; Varank, G. Performance of Electrocoagulation and Electro-Fenton Processes for Treatment of Nanofiltration Concentrate of Biologically Stabilized Landfill Leachate. J. Water Process. Eng. 2019, 31, 100863. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A Comprehensive Review on Contaminants Removal from Pharmaceutical Wastewater by Electrocoagulation Process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Jia, H.; Guo, J.; Meng, X.; Wang, J. Electrochemical Methods for Landfill Leachate Treatment: A Review on Electrocoagulation and Electrooxidation. Sci. Total Environ. 2022, 806, 150529. [Google Scholar] [CrossRef]
- Ding, J.; Wei, L.; Huang, H.; Zhao, Q.; Hou, W.; Kabutey, F.T.; Yuan, Y.; Dionysiou, D.D. Tertiary Treatment of Landfill Leachate by an Integrated Electro-Oxidation/Electro-Coagulation/Electro-Reduction Process: Performance and Mechanism. J. Hazard. Mater. 2018, 351, 90–97. [Google Scholar] [CrossRef]
- Särkkä, H.; Bhatnagar, A.; Sillanpää, M. Recent Developments of Electro-Oxidation in Water Treatment—A Review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- OECD. Test Guideline 208: Terrestrial Plant Test-Seedling Emergence and Seedling Growth Test. In Guidelines for the Testing of Chemicals, Terrestrial Plant Test Seedling Emergence and Seedling Growth Test; OECD Publishing: Berlin, Germany; Mexico City, Mexico; Tokyo, Japan; Washington, DC, USA, 2006; Volume 227, pp. 1–21. [Google Scholar]
- USEPA Ecological Effects Test Guidelines OPPTS (850.4200): Seed Germination/Root Elongation Toxicity Test. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100RF5I.PDF?Dockey=P100RF5I.PDF (accessed on 6 December 2020).
- ASTM. Chemical Effects on the Germination and Early Growth of Terrestrial Plants STP19052S; ASTM: West Conshohocken, PA, USA, 1990; Available online: https://www.astm.org/stp19052s.html (accessed on 5 December 2023).
- ISO 11269-2; Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants. ISO: Vernier/Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/51382.html (accessed on 3 December 2023).
- Control Bio, Control Biológico [Biological Control]. Available online: https://controlbio.es/es/ (accessed on 6 December 2023).
- Chitimus, D.; Nedeff, V.; Mosnegutu, E.; Barsan, N.; Irimia, O.; Nedeff, F. Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites Australis (Common Reed) with Heavy Metals. Sustainability 2023, 15, 8729. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Otaibi, T.G.; El-Sheikh, M.A.; Al-Ghanayem, A.A.; Ameen, F. Accumulation of Heavy Metals in a Macrophyte Phragmites Australis: Implications to Phytoremediation in the Arabian Peninsula Wadis. Environ. Monit. Assess. 2020, 192, 202. [Google Scholar] [CrossRef]
- Stadler, A.; Michaelis, M.A. Chemist’s Guide to Sample Preparation, 3rd ed.; Anton Paar Publishing: Graz, Austria, 2021; Available online: https://www.anton-paar.com/corp-en/a-chemists-guide-to-sample-preparation/ (accessed on 6 December 2023).
- Poblete, R.; Pérez, N. Use of Sawdust as Pretreatment of Photo-Fenton Process in the Depuration of Landfill Leachate. J. Environ. Manag. 2020, 253, 109697. [Google Scholar] [CrossRef]
- Siti Nor, F.Z.; Hamidi, A.A. Characteristic of Leachate at Alor Pongsu Landfill Site, Perak, Malaysia: A Comparative Study. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012013. [Google Scholar]
- Hussein, M.; Yoneda, K.; Zaki, Z.M.; Amir, A. Leachate Characterizations and Pollution Indices of Active and Closed Unlined Landfills in Malaysia. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100232. [Google Scholar] [CrossRef]
- Wijekoon, P.; Koliyabandara, P.A.; Cooray, A.T.; Lam, S.S.; Athapattu, B.C.L.; Vithanage, M. Progress and Prospects in Mitigation of Landfill Leachate Pollution: Risk, Pollution Potential, Treatment and Challenges. J. Hazard. Mater. 2022, 421, 126627. [Google Scholar] [CrossRef] [PubMed]
- Naveen, B.P.; Sivapullaiah, P.V.; Sitharam, T.G. Effect of Aging on the Leachate Characteristics from Municipal Solid Waste Landfill. Jpn. Geotech. Soc. Spec. Publ. 2016, 2, 1940–1945. [Google Scholar] [CrossRef]
- Abunama, T.; Moodley, T.; Abualqumboz, M.; Kumari, S.; Bux, F. Variability of Leachate Quality and Polluting Potentials in Light of Leachate Pollution Index (LPI)—A Global Perspective. Chemosphere 2021, 282, 131119. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.K.; Kumar, M. Sequential Coagulation/Flocculation and Microwave-Persulfate Processes for Landfill Leachate Treatment: Assessment of Bio-Toxicity, Effect of Pretreatment and Cost-Analysis. Waste Manag. 2019, 85, 18–29. [Google Scholar] [CrossRef]
- Ibrahim, A.; Yaser, A.Z. Colour Removal from Biologically Treated Landfill Leachate with Tannin-Based Coagulant. J. Environ. Chem. Eng. 2019, 7, 103483. [Google Scholar] [CrossRef]
- Singh, A.; Dash, R.R. Nitrogen Removal from Landfill Leachate. In Landfill Leachate Management; IWA Publishing: London, UK, 2023; p. 106. [Google Scholar]
- Fudala-Ksiazek, S.; Sobaszek, M.; Luczkiewicz, A.; Pieczynska, A.; Ofiarska, A.; Fiszka-Borzyszkowska, A.; Sawczak, M.; Ficek, M.; Bogdanowicz, R.; Siedlecka, E.M. Influence of the Boron Doping Level on the Electrochemical Oxidation of Raw Landfill Leachates: Advanced Pre-Treatment Prior to the Biological Nitrogen Removal. Chem. Eng. J. 2018, 334, 1074–1084. [Google Scholar] [CrossRef]
- De Battisti, A.; Martínez-Huitle, C.A. Electrocatalysis in Wastewater Treatment. Quim. Nova 2018, 34, 119–131. [Google Scholar]
- Sutton, R.; Sposito, G. Molecular Structure in Soil Humic Substances: The New View. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Piccolo, A.; Naedi, S.; Concheri, G. Macromolecular Changes of Humic Substances Induced by Interaction with Organic Acids. Eur. J. Soil Sci. 1996, 47, 319–328. [Google Scholar] [CrossRef]
- Schellekens, J.; Buurman, P.; Kalbitz, K.; Zomeren, A.V.; Vidal-Torrado, P.; Cerli, C.; Comans, R.N. Molecular Features of Humic Acids and Fulvic Acids from Contrasting Environments. Environ. Sci. Technol. 2017, 51, 1330–1339. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation Process in Water Treatment: A Review of Electrocoagulation Modeling Approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on Landfill Leachate Treatment by Electrochemical Oxidation: Drawbacks, Challenges and Future Scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Wang, S.; Zhao, Q.; Wei, L.; Huang, H.; Yuan, Y.; Dionysiou, D. Electrochemical Treatment of Bio-Treated Landfill Leachate: Influence of Electrode Arrangement, Potential, and Characteristics. Chem. Eng. J. 2018, 344, 34–41. [Google Scholar] [CrossRef]
- Yildiz, Y.Ş.; Koparal, A.S.; Irdemez, Ş.; Keskinler, B. Electrocoagulation of Synthetically Prepared Waters Containing High Concentration of NOM Using Iron Cast Electrodes. J. Hazard. Mater. 2007, 139, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.A.Q.; Ferro, S.; Martínez-Huitle, C.A.; Vong, Y.M. Boron Doped Diamond Electrode for the Wastewater Treatment. J. Braz. Chem. Soc. 2006, 17, 227–236. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Zhong, C.; Liu, M.; Yang, L.; He, J.; Sun, Y.; Zhao, C.; Wang, D. Treatment of Landfill Leachate by Coagulation: A Review. Sci. Total Environ. 2024, 912, 169294. [Google Scholar] [CrossRef]
- Yang, Y.H.; Zhang, H.Y. Effect of Citric Acid on Aluminum Toxicity in the Growth of Mungbean Seedlings. J. Plant Nutr. 1998, 21, 1037–1044. [Google Scholar] [CrossRef]
- Storck, T.R.; Canabarro, M.I.; Silvestri, S.; Piccoli, A.L.; Ames, J.; Loro, V.L.; Zanella, R.; Tassinari, A.; Tiecher, T.L.; Brunetto, G. Toxicity Evaluation of Landfill Leachate after Treatment by Simple Distillation Using Danio Rerio Biomarkers. Process Saf. Environ. Prot. 2023, 174, 243–252. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, L.; Liang, H.; Xu, A.; Li, Y.; Nabi, M.; Wang, H.; Hu, J.; Gao, D. Efficient Nitrogen Removal from Mature Landfill Leachate by Single-Stage Partial-Nitritation Anammox Using Expanded Granular Sludge Bed. J. Environ. Manag. 2023, 344, 118460. [Google Scholar] [CrossRef]
- Bicelli, L.G.; Giordani, A.; Augusto, M.R.; Okada, D.Y.; de Moura, R.B.; Vich, D.V.; Contrera, R.C.; Cano, V.; de Souza, T.S.O. Microbial Interactions and Nitrogen Removal Performance in an Intermittently Rotating Biological Contactor Treating Mature Landfill Leachate. Bioresour. Technol. 2023, 389, 129797. [Google Scholar] [CrossRef]
- Fuentes, A. Phytotoxicity and Heavy Metals Speciation of Stabilised Sewage Sludges. J. Hazard. Mater. 2004, 108, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Arunbabu, V.; Indu, K.S.; Ramasamy, E.V. Leachate Pollution Index as an Effective Tool in Determining the Phytotoxicity of Municipal Solid Waste Leachate. Waste Manag. 2017, 68, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Poblete, R.; Cortes, E.; Bakit, J.; Luna-Galiano, Y. Landfill Leachate Treatment Using Combined Fish Scales Based Activated Carbon and Solar Advanced Oxidation Processes. Process Saf. Environ. Prot. 2019, 123, 253–262. [Google Scholar] [CrossRef]
- Li, G.; Chen, J.; Yan, W.; Sang, N. A Comparison of the Toxicity of Landfill Leachate Exposure at the Seed Soaking and Germination Stages on Zea mays L. (Maize). J. Environ. Sci. 2017, 55, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-R.; Chen, Z.-F.; Liao, X.-L.; Liu, Q.-Y.; Zhou, J.-M.; Ou, S.-P.; Cai, Z. Identifying Potential Toxic Organic Substances in Leachates from Tire Wear Particles and Their Mechanisms of Toxicity to Scenedesmus Obliquus. J. Hazard. Mater. 2023, 458, 132022. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.L.; Bitton, G.; Townsend, T. Heavy Metal Binding Capacity (HMBC) of Municipal Solid Waste Landfill Leachates. Chemosphere 2005, 60, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Bolobajev, J.; Kattel, E.; Viisimaa, M.; Goi, A.; Trapido, M.; Tenno, T.; Dulova, N. Reuse of Ferric Sludge as an Iron Source for the Fenton-Based Process in Wastewater Treatment. Chem. Eng. J. 2014, 255, 8–13. [Google Scholar] [CrossRef]
- Kaur, N.; Erickson, T.E.; Ball, A.S.; Ryan, M.H. A Review of Germination and Early Growth as a Proxy for Plant Fitness under Petrogenic Contamination—Knowledge Gaps and Recommendations. Sci. Total Environ. 2017, 603–604, 728–744. [Google Scholar] [CrossRef]
- Kalčíková, G.; Zagorc-Končan, J.; Zupančič, M.; Gotvajn, A.Ž. Variation of Landfill Leachate Phytotoxicity Due to Landfill Ageing. J. Chem. Technol. Biotechnol. 2012, 87, 1349–1353. [Google Scholar] [CrossRef]
- Alcantar, R.F. Evolución de Las Características Físico-Químicas Del Lixiviado Generado en El Relleno Sanitario Bordo Poniente [Evolution of the Physical-Chemical Characteristics of Leachate Generated in Bordo Poniente Landfill]. Bachelor’s Thesis, UNAM, Mexico City, Mexico, 2015. [Google Scholar]
- Parra, N.; Ovando, E. Comportamiento de un Sistema de Impermeabilización con Geomembrana en Relleno [Behavior of a Geomembrane Waterproofing System]; Mexico City. 2011. Available online: https://www.iingen.unam.mx/es-mx/Investigacion/Proyecto/Paginas/Comportamientodeunsistemadeimpermeabilizacion.aspx (accessed on 8 December 2023).
- NYSDEC New York State Brownfield Cleanup Program Development of Soil Cleanup Objectives Technical Support Document; New York. 2006. Available online: https://extapps.dec.ny.gov/docs/remediation_hudson_pdf/techsuppdoc.pdf (accessed on 7 January 2023).
- Saadani, O.; Fatnassi, I.C.; Chiboub, M.; Abdelkrim, S.; Barhoumi, F.; Jebara, M.; Jebara, S.H. In Situ Phytostabilisation Capacity of Three Legumes and Their Associated Plant Growth Promoting Bacteria (PGPBs) in Mine Tailings of Northern Tunisia. Ecotoxicol. Environ. Saf. 2016, 130, 263–269. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Aiman Hasan, S.; Hayat, S.; Aiman Hasan, S.; Fariduddin, Q.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium-Induced Changes in the Growth and Carbonic Anhydrase Activity of Chickpea Cadmium: Toxicity and Tolerance in Plants. J. Environ. Biol. 2009, 30, 165–174. [Google Scholar]
- Yan, L.; Riaz, M.; Li, S.; Cheng, J.; Jiang, C. Harnessing the Power of Exogenous Factors to Enhance Plant Resistance to Aluminum Toxicity; a Critical Review. Plant Physiol. Biochem. 2023, 203, 108064. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, B.R.; Sheri, V.; Kumar, M.; Zhang, Z.; Zhang, B. The Update and Transport of Aluminum Nanoparticles in Plants and Their Biochemical and Molecular Phototoxicity on Plant Growth and Development: A Systematic Review. Environ. Pollut. 2024, 340, 122875. [Google Scholar] [CrossRef] [PubMed]
- Gemin, L.G.; de Lara, G.B.; Mógor, Á.F.; Mazaro, S.M.; Sant’Anna-Santos, B.F.; Mógor, G.; Amatussi, J.D.O.; Cordeiro, E.C.N.; Marques, H.M.C. Polysaccharides Combined to Copper and Magnesium Improve Tomato Growth, Yield, Anti-Oxidant and Plant Defense Enzymes. Sci. Hortic. 2023, 310, 111758. [Google Scholar] [CrossRef]
- Wang, S.; Gariepy, Y.; Adekunle, A.; Raghavan, V. Influence of Iron Fertilizer Form and Concentration on Bioelectricity and Methane Emission from Hydroponic Plant Microbial Fuel Cells. J. Clean. Prod. 2023, 430, 139676. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, T.J.; Ojeda-Barrios, D.L.; Blanco-Macías, F.; Valdez-Cepeda, R.D.; Parra-Quezada, R. Urease and Nickel in Plant Physiology. Rev. Chapingo Ser. Hortic. 2016, 22, 69–81. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, P.K.; Singh, R.P. Application of Plant Growth Promoting Microbes to Enrich Zinc in Potato for Nutritional Security and Sustainable Agriculture. Rhizosphere 2023, 25, 100665. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A. A Review on Plant Annexins: The Calcium Binding Proteins with Multifaceted Roles in Plant Growth, Development and Stress Tolerance. S. Afr. J. Bot. 2023, 162, 108–114. [Google Scholar] [CrossRef]
- John, S.; Apelt, F.; Kumar, A.; Acosta, I.F.; Bents, D.; Annunziata, M.G.; Fichtner, F.; Gutjahr, C.; Mueller-Roeber, B.; Olas, J.J. Transcription Factor HSFA7b Controls Thermomemory at the Shoot Apical Meristem by Regulating Ethylene Biosynthesis and Signaling in Arabidopsis. Plant Commun. 2023, 100743. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, L.; Chen, E.; Lei, X.; Ren, J.; Yang, Y.; Xiao, B.; Gong, C. Magnesium Transporter CsMGT10 of Tea Plants Plays a Key Role in Chlorosis Leaf Vein Greening. Plant Physiol. Biochem. 2023, 201, 107842. [Google Scholar] [CrossRef] [PubMed]
- He, M.Y.; Ren, T.X.; Jin, Z.D.; Deng, L.; Liu, H.J.; Cheng, Y.Y.; Li, Z.Y.; Liu, X.X.; Yang, Y.; Chang, H. Precise Analysis of Potassium Isotopic Composition in Plant Materials by Multi-Collector Inductively Coupled Plasma Mass Spectrometry. Spectrochim Acta Part B At. Spectrosc. 2023, 209, 106781. [Google Scholar] [CrossRef]
- Nong, H.; Liu, J.; Chen, J.; Zhao, Y.; Wu, L.; Tang, Y.; Liu, W.; Yang, G.; Xu, Z. Woody Plants Have the Advantages in the Phytoremediation Process of Manganese Ore with the Help of Microorganisms. Sci. Total Environ. 2023, 863, 160995. [Google Scholar] [CrossRef]
- Yang, S.; Liang, S.; Yi, L.; Xu, B.; Cao, J.; Guo, Y.; Zhou, Y. Heavy Metal Accumulation and Phytostabilization Potential of Dominant Plant Species Growing on Manganese Mine Tailings. Front. Environ. Sci. Eng. 2014, 8, 394–404. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Liang, X.; Dai, P.; Zhang, Y.; Li, B.; Lin, X.; Sun, C. Enhancement of Polyphenolic Metabolism as an Adaptive Response of Lettuce (Lactuca Sativa) Roots to Aluminum Stress. Environ. Pollut. 2020, 261, 114230. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological Essence of Magnesium in Plants, and Its Widespread Deficiency in the Farming System of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- UME. Magnesium for Crop Production; University of Minnesota Extension: St. Paul, MN, USA, 2023; Available online: https://extension.umn.edu/micro-and-secondary-macronutrients/magnesium-crop-production (accessed on 1 December 2023).
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Integri, El Magnesio En La Nutrición Vegetal [Magnesium in Plant Nutrition]; Mexico City. 2023. Available online: https://www.intagri.com/articulos/nutricion-vegetal/el-manganeso-en-la-nutricion-vegetal (accessed on 26 November 2023).


| Parameter | Raw Leachate | Electro-Coagulation Effluent | Electro-Oxidation Effluent |
|---|---|---|---|
| pH | 8.4 ± 0.1 | 9.5 ± 0.1 | 5.6 ± 0.1 |
| Biodegradability index | 0.094 | 0.26 | 0.48 |
| Electrical conductivity | 8.5 ± 1 | 2.2 ± 0.5 | 1.7 ± 0.4 |
| Chlorides | 6.7 ± 0.1 | 3.6 ± 0.1 | 1.9 ± 0.4 |
| BOD5 | 0.32 ± 0.01 | 0.338 ± 0.01 | 0.288 ± 0.01 |
| Color | 3200 ± 90 | 200 ± 10 | 20 ± 1 |
| DOC | 1.2 ± 0.2 | 0.36 ± 0.01 | 0.16 ± 0.01 |
| NH3-N | 0.66 ± 0.03 | 0.33 ± 0.01 | 0.12 ± 0.01 |
| Total COD | 3.4 ± 0.1 | 1.3 ± 0.5 | 0.6 ± 0.01 |
| Soluble COD | 1.77 ± 0.1 | 0.97 ± 0.2 | 0.4 ± 0.1 |
| Biodegradable COD | 0.87 ± 0.04 | 0.49 ± 0.1 | 0.35 ± 0.01 |
| Non-biodegradable soluble COD | 0.89 ± 0.04 | 0.48 ± 0.02 | 0.052 ± 0.02 |
| Humic acid | 1.94 ± 0.04 | 0.61 ± 0.03 | 0.37 ± 0.02 |
| Fulvic acid | 0.77 ± 0.03 | 0.29 ± 0.01 | 0.13 ± 0.01 |
| Hydrophilic fraction | 0.87 ± 0.04 | 0.34 ± 0.01 | 0.1 ± 0.01 |
| Germination Rate (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed | Raw leachate (dilutions in percent) | |||||||||||
| Neutral pH | Unadjusted pH (8.4) | |||||||||||
| Control | 2.5 | 10 | 30 | 60% | 90% | Control | 2.5% | 10 | 30 | 60 | 90 | |
| L. sativa | 97 ± 4 | 97% ± 4 | 97 ± 3 | 52 ± 2 | 24 ± 1 | 2 ± 0.1 | 92 ± 4 | 94 ± 3 | 96 ± 4 | 40 ± 2 | 12 ± 0.5 | 0.8 ± 0.01 |
| C. sativus | 97% ± 4 | 97% ± 3 | 97 ± 4 | 62% ± 3 | 23 ± 1 | 2 ± 0.1 | 93 ± 3 | 93 ± 2 | 95 ± 4 | 54 ± 2 | 15 ± 0.5 | 0.0 |
| P. vulgaris | 98% ± 4 | 97% ± 4 | 97 ± 4 | 58% ± 3 | 33 ± 1 | 0 | 93 ± 4 | 95 ± 2 | 95 ± 4 | 48 ± 2 | 27 ± 0.5 | 0.0 |
| Seed | Electro-coagulation effluent (dilutions in percent) | |||||||||||
| Neutral pH | Unadjusted pH (9.5) | |||||||||||
| Control | 2.5 | 10 | 30 | 60 | 90 | Control | 2.5 | 10 | 30 | 60 | 90 | |
| L. sativa | 97 ± 4 | 97 ± 4 | 97 ± 4 | 55 ± 0.1 | 26 ± 0.1 | 7 ± 0.3 | 95 ± 4 | 96 ± 3 | 94 ± 4 | 51 ± 2 | 20 ± 1 | 5 ± 0.2 |
| C. sativus | 98 ± 4 | 98 ± 4 | 97 ± 4 | 67 ± 0.3 | 28 ± 0.1 | 8 ± 0.4 | 95 ± 3 | 95 ± 3 | 93 ± 4 | 60 ± 3 | 20 ± 1 | 5 ± 0.2 |
| P. vulgaris | 98 ± 3 | 97 ± 4 | 98 ± 3 | 63 ± 0.3 | 42 ± 0.2 | 8 ± 0.4 | 95 ± 3 | 93 ± 3 | 95 ± 3 | 60 ± 2 | 38 ± 1 | 5 ± 0.1 |
| Seed | Electro-oxidation effluent (dilutions in percent) | |||||||||||
| Neutral pH | Unadjusted pH (5.6) | |||||||||||
| Control | 2.5 | 10 | 30 | 60. | 90. | Control | 2.5 | 10 | 30 | 60 | 90 | |
| L. sativa | 99 ± 5 | 99 ± 4 | 97 ± 4 | 61 ± 2 | 32 ± 1 | 12.5 ± 0.5 | 98 ± 5 | 98 ± 4 | 97 ± 4 | 57 ± 1 | 29 ± 1 | 10 ± 0.5 |
| C. sativus | 100 ± 4 | 100 ± 4 | 98 ± 4 | 72 ± 3 | 40 ± 2 | 15.0 ± 0.5 | 98 ± 4 | 98 ± 3 | 95 ± 4 | 67 ± 3 | 33 ± 1 | 11 ± 0.5 |
| P. vulgaris | 100 ± 4 | 98 ± 4 | 100 ± 4 | 72 ± 3 | 53 ± 2 | 21.7 ± 1 | 98 ± 4 | 95 ± 4 | 97 ± 5 | 65 ± 3 | 47 ± 2 | 18 ± 1 |
| Half Maximal Effective Concentration (%) | ||
|---|---|---|
| Seed | Raw leachate | |
| pH 7 | pH 8.4 | |
| L. sativa | 2.1 ± 0.1 | 1.7 ± 0.1 |
| C. sativus | 2.3 ± 0.1 | 2.0. ± 0.1 |
| P. vulgaris | 2.9 ± 0.1 | 1.6 ± 0.1 |
| Seed | Electro-coagulation effluent | |
| pH 7 | pH 9.5 | |
| L. sativa | 18.9 ± 1 | 16.6 ± 1 |
| C. sativa | 20.5 ± 1 | 18.1 ± 2 |
| P. vulgaris | 22.8 ± 2 | 21.1 ± 1 |
| Seed | Electro-oxidation effluent | |
| pH 7 | pH 5.6 | |
| L. sativa | 49.8 ± 2 | 47.0 ± 2 |
| C. sativus | 55.7 ± 2 | 50.3 ± 2 |
| P. vulgaris | 62.0 ± 3 | 56.2 ± 2 |
| Seed | Dilution (%) | Transfer Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ba | Ca | Cu | Fe | K | Mg | Mn | Ni | Zn | ||
| L. sativa | 0 | 0.81 | 0.44 | 1.33 | 0.65 | 0.13 | 0.85 | 0.35 | 0.24 | 0.11 | 0.78 |
| 2.5 | 0.87 | 0.46 | 1.36 | 0.65 | 0.14 | 0.90 | 0.37 | 0.25 | 0.11 | 0.82 | |
| 10 | 0.93 | 0.48 | 1.36 | 0.70 | 0.15 | 0.95 | 0.39 | 0.26 | 0.11 | 0.87 | |
| 30 | 0.95 | 0.52 | 1.34 | 0.87 | 0.15 | 1.00 | 0.35 | 0.21 | 0.12 | 0.93 | |
| 60 | 0.99 | 0.54 | 1.41 | 0.91 | 0.15 | 1.03 | 0.36 | 0.22 | 0.12 | 0.96 | |
| 90 | 1.00 | 0.54 | 1.43 | 0.91 | 0.16 | 1.04 | 0.36 | 0.23 | 0.14 | 0.97 | |
| C. sativus | 0 | 0.76 | 0.62 | 3.12 | 0.78 | 0.06 | 0.58 | 0.39 | 0.18 | 0.05 | 0.67 |
| 2.5 | 0.91 | 0.63 | 3.09 | 0.87 | 0.07 | 0.66 | 0.40 | 0.19 | 0.05 | 0.68 | |
| 10 | 1.08 | 0.78 | 3.13 | 0.87 | 0.10 | 0.72 | 0.42 | 0.15 | 0.05 | 0.69 | |
| 30 | 1.13 | 0.95 | 3.10 | 0.91 | 0.11 | 0.87 | 0.34 | 0.16 | 0.07 | 0.71 | |
| 60 | 1.25 | 1.04 | 3.14 | 0.91 | 0.12 | 0.95 | 0.35 | 0.15 | 0.08 | 0.75 | |
| 90 | 1.37 | 1.15 | 3.15 | 0.91 | 0.13 | 1.05 | 0.37 | 0.22 | 0.09 | 0.82 | |
| P. vulgaris | 0 | 0.20 | 0.45 | 0.91 | 0.39 | 0.02 | 0.41 | 0.17 | 0.06 | 0.05 | 0.56 |
| 2.5 | 0.21 | 0.51 | 0.93 | 0.39 | 0.02 | 0.44 | 0.17 | 0.06 | 0.05 | 0.65 | |
| 10 | 0.23 | 0.57 | 1.00 | 0.61 | 0.02 | 0.53 | 0.18 | 0.07 | 0.05 | 0.70 | |
| 30 | 0.27 | 0.57 | 0.98 | 0.61 | 0.03 | 0.52 | 0.14 | 0.09 | 0.06 | 0.71 | |
| 60 | 0.30 | 0.59 | 1.04 | 0.70 | 0.03 | 0.58 | 0.16 | 0.10 | 0.06 | 0.77 | |
| 90 | 0.33 | 0.66 | 1.06 | 0.74 | 0.03 | 0.63 | 0.18 | 0.11 | 0.07 | 0.86 | |
| Seed | Dilution (%) | Enrichment Coefficient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ba | Ca | Cu | Fe | K | Mg | Mn | Ni | Zn | ||
| L. sativa | 2.5 | 1.07 | 1.05 | 1.03 | 1.00 | 1.05 | 1.05 | 1.05 | 1.05 | 1.00 | 1.05 |
| 10 | 1.15 | 1.09 | 1.02 | 1.07 | 1.11 | 1.11 | 1.11 | 1.10 | 1.07 | 1.11 | |
| 30 | 1.16 | 1.19 | 1.01 | 1.33 | 1.12 | 1.17 | 0.98 | 0.90 | 1.13 | 1.18 | |
| 60 | 1.22 | 1.23 | 1.06 | 1.40 | 1.16 | 1.21 | 1.03 | 0.95 | 1.13 | 1.22 | |
| 90 | 1.23 | 1.23 | 1.08 | 1.40 | 1.16 | 1.21 | 1.03 | 0.97 | 1.27 | 1.24 | |
| C. sativus | 2.5 | 1.20 | 1.02 | 0.99 | 1.11 | 1.09 | 1.13 | 1.05 | 1.01 | 1.00 | 1.02 |
| 10 | 1.41 | 1.25 | 1.01 | 1.11 | 1.66 | 1.23 | 1.08 | 0.80 | 1.00 | 1.03 | |
| 30 | 1.49 | 1.52 | 1.00 | 1.17 | 1.80 | 1.48 | 0.88 | 0.87 | 1.43 | 1.06 | |
| 60 | 1.64 | 1.67 | 1.01 | 1.17 | 1.98 | 1.63 | 0.92 | 0.84 | 1.57 | 1.12 | |
| 90 | 1.80 | 1.85 | 1.01 | 1.17 | 2.18 | 1.80 | 0.96 | 1.18 | 1.71 | 1.23 | |
| P. vulgaris | 2.5 | 1.04 | 1.14 | 1.03 | 1.00 | 1.32 | 1.07 | 1.00 | 1.07 | 1.00 | 1.17 |
| 10 | 1.13 | 1.27 | 1.10 | 1.56 | 1.37 | 1.29 | 1.09 | 1.24 | 1.00 | 1.26 | |
| 30 | 1.33 | 1.27 | 1.08 | 1.56 | 1.59 | 1.28 | 0.83 | 1.59 | 1.29 | 1.28 | |
| 60 | 1.46 | 1.32 | 1.15 | 1.78 | 1.75 | 1.41 | 0.97 | 1.72 | 1.29 | 1.39 | |
| 90 | 1.61 | 1.48 | 1.17 | 1.89 | 1.92 | 1.55 | 1.12 | 1.90 | 1.43 | 1.54 | |
| Parameter | PQL | Wavelength (nm) | Concentration (mg kg−1) | Maximum Recommended Value (mg kg−1) * | Parameter | PQL | Wavelength (nm) | Concentration (mg kg−1) | Maximum Recommended Value (mg kg−1) * |
|---|---|---|---|---|---|---|---|---|---|
| Ag | 0.6 | 328 | <0.6 | K | 62.5 | 766 | 35,600 ± 10 | ||
| Al | 6.3 | 396 | 3201 ± 110 | Mg | 0.6 | 280 | 10,615 ± 550 | ||
| As | 6.3 | 194 | <6 | 16 | Mn | 0.6 | 258 | 486 ± 10 | |
| Ba | 0.6 | 233 | 98 ± 4 | 350 | Ni | 3.1 | 232 | 140 | |
| Be | 0.6 | 313 | <0.6 | Pb | 6.3 | 220 | 7 ± 0.20 | 400 | |
| Ca | 15.6 | 318 | 2268 ± 110 | Se | 6.3 | 196 | <6 | ||
| Cd | 0.6 | 226 | 2 ± 0.05 | 2.5 | Ti | 0.6 | 336 | 1184 ± 50 | |
| Co | 3.1 | 229 | 13 ± 0.06 | Tl | 6.3 | 191 | <6 | ||
| Cu | 3.1 | 327 | 23 ± 1 | 270 | V | 0.6 | 312 | 50 ± 2 | |
| Fe | 3.1 | 238 | 24,125 ± 1120 | Zn | 0.6 | 2104 | 97 ± 4 | 2200 |
| Parameter | Al | Ba | Ca | Cu | Fe | K | Mg | Mn | Ni | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed | Wavelength (nm) | 393 | 233 | 318 | 327 | 238 | 766 | 280 | 258 | 232 | 214 |
| PQL | 6.3 | 0.6 | 15.6 | 3.1 | 3.1 | 62.5 | 0.6 | 0.6 | 3.1 | 0.6 | |
| Dilution (%) | Concentration (mg kg−1) | ||||||||||
| L. sativa | 0 | 2599 ± 130 | 43 ± 2 | 3013 ± 150 | 15 ± 1 | 3222 ± 160 | 30,399 ± 1500 | 3739 ± 140 | 115 ± 5 | 15 ± 1 | 76 ± 3 |
| 2.5 | 2788 ± 150 | 45 ± 2 | 3093 ± 140 | 15 ± 1 | 3391 ± 140 | 31,999 ± 1400 | 3936 ± 160 | 121 ± 6 | 15 ± 1 | 80 ± 2 | |
| 10 | 2987 ± 180 | 47 ± 2 | 3082 ± 160 | 16 ± 1 | 3570 ± 120 | 33,683 ± 1600 | 4143 ± 210 | 127 ± 5 | 16 ± 1 | 84 ± 3 | |
| 30 | 3025 ± 150 | 51 ± 1 | 3037 ± 140 | 20 ± 1 | 3609 ± 120 | 35,452 ± 110 | 3681 ± 140 | 104 ± 4 | 17 ± 1 | 90 ± 3 | |
| 60 | 3176 ± 140 | 53 ± 1 | 3189 ± 120 | 21 ± 1 | 3739 ± 110 | 36,724 ± 120 | 3865 ± 150 | 109 ± 5 | 17 ± 1 | 93 ± 4 | |
| 90 | 3192 ± 150 | 53 ± 1 | 3247 ± 150 | 21 ± 1 | 3753 ± 160 | 36,851 ± 110 | 3848 ± 160 | 111 ± 5 | 19 ± 2 | 94 ± 4 | |
| C. sativus | 0 | 2442 ± 120 | 61 ± 3 | 7067 ± 380 | 18 ± 1 | 1457 ± 1.1 | 20,805 ± 80 | 4094 ± 210 | 89 ± 4 | 7 ± 1 | 65 ± 3 |
| 2.5 | 2919 ± 80 | 62 ± 3 | 7018 ± 270 | 20 ± 1 | 1591 ± 0.6 | 23,552 ± 110 | 4287 ± 180 | 90 ± 4 | 7 ± 1 | 66 ± 3 | |
| 10 | 3442 ± 150 | 76 ± 6 | 7108 ± 480 | 20 ± 1 | 2417 ± 110 | 25,532 ± 120 | 4413 ± 120 | 71 ± 3 | 7 ± 1 | 67 ± 2 | |
| 30 | 3631 ± 140 | 93 ± 4 | 7035 ± 300 | 21 ± 1 | 2626 ± 90 | 30,868 ± 150 | 3595 ± 140 | 77 ± 4 | 10 ± 4 | 69 ± 2 | |
| 60 | 3994 ± 120 | 102 ± 4 | 7113 ± 320 | 21 ± 1 | 2889 ± 160 | 33,955 ± 140 | 3754 ± 170 | 75 ± 4 | 11 ± 5 | 73 ± 3 | |
| 90 | 4393 ± 200 | 113 ± 5 | 7139 ± 410 | 21 ± 1 | 3178 ± 180 | 37,350 ± 160 | 3950 ± 200 | 105 ± 5 | 12 ± 5 | 80 ± 3 | |
| P. vulgaris | 0 | 654 ± 40 | 44 ± 2 | 2058 ± 140 | 9 ± 1 | 422 ± 300 | 14,557 ± 700 | 1753 ± 120 | 29 ± 1 | 7 ± 3 | 54 ± 2 |
| 2.5 | 682 ± 30 | 50 ± 2 | 2117 ± 160 | 9 ± 1 | 557 ± 40 | 15,518 ± 100 | 1753 ± 180 | 31 ± 1 | 7 ± 3 | 63 ± 2 | |
| 10 | 736 ± 30 | 56 ± 2 | 2264 ± 100 | 14 ± 1 | 578 ± 30 | 18,838 ± 110 | 1910 ± 80 | 36 ± 1 | 7 ± 2 | 68 ± 2 | |
| 30 | 868 ± 40 | 56 ± 2 | 2225 ± 110 | 14 ± 1 | 670 ± 20 | 18,627 ± 80 | 1447 ± 110 | 46 ± 2 | 9 ± 2 | 69 ± 3 | |
| 60 | 955 ± 40 | 58 ± 2 | 2358 ± 80 | 16 ± 1 | 737 ± 30 | 20,489 ± 1000 | 1692 ± 120 | 50 ± 2 | 9 ± 4 | 75 ± 3 | |
| 90 | 1050 ± 50 | 65 ± 2 | 2413 ± 120 | 17 ± 1 | 811 ± 40 | 22,538 ± 1000 | 1961 ± 140 | 55 ± 2 | 10 ± 1 | 83 ± 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cruz, A.; Rojas-Valencia, M.N. Assessment of Phytotoxicity in Untreated and Electrochemically Treated Leachates through the Analysis of Early Seed Growth and Inductively Coupled Plasma-Optical Emission Spectroscopy Characterization. Horticulturae 2024, 10, 67. https://doi.org/10.3390/horticulturae10010067
Martínez-Cruz A, Rojas-Valencia MN. Assessment of Phytotoxicity in Untreated and Electrochemically Treated Leachates through the Analysis of Early Seed Growth and Inductively Coupled Plasma-Optical Emission Spectroscopy Characterization. Horticulturae. 2024; 10(1):67. https://doi.org/10.3390/horticulturae10010067
Chicago/Turabian StyleMartínez-Cruz, Alfredo, and María Neftalí Rojas-Valencia. 2024. "Assessment of Phytotoxicity in Untreated and Electrochemically Treated Leachates through the Analysis of Early Seed Growth and Inductively Coupled Plasma-Optical Emission Spectroscopy Characterization" Horticulturae 10, no. 1: 67. https://doi.org/10.3390/horticulturae10010067
APA StyleMartínez-Cruz, A., & Rojas-Valencia, M. N. (2024). Assessment of Phytotoxicity in Untreated and Electrochemically Treated Leachates through the Analysis of Early Seed Growth and Inductively Coupled Plasma-Optical Emission Spectroscopy Characterization. Horticulturae, 10(1), 67. https://doi.org/10.3390/horticulturae10010067







