Genome-Wide Identification of the DGK Gene Family in Kiwifruit (Actinidia valvata Dunn) and an Expression Analysis of Their Responses to Waterlogging Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of DGK Genes in A. valvata
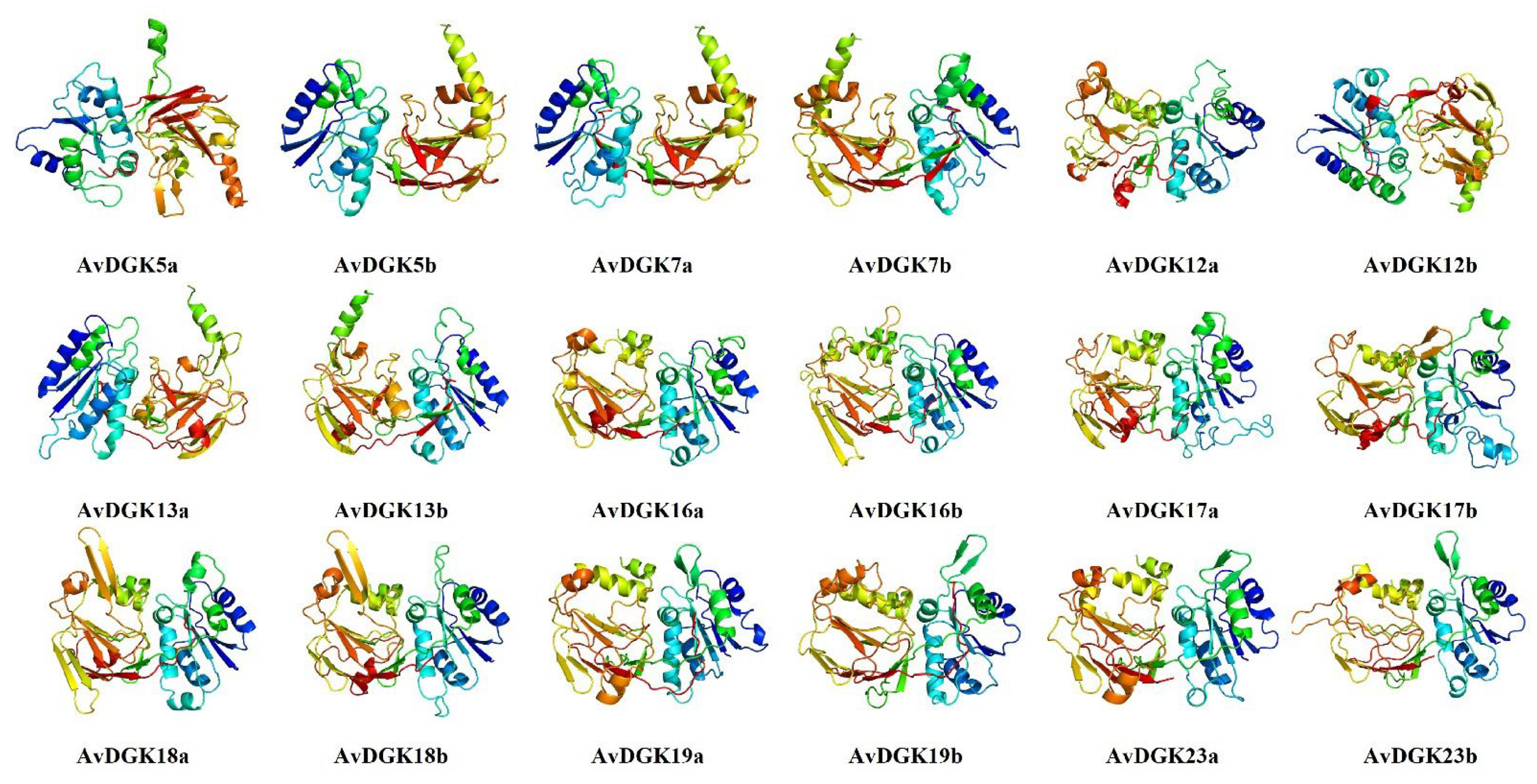
2.2. Physiochemical Properties, Protein Secondary Structure, and 3D Modeling of AvDGK Proteins
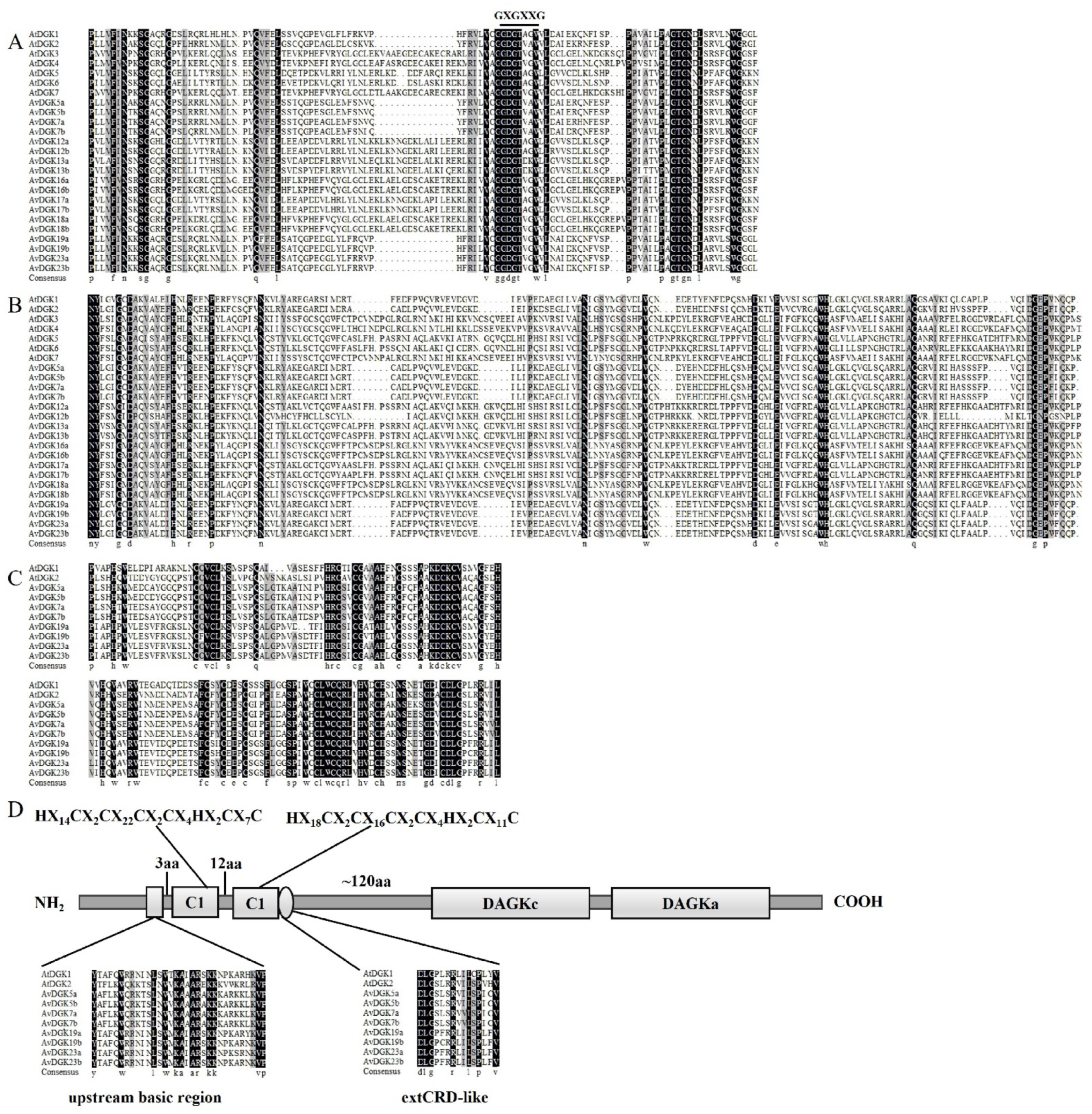
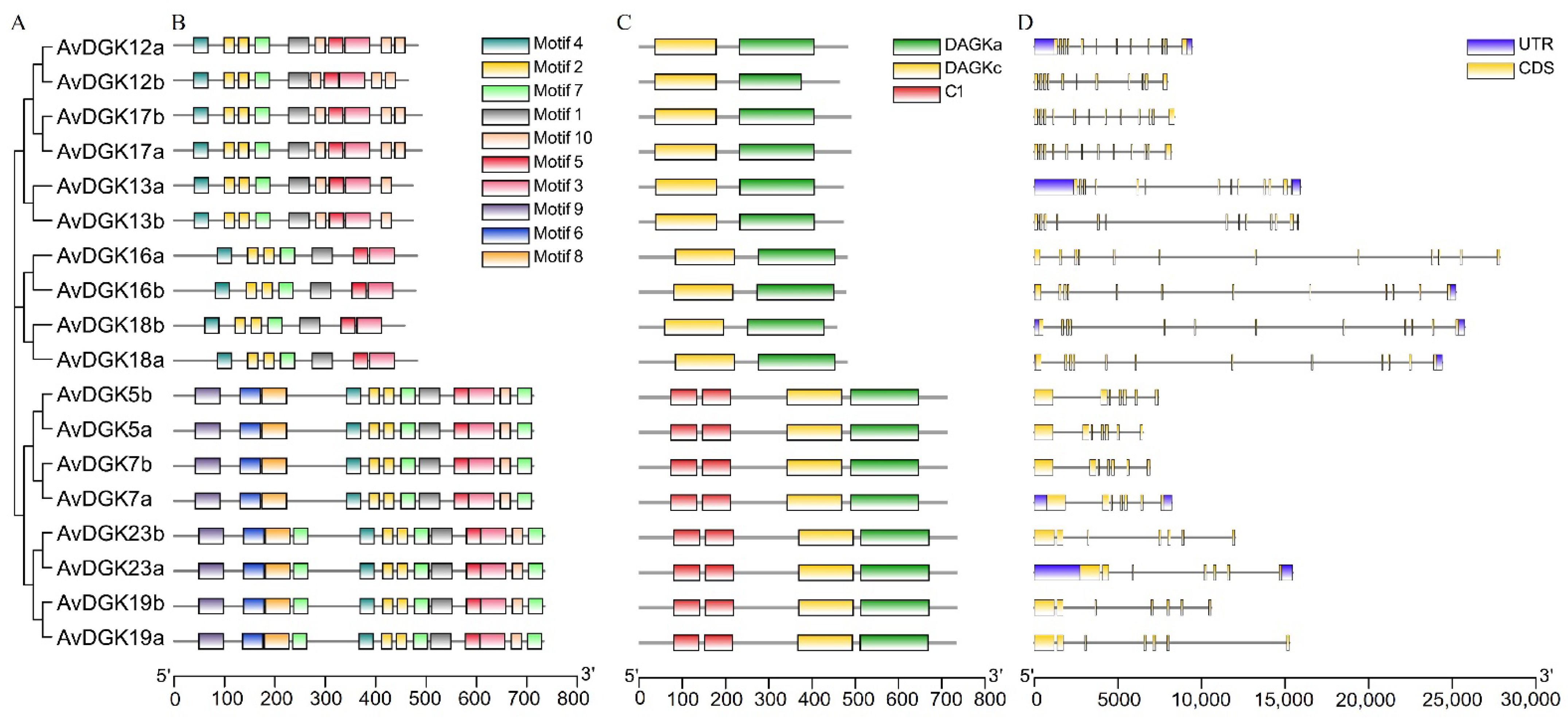
2.3. Phylogenetic Analyses and Multiple Sequence Alignment of AvDGK Proteins
2.4. Analysis of Gene Structures and Conserved Motifs
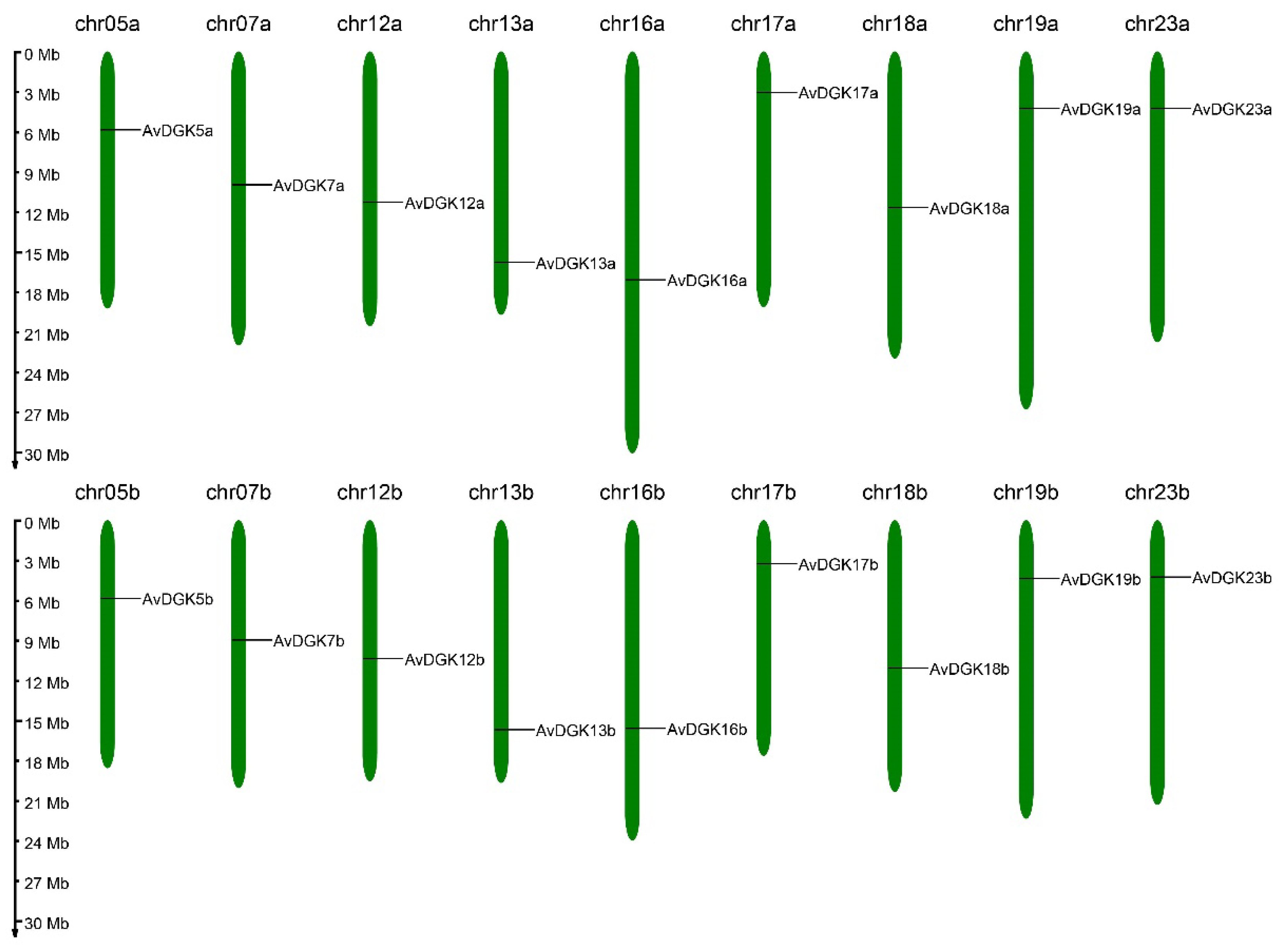
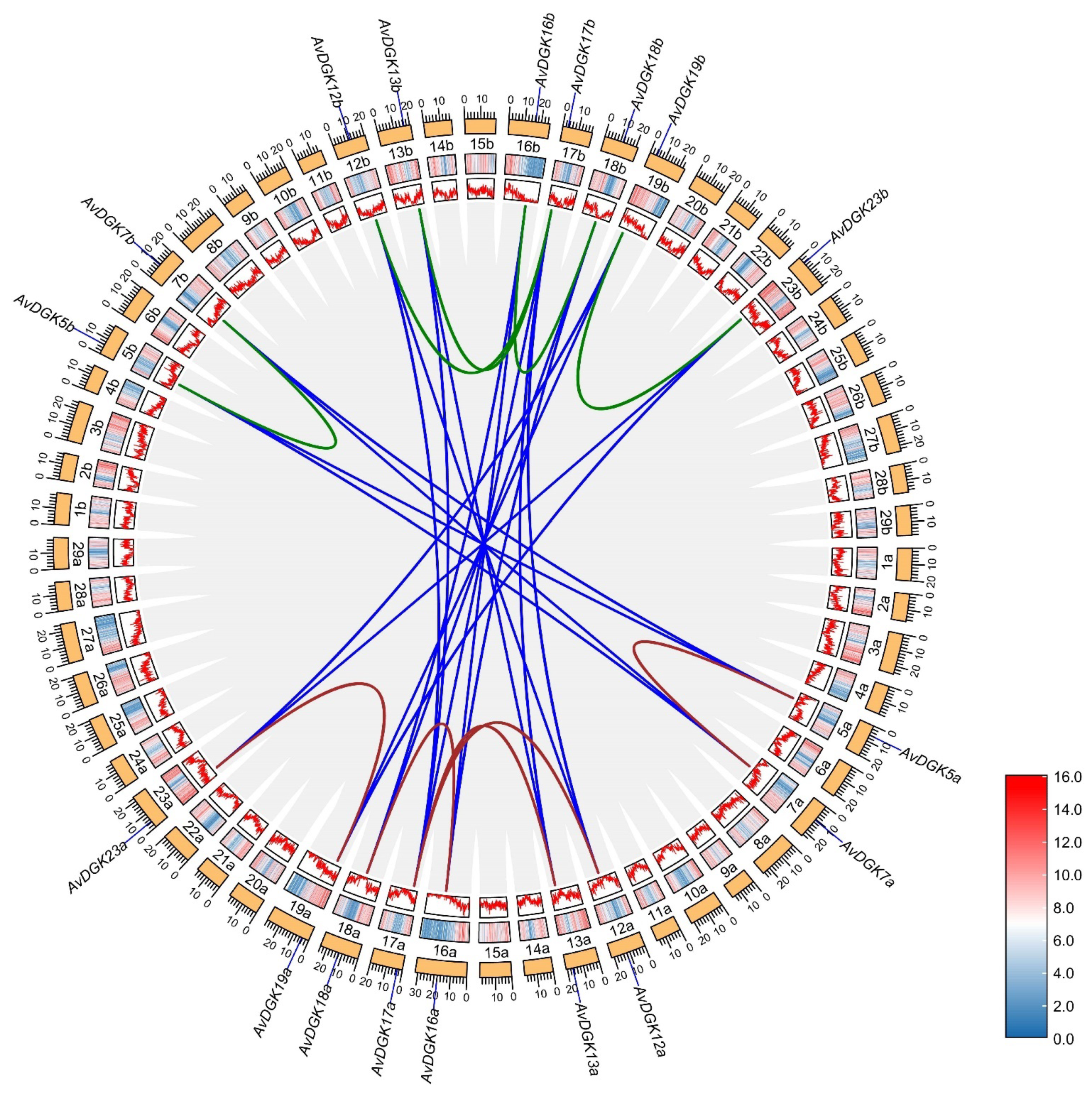
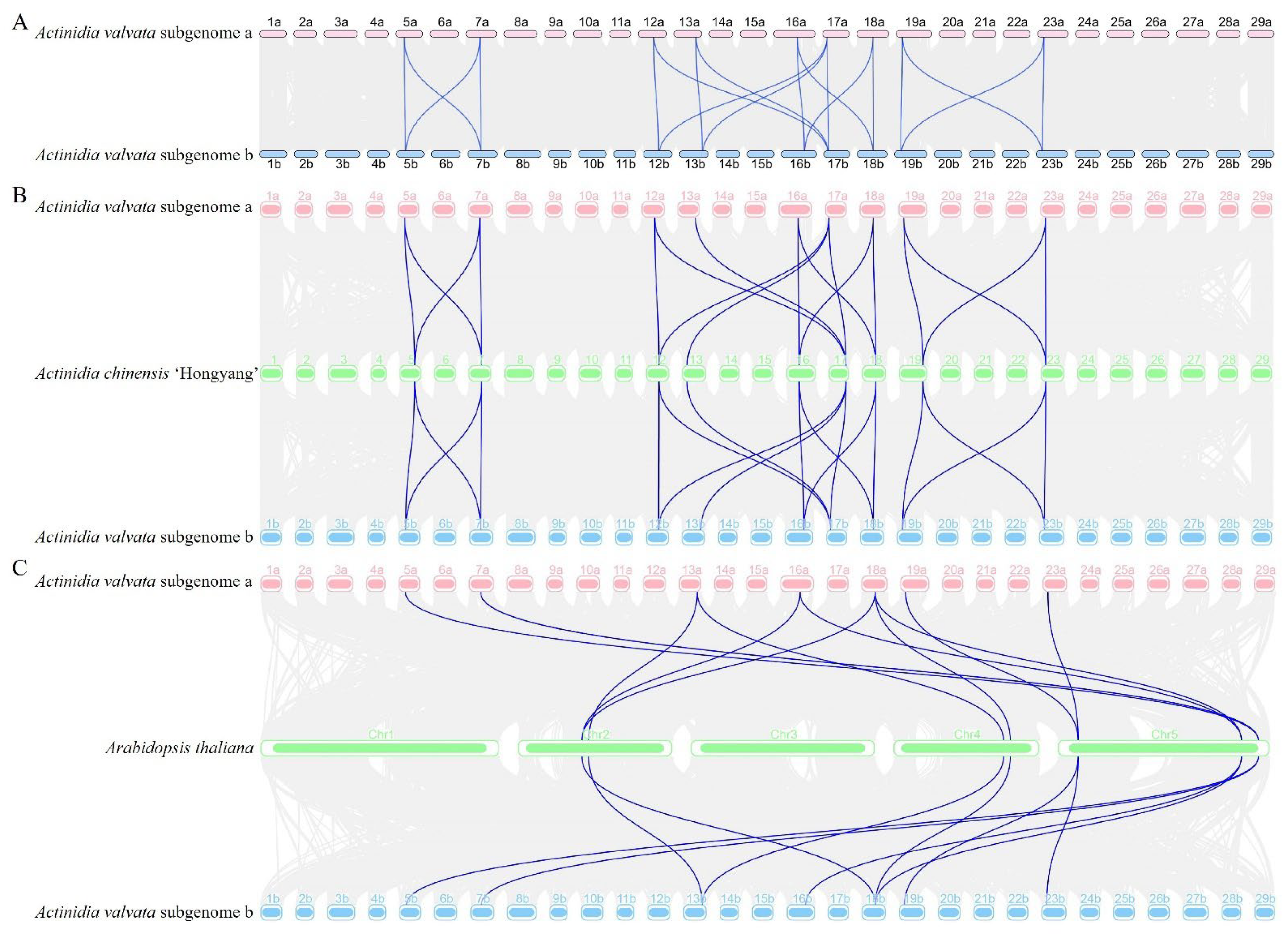
2.5. Chromosome Location, Gene Duplication, and a Collinearity Analysis of AvDGK Genes
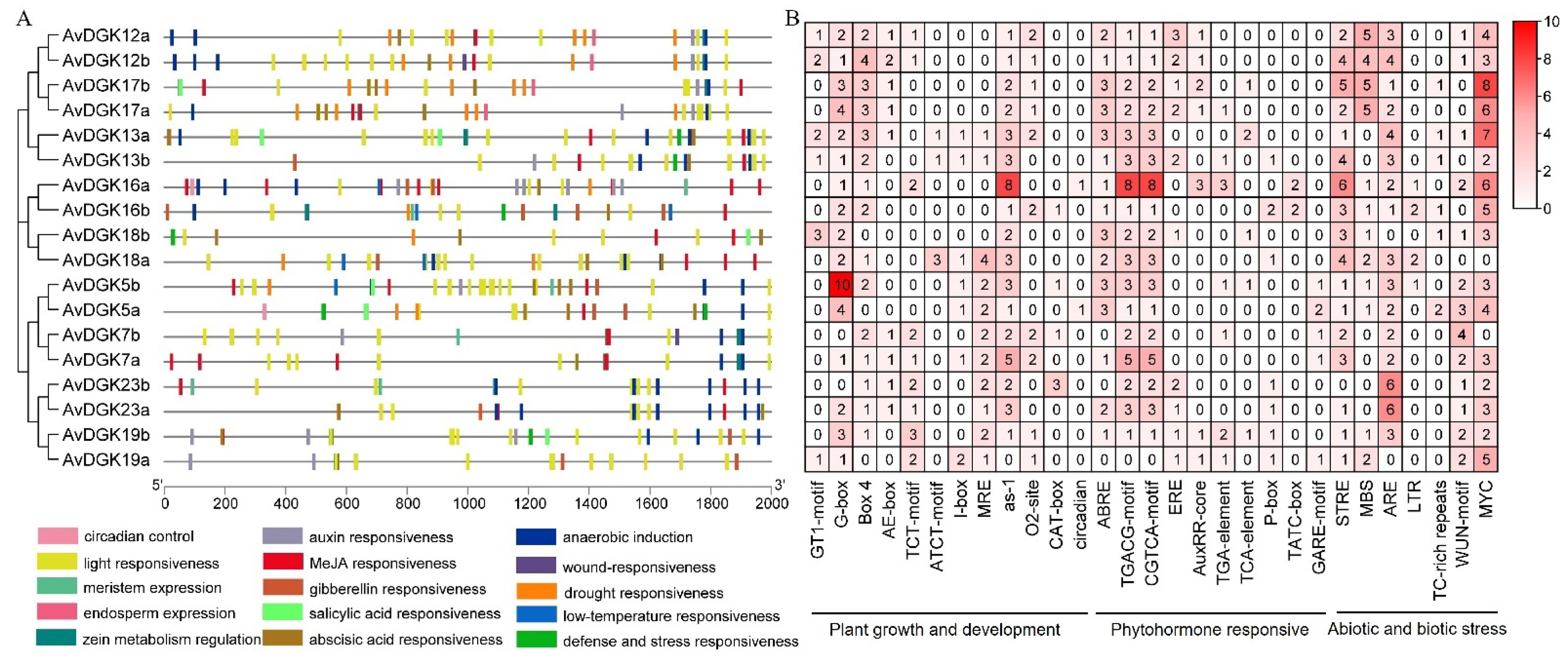
2.6. Cis-Regulatory Element Prediction and an Analysis of Promoters
2.7. Gene Expression Analysis of AvDGKs
2.8. Plant Materials and Treatments
2.9. RNA Extraction and qRT-PCR Analysis
2.10. Statistical Analysis
3. Results
3.1. Genome-Wide Identification of DGK Genes in A. valvata
3.2. Phylogenetic Analysis and Multiple Sequence Alignment of DGK Genes
3.3. Functional Domain, Secondary Structure, and 3D Modeling of AvDGKs
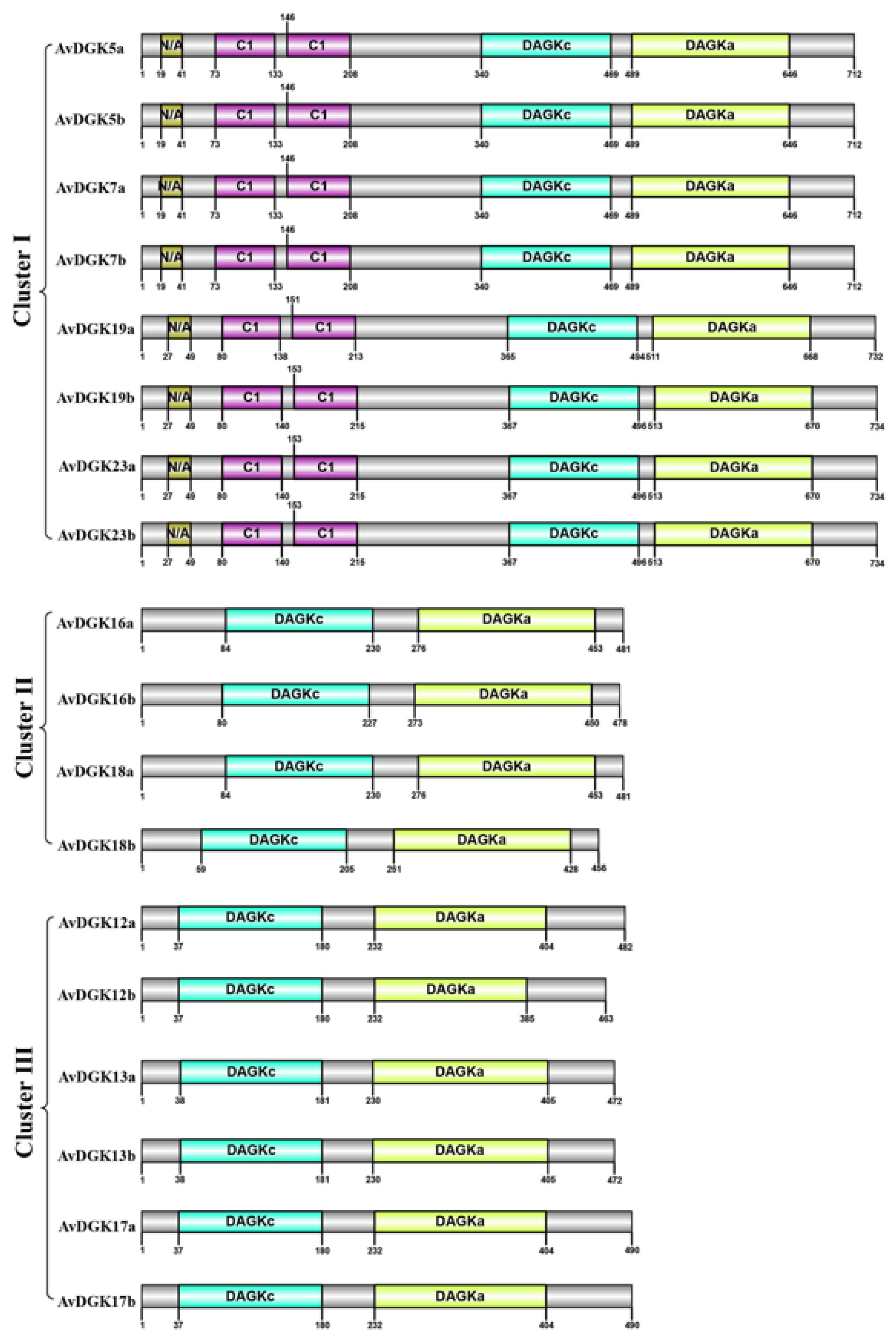
3.4. Gene Structure, Domain, and Conserved Motif Analysis of AvDGKs
3.5. Synteny and Gene Duplication Analysis of AvDGKs
3.6. Analysis of Cis-Elements in the Promoters of AvDGK Genes
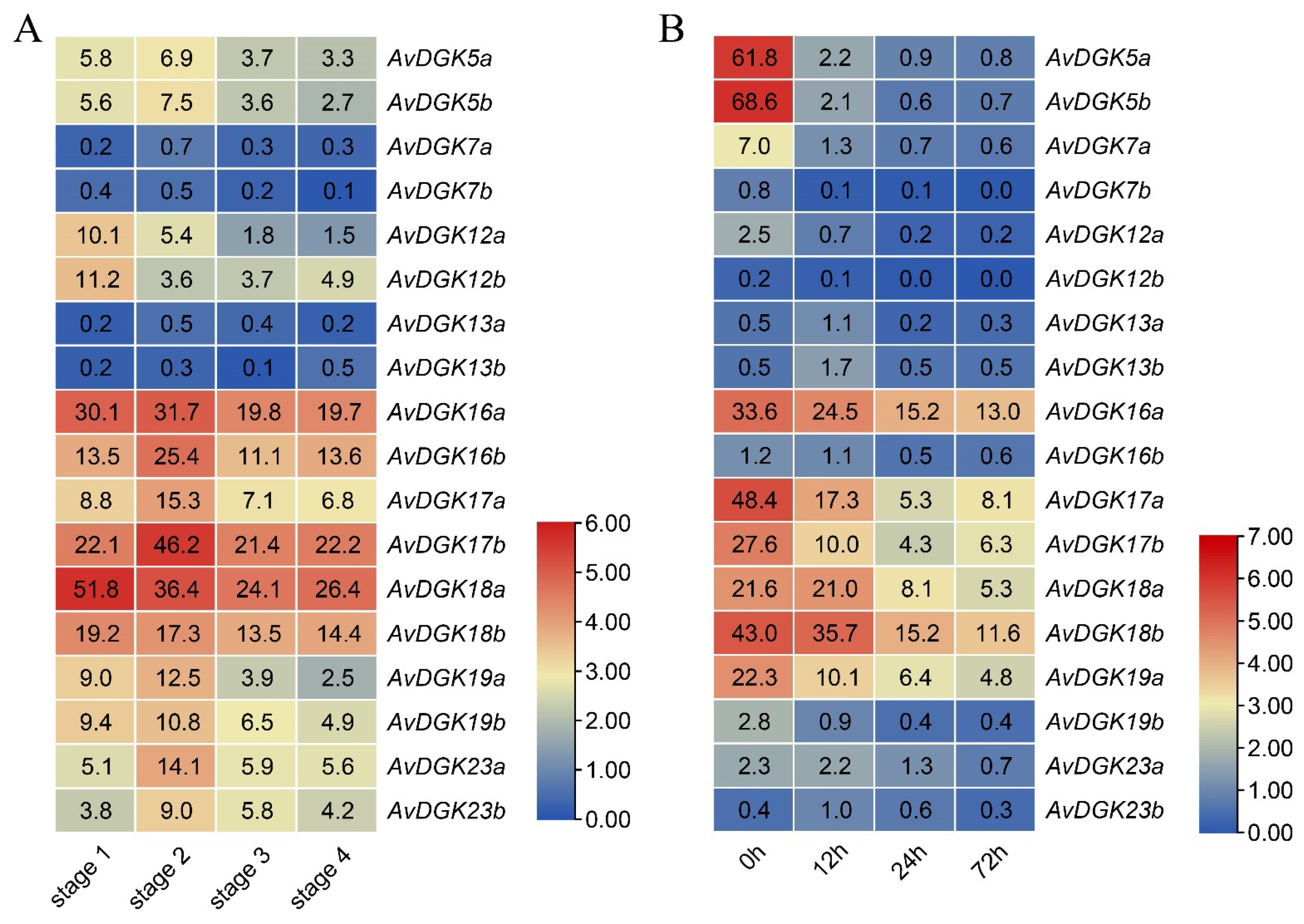
3.7. Expression Patterns of AvDGKs in Kiwifruit
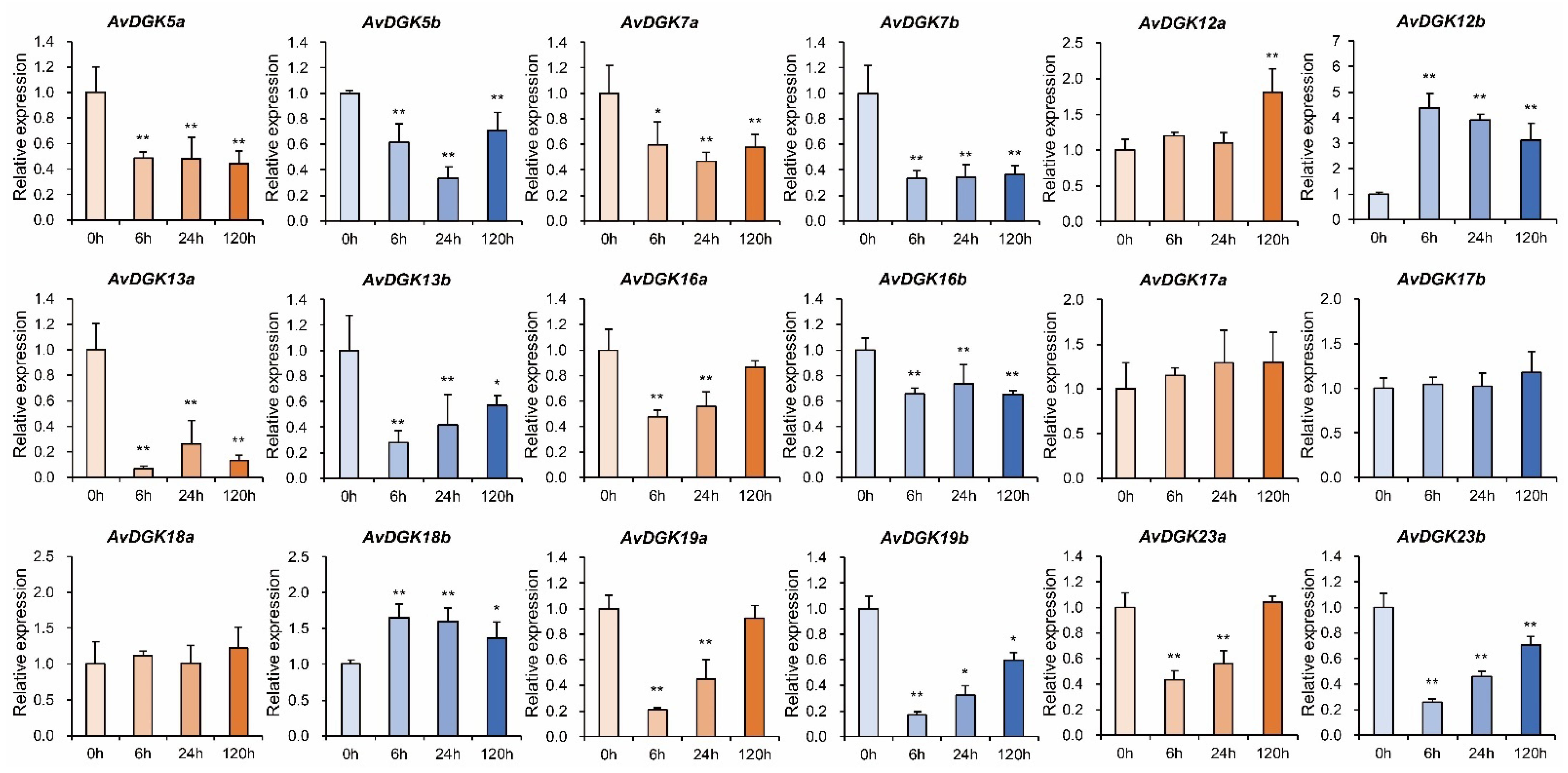
3.8. RT-qPCR of AvDGKs under Waterlogging Stress at Different Times
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, S.; Kim, S.; Li, J.; Tang, S.; Wang, X. Phosphatidic acid signaling and function in nuclei. Prog. Lipid Res. 2024, 93, 101267. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ma, X.; Zhang, C.; Kim, S.; Li, B.; Xie, Y.; Yeo, I.; Thapa, H.; Chen, S.; Devarenne, T.P.; et al. Dual phosphorylation of DGK5-mediated PA burst regulates ROS in plant immunity. Cell 2024, 187, 609–623.e21. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Lu, S.; Fadlalla, T.; Iqbal, S.; Yue, H.; Yang, B.; Hong, Y.; Wang, X.; Guo, L. The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog. Lipid Res. 2022, 86, 101158. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, S.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wang, X. Phosphatidic acid: An emerging versatile class of cellular mediators. Essays Biochem. 2020, 64, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Testerink, C.; Munnik, T. Phosphatidic acid: A multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005, 10, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Liao, K.; Wang, L.; Shi, L.; Zhang, Y.; Xu, L.; Zhou, Y.; Li, J.; Chen, Y.; Chen, Q.; et al. Calcium-dependent activation of CPK12 facilitates its cytoplasm-to-nucleus translocation to potentiate plant hypoxia sensing by phosphorylating ERF-VII transcription factors. Mol. Plant 2023, 16, 979–998. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Premkumar, A.; Rasmussen, U.; Schulz, A.; Lager, I. Phospholipases AtPLDζ1 and AtPLDζ2 function differently in hypoxia. Physiol. Plant. 2018, 162, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, Y.; Kretynin, S.; Bukhonska, Y.; Pokotylo, I.; Ruelland, E.; Martinec, J.; Kravets, V. Phosphatidic acid in plant hormonal signaling: From target proteins to membrane conformations. Int. J. Mol. Sci. 2022, 23, 3227. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gu, Y.; Lee, Y.; Yang, Z.; Lee, Y. Phosphatidic acid induces leaf cell death in Arabidopsis by activating the rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol. 2004, 134, 129–136. [Google Scholar] [CrossRef]
- Scholz, P.; Pejchar, P.; Fernkorn, M.; Škrabálková, E.; Pleskot, R.; Blersch, K.; Munnik, T.; Potocký, M.; Ischebeck, T. DIACYLGLYCEROL KINASE 5 regulates polar tip growth of tobacco pollen tubes. New Phytol. 2022, 233, 2185–2202. [Google Scholar] [CrossRef] [PubMed]
- Kue Foka, I.C.; Ketehouli, T.; Zhou, Y.; Li, X.; Wang, F.; Li, H. The emerging roles of diacylglycerol kinase (DGK) in plant stress tolerance, growth, and development. Agronomy 2020, 10, 1375. [Google Scholar] [CrossRef]
- Escobar Sepúlveda, H.F.; Trejo Téllez, L.I.; Pérez Rodríguez, P.; Hidalgo Contreras, J.V.; Gómez Merino, F.C. Diacylglycerol kinases are widespread in higher plants and display inducible gene expression in response to beneficial elements, metal, and metalloid ions. Front. Plant Sci. 2017, 8, 227478. [Google Scholar] [CrossRef] [PubMed]
- Arisz, S.A.; Testerink, C.; Munnik, T. Plant PA signaling via diacylglycerol kinase. Biochim. Biophys. Acta 2009, 1791, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Chen, C.; Jing, W.; Zhang, Q.; Wang, H.; Wang, R.; Zhang, W. The rice diacylglycerol kinase family: Functional analysis using transient RNA interference. Front. Plant Sci. 2012, 3, 21372. [Google Scholar] [CrossRef] [PubMed]
- Yeken, M.Z.; Özer, G.; Çiftçi, V. Genome-wide identification and expression analysis of DGK (diacylglycerol kinase) genes in common bean. J. Plant Growth Regul. 2023, 42, 2558–2569. [Google Scholar] [CrossRef]
- Tang, F.; Xiao, Z.; Sun, F.; Shen, S.; Chen, S.; Chen, R.; Zhu, M.; Zhang, Q.; Du, H.; Lu, K.; et al. Genome-wide identification and comparative analysis of diacylglycerol kinase (DGK) gene family and their expression profiling in Brassica napus under abiotic stress. BMC Plant Biol. 2020, 20, 473. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Si, X.; Jia, Y.; Zhang, H.; Tian, S.; Li, W.; Zhang, K.; Pan, Y. Genomic profiling and expression analysis of the diacylglycerol kinase gene family in heterologous hexaploid wheat. PeerJ 2021, 9, e12480. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, Y.; Shao, Y.; Li, M.; Ma, F. Comprehensive genomic analysis and expression profiling of diacylglycerol kinase gene family in Malus prunifolia (Willd.) Borkh. Gene 2015, 561, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Carther, K.F.I.; Ketehouli, T.; Ye, N.; Yang, Y.H.; Wang, N.; Dong, Y.Y.; Yao, N.; Liu, X.M.; Liu, W.C.; Li, X.W.; et al. Comprehensive genomic analysis and expression profiling of diacylglycerol kinase (DGK) gene family in soybean (Glycine max) under abiotic stresses. Int. J. Mol. Sci. 2019, 20, 1361. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhao, C.; He, L.; Yan, B.; Dong, J.; Li, Z.; Yang, K.; Xu, J. Genome-wide identification and abiotic stress responses of DGK gene family in maize. J. Plant Biochem. Biotechnol. 2018, 27, 156–166. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Z.; Yang, M.; Gu, L. Genome-wide identification and characterization of the diacylglycerol kinase (DGK) gene family in Populus trichocarpa. Physiol. Mol. Plant Pathol. 2023, 127, 102121. [Google Scholar] [CrossRef]
- Gómez Merino, F.C.; Brearley, C.A.; Ornatowska, M.; Abdel Haliem, M.E.F.; Zanor, M.I.; Mueller Roeber, B. AtDGK2, a novel diacylglycerol kinase from Arabidopsis thaliana, phosphorylates 1-stearoyl-2-arachidonoyl-sn-glycerol and 1,2-dioleoyl-sn-glycerol and exhibits cold-inducible gene expression. J. Biol. Chem. 2004, 279, 8230–8241. [Google Scholar] [CrossRef] [PubMed]
- Vaultier, M.N.; Cantrel, C.; Guerbette, F.; Boutté, Y.; Vergnolle, C.; Çiçek, D.; Bolte, S.; Zachowski, A.; Ruelland, E. The hydrophobic segment of Arabidopsis thaliana cluster I diacylglycerol kinases is sufficient to target the proteins to cell membranes. FEBS Lett. 2008, 582, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Snedden, W.A.; Blumwald, E. Alternative splicing of a novel diacylglycerol kinase in tomato leads to a calmodulin-binding isoform. Plant J. 2000, 24, 317–326. [Google Scholar] [CrossRef]
- Gómez Merino, F.C.; Arana Ceballos, F.A.; Trejo Téllez, L.I.; Skirycz, A.; Brearley, C.A.; Dörmann, P.; Mueller Roeber, B. Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases (DGKs), displays unique biochemical features and saturates at low substrate concentration. J. Biol. Chem. 2005, 280, 34888–34899. [Google Scholar] [CrossRef]
- Yuan, S.; Kim, S.; Deng, X.; Hong, Y.; Wang, X. Diacylglycerol kinase and associated lipid mediators modulate rice root architecture. New Phytol. 2019, 223, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, S.; Kim, S.; Wang, X. Lipid phosphorylation by a diacylglycerol kinase suppresses ABA biosynthesis to regulate plant stress responses. Mol. Plant 2024, 17, 342–358. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, D.; Yu, W.; Shi, L.; Zhang, Y.; Lai, Y.; Huang, L.; Qi, H.; Chen, Q.; Yao, N.; et al. Phosphatidic acid modulates MPK3- and MPK6-mediated hypoxia signaling in Arabidopsis. Plant Cell 2021, 34, 889–909. [Google Scholar] [CrossRef]
- Huo, L.; Wang, H.; Wang, Q.; Gao, Y.; Xu, K.; Sun, X. Exogenous treatment with melatonin enhances waterlogging tolerance of kiwifruit plants. Front. Plant Sci. 2022, 13, 1081787. [Google Scholar] [CrossRef]
- Wurms, K.V.; Reglinski, T.; Buissink, P.; Ah Chee, A.; Fehlmann, C.; McDonald, S.; Cooney, J.; Jensen, D.; Hedderley, D.; McKenzie, C.; et al. Effects of drought and flooding on phytohormones and abscisic acid gene xxpression in Kiwifruit. Int. J. Mol. Sci. 2023, 24, 7580. [Google Scholar] [CrossRef]
- Huang, H. Systematics and genetic variation of Actinidia. In Kiwifruit; Academic Press: San Diego, CA, USA, 2016; Chapter 1; pp. 9–44. [Google Scholar] [CrossRef]
- Ferguson, A.R.; Huang, H. Genetic resources of Kiwifruit: Domestication and breeding. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Chapter 1; pp. 1–121. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Soejarto, D.D. Advances in the study of the systematics of Actinidia Lindley. Front. Biol. China 2009, 4, 55–61. [Google Scholar] [CrossRef]
- Gao, M.; Gai, C.; Li, X.; Feng, X.; Lai, R.; Song, Y.; Zeng, R.; Chen, D.; Chen, Y. Waterlogging tolerance of Actinidia valvata Dunn is associated with high activities of pyruvate decarboxylase, alcohol dehydrogenase and antioxidant enzymes. Plants 2023, 12, 2872. [Google Scholar] [CrossRef]
- Bai, D.; Li, Z.; Hu, C.; Zhang, Y.; Muhammad, A.; Zhong, Y.; Fang, J. Transcriptome-wide identification and expression analysis of ERF family genes in Actinidia valvata during waterlogging stress. Sci. Hortic. 2021, 281, 109994. [Google Scholar] [CrossRef]
- Li, Z.; Bai, D.; Zhong, Y.; Abid, M.; Qi, X.; Hu, C.; Fang, J. Physiological responses of two contrasting Kiwifruit (Actinidia spp.) rootstocks against waterlogging stress. Plants 2021, 10, 2586. [Google Scholar] [CrossRef]
- Bai, D.; Zhong, Y.; Gu, S.; Qi, X.; Sun, L.; Lin, M.; Wang, R.; Li, Y.; Hu, C.; Fang, J. AvERF73 positively regulates waterlogging tolerance in kiwifruit by participating in hypoxia response and mevalonate pathway. Hortic. Plant J. 2024; in press. [Google Scholar] [CrossRef]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2011, 40, D1202–D1210. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, D412–D419. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.; Shen, H. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.; Ma, J.; Luo, X.; Nie, P.; Zuo, Z.; Lahrmann, U.; Zhao, Q.; Zheng, Y.; Zhao, Y.; et al. IBS: An illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015, 31, 3359–3361. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2011, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhao, X.Q.; Wang, J.; Wong, G.K.; Yu, J. KaKs_Calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Fan, C.; Sun, J.; Chang, Y.; Lu, J.; Sun, J.; Wang, C.; Liu, J. Genome-wide Identification, evolution, and expression analysis of the TCP gene family in rose (Rosa chinensis Jacq.). Horticulturae 2022, 8, 961. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Arisz, S.A.; van Wijk, R.v.; Roels, W.; Zhu, J.; Haring, M.A.; Munnik, T. Rapid phosphatidic acid accumulation in response to low temperature stress in Arabidopsis is generated through diacylglycerol kinase. Front. Plant Sci. 2013, 4, 20590. [Google Scholar] [CrossRef]
- Shen, L.; Zhuang, B.; Wu, Q.; Zhang, H.; Nie, J.; Jing, W.; Yang, L.; Zhang, W. Phosphatidic acid promotes the activation and plasma membrane localization of MKK7 and MKK9 in response to salt stress. Plant Sci. 2019, 287, 110190. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, Z.; Shi, M.; Zhou, Y.; Huo, L.; Li, X.; Xu, K. Comparative transcriptome provides insight into responding mechanism of waterlogging stress in Actinidia valvata Dunn. Gene 2022, 845, 146843. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, Y.; Bai, D.; Lin, M.; Qi, X.; Fang, J. Comparative analysis of physiological traits of three Actinidia valvata Dunn genotypes during waterlogging and post-waterlogging recovery. Hortic. Environ. Biotechnol. 2020, 61, 825–836. [Google Scholar] [CrossRef]
- Li, Z.; Bai, D.; Zhong, Y.; Lin, M.; Sun, L.; Qi, X.; Hu, C.; Fang, J. Full-Length transcriptome and RNA-Seq analyses reveal the mechanisms underlying waterlogging tolerance in Kiwifruit (Actinidia valvata). Int. J. Mol. Sci. 2022, 23, 3237. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; O’Hely, M.; Walsh, B.; Force, A. The probability of preservation of a newly arisen gene duplicate. Genetics 2001, 159, 1789–1804. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Wolf, Y.I.; Sorokin, A.V.; Mirkin, B.G.; Koonin, E.V. Remarkable interkingdom conservation of intron positions and massive, lineage-specific intron loss and gain in eukaryotic evolution. Curr. Biol. 2003, 13, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Castillo, M.C.; Gayubas, B. The hypoxia–reoxygenation stress in plants. J. Exp. Bot. 2020, 72, 5841–5856. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qin, G.; Tian, S. Regulatory network of fruit ripening: Current understanding and future challenges. New Phytol. 2020, 228, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Verma, T.; Kapoor, D. Ethylene and regulation of metabolites in plants. In Ethylene in Plant Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; Chapter 3; pp. 32–48. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Smith, D.L.; Green, K.C. Characterization of a phospholipase Dα cDNA from tomato fruit. Biochem. Soc. Trans. 2000, 28, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Liu, D.; Chu, M.; Liu, X.; Wei, Y.; Che, X.; Zhu, L.; He, L.; Xu, J. Dynamic and adaptive membrane lipid remodeling in leaves of sorghum under salt stress. Crop J. 2022, 10, 1557–1569. [Google Scholar] [CrossRef]
- Xu, L.; Pan, R.; Zhou, M.; Xu, Y.; Zhang, W. Lipid remodelling plays an important role in wheat (Triticum aestivum) hypoxia stress. Funct. Plant Biol. 2020, 47, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, Y.; Tao, F.; Yang, S.; Li, W. Submergence induced changes of molecular species in membrane lipids in Arabidopsis thaliana. Plant Divers. 2016, 38, 156–162. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, Y.; Chen, Q.; Xiao, S. New insights into the role of lipids in plant hypoxia responses. Prog. Lipid Res. 2021, 81, 101072. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Gene ID | CDS Length (bp) | Number of Amino Acids (aa) | Molecular Weight (kDa) | pI | Subcellular Localization |
|---|---|---|---|---|---|---|
| AvDGK5a | Ava05g00367 | 2139 | 712 | 79.50 | 8.62 | Nucleus |
| AvDGK5b | Avb05g00366 | 2139 | 712 | 79.31 | 8.74 | Nucleus |
| AvDGK7a | Ava07g00406 | 2139 | 712 | 79.24 | 8.14 | Nucleus |
| AvDGK7b | Avb07g00371 | 2139 | 712 | 79.39 | 8.14 | Nucleus |
| AvDGK12a | Ava12g00623 | 1449 | 482 | 54.05 | 8.57 | Chloroplast. Cytoplasm. Nucleus |
| AvDGK12b | Avb12g00588 | 1392 | 463 | 51.84 | 7.06 | Cytoplasm |
| AvDGK13a | Ava13g01333 | 1419 | 472 | 53.55 | 9.16 | Chloroplast |
| AvDGK13b | Avb13g01246 | 1419 | 472 | 53.28 | 8.68 | Chloroplast. Cytoplasm. Nucleus |
| AvDGK16a | Ava16g01104 | 1446 | 481 | 53.40 | 6.84 | Chloroplast |
| AvDGK16b | Avb16g01069 | 1437 | 478 | 53.14 | 6.87 | Chloroplast |
| AvDGK17a | Ava17g00287 | 1473 | 490 | 54.90 | 6.39 | Chloroplast. Cytoplasm |
| AvDGK17b | Avb17g00295 | 1473 | 490 | 54.95 | 6.31 | Cytoplasm. Nucleus |
| AvDGK18a | Ava18g00909 | 1446 | 481 | 53.53 | 6.72 | Chloroplast |
| AvDGK18b | Avb18g00884 | 1371 | 456 | 50.84 | 6.41 | Chloroplast |
| AvDGK19a | Ava19g00482 | 2199 | 732 | 80.77 | 6.32 | Nucleus |
| AvDGK19b | Avb19g00480 | 2205 | 734 | 81.06 | 6.50 | Nucleus |
| AvDGK23a | Ava23g00486 | 2205 | 734 | 80.91 | 6.44 | Nucleus |
| AvDGK23b | Avb23g00493 | 2205 | 734 | 80.83 | 6.41 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, C.; Wang, F.; Liu, S.; Gao, J.; Li, J.; Mo, Q.; Ye, K.; Qi, B.; Gong, H. Genome-Wide Identification of the DGK Gene Family in Kiwifruit (Actinidia valvata Dunn) and an Expression Analysis of Their Responses to Waterlogging Stress. Horticulturae 2024, 10, 310. https://doi.org/10.3390/horticulturae10040310
Zhang M, Liu C, Wang F, Liu S, Gao J, Li J, Mo Q, Ye K, Qi B, Gong H. Genome-Wide Identification of the DGK Gene Family in Kiwifruit (Actinidia valvata Dunn) and an Expression Analysis of Their Responses to Waterlogging Stress. Horticulturae. 2024; 10(4):310. https://doi.org/10.3390/horticulturae10040310
Chicago/Turabian StyleZhang, Meijuan, Cuixia Liu, Faming Wang, Shibiao Liu, Jianyou Gao, Jiewei Li, Quanhui Mo, Kaiyu Ye, Beibei Qi, and Hongjuan Gong. 2024. "Genome-Wide Identification of the DGK Gene Family in Kiwifruit (Actinidia valvata Dunn) and an Expression Analysis of Their Responses to Waterlogging Stress" Horticulturae 10, no. 4: 310. https://doi.org/10.3390/horticulturae10040310
APA StyleZhang, M., Liu, C., Wang, F., Liu, S., Gao, J., Li, J., Mo, Q., Ye, K., Qi, B., & Gong, H. (2024). Genome-Wide Identification of the DGK Gene Family in Kiwifruit (Actinidia valvata Dunn) and an Expression Analysis of Their Responses to Waterlogging Stress. Horticulturae, 10(4), 310. https://doi.org/10.3390/horticulturae10040310








