Effect of Different Intensities of Leaf Removal on Tomato Development and Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Trials
- –
- Leaf removal control (LRC): all leaves were removed from the base to 2 leaves below the truss close to harvest, which we identified as the reference truss (T0).
- –
- Leaf removal 1 (LR1): all leaves were removed from the base to 2 leaves below truss T1, the next truss above T0.
- –
- Leaf removal 2 (LR2): leaves were removed from the base to 2 leaves below T2 (second truss above T0).
- –
- Leaf removal 4 (LR4): leaves were removed from the base to 2 leaves below T4 (fourth truss above T0).
- –
- Intense leaf removal (LRI): between 10 and 12 leaves per stem were kept.
- –
- Trial 1: The effect of two leaf removal intensities (LR1 and LR2) was tested against control LRC. Leaf removal was carried out on a monthly basis, during the 08/09 season.
- –
- Trial 2: The effect of two leaf removal intensities (LR2 and LR4) was tested against control LRC. Leaf removal was carried out at two frequencies: monthly and fortnightly, during the 09/10 season.
- –
- Trial 3: The effect of intense leaf removal (LRI) was compared to control LRC. Leaf removal was carried out fortnightly, during the 10/11 season (Figure 2).
2.2. Data Studied
2.2.1. Climate Variables
2.2.2. Crop Ecophysiological Parameters
- –
- Number of leaves emitted and removed. Measurements were taken monthly or fortnightly, depending on the leaf removal treatment, by counting the new leaves emitted on the plant since the last one marked in the previous measurement and the leaves removed according to the treatment tested.
- –
- Biomass produced by destructive methods during various crop stages, sorted by stems, leaves, shoots, flowers and fruits.
- –
- Leaf area index (LAI) [13]. A Licor 3100C leaf area meter was used. Leaves with more than 50% of the surface area showing symptoms of senescence were not measured.
- –
- Number of trusses, flowers and fruits emitted, counting both viable and aborted fruit.
- –
- Specific leaf area (SLA), determined by the ratio between the leaf area sampled (LA) and its corresponding dry weight.
- –
- Radiation use efficiency (RUE, also referred to as light use efficiency LUE), calculated as the ratio between the biomass produced by the crop and the incident radiation during the same period.
- –
- Net assimilation rate (NAR), defined as the rate of dry matter production per unit leaf area. It was related to the incident radiation in the same measurement period.
2.2.3. Yield Components
- –
- Commercial and non-commercial yields.
- –
- Distribution by size of commercial fruit, 3M (35–47 mm), 2M (47–57 mm), M (57–67 mm), G (67–82 mm) and 2G (82–102 mm).
- –
- Harvest index (HI), calculated as the ratio of fruit yield, expressed in dry weight, to the total biomass obtained.
2.3. Statistical Analysis
3. Results
3.1. Climate Variables
3.2. Ecophysiological Variables
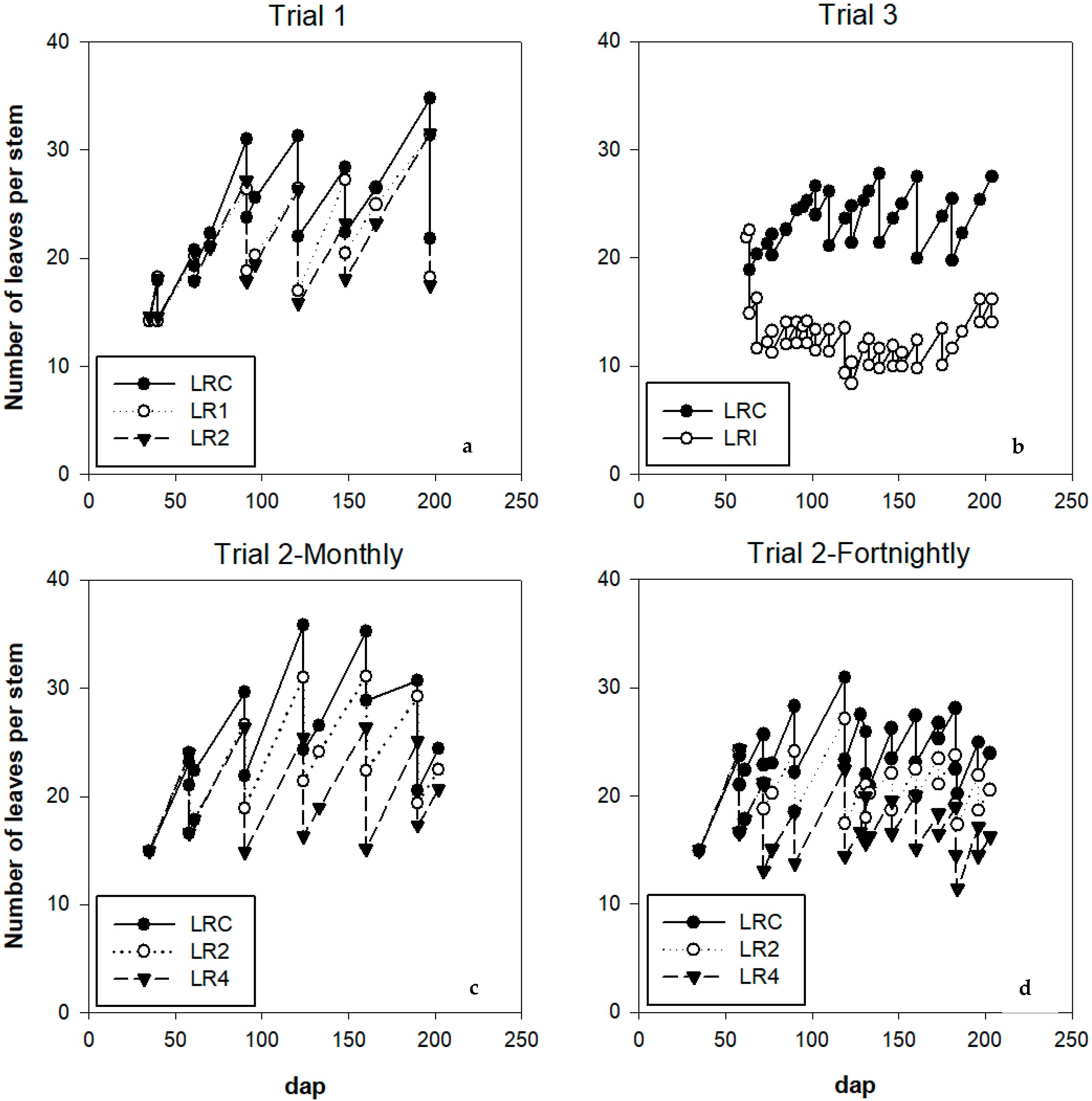
3.2.1. Leaves
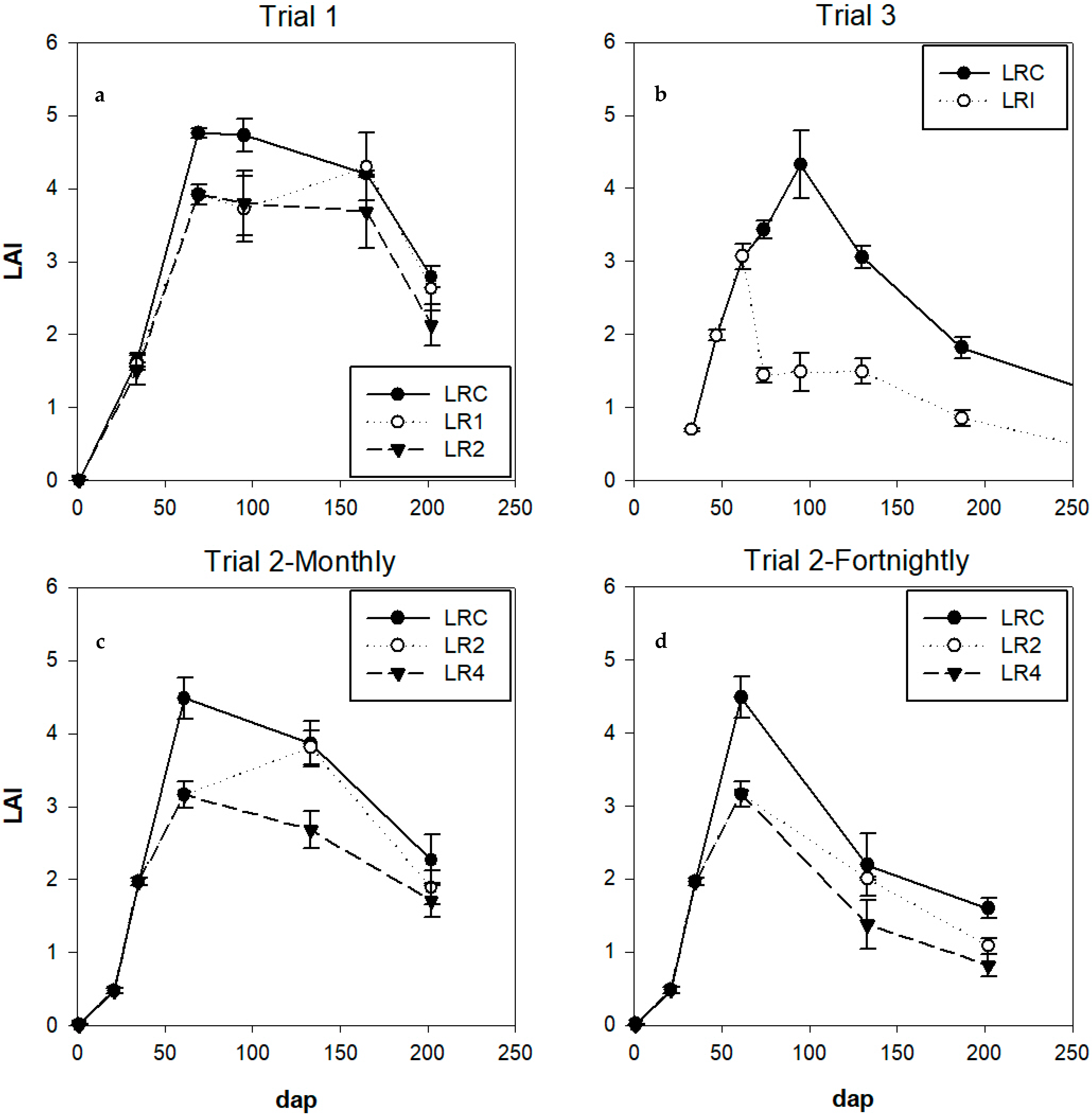
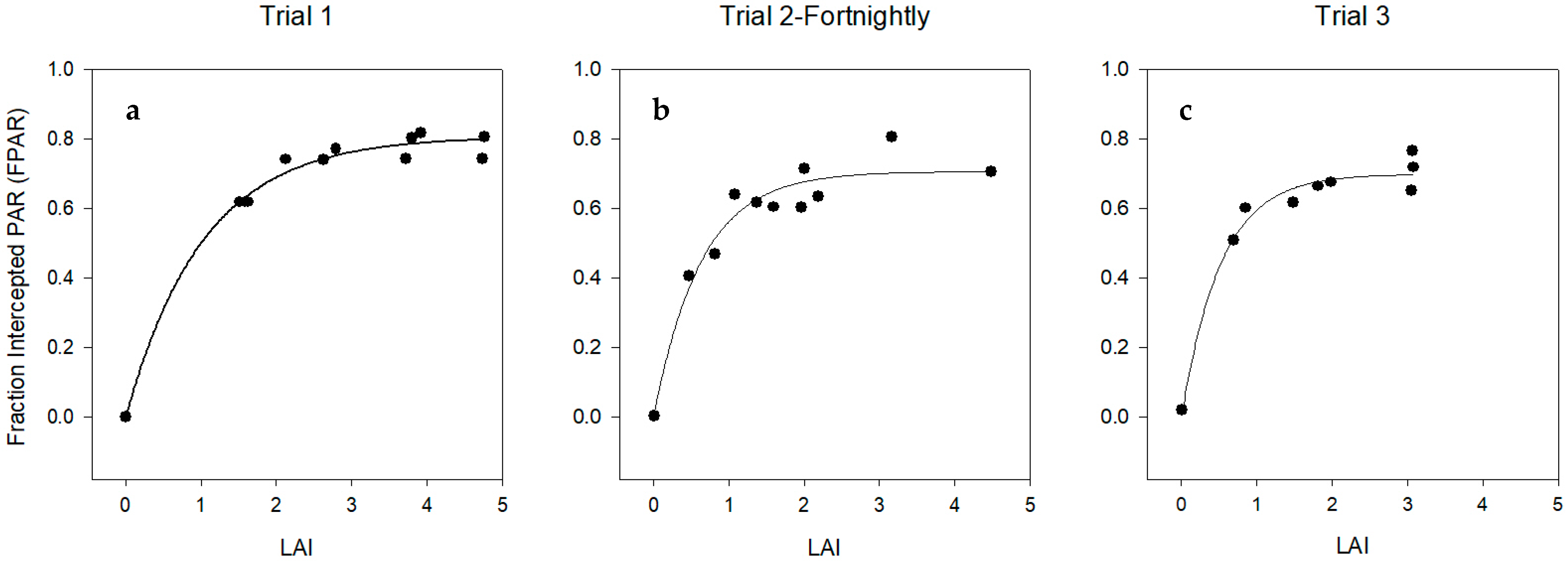
Leaf Area Index (LAI) and Specific Leaf Area (SLA)
3.2.2. Trusses
3.2.3. Biomass
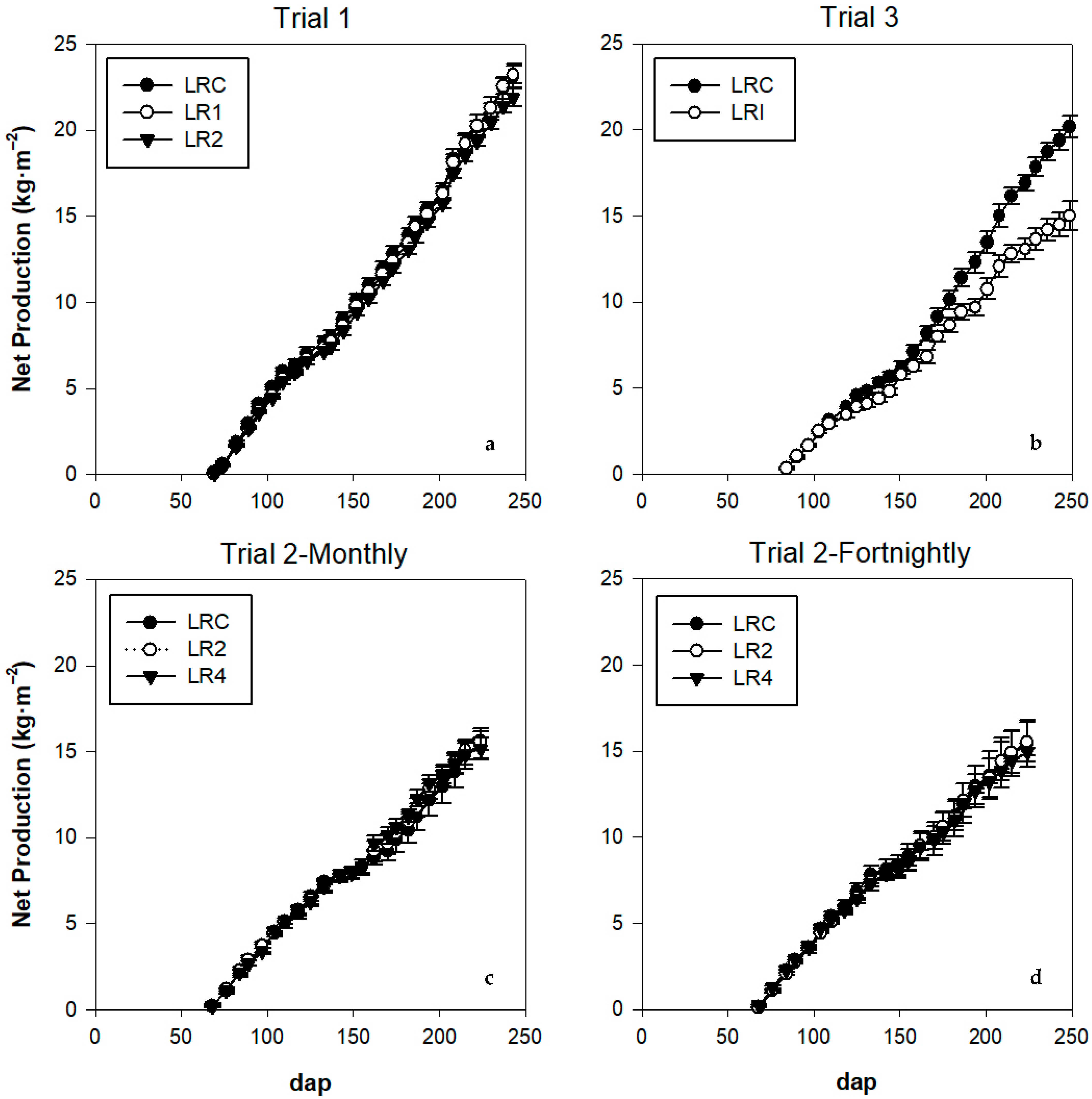
Biomass Production
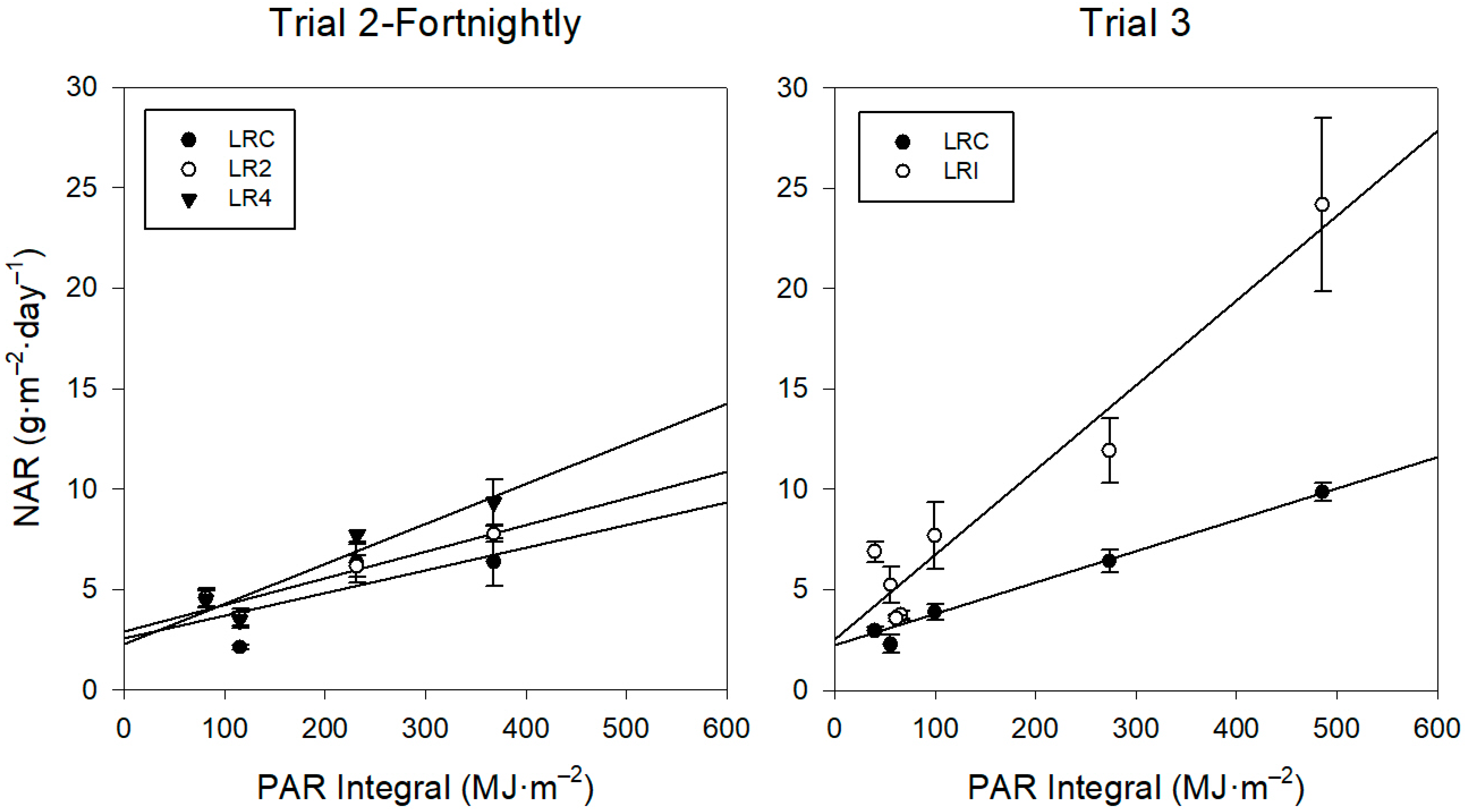
Radiation Use Efficiency (RUE)
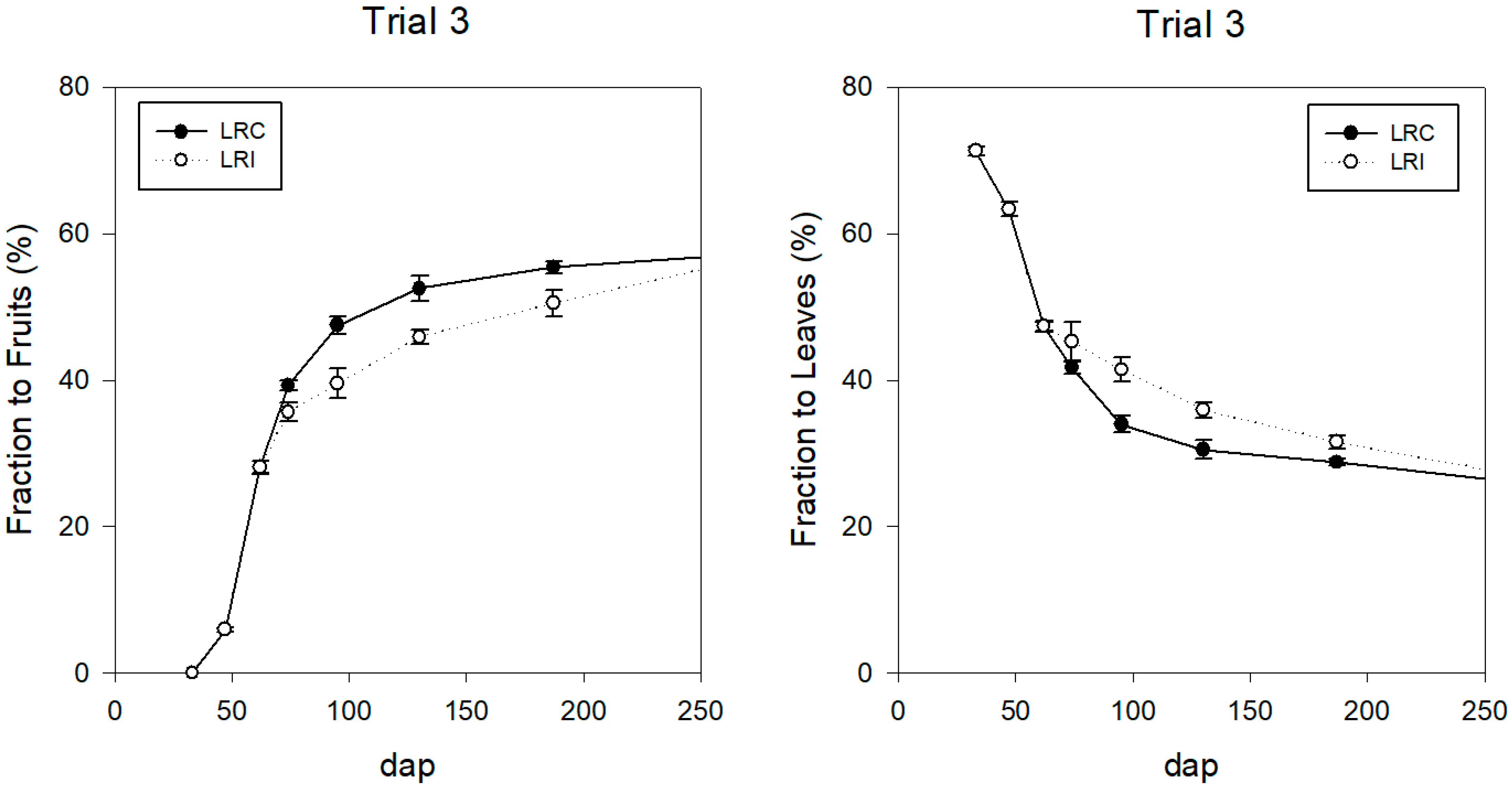
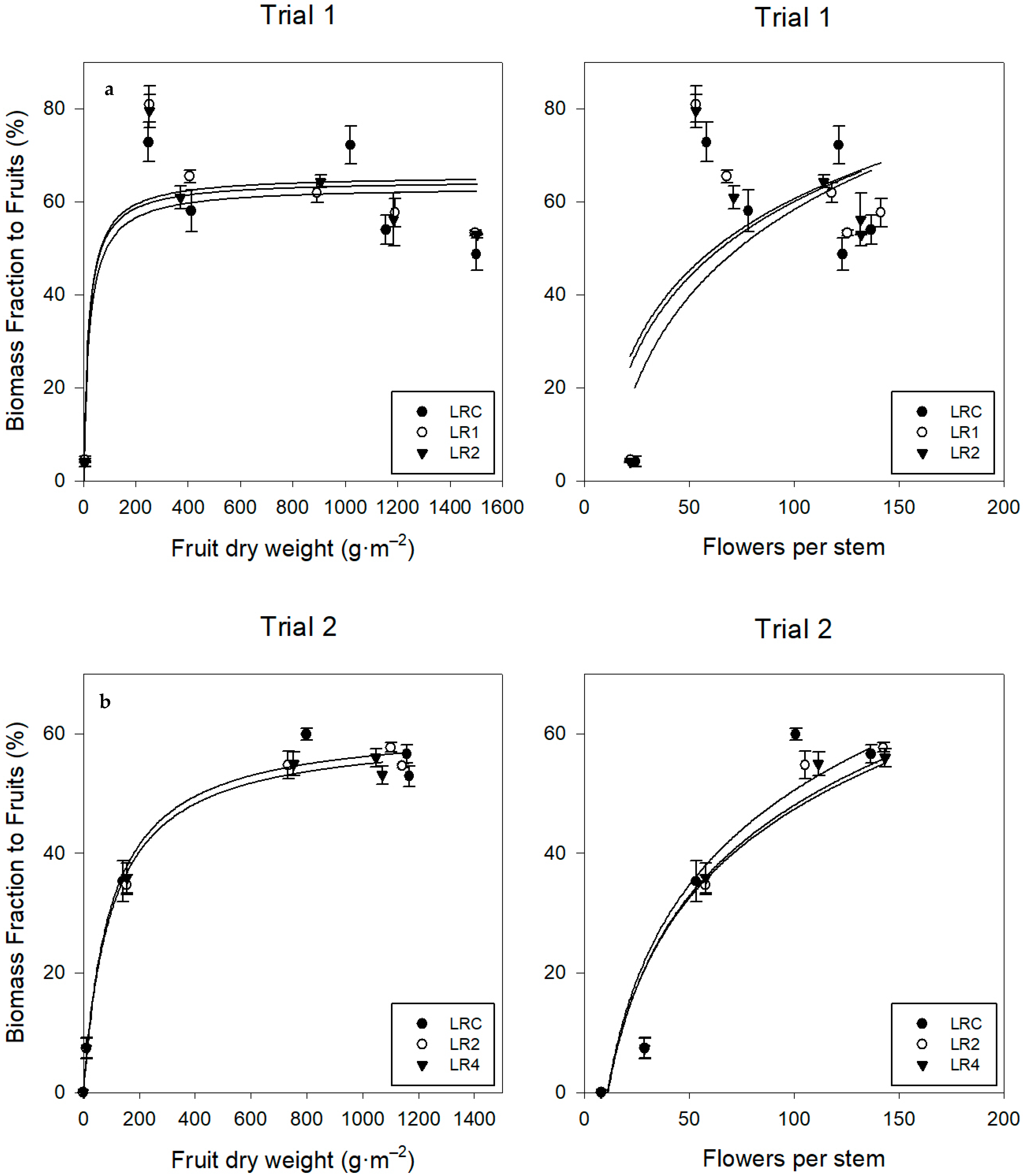
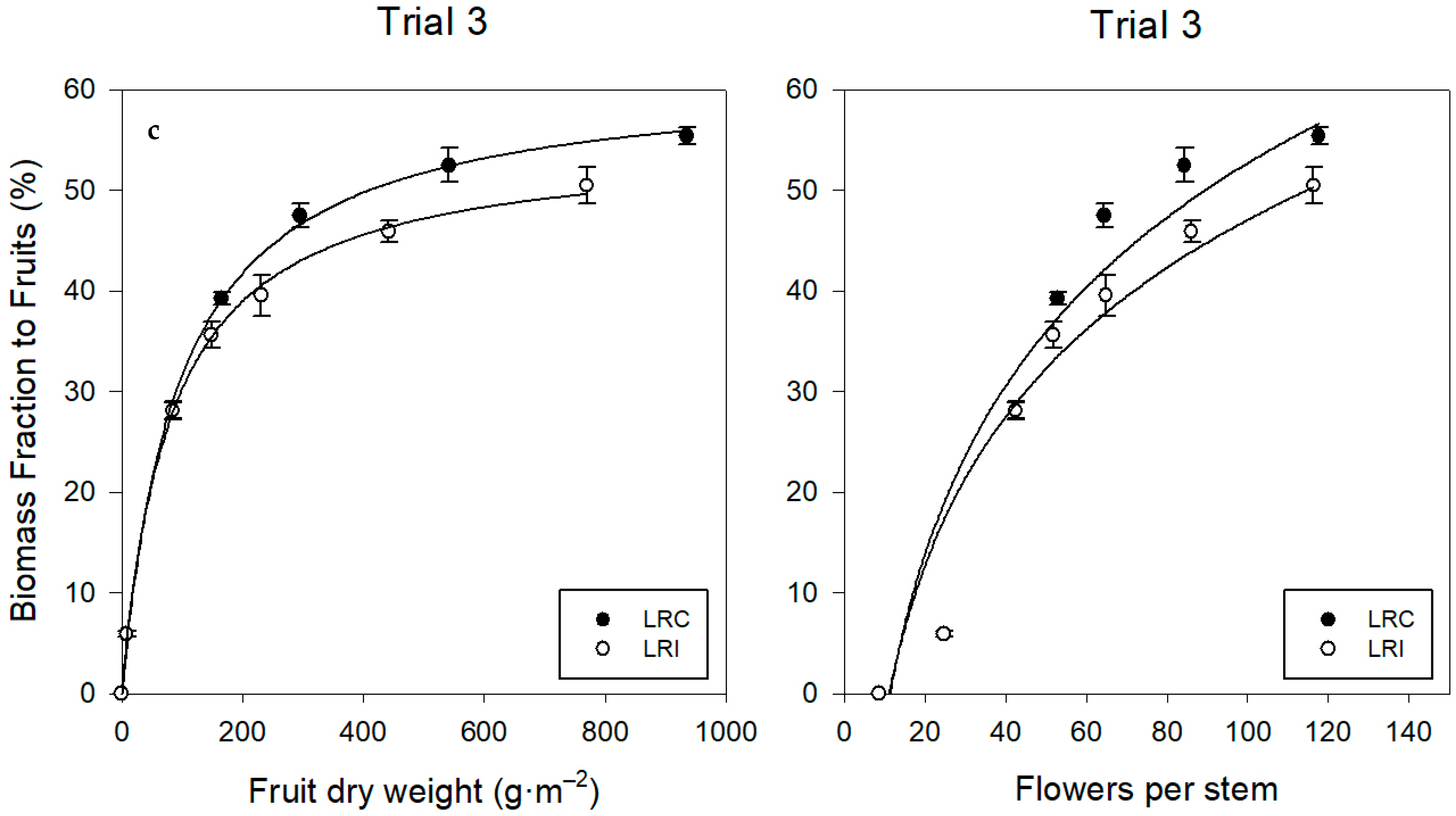
Assimilate Distribution
3.3. Yield Components
3.3.1. Fruit Yield and Size
3.3.2. Harvest Index
4. Discussion
4.1. Leaves
4.1.1. Number of Leaves
4.1.2. Leaf Area Index (LAI) and Specific Leaf Area (SLA)
4.2. Trusses and Flowers
4.3. Biomass
4.3.1. Biomass Production
4.3.2. Radiation Use Efficiency (RUE) and Net Assimilation Rate (NAR)
4.3.3. Assimilate Distribution
4.4. Yield Components
4.4.1. Fruit Yield and Size
4.4.2. Harvest Index
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.P. Tolerance of tomato to manual defoliation. Proc. Fla. State Hortic. Soc. 1979, 92, 99–100. [Google Scholar]
- Tanaka, A.; Fujita, K. Nutrio-physiological studies on the tomato plant. Soil Sci. Plant Nutr. 1974, 20, 305–315. [Google Scholar] [CrossRef]
- Picken, A.J.F.; Stewart, K.; Klapwijk, D. 1986. Germination and vegetative development. In The Tomato Crop: A Scientific Basis for Improvement; Atherton, J.G., Rudich, J., Eds.; Chapman & Hall Ltd.: London, UK; New York, NY, USA, 1986; pp. 111–166. [Google Scholar]
- Andriolo, J.L.; Falcão, L.L.; Duarte, T.S.; Skrebsky, E.C. Defoliation of greenhouse tomato plants and its effects on dry matter accumulation and distribution to fruits. Acta Hortic. 2001, 559, 123–126. [Google Scholar] [CrossRef]
- Kinet, J.M. Effect of defoliation and growth substances on the development of the inflorescence in tomato. Sci. Hortic. 1977, 6, 27–35. [Google Scholar] [CrossRef]
- Wolk, J.O.; Kretchman, D.W.; Ortega, D.G. Response of tomato to defoliation. J. Am. Soc. Hortic. Sci. 1983, 108, 536–540. [Google Scholar] [CrossRef]
- Heuvelink, E. Developmental processes. In Tomatoes; Heuvelink, E., Ed.; CABI Publishing: Oxford, UK, 2005; pp. 53–83. [Google Scholar]
- Acock, B.; Charles-Edwards, A.; Fitter, D.J.; Hand, D.W.; Ludwig, L.J.; Wilson, W.J.; Withers, A.C. The contribution of leaves from different levels within a tomato crop to canopy net photosynthesis: An experimental examination of two canopy models. J. Exp. Bot. 1978, 29, 815–827. [Google Scholar] [CrossRef]
- Grimaldi, C.A.; Carbone, A.; Garbi, M.; García, N.; Barrenechea, M.; Martínez, S.; Giménez, D. Incidence of leaf removal on the redistribution of assimilates in tomato (Lycopersicon esculentum Mill.). Acta Hortic. 2003, 614, 567–571. [Google Scholar] [CrossRef]
- Martínez, S.; Grimaldi, M.C.; Garbi, M.; Artur, M. Defoliation effect at three phenological stages upon tomato (Lycopersicon esculentum Mill.) yield under greenhouse. Agric. Técnica 2001, 61, 522–526. [Google Scholar]
- Xiao, S.; Heuvelink, E. Effect of synchronous leaf pruning on optimal distribution of dry matter in greenhouse tomato. Acta Hortic. Sin. 2005, 32, 510–512. [Google Scholar]
- Xiao, S.; Van der Ploeg, A.; Bakker, M.; Heuvelink, E. Two instead of three leaves between tomato trusses: Measured and simulated effects on partitioning and yield. Acta Hortic. 2004, 654, 303–309. [Google Scholar] [CrossRef]
- Watson, D.J. Comparative physiological studies on the growth of field crops: I. Variation in net assimilation rate and leaf area between species and varieties within and between years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- Russell, G.; Jarvis, P.G.; Monteith, J.L. Absorption of Radiation by Canopies and Stand Growth. In Plant Canopies: Their Growth Form and Function; Cambridge University Press: Cambridge, UK, 1989; pp. 21–39. [Google Scholar]
- Monsi, M.; Saeki, Y.T. Uber den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Jpn. J. Bot. 1953, 14, 22–52. [Google Scholar]
- Heuvelink, E.; Buiskoll, R.P.M. Influence of sink-source interaction on dry matter production in tomato. Ann. Bot. 1995, 75, 381–389. [Google Scholar] [CrossRef]
- De Koning, A.N.M. Growth of a tomato crop: Measurements for model validation. Acta Hortic. 1993, 328, 141–146. [Google Scholar] [CrossRef]
- Sandri, M.A.J.; Andriolo, J.L.; Witter, M.; Ross, T.D. High density of defoliated tomato plants in protected cultivation and its effects on development of trusses and fruits. Hortic. Bras. 2002, 20, 485–489. [Google Scholar] [CrossRef]
- Heuvelink, E.; Marcelis, L.F.M. Influence of assimilate supply on leaf formation in sweet pepper and tomato. J. Hortic. Sci. 1996, 71, 405–414. [Google Scholar] [CrossRef]
- Shishido, Y.; Kumakara, H.; Hori, Y. Changes in source-sink interaction by defoliation and darkening of source and sink leaf in tomato (Lycopersicon esculentum Mill.). J. Jpn. Soc. Hortic. Sci. 1993, 62, 95–102. [Google Scholar] [CrossRef]
- Aung, L.H.; Kelly, W.C. Influence of defoliation on vegetative, floral and fruit development in tomatoes (Lycopersicum esculentum Mill.). Proc. Am. Soc. Hortic. Sci. 1966, 89, 563–570. [Google Scholar]
- Hocking, P.J.; Steer, B.T. The distribution and identity of assimilates in tomato with special reference to stem reserves. Ann. Bot. 1994, 73, 315–325. [Google Scholar] [CrossRef]
- Hurd, R.G.; Thornley, J.H.M. An analysis of the growth of young tomato plants in water culture at different light integrals and CO2 concentrations. I: Physiological aspects. Ann. Bot. 1974, 38, 375–388. [Google Scholar] [CrossRef]
- Marcelis, L.F.M. Sink strength as a determinant of dry matter partitioning in the whole plant. J. Exp. Bot. 1996, 47, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Kinet, J.M. Effect of light conditions on the development of the inflorescence in tomato. Sci. Hortic. 1977, 6, 15–26. [Google Scholar] [CrossRef]
- Ho, L.C. Tomato. In Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 709–728. [Google Scholar]
- Medrano, E. Gestión de Riego en Cultivo de Pepino “Cucumis sativus L.” en Sustrato: Evaluación de la Transpiración Durante la Ontogenia. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 1999. [Google Scholar]
- Heuvelink, E.; Bakkers, M.J.; Elings, A.; Kaarsemaker, R.C.; Marcelis, L.F.M. Effect of leaf area on tomato yield. Acta Hortic. 2005, 691, 43–50. [Google Scholar] [CrossRef]
- Adams, S.R.; Woodward, G.C.; Valdes, V.M. The effects of leaf removal and of modifying temperature set points with solar radiation on tomato yields. J. Hortic. Sci. Biotechnol. 2002, 77, 733–738. [Google Scholar] [CrossRef]
- Lorenzo, P. Intercepción de luz, Bioproductividad e Intercambios Gaseosos Durante la Ontogenia de un Cultivo Invernal de Pepino “Cucumis sativus L”. en Almería. Ph.D. Thesis, Universidad de Barcelona, Barcelona, Spain, 1994. [Google Scholar]
- Pettersen, R.I.; Torre, S.; Gislerod, H.R. Effects of leaf aging and light duration on photosynthetic characteristics in a cucumber canopy. Sci. Hortic. 2010, 125, 82–87. [Google Scholar] [CrossRef]
- Bolaños, J.A.; Hsiao, T.C. Photosynthetic and respiratory characterization of field grown tomato. Photosynth. Res. 1991, 28, 31–32. [Google Scholar] [CrossRef]
- Dueck, T.A.; Grashoff, C.; Broekhuijsen, G.; Marcelis, L.F.M. Efficency of light energy used by leaves situated in different levels of a sweet pepper canopy. Act. Hortic. 2006, 711, 201–205. [Google Scholar] [CrossRef]
- Shishido, Y.C.; Yun, C.J.; Yuhashi, T.; Seyama, N.; Imada, S. Changes in photosynthesis, translocation and distribution of C-14 assimilates during leaf development and the rate of contribution of each leaf to fruit growth in tomato. J. Jpn. Soc. Hortic. Sci. 1991, 59, 771–779. [Google Scholar] [CrossRef][Green Version]
- Xu, H.L.; Gauthier, L.; Desjardins, Y.; Gosselin, A. Photosynthesis in leaves, fruits, stem and petioles of greenhouse-grown tomato plants. Photosynthetica 1997, 33, 113–123. [Google Scholar] [CrossRef]
- Matsuda, R.; Suzuki, K.; Nagano, A.; Higashide, T.; Takaichi, M. Responses of leaf photosynthesis and plant growth to altered source-sink balance in a japanese and a dutch tomato cultivar. Sci. Hortic. 2011, 127, 520–527. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Heuvelink, E. Evaluation of a dynamic simulation model for tomato crop growth and development. Ann. Bot. 1998, 83, 413–422. [Google Scholar] [CrossRef]
- Starck, Z. Photosynthesis and endogenous regulation of the source-sink relation in tomato plants. Photosynthetica 1983, 17, 1–11. [Google Scholar]





| Parameter | Trial 1 | Trial 2 | Trial 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LRC | LR1 | LR2 | LRC | LR2 | LR4 | LRC | LRI | ||
| crop duration (days) | 252 | 202 | 270 | ||||||
| total emitted leaves | 60.6 a | 60.4 a | 62.5 a | M | 66.5 a | 65.5 a | 65.9 a | 83.5 a | 81.8 a |
| F | 67.6 a | 66.1 a | 65.1 a | ||||||
| average leaves present | 24.4 a | 21.5 b | 20.4 b | M | 24.4 a | 21.5 b | 19.1 c | 23.4 a | 12.7 b |
| F | 22.1 b | 19.2 c | 15.4 d | ||||||
| average leaves pruned per removal | 6.8 b | 7.3 ab | 7.5 a | M | 7.6 b | 8.7 ab | 9.4 a | 5.3 a | 2.9 b |
| F | 4.0 c | 4.4 c | 5.0 c | ||||||
| Trial 1 | Trial 2 | Trial 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LRC | LR1 | LR2 | LRC | LR2 | LR4 | LRC | LRI | ||
| Crop duration (days) | 252 | 202 | 270 | ||||||
| Total flower number | 136.8 a | 141.4 a | 131.8 a | M | 137.8 a | 145.5 a | 136.7 a | 155.1 a | 146.8 a |
| F | 136.7 a | 142.6 a | 143.4 a | ||||||
| Viable flower number | 123.8 a | 127.6 a | 117.9 a | M | 112.7 a | 127.3 a | 115.2 a | 129.8 a | 108.3 a |
| F | 119.2 a | 121.2 a | 125.4 a | ||||||
| TRIAL 1 | TRIAL 2 | TRIAL 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LRC | LR1 | LR2 | LRC | LR2 | LR4 | LRC | LRI | ||
| Crop duration (days) | 252 | 202 | 270 | ||||||
| Biomass production | 3120 a | 2810 a | 2841 a | M | 2282 a | 2187 a | 2025 a | 2626 a | 2237 b |
| F | 2218 a | 2091 a | 2017 a | ||||||
| Trial 1 | Trial 2 | Trial 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LRC | LR1 | LR2 | LRC | LR2 | LR4 | LRC | LRI | |||
| Stem | % g·m−2 | 11.9 352.7 | 10.4 291.1 | 10.7 305.7 | M | 17.1 390.2 | 9.8 213.9 | 9.7 197.2 | 10.9 286.5 | 11.2 251.0 |
| F | 11.5 258.1 | 10.2 212.5 | 10.5 211.7 | |||||||
| Leaves | % g·m−2 | 26.6 786.6 | 26.1 729.2 | 26.4 751.9 | M | 20.0 456.0 | 25.2 550.3 | 27.5 556.1 | 25.9 679.1 | 26.5 595.0 |
| F | 25.9 580.7 | 24.7 515.7 | 26.7 539.0 | |||||||
| Side shoots | % g·m−2 | 7.8 230.6 | 7.5 209.3 | 7.4 211.3 | M | 1.0 23.7 | 1.2 25.4 | 0.9 19.0 | 3.0 79.2 | 3.0 68.0 |
| F | 1.0 21.8 | 1.0 20.5 | 0.9 18.4 | |||||||
| Flowers | % g·m−2 | 2.9 84.5 | 2.6 71.4 | 2.6 74.1 | M | 2.2 49.8 | 2.5 54.2 | 2.3 46.4 | 2.9 76.0 | 2.9 64.0 |
| F | 2.5 55.7 | 2.8 58.7 | 2.9 57.8 | |||||||
| Fruits | % g·m−2 | 50.8 1500.8 | 53.5 1496.9 | 52.8 1503.0 | M | 59.7 1362.8 | 61.4 1343.1 | 59.6 1206.4 | 57.3 1505.4 | 56.4 1264.8 |
| F | 59.1 1322.3 | 61.4 1282.8 | 59.0 1191.5 | |||||||
| Trial 1 | Trial 2 | Trial 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| LRC | LR1 | LR2 | LRC | LR2 | LR4 | LRC | LRI | |
| Net | 23.3 a | 23.5 a | 23.1 a | 15.4 a | 15.6 a | 15.1 a | 20.8 a | 15.3 b |
| Non-commercial | 3.0 a | 3.1 a | 3.0 a | 7.1 a | 6.3 ab | 5.0 b | 6.4 a | 7.3 a |
| Total | 26.3 a | 26.6 a | 26.2 a | 22.5 a | 21.8 a | 20.1 b | 27.2 a | 22.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raya, V.; Parra, M.; Cid, M.d.C.; Santos, B.; Ríos, D. Effect of Different Intensities of Leaf Removal on Tomato Development and Yield. Horticulturae 2024, 10, 1136. https://doi.org/10.3390/horticulturae10111136
Raya V, Parra M, Cid MdC, Santos B, Ríos D. Effect of Different Intensities of Leaf Removal on Tomato Development and Yield. Horticulturae. 2024; 10(11):1136. https://doi.org/10.3390/horticulturae10111136
Chicago/Turabian StyleRaya, Vanesa, Margarita Parra, María del Carmen Cid, Belarmino Santos, and Domingo Ríos. 2024. "Effect of Different Intensities of Leaf Removal on Tomato Development and Yield" Horticulturae 10, no. 11: 1136. https://doi.org/10.3390/horticulturae10111136
APA StyleRaya, V., Parra, M., Cid, M. d. C., Santos, B., & Ríos, D. (2024). Effect of Different Intensities of Leaf Removal on Tomato Development and Yield. Horticulturae, 10(11), 1136. https://doi.org/10.3390/horticulturae10111136





