Optimizing Greenhouse Cucumber Fertigation Through Grafting: Improving Yield, Bioactive Compounds, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Production
Grafting Procedure
2.2. Conditions and Agrotechnical Practices in Greenhouse Experiment
2.3. Measurement Procedures
2.3.1. Yield Measurement
2.3.2. Sample Size for Quality Assessment
2.3.3. Dry Matter
2.3.4. Total Phenolics Content
2.3.5. Total Flavonoid Content
2.3.6. Antioxidant Activity Tests (DPPH, FRAP, and ABTS)
2.4. Statistical Data Analysis
3. Results
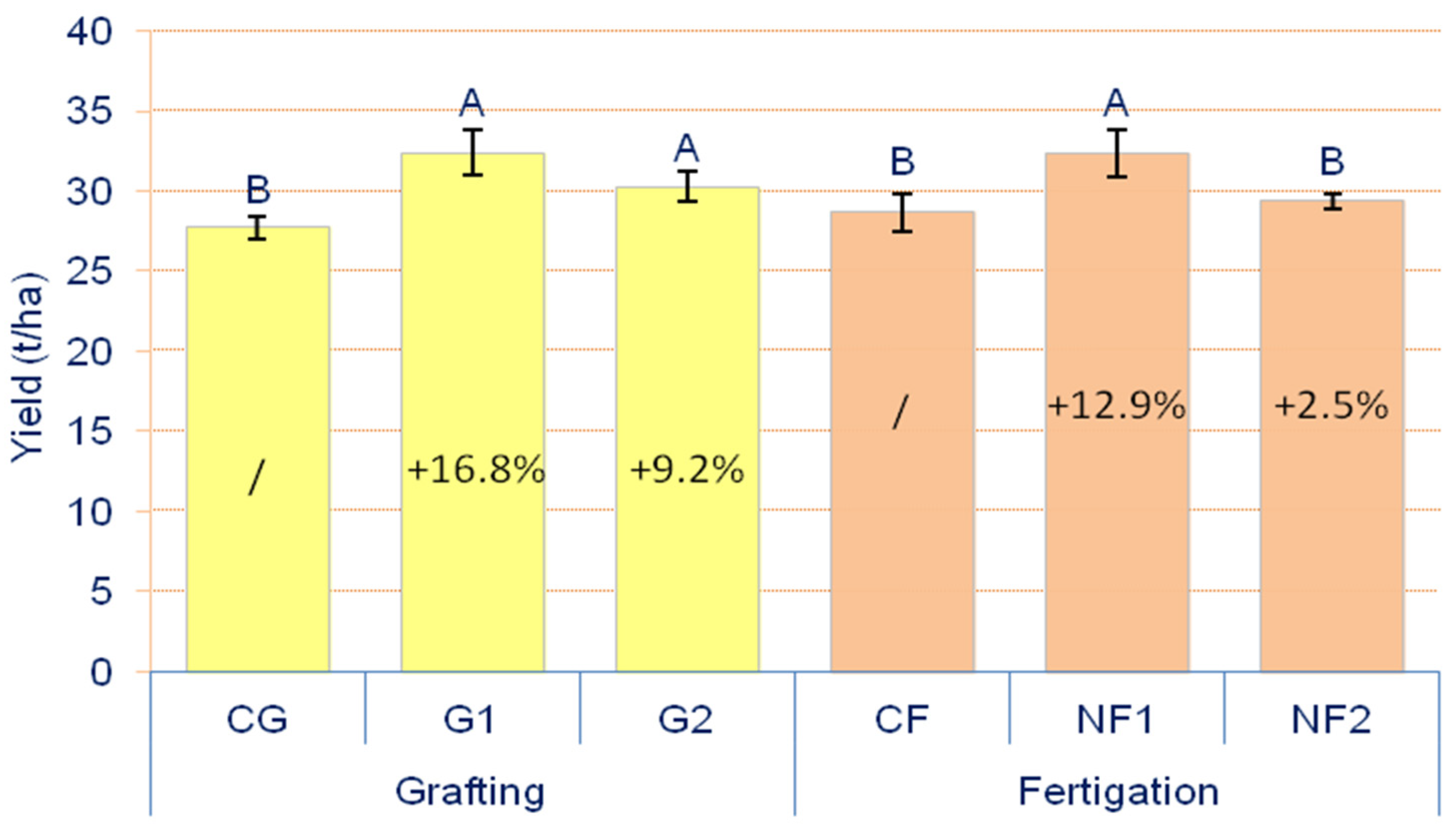
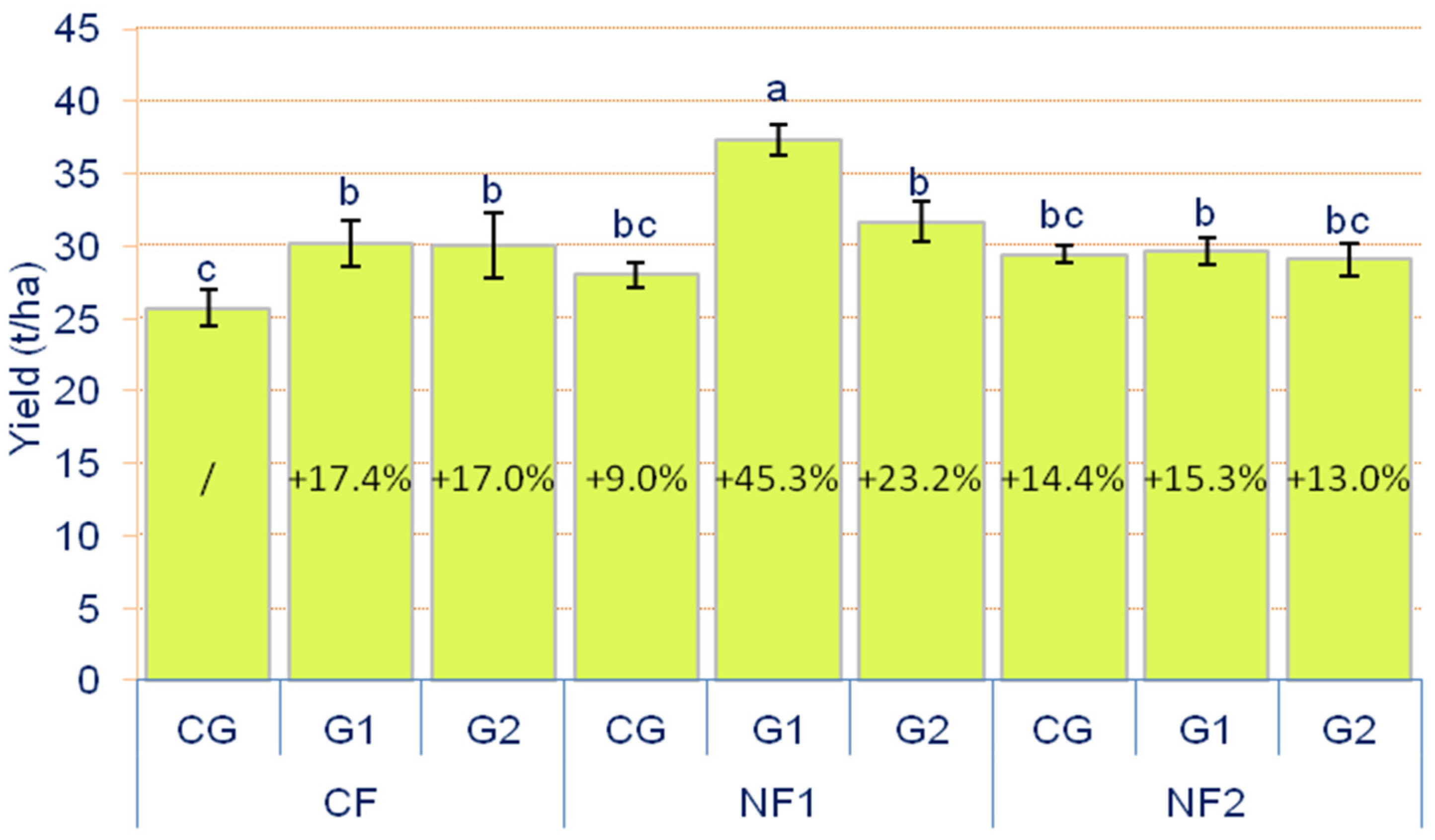
3.1. Yield of Cucumber
3.2. Dry Matter (DM)
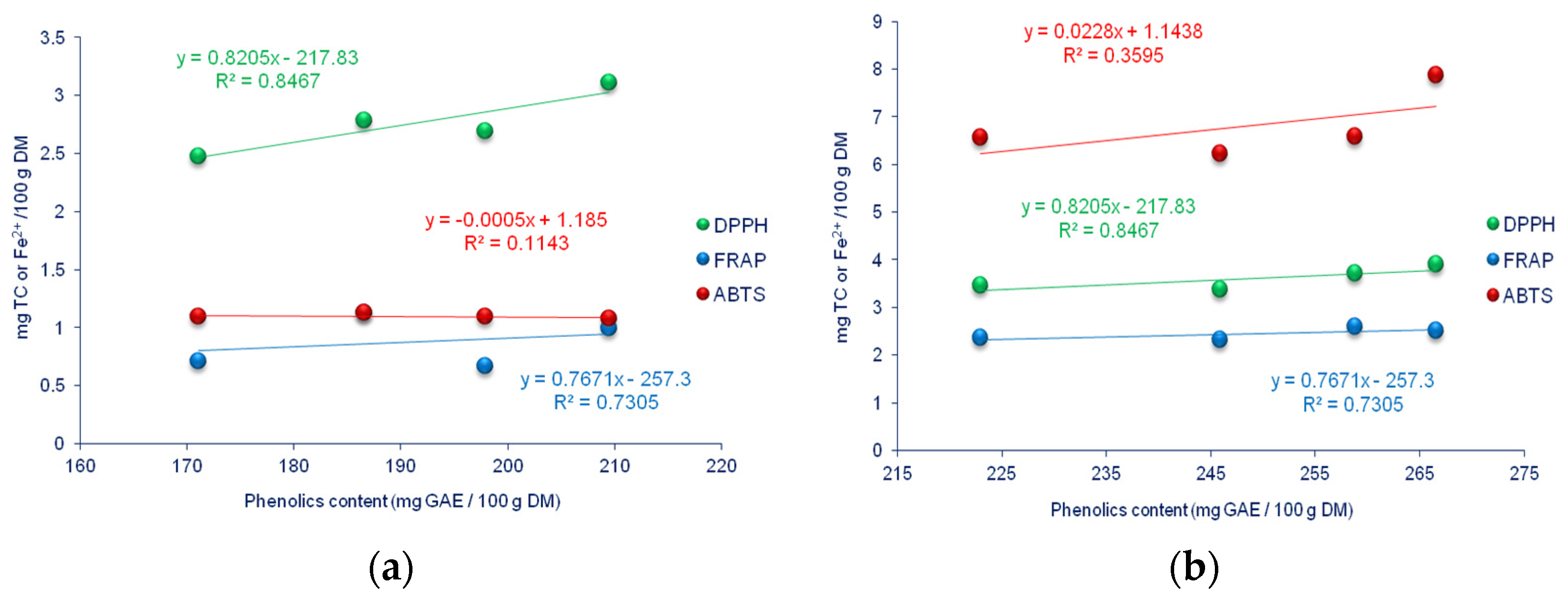
3.3. Phenolics Content
3.4. Flavonoids Content
3.5. Indicators of Antioxidative Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsadon, A.; Al-Helal, I.; Ibrahim, A.; Abdel-Ghany, A.; Al-Zaharani, S.; Ashour, T. The effects of plastic greenhouse covering on cucumber (Cucumis sativus L.) growth. Ecol. Eng. 2016, 87, 305–312. [Google Scholar] [CrossRef]
- Čepulienė, R.; Butkevičienė, L.M.; Skinulienė, L.; Steponavičienė, V. Response of cucumbers (Cucumis sativus L.) to waste wood fiber substrates and additional nitrogen fertilization. Plants 2022, 11, 3464. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, P.; Ladika, G.; Tsiantas, K.; Kritsi, E.; Tsiaka, T.; Cavouras, D.; Zoumpoulakis, P.; Sinanoglou, V.J. Quality assessment of greenhouse-cultivated cucumbers (Cucumis sativus) during storage using instrumental and image analyses. Appl. Sci. 2024, 14, 8676. [Google Scholar] [CrossRef]
- Al-Rashdi, S.; Al-Subhi, N.; Al-Dairi, M.; Pathare, P.B. Effect of a Moringa oil–Beeswax edible coating on the shelf-life and quality of fresh cucumber. Processes 2024, 12, 1148. [Google Scholar] [CrossRef]
- Swamy, K.R.M. Origin, distribution, taxonomy, botanical description, genetics, genetic diversity and breeding of cucumber (Cucumis sativus L.). Int. J. Dev. Res. 2023, 13, 61542–61559. [Google Scholar] [CrossRef]
- Ji, L.; Wu, J.; Gao, W.; Wei, J.; Yang, J.; Guo, C. Antioxidant capacity of different fractions of vegetables and correlation with the contents of ascorbic acid, phenolics, and flavonoids. J. Food Sci. 2011, 76, C1257–C1261. [Google Scholar] [CrossRef]
- Chinatu, L.N.; Onwuchekwa-Henry, C.B.; Okoronkwo, C.M. Assessment of yield and yield components of cucumber (Cucumis sativus L.) in Southeastern Nigeria. Int. J. Agric. Earth Sci. 2017, 3, 35–44. Available online: https://www.iiardjournals.org/get/IJAES/VOL.%203%20NO.%201%202017/Assessment%20of%20Yield.pdf (accessed on 18 June 2024).
- Kapusta-Duch, J.; Leszczyńska, T.; Borczak, B. Influence of packages on nutritional quality of pickled chilled stored cucumbers. Ecol. Chem. Eng. A 2016, 23, 357–371. [Google Scholar] [CrossRef]
- Tamme, T.; Reinik, M.; Roasto, M.; Meremäe, K.; Kiis, A. Nitrate in leafy vegetables, culinary herbs, and cucumber grown under cover in Estonia: Content and intake. Food Addit. Contam. 2010, 3, 108–113. [Google Scholar] [CrossRef]
- Chung, S.W.C.; Tran, J.C.H.; Tong, K.S.K.; Chen, M.Y.Y.; Xiao, Y.; Ho, Y.Y.; Chan, C.H.Y. Nitrate and nitrite levels in commonly consumed vegetables in Hong Kong. Food Addit. Contam. Part B 2011, 4, 34–41. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Colla, G.; Rea, E. Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. HortScience 2008, 43, 730–736. [Google Scholar] [CrossRef]
- Colla, G.; Suãrez, C.M.C.; Cardarelli, M.; Rouphael, Y. Improving nitrogen use efficiency in melon by grafting. HortScience 2010, 45, 559–565. [Google Scholar] [CrossRef]
- Sallaku, G.; Sandén, H.; Babaj, I.; Kaciu, S.; Balliu, A.; Rewald, B. Specific nutrient absorption rates of transplanted cucumber seedlings are highly related to RGR and influenced by grafting method, AMF inoculation and salinity. Sci. Hortic. 2019, 243, 177–188. [Google Scholar] [CrossRef]
- El-Sayed, S.; Ahmed, A.; El-Eslamboly, A.; Abdeldaym, E. Application of grafting as a tool for improving morphological and physiological traits of cucumber plants grown under net-house conditions. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 439–453. Available online: https://ikprress.org/index.php/PCBMB/article/view/6431/5793 (accessed on 20 June 2024).
- Lee, J.M.; Kubota, C.; Tsao, J.S.; Bie, Z.; Echevarria, P.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 27, 93–105. [Google Scholar] [CrossRef]
- Helaly, A.A.; Abdelghaffar, M.S.; Abualhamd, A.S.; Alabd, M.T.G. Effects of different rootstocks on growth, biochemical and molecular changes in grafted cucumber (Cucumis sativus L.). J. Agric. Res. Dev. 2019, 33, 1. Available online: https://www.fayoum.edu.eg/Agri/conference3/pdf/Researches/E/4.pdf (accessed on 20 June 2024).
- Sarafi, E.; Siomos, A.; Tsouvaltzis, P.; Chatzissavvidis, C.; Therios, I. Boron toxicity effects on grafted and non-grafted pepper (Capsicum annuum) plants. J. Soil Sci. Plant Nutr. 2017, 17, 441–460. [Google Scholar] [CrossRef][Green Version]
- Qaryouti, M.M.; Qawasmi, W.; Hamdan, H.; Edwan, M. Tomato fruit yield and quality as affected by grafting and growing system. Acta Hortic. 2007, 741, 199–206. [Google Scholar] [CrossRef]
- Nicoletto, C.; Tosini, F.; Sambo, P. Effect of grafting and ripening conditions on some qualitative traits of ‘Cuore di Bue’ tomato fruits. J. Sci. Food Agric. 2013, 93, 1397–1403. [Google Scholar] [CrossRef]
- Mavlyanova, F.R.; Lyan, E.E.; Karimov, A.B.; Dubinin, V.B. The vegetative grafting effect on increasing tomato fruit quality. IOP Conf. Ser. Earth Environ. Sci. 2020, 613, 012077. [Google Scholar] [CrossRef]
- Kolešla, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Maksimović, I. Grafting influence on the weight and quality of tomato fruit under salt sress. Ann. Appl. Biol. 2018, 172, 187–196. [Google Scholar] [CrossRef]
- Vinković-Vrček, I.; Samobor, V.; Bojić, M.; Medić-Šarić, M.; Vukobratović, M.; Erhatić, R.; Horvat, D.; Matotan, Z. The effect of grafting on the antioxidant properties of tomato (Solanum lycopersicum L.). Span. J. Agric. Res. 2011, 9, 844–851. [Google Scholar] [CrossRef]
- López-Marín, J.; Gonzáles, A.; Pérez-Alfocea, F.; Egea-Gilabert, C.; Fernández, A.J. Grafting is an efficient alternative to shading screens to alleviate thermal stress in greenhouse-grown pepper. Sci. Hortic. 2013, 149, 39–46. [Google Scholar] [CrossRef]
- Lazić, B.; Marković, V.; Đurovka, M.; Ilin, Ž. Vegetable; Faculty of Agriculture, University of Novi Sad: Novi Sad, Serbia, 2001; pp. 394–409. [Google Scholar]
- Arshad, I.; Ali, W.; Khan, A.Z. Effect of different levels of NPK fertilizers on the growth and yield of greenhouse cucumber (Cucumis sativus) by using drip irrigation technology. Int. J. Res. 2014, 8, 650–660. Available online: https://journals.pen2print.org/index.php/ijr/article/viewFile/534/229 (accessed on 22 June 2024).
- Jilani, M.; Bakar, A.; Waseem, K.; Kiran, M. Effect of different levels of NPK on the growth and yield of cucumber (Cucumis sativus) under the plastic tunnel. J. Agric. Soc. Sci. 2009, 5, 99–101. Available online: https://www.researchgate.net/profile/Kashif-Waseem/publication/242418561_Effect_of_Different_Levels_of_NPK_on_the_Growth_and_Yield_of_Cucumber_Cucumis_sativus_Under_the_Plastic_Tunnel/links/544f9f690cf26dda089207d5/Effect-of-Different-Levels-of-NPK-on-the-Growth-and-Yield-of-Cucumber-Cucumis-sativus-Under-the-Plastic-Tunnel.pdf (accessed on 22 June 2024).
- Diana, M.; Lazureanu, A.; Gogoasa, I.; Poiana, M.A.; Harmanescu, M.; Gergen, I. Influence of NPK fertilization on nutritional quality of tomatoes. Bull. Univ. Agric. Sci. Vet. Med. Hortic. 2007, 64, 1454–2382. Available online: https://journals.usamvcluj.ro/index.php/horticulture/article/view/1946 (accessed on 22 June 2024).
- Morais, M.; Martins, P.; Serafim, M.; Zeviani, W.; Araujo, K.; Gillio, T.; Campos, R.A.; Neves, L. Fruit yield and antioxidant activity of pepper genotypes subjected to nitrogen doses. J. Agric. Sci. 2019, 11, 139. [Google Scholar] [CrossRef]
- Uysal, N.; Tuzel, Y.; Oztekin, G.; Tuzel, I. Effects of different rootstocks on greenhouse cucumber production. Acta Hortic. 2012, 927, 281–289. [Google Scholar] [CrossRef]
- Noor, R.S.; Wang, Z.; Umair, M.; Yaseen, M.; Ameen, M.; Rehman, S.-U.; Khan, M.U.; Imran, M.; Ahmed, W.; Sun, Y. Interactive effects of grafting techniques and scion-rootstocks combinations on vegetative growth, yield and quality of cucumber (Cucumis sativus L.). Agronomy 2019, 9, 288. [Google Scholar] [CrossRef]
- Khapte, P.S.; Kumar, P.; Panwar, N.R.; Burman, U.; Rouphael, Y.; Kumar, P. Combined influence of grafting and type of protected environment structure on agronomic and physiological traits of single- and cluster-fruit-bearing cucumber hybrids. Agronomy 2021, 11, 1604. [Google Scholar] [CrossRef]
- Bayoumi, Y.; Abd-Alkarim, E.; El-Ramady, H.; El-Aidy, F.; Hamed, E.-S.; Taha, N.; Prohens, J.; Rakha, M. Grafting improves fruit yield of cucumber plants grown under combined heat and soil salinity stresses. Horticulturae 2021, 7, 61. [Google Scholar] [CrossRef]
- Cui, J.B.; Niu, Q.W.; Du, D.Y.; Zhang, Q. Response of yield and nitrogen use efficiency to aerated irrigation and N application rate in greenhouse cucumber. Sci. Hortic. 2020, 265, 109220. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Ni, H.; Ren, S.; Li, N.; Wu, Y.; Yang, Y.; Liu, Y.; Liu, Z.; Liu, Y.; et al. Effect of fertilization combination on cucumber quality and soil microbial community. Front. Microbiol. 2023, 14, 1122278. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Zhang, M.; Li, D.; Jiang, Y.; Yao, W.; Zhang, Z. Yield, quality, and water and fertilizer partial productivity of cucumber as influenced by the interaction of water, nitrogen, and magnesium. Agronomy 2023, 13, 772. [Google Scholar] [CrossRef]
- Family Farm Koprivica, Čelarevo, Serbia. Available online: https://rasadnikpovrcakoprivica.rs/ (accessed on 25 June 2024).
- Regulation on Food Analysis “Official Gazette SFRY 29/83”. Available online: http://demo.paragraf.rs/demo/combined/Old/t/t2004_09/t09_0137.htm (accessed on 29 June 2024).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of Plant Analysis; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Vakula, A.; Tepić Horecki, A.; Pavlić, B.; Jokanović, M.; Ognjanov, V.; Milović, M.; Teslić, N.; Parpinello, G.; Decleer, M.; Šumić, Z. Application of different techniques on stone fruit (Prunus spp.) drying and assessment of physical, chemical and biological properties: Characterization of dried fruit properties. J. Food Process. Preserv. 2020, 45, e15158. [Google Scholar] [CrossRef]
- Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Milić, A.; Šumić, Z.; Žunić, D.; Adamović, B.; Ilin, Ž. Biostimulants improve bulb yield, concomitantly affecting the total phenolics, flavonoids, and antioxidant capacity of onion (Allium cepa). Horticulturae 2024, 10, 391. [Google Scholar] [CrossRef]
- Amerian, M.; Palangi, A.; Gohari, G.; Ntatsi, G. Enhancing salinity tolerance in cucumber through selenium biofortification and grafting. BMC Plant Biol. 2024, 24, 24. [Google Scholar] [CrossRef]
- Vanlay, M.; Samnang, S.; Jung, H.-J.; Choe, P.; Kang, K.K.; Nou, I.-S. Interspecific and intraspecific hybrid rootstocks to improve horticultural traits and soil-borne disease resistance in tomato. Genes 2022, 13, 1468. [Google Scholar] [CrossRef] [PubMed]
- Vojnović, Đ. Effects of Biostimulants and Nitrogen on Yield and Quality of Onion. Ph.D. Thesis, Faculty of Agriculture, University of Novi Sad, Novi Sad, Serbia, 2023. [Google Scholar]
- Zhang, H.; Li, X.; Zhang, S.; Yin, Z.; Zhu, W.; Li, J.; Meng, L.; Zhong, H.; Xu, N.; Wu, Y.; et al. Rootstock alleviates salt stress in grafted mulberry seedlings: Physiological and PSII function responses. Front. Plant Sci. 2018, 9, 1806. [Google Scholar] [CrossRef]
- Özdemir, A.E.; Çandır, E.; Yetişir, H.; Aras, V.; Arslan, Ö.; Baltaer, Ö.; Üstün, D.; Ünlü, M. Effects of rootstocks on storage and shelf life of grafted watermelons. J. Appl. Bot. Food Qual. 2016, 89, 191–201. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sánchez, E.; Carvajal-Millán, E.; Muñoz-Márquez, E.; Guevara-Aguilar, A. Characterization of the nutraceutical quality and antioxidant activity in bell pepper in response to grafting. Molecules 2013, 18, 15689–15703. [Google Scholar] [CrossRef]
- Lecholocholo, N.; Shoko, T.; Manhivi, V.E.; Akinola, S.A.; Maboko, M.M.; Sivakumar, D. Impact of different rootstocks on antioxidant properties and volatile profile of honeydew melons (Cucumis melo L.) during postharvest storage. Agronomy 2022, 12, 2498. [Google Scholar] [CrossRef]
- Putra, P.R.; Mediartha, K.I.; Setiawan, D.A.M.; Sujai, N.A.P.; Arista, A.R.; Kandi, P.R.; Aisyah, I.S.; Nurcholis, W. Effects of NPK fertilizer on growth, phytochemical content and antioxidant activity of purslane (Portulaca grandiflora). Curr. Appl. Sci. Technol. 2024, 24, e0257237. [Google Scholar] [CrossRef]
- Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Karadžić Banjac, M.; Kovačević, S.; Daničić, T.; Podunavac-Kuzmanović, S.; Ilin, Ž. Onion (Allium cepa L.) yield and quality depending on biostimulants and nitrogen fertilization-a chemometric perspective. Processes 2023, 11, 684. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative proteomic analysis of the graft unions in hickory (Carya cathayensis) provides insights into response mechanisms to grafting process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L.; Garcia-Flores, L.; Villano, D.; Medina, S.; Gil-Izquierdo, A. Rootstock effect on serotonin and nutritional quality of tomatoes produced under low temperature and light conditions. J. Food Compos. Anal. 2016, 46, 50–59. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhungana, K.S.; Kim, I.; Shin, D. Effect of foliar application of potassium fertilizers on soybean plants under salinity stress. J. Saudi Soc. Agric. Sci. 2020, 19, 261–269. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, Q.; Bi, J.; Zhang, G.; Zhang, P. The effects of potassium fertilizer on the active constituents and metabolites of bulbs from Lilium davidii var. unicolor. Horticulturae 2023, 9, 1216. [Google Scholar] [CrossRef]
- Sarwar, M.; Patra, K.J.; Ali, A.; Maqbool, M.; Arshad, I.M. Effect of compost and NPK fertilizer on improving biochemical and antioxidant properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 62–66. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, H.B.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B 2016, 159, 1–7. [Google Scholar] [CrossRef]
- Greathouse, J.; Henning, S.; Soendergaard, M. Effect of grafting rootstock on the antioxidant capacity and content of heirloom tomatoes (Solanum lycopersicum L.) in hydroponic culture. Plants 2021, 10, 965. [Google Scholar] [CrossRef]
- Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Žunić, D.; Adamović, B.; Milić, A.; Šumić, Z.; Sabadoš, V.; Ilin, Ž. Biostimulants affect differently biomass and antioxidant status of onion (Allium cepa) depending on production method. Horticulturae 2023, 9, 1345. [Google Scholar] [CrossRef]

| Phase | Fertilizer Type | Weigh Fertilizer (g) per Plant/Week (Active Ingredients of Fertilizer (g) per Plant/Week) | ||
|---|---|---|---|---|
| CF | NF1 | NF2 | ||
| P_1 | NPK 8:16:24 (K-Adriatica S.p.A.®, Loreo, Italy) | 8 (0.64 N + 1.28 P + 1.92 K) | 0 | 0 |
| AN 33.5% N (Petrokemija®, Kutina, Croatia) | 5 (1.67 N) | 0 | 0 | |
| P_2 | NPK 8:16:24 (K-Adriatica S.p.A.®, Loreo, Italy) | 4 (0.32 N + 0.64 K + 0.96 P) | 0 | 0 |
| FerticareTM 15:30:15 (Yara®, Oslo, Norway) | 0 | 0.5 (0.075 N + 0.15 P + 0.075 K) | 1 (0.15 N + 0.30 P + 0.15 K) | |
| CaNO3 (Van Iperen®, Westmass, The Netherlands) | 0 | 1 (0.155 Ca + 0.263 N) | 2 (0.31 Ca + 0.52 N) | |
| P_3 | NPK 8:16:24 (K-Adriatica S.p.A.®, Loreo, Italy) | 4 (0.32 N + 0.64 K + 0.96 P) | 0 | 0 |
| NPK 12:11:18 (Yara®, Oslo, Norway) | 0 | 0.5 (0.06 N + 0.055 P + 0.09 K) | 1 (0.12 N + 0.11 P + 0.18 K) | |
| KNO3 (Yara®, Oslo, Norway) | 0 | 0.5 (0.23 K + 0.065 N) | 1 (0.46 K + 0.13 N) | |
| CaNO3 (Van Iperen®, Westmass, The Netherlands) | 0 | 1 (0.15 Ca + 0.26 N) | 2 (0.31 Ca + 0.52 N) | |
| P_4 | NPK 8:16:24 (K-Adriatica S.p.A.®, Loreo, Italy) | 4 (0.32 N + 0.64 P + 0.96 K) | 0 | 0 |
| NPK 12:11:18 (Yara®, Oslo, Norway) | 0 | 1.0 (0.12 N + 0.11 P + 0.18 K) | 2 (0.24 N + 0.22 P + 0.36 K) | |
| CaNO3 (Van Iperen®, Westmass, The Netherlands) | 0 | 1.0 (0.15 Ca + 0.26 N) | 2 (0.31 Ca + 0.52 N) | |
| P_5 | NPK 8:16:24 (K-Adriatica S.p.A.®, Loreo, Italy) | 5 (0.4 N + 0.8 P + 1.2 K) | 0 | 0 |
| FerticareTM 28:8:16 II (Yara®, Oslo, Norway) | 0 | 1 (0.28 N + 0.08 P + 0.16 K) | 2 (0.56 N + 0.16 P + 0.32 K) | |
| NPK 12:11:18 (Yara®, Oslo, Norway) | 0 | 0.5 (0.06 N + 0.055 P + 0.09 K) | 1 (0.12 N + 0.11 P + 0.18 K) | |
| KNO3 (Yara®, Oslo, Norway) | 0 | 1 (0.46 K + 0.13 N) | 2 (0.92 K + 0.26 N) | |
| CaNO3 (Van Iperen®, Westmass, The Netherlands) | 0 | 1 (0.15 Ca + 0.26 N) | 2 (0.31 Ca + 0.52 N) | |
| Pesticides | Commercial Name | Active Ingredient | Application Rate | Target Diseases/Pests |
|---|---|---|---|---|
| Fungicides | Quadris® (Syngenta, Basel, Switzerland) | Azoxystrobin 250 g/L | 7.5 mL/10 L water | Pseudoperonospora cubensis; Erysiphae cichoracearum |
| Switch® 62.5 WG (Syngenta, Basel, Switzerland) | Cyprodinil 375 g/kg + fludioxonil 250 g/kg | 10 g/10 L water | Botrytis cinerea | |
| Insecticides | Actara® 25 WG (Syngenta, Basel, Switzerland) | Thiamethoxam 250 g/kg | 3.5 g/10 L water | Thripidae; Aphididae |
| Teppeki® (Belchim, Londerzeel, Belgium) | Flonicamid 500 g/kg | 1.5 g/10 L water | Trialeurodes vaporariorum | |
| Apollo® 50 SC (Adama, Beijing China) | Clofentezine 500 g/L | 3 mL/10 L water | Tetranychus urticae |
| Fertigation | Grafting | Flesh | Relative Change (%) | Peel | Relative Change (%) |
|---|---|---|---|---|---|
| CF | CG | 4.29 ± 0.15 a | / | 5.53 ± 0.09 cd | / |
| G1 | 4.00 ± 0.06 ab | −6.75 | 6.60 ± 0.15 a | +19.34 | |
| G2 | 3.85 ± 0.02 bc | −10.25 | 5.64 ± 0.21 cd | +1.98 | |
| NF1 | CG | 3.57 ± 0.09 c–e | −16.78 | 5.59 ± 0.09 cd | +1.08 |
| G1 | 3.80 ± 0.16 b–d | −11.42 | 6.83 ± 0.12 a | +23.50 | |
| G2 | 3.83 ± 0.01 bc | −10.72 | 5.86 ± 0.10 bc | +5.96 | |
| NF2 | CG | 3.77 ± 0.07 b–d | −12.12 | 5.76 ± 0.00 bc | +4.15 |
| G1 | 3.38 ± 0.16 e | −21.21 | 5.32 ± 0.05 d | −3.79 | |
| G2 | 3.52 ± 0.09 de | −17.94 | 6.14 ± 0.10 b | +11.03 |
| Fertigation | Grafting | Flesh | Relative Change (%) | Peel | Relative Change (%) |
|---|---|---|---|---|---|
| CF | CG | 171.08 ± 1.93 e | / | 269.65 ± 0.96 a | / |
| G1 | 183.48 ± 1.17 cd | +7.24 | 222.91 ± 1.39 e | −17.33 | |
| G2 | 186.24 ± 2.43 c | +8.86 | 245.87 ± 0.83 c | −8.81 | |
| NF1 | CG | 197.86 ± 2.16 b | +15.65 | 258.83 ± 1.67 b | −4.01 |
| G1 | 186.61 ± 3.26 c | +9.07 | 221.99 ± 0.93 e | −17.67 | |
| G2 | 172.65 ± 2.81 e | +0.91 | 246.52 ± 1.38 c | −8.57 | |
| NF2 | CG | 184.56 ± 3.07 c | +7.87 | 242.29 ± 2.32 c | −10.14 |
| G1 | 209.49 ± 2.28 a | +22.45 | 266.55 ± 0.99 a | −1.14 | |
| G2 | 176.53 ± 2.19 de | +3.18 | 237.30 ± 1.89 d | −11.99 |
| Fertigation | Grafting | Flesh | Relative Change (%) | Peel | Relative Change (%) |
|---|---|---|---|---|---|
| CF | CG | 285.91 ± 7.60 f | / | 1205.87 ± 10.20 cd | / |
| G1 | 298.49 ± 7.06 f | +4.39 | 1048.35 ±19.61 g | −13.06 | |
| G2 | 330.84 ± 4.23 e | +15.71 | 1240.16 ± 7.64 c | +2.84 | |
| NF1 | CG | 425.22 ± 4.56 cd | +48.72 | 1309.55 ± 14.57 b | +8.59 |
| G1 | 455.85 ± 7.43 bc | +59.43 | 1124.47 ± 2.38 e | −6.75 | |
| G2 | 456.54 ± 25.90 bc | +59.67 | 1172.39 ± 17.38 d | −2.77 | |
| NF2 | CG | 480.47 ± 4.32 ab | +68.04 | 1174.73 ± 17.66 d | −2.58 |
| G1 | 506.92 ± 4.81 a | +77.30 | 1386.44 ± 16.22 a | +14.97 | |
| G2 | 398.86 ± 9.25 d | +39.50 | 1118.93 ± 7.96 e | −7.20 |
| Fertigation | Grafting | DPPH | Relative Change (%) | FRAP | Relative Change (%) | ABTS | Relative Change (%) |
|---|---|---|---|---|---|---|---|
| CF | CG | 2.47 ± 0.01 d | / | 0.71 ± 0.00 ef | / | 1.09 ± 0.07 d | / |
| G1 | 2.63 ± 0.03 c | +6.47 | 0.86 ± 0.01 c | +21.12 | 1.53 ± 0.01 a | +40.36 | |
| G2 | 2.58 ± 0.02 bc | +4.45 | 0.74 ± 0.01 de | +4.22 | 1.56 ± 0.03 a | +43.11 | |
| NF1 | CG | 2.69 ±0.09 bc | +8.90 | 0.67 ± 0.01 f | −5.63 | 1.09 ± 0.07 d | 00.00 |
| G1 | 2.78 ± 0.03 b | +12.55 | 1.13 ± 0.00 a | +59.15 | 1.13 ± 0.01 cd | +3.66 | |
| G2 | 2.59 ± 0.05 cd | +4.85 | 0.86 ± 0.00 c | +21.12 | 1.25 ± 0.08 bc | +14.67 | |
| NF2 | CG | 2.82 ± 0.06 b | +14.17 | 0.77 ± 0.01 d | +8.45 | 1.30 ± 0.01 b | +19.26 |
| G1 | 3.11 ± 0.02 a | +25.91 | 0.99 ± 0.02 b | +39.43 | 1.08 ± 0.08 d | −0.91 | |
| G2 | 2.81 ± 0.03 b | +13.76 | 0.85 ± 0.01 c | +17.71 | 1.35 ± 0.01 b | +23.85 |
| Fertigation | Grafting | DPPH | Relative Change (%) | FRAP | Relative Change (%) | ABTS | Relative Change (%) |
|---|---|---|---|---|---|---|---|
| CF | CG | 3.00 ± 0.03 e | / | 2.41 ± 0.01 d | / | 5.07 ± 0.03 g | / |
| G1 | 3.46 ± 0.02 c | +15.33 | 2.36 ± 0.00 e | −2.07 | 6.55 ± 0.05 d | +29.19 | |
| G2 | 3.37 ± 0.02 cd | +12.33 | 2.31 ± 0.00 f | −4.14 | 6.23 ± 0.02 e | +22.87 | |
| NF1 | CG | 3.70 ± 0.04 b | +23.33 | 2.59 ± 0.00 c | +7.46 | 6.58 ± 0.03 d | +29.78 |
| G1 | 3.80 ± 0.09 ab | +26.66 | 2.60 ± 0.01 c | +7.88 | 7.65 ± 0.02 b | +50.88 | |
| G2 | 3.41 ± 0.01 c | +13.66 | 2.76 ± 0.01 b | +14.52 | 6.78 ± 0.05 c | +33.72 | |
| NF2 | CG | 3.26 ± 0.04 d | +8.66 | 2.43 ± 0.00 e | +0.82 | 6.22 ± 0.02 f | +22.68 |
| G1 | 3.90 ± 0.01 a | +30.00 | 2.50 ± 0.01 d | +3.73 | 7.87 ± 0.01 a | +55.22 | |
| G2 | 3.86 ± 0.01 a | +28.66 | 3.23 ± 0.01 a | +34.02 | 7.81 ± 0.08 a | +54.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojnović, Đ.; Maksimović, I.; Koprivica, G.; Tepić Horecki, A.; Milić, A.; Adamović, B.; Šumić, Z.; Ilin, Ž. Optimizing Greenhouse Cucumber Fertigation Through Grafting: Improving Yield, Bioactive Compounds, and Antioxidant Activity. Horticulturae 2024, 10, 1135. https://doi.org/10.3390/horticulturae10111135
Vojnović Đ, Maksimović I, Koprivica G, Tepić Horecki A, Milić A, Adamović B, Šumić Z, Ilin Ž. Optimizing Greenhouse Cucumber Fertigation Through Grafting: Improving Yield, Bioactive Compounds, and Antioxidant Activity. Horticulturae. 2024; 10(11):1135. https://doi.org/10.3390/horticulturae10111135
Chicago/Turabian StyleVojnović, Đorđe, Ivana Maksimović, Gabrijela Koprivica, Aleksandra Tepić Horecki, Anita Milić, Boris Adamović, Zdravko Šumić, and Žarko Ilin. 2024. "Optimizing Greenhouse Cucumber Fertigation Through Grafting: Improving Yield, Bioactive Compounds, and Antioxidant Activity" Horticulturae 10, no. 11: 1135. https://doi.org/10.3390/horticulturae10111135
APA StyleVojnović, Đ., Maksimović, I., Koprivica, G., Tepić Horecki, A., Milić, A., Adamović, B., Šumić, Z., & Ilin, Ž. (2024). Optimizing Greenhouse Cucumber Fertigation Through Grafting: Improving Yield, Bioactive Compounds, and Antioxidant Activity. Horticulturae, 10(11), 1135. https://doi.org/10.3390/horticulturae10111135








