Light Modulation of Photosynthate Accumulation in Microgreens Grown in a Controlled Environment During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions
2.2. Phytochemical Analysis
2.3. Statistical Analysis
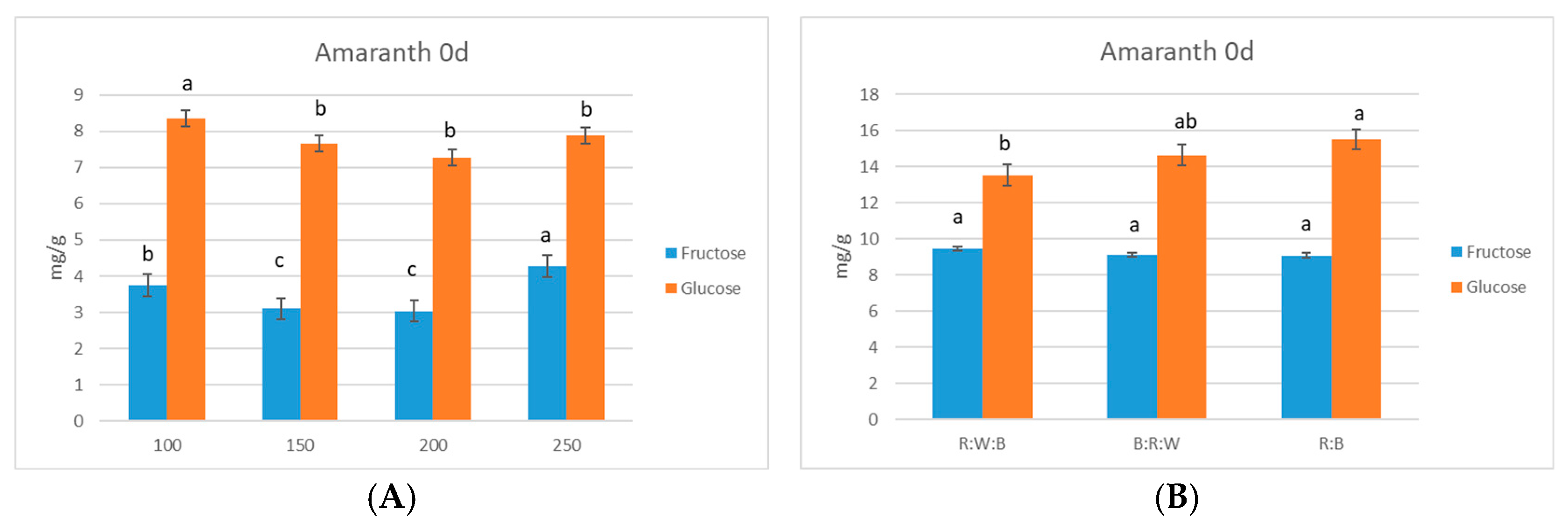
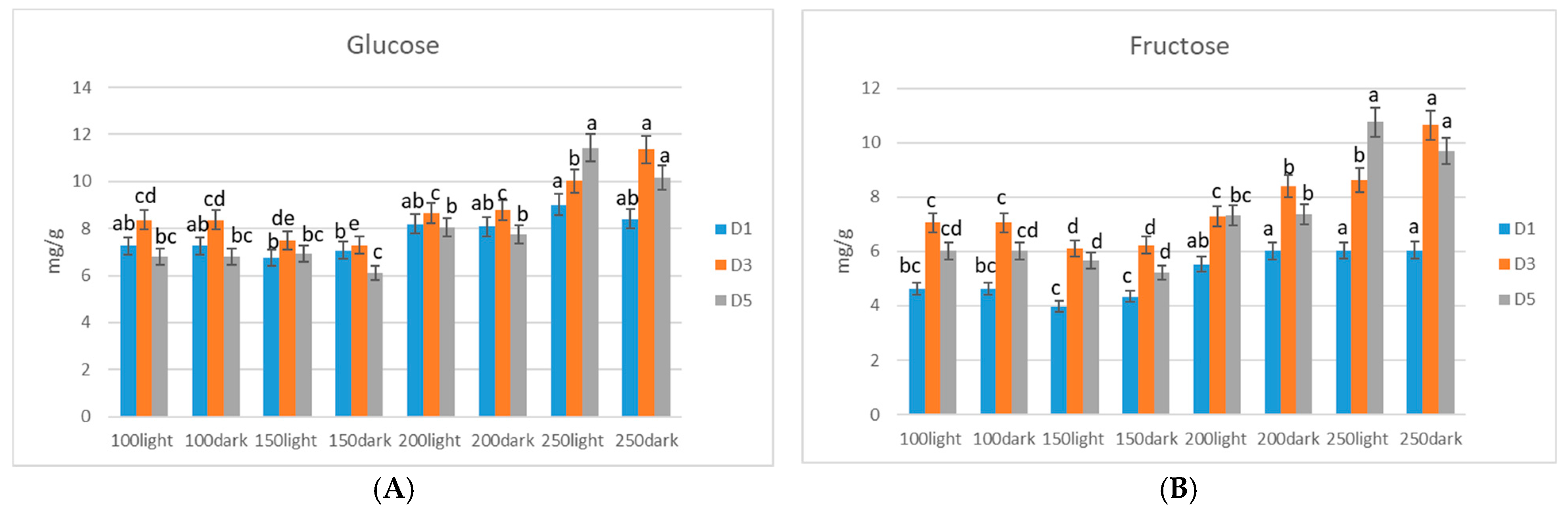
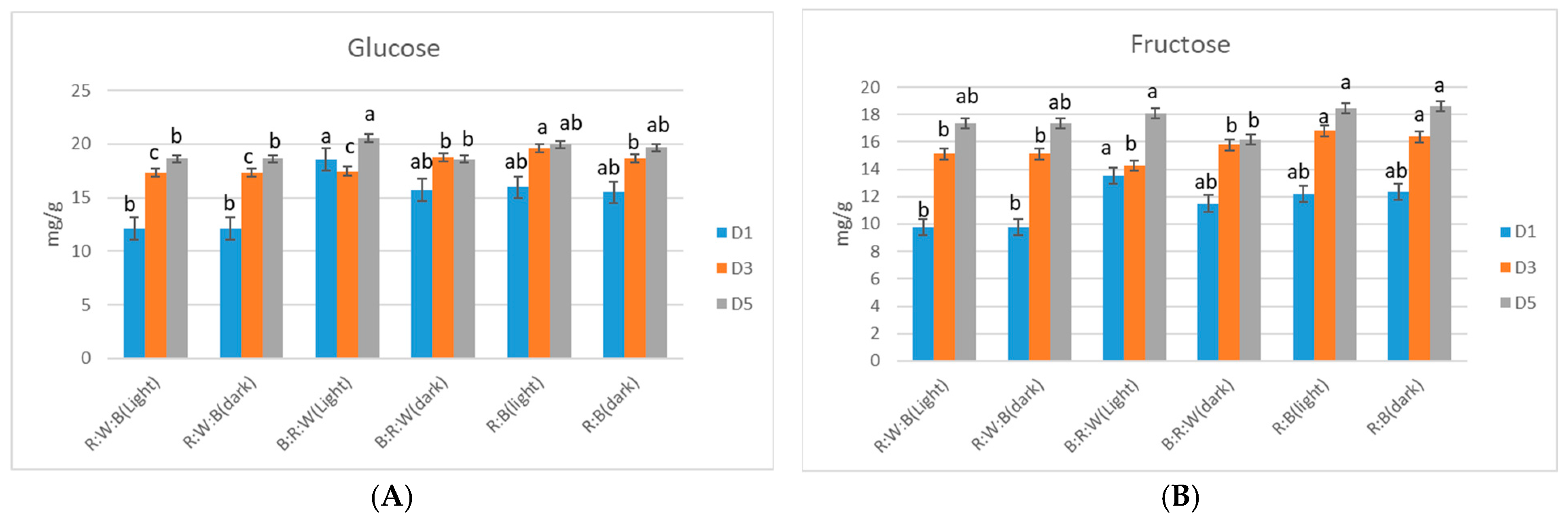
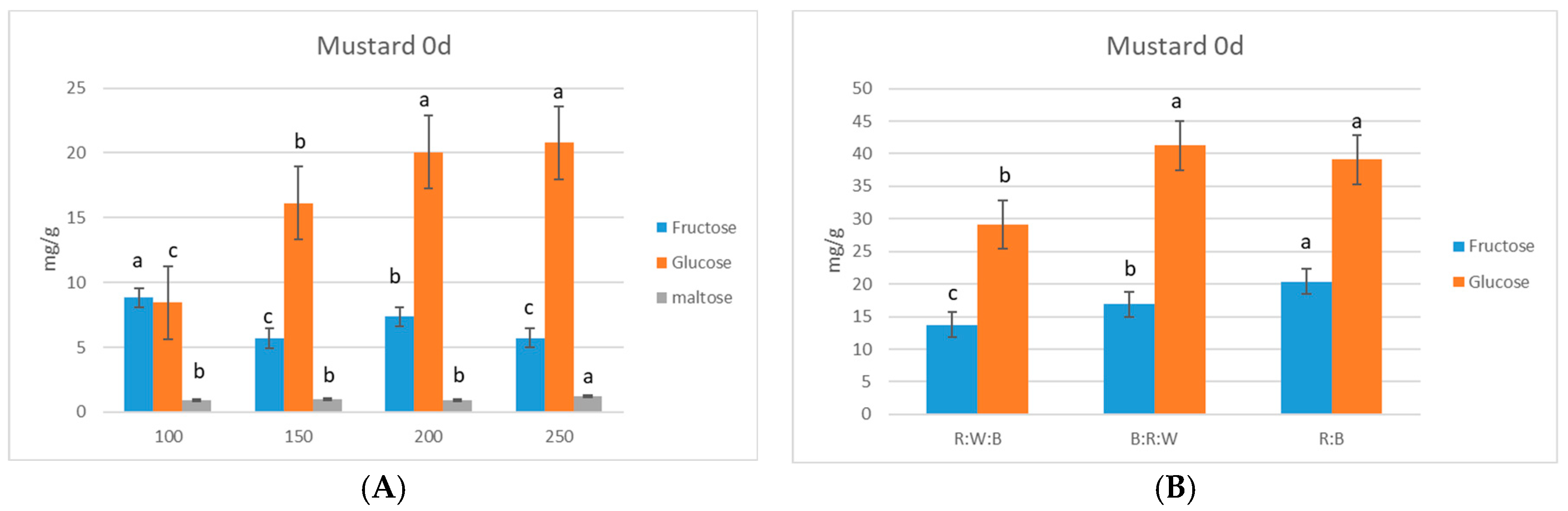
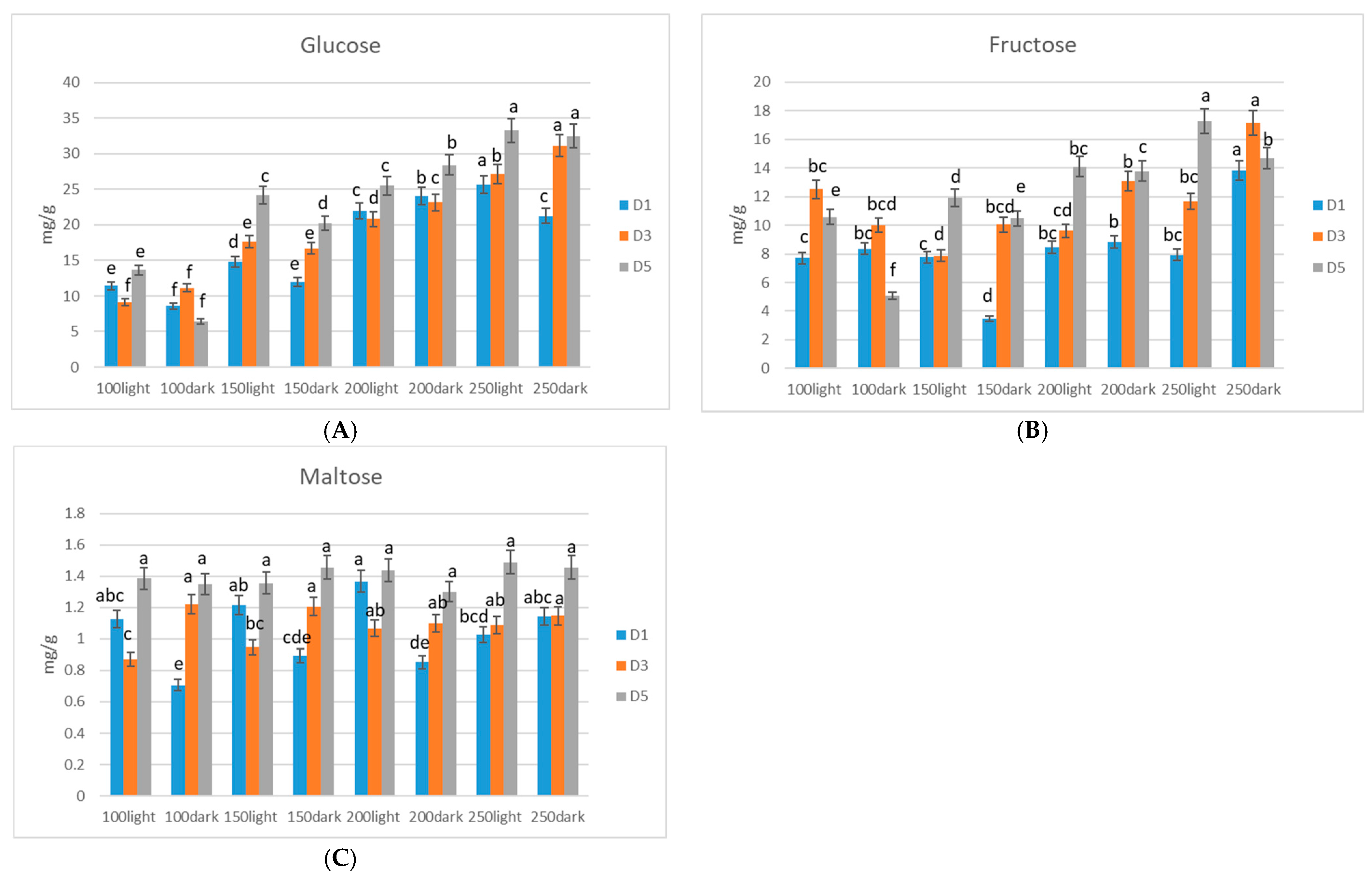
3. Results
Sugar Accumulation in Microgreens
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pescarini, H.B.; Silva, V.G.D.; Mello, S.d.C.; Purquerio, L.F.V.; Sala, F.C.; Zorzeto Cesar, T.Q. Updates on Microgreens Grown under Artificial Lighting: Scientific Advances in the Last Two Decades. Horticulturae 2023, 9, 864. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Lau, T.Q.; Tang, V.T.H.; Kasedo, J. Influence of Soil and Light Condition on the Growth and Antioxidants Content of Amaranthus Cruentus (Red Amaranth) Microgreen. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012051. [Google Scholar]
- Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Sharma, S. Microgreens: A Nutrient-Rich Crop That Can Diversify the Food System. Int. J. Pure Appl. Biosci 2018, 6, 182–186. [Google Scholar] [CrossRef]
- Eliseeva, L.G.; Simina, D.V. Microgreens: A Newly Emerging Product, Aspects, Prospects, and Disadvantages. Вестник Вoрoнежскoгo Гoсударственнoгo Университета Инженерных Технoлoгий 2021, 83, 102–107. [Google Scholar]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, Shelf Life, and Bioactive Components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop: HS1164, rev. 9/2020. Edis 2020, 2020, 5. [Google Scholar] [CrossRef]
- Priti; Sangwan, S.; Kukreja, B.; Mishra, G.P.; Dikshit, H.K.; Singh, A.; Aski, M.; Kumar, A.; Taak, Y.; Stobdan, T.; et al. Yield Optimization, Microbial Load Analysis, and Sensory Evaluation of Mungbean (Vigna radiata L.), Lentil (Lens culinaris subsp. culinaris), and Indian Mustard (Brassica juncea L.) Microgreens Grown under Greenhouse Conditions. PLoS ONE 2022, 17, e0268085. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and Correlation of Sensory Attributes and Chemical Compositions of Emerging Fresh Produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Senevirathne, G.I.; Gama-Arachchige, N.S.; Karunaratne, A.M. Germination, Harvesting Stage, Antioxidant Activity, and Consumer Acceptance of Ten Microgreens. Ceylon J. Sci. 2019, 48, 91–96. [Google Scholar] [CrossRef]
- Gamel, T.H.; Linssen, J.P.; Mesallam, A.S.; A Damir, A.; A Shekib, L. Effect of Seed Treatments on the Chemical Composition of Two Amaranth Species: Oil, Sugars, Fibres, Minerals, and Vitamins. J. Sci. Food Agric. 2006, 86, 82–89. [Google Scholar] [CrossRef]
- Becker, R.; Wheeler, E.L.; Lorenz, K.; Stafford, A.E.; Grosjean, O.K.; Betschart, A.A.; Saunders, R.M. A Compositional Study of Amaranth Grain. J. Food Sci. 1981, 46, 1175–1180. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Pant, U.; Bhajan, R.; Singh, A.; Kulshesthra, K.; Singh, A.K.; Punetha, H. Green Leafy Mustard: A Healthy Alternative. Electron. J. Plant Breed. 2020, 11, 267–270. [Google Scholar]
- Munshi, S.K.; Kochhar, A. Carbohydrate Metabolism in the Siliqua Relating to Oil-Filling in Mustard Seeds. J. Agron. Crop Sci. 1994, 172, 126–136. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Mao, S.; Wu, Q.; Tian, Y.; Wang, F.; Wang, P.; Huang, K.; Wu, Q. Variation Characteristics of Glucosinolate Contents in Leaf Mustard (Brassica juncea). Agronomy 2022, 12, 2287. [Google Scholar] [CrossRef]
- Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patané, C. Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Meas, S.; Luengwilai, K.; Thongket, T. Enhancing Growth and Phytochemicals of Two Amaranth Microgreens by LEDs Light Irradiation. Sci. Hortic. 2020, 265, 109204. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Samuolienė, G.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Novičkovas, A.; Duchovskis, P. Response of Mustard Microgreens to Different Wavelengths and Durations of UV-A LEDs. Front. Plant Sci. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Fotelli, M.; Ilic, Z.S.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Długosz-Grochowska, O.; Kołton, A.; Zupnik, M. Effects of LED supplemental lighting on yield and some quality parameters of lamb’s lettuce grown in two winter cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Laužikė, K.; Samuolienė, G. The Distinct Impact of Multi-Color LED Light on Nitrate, Amino Acid, Soluble Sugar, and Organic Acid Contents in Red and Green Leaf Lettuce Cultivated in Controlled Environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef]
- Jones-Baumgardt, C.; Llewellyn, D.; Ying, Q.; Zheng, Y. Intensity of Sole-Source Light-Emitting Diodes Affects Growth, Yield, and Quality of Brassicaceae Microgreens. HortScience 2019, 54, 1168–1174. [Google Scholar] [CrossRef]
- Liu, K.; Gao, M.; Jiang, H.; Ou, S.; Li, X.; He, R.; Li, Y.; Liu, H. Light Intensity and Photoperiod Affect Growth and Nutritional Quality of Brassica Microgreens. Molecules 2022, 27, 883. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential Effects of Low Light Intensity on Broccoli Microgreens Growth and Phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars: Metabolism, Sensing, and Abiotic Stress: A Complex Network in the Life of Plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Zaitoun, M.; Ghanem, M.; Harphoush, S. Sugars: Types and Their Functional Properties in Food and Human Health. Int. J. Public Health Res. 2018, 6, 93–99. [Google Scholar]
- Edwards, C.H.; Rossi, M.; Corpe, C.P.; Butterworth, P.J.; Ellis, P.R. The Role of Sugars and Sweeteners in Food, Diet, and Health: Alternatives for the Future. Trends Food Sci. Technol. 2016, 56, 158–166. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Antioxidant Activity and Chemical Properties of Crude and Fractionated Maillard Reaction Products Derived from Four Sugar–Amino Acid Maillard Reaction Model Systems. Ann. N. Y. Acad. Sci. 2008, 1126, 220–224. [Google Scholar] [CrossRef]
- Christie, J.M.; Briggs, W.R. Blue Light Sensing in Higher Plants. J. Biol. Chem. 2001, 276, 11457–11460. [Google Scholar] [CrossRef] [PubMed]
- Boccalandro, H.E.; Giordano, C.V.; Ploschuk, E.L.; Piccoli, P.N.; Bottini, R.; Casal, J.J. Phototropins but Not Cryptochromes Mediate the Blue Light-Specific Promotion of Stomatal Conductance, While Both Enhance Photosynthesis and Transpiration under Full Sunlight. Plant Physiol. 2012, 158, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Olle, M.; Alsiņa, I. Influence of Wavelength of Light on Growth, Yield and Nutritional Quality of Greenhouse Vegetables. Proc. Latv. Acad. Sciences. Sect. B. Nat. Exact Appl. Sci. 2019, 73, 1–9. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Duchovskis, P. Nutrient Levels in Brassicaceae Microgreens Increase under Tailored Light-Emitting Diode Spectra. Front. Plant Sci. 2019, 10, 1475. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Frąszczak, B.; Sosnowska, D.; Kajszczak, D.; Szymczak, K.; Bonikowski, R. LED Light Quality Affected Bioactive Compounds, Antioxidant Potential, and Nutritional Value of Red and White Cabbage Microgreens. Appl. Sci. 2023, 13, 5435. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, S.; Piškor, A.; Melnjak, A.; Mikuška, A.; Šrajer Gajdošik, M.; Begović, L. Antioxidant Capacity and Shelf Life of Radish Microgreens Affected by Growth Light and Cultivars. Horticulturae 2023, 9, 76. [Google Scholar] [CrossRef]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Ahmed, M.A.; Ali, W.M.; Kalaji, H.M.; Chen, F. Effects of Light Spectrum on Morpho-Physiological Traits of Grafted Tomato Seedlings. PLoS ONE 2021, 16, e0250210. [Google Scholar] [CrossRef] [PubMed]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of Light Quality on Growth and Vegetable Quality in Leaf Lettuce, Spinach and Komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-Red Light Is Needed for Efficient Photochemistry and Photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Rehana, S.; Ahmed, F.; Zeba, N.; Husna, A.; Hossain, F. Effect of Sunlight and Artificial Light on Micropropagation of Potato (Solanum tuberosum L.) Plantlets. Arch. Agric. Environ. Sci. 2018, 3, 151–156. [Google Scholar] [CrossRef][Green Version]
- Henry, C.; Watson-Lazowski, A.; Oszvald, M.; Griffiths, C.; Paul, M.J.; Furbank, R.T.; Ghannoum, O. Sugar Sensing Responses to Low and High Light in Leaves of the C4 Model Grass Setaria viridis. J. Exp. Bot. 2020, 71, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, A.; Zięba, P.; Gabryś, H. Sugar and Light Effects on the Condition of the Photosynthetic Apparatus of Arabidopsis thaliana Cultured in Vitro. J. Plant Growth Regul. 2012, 31, 90–101. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Xie, Z.K.; Yu, L.L.; Wang, Q. Effect of Light Exposure on Sensorial Quality, Concentrations of Bioactive Compounds and Antioxidant Capacity of Radish Microgreens During Low Temperature Storage. Food Chem. 2014, 151, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Craver, J.K.; Gerovac, J.R.; Lopez, R.G.; Kopsell, D.A. Light Intensity and Light Quality from Sole-Source Light-Emitting Diodes Impact Phytochemical Concentrations within Brassica Microgreens. J. Am. Soc. Hortic. Sci. 2017, 142, 3–12. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskaitė, J.; Sirtautas, R.; Novičkovas, A.; Duchovskis, P. Effect of Supplementary Pre-Harvest LED Lighting on the Antioxidant and Nutritional Properties of Green Vegetables. Acta Hortic. 2013, 1009, 61–67. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudžinskaitė, I.; Laužikė, K.; Pukalskas, A.; Samuoliene, G. Light Modulation of Photosynthate Accumulation in Microgreens Grown in a Controlled Environment During Storage. Horticulturae 2025, 11, 176. https://doi.org/10.3390/horticulturae11020176
Gudžinskaitė I, Laužikė K, Pukalskas A, Samuoliene G. Light Modulation of Photosynthate Accumulation in Microgreens Grown in a Controlled Environment During Storage. Horticulturae. 2025; 11(2):176. https://doi.org/10.3390/horticulturae11020176
Chicago/Turabian StyleGudžinskaitė, Ieva, Kristina Laužikė, Audrius Pukalskas, and Giedrė Samuoliene. 2025. "Light Modulation of Photosynthate Accumulation in Microgreens Grown in a Controlled Environment During Storage" Horticulturae 11, no. 2: 176. https://doi.org/10.3390/horticulturae11020176
APA StyleGudžinskaitė, I., Laužikė, K., Pukalskas, A., & Samuoliene, G. (2025). Light Modulation of Photosynthate Accumulation in Microgreens Grown in a Controlled Environment During Storage. Horticulturae, 11(2), 176. https://doi.org/10.3390/horticulturae11020176






