Early Generation Selection of Potato Breeding Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. True Potato Seed Planting
2.3. Growing of Minitubers in the Greenhouse
2.4. Phenotypic Data Measurements
2.5. DNA Extraction
2.6. Molecular Markers
2.7. PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Molecular Analysis of Potato Genotypes for Tuber Shape Traits Using Molecular Markers
3.2. Molecular Screening and Phenotypic Data of Potato Breeding Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsch, C.N.; Hirsch, C.D.; Felcher, K.; Coombs, J.; Zarka, D.; Deynze, A.V.; Jong, W.D.; Veilleux, R.E.; Jansky, S.; Bethke, P.; et al. Retrospective View of North American Potato (Solanum tuberosum L.) Breeding in the 20th and 21st Centuries. G3 Genes|Genomes|Genetics 2013, 3, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Daurov, D.; Daurova, A.; Karimov, A.; Tolegenova, D.; Volkov, D.; Raimbek, D.; Zhambakin, K.; Shamekova, M. Determining Effective Methods of Obtaining Virus-Free Potato for Cultivation in Kazakhstan. Am. J. Potato Res. 2020, 97, 367–375. [Google Scholar] [CrossRef]
- Daurov, D.; Argynbayeva, A.; Daurova, A.; Zhapar, K.; Sapakhova, Z.; Zhambakin, K.; Shamekova, M. Monitoring the Spread of Potato Virus Diseases in Kazakhstan. Am. J. Potato Res. 2023, 100, 63–70. [Google Scholar] [CrossRef]
- Daurov, D.; Daurova, A.; Sapakhova, Z.; Kanat, R.; Akhmetzhanova, D.; Abilda, Z.; Toishimanov, M.; Raissova, N.; Otynshiyev, M.; Zhambakin, K.; et al. The Impact of the Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers. Agronomy 2024, 14, 1782. [Google Scholar] [CrossRef]
- Bureau of National Statistics of the Republic of Kazakhstan. Available online: https://stat.gov.kz/en/ (accessed on 12 August 2024).
- Islam, M.M.; Naznin, S.; Naznin, A.; Uddin, M.N.; Amin, M.N.; Rahman, M.M.; Tipu, M.M.H.; Alsuhaibani, A.M.; Gaber, A.; Ahmed, S. Dry Matter, Starch Content, Reducing Sugar, Color and Crispiness are Key Parameters of Potatoes Required for Chip Processing. Horticulturae 2022, 8, 362. [Google Scholar] [CrossRef]
- Sliwka, J.; Jakuczun, H.; Lebecka, R.; Marczewski, W.; Gebhardt, C.; Zimnoch-Guzowska, E. Tagging QTLs for Late Blight Resistance and Plant Maturity from Diploid Wild Relatives in A Cultivated Potato (Solanum tuberosum L.) Background. Theor. Appl. Genet. 2007, 115, 101–112. [Google Scholar] [CrossRef]
- Hui, Z.M.; Xu, J.F.; Jian, Y.Q.; Bian, C.S.; Duan, S.G.; Hu, J.; Li, G.C.; Jin, L.P. 2b-RAD Based Maturity Associated Molecular Marker Identification in Tetraploid Potato (Solanum tuberosum L.). Acta Agronomica Sinica 2022, 48, 2274–2284. Available online: https://zwxb.chinacrops.org/EN/10.3724/SP.J.1006.2022.14138 (accessed on 12 August 2024).
- Huang, W.; Dong, J.; Zhao, X.; Zhao, Z.; Li, C.; Li, J.; Song, B. QTL Analysis of Tuber Shape in A Diploid Potato Population. Front. Plant Sci. 2022, 13, 1046287. [Google Scholar] [CrossRef]
- Stark, J.C.; Love, S.L.; Knowles, N.R. Tuber quality. In Potato Production Systems; Stark, J., Thornton, M., Nolte, P., Eds.; Springer: Cham, Switzerland, 2020; pp. 479–497. [Google Scholar] [CrossRef]
- Neilson, J.A.D.; Smith, A.M.; Mesina, L.; Vivian, R.; Smienk, S.; De Koyer, D. Potato Tuber Shape Phenotyping Using RGB Imaging. Agronomy 2021, 11, 1781. [Google Scholar] [CrossRef]
- Fan, G.; Wang, Q.; Xu, J.; Chen, N.; Zhu, W.; Duan, S.; Yang, X.; De Jong, W.S.; Guo, Y.; Jin, L.; et al. Fine Mapping and Candidate Gene Prediction of Tuber Shape Controlling Ro Locus Based on Integrating Genetic and Transcriptomic Analyses in Potato. Int. J. Mol. Sci. 2022, 23, 1470. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Yu, Q.; Liu, R.; Wang, Z.Y.; Sun, Y. AtOFPs Regulate Cell Elongation by Modulating Microtubule Orientation Via Direct Interaction with TONNEAU2. Plant Sci. 2020, 292, 110405. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Whitworth, J.; Novy, R.G. QTL Identified That Influence Tuber Length-Width Ratio, Degree of Flatness, Tuber Size, and Specific Gravity in a Russet-Skinned, Tetraploid Mapping Population. Front. Plant Sci. 2024, 15, 1343632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zou, M.; Deng, K.; Xia, C.; Jiang, S.; Zhang, C.; Ma, Y.; Dong, X.; He, M.; Na, T.; et al. Insights into The Genetic Determination of Tuber Shape and Eye Depth in Potato Natural Population Based on Autotetraploid Potato Genome. Front. Plant Sci. 2023, 14, 1080666. [Google Scholar] [CrossRef] [PubMed]
- Arefi, A.; Hensel, O.; Sturm, B. Intelligent Potato Frying: Time to Say Goodbye to the “Good Old” Processing Strategies. Therm. Sci. Eng. Prog. 2022, 34, 101389. [Google Scholar] [CrossRef]
- Sobol, Z.; Jakubowski, T.; Surma, M. Effect of Potato Tuber Exposure to UV-C Radiation and Semi-Product Soaking in Water on Acrylamide Content in French Fries Dry Matter. Sustainability 2020, 12, 3426. [Google Scholar] [CrossRef]
- Chen, N.; Zhu, W.; Xu, J.; Duan, S.; Bian, C.; Hu, J.; Wang, W.; Li, G.; Jin, L. Molecular Marker Development and Primary Physical Map Construction for The Tuber Shape Ro Gene Locus in Diploid Potato (Solanum tuberosum L.). Mol. Breed. 2019, 39, 6. [Google Scholar] [CrossRef]
- Goo, Y.M.; Han, E.H.; Jeong, J.C.; Kwak, S.S.; Yu, J.; Kim, Y.H.; Ahn, M.J.; Lee, S.W. Overexpression of the Sweet Potato Ibor Gene Results in the Increased Accumulation of Carotenoid and Confers Tolerance to Environmental Stresses in Transgenic Potato. Comptes Rendus Biol. 2015, 338, 12–20. [Google Scholar] [CrossRef]
- Galpaz, N.; Ronen, G.; Khalfa, Z.; Zamir, D.; Hirschberg, J. Achromoplast-Specific Carotenoid Biosynthesis Pathway is Revealed by Cloning of The Tomato White-Flower Locus. Plant Cell 2006, 18, 1947–1960. [Google Scholar] [CrossRef]
- Sharma, S.K.; McLean, K.; Hedley, P.E.; Dale, F.; Daniels, S.; Bryan, J.G. Genotyping-By-Sequencing Targets Genic Regions and Improves Resolution of Genome-Wide Association Studies in Autotetraploid Potato. Theor. Appl. Genet. 2024, 137, 180. [Google Scholar] [CrossRef]
- Dutt, S.; Devi, K.; Chauhan, M. Analysis of Carotenoids Metabolism Genes Landscape in Potato and Cloning of Zeaxanthin Epoxidase Gene. Potato J. 2023, 50, 132–148. Available online: https://epubs.icar.org.in/index.php/PotatoJ/article/view/138194 (accessed on 12 August 2024).
- Römer, S.; Lübeck, J.; Kauder, F.; Steiger, S.; Adomat, C.; Sandmann, G. Genetic Engineering of a Zeaxanthin-Rich Potato by Antisense Inactivation and Co-Suppression of Carotenoid Epoxidation. Metab. Eng. 2002, 4, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.L.; Ducreux, L.; Griffiths, D.W.; Stewart, D.; Davies, H.V.; Taylor, M.A. Carotenogenesis During Tuber Development and Storage in Potato. J. Exp. Bot. 2004, 55, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Polivanova, O.B.; Gins, E.M.; Moskalev, E.A.; Voinova, M.S.; Koroleva, A.K.; Semenov, A.Z.; Sivolapova, A.B.; Ivanova, A.S.; Kazakov, O.G.; Simakov, E.A.; et al. Quality Evaluation, Phytochemical Characteristics and Estimation of Beta-Carotene Hydroxylase 2 (Chy2) Alleles of Interspecific Potato Hybrids. Agronomy 2021, 11, 1619. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Rejman, K.; Kaczorowska, J.; Laskowski, W. Vegetables, Potatoes and Their Products as Sources of Energy and Nutrients to the Average Diet in Poland. Int. J. Environ. Res. Public Health 2021, 18, 3217. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Ma, Z.; Chen, H.; Gao, H. Toward an Understanding of Potato Starch Structure, Function, Biosynthesis, and Applications. Food Front. 2023, 4, 980–1000. [Google Scholar] [CrossRef]
- Raj, N.; Dalal, N.; Bisht, V.; Dhakar, U. Potato Starch: Novel Ingredient for Food Industry. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1718–1724. [Google Scholar] [CrossRef]
- Tessema, L.; Mohammed, W.; Abebe, T. Evaluation of Potato (Solanum tuberosum L.) Varieties for Yield and Some Agronomic Traits. Open Agric. 2020, 5, 63–74. [Google Scholar] [CrossRef]
- Sergeeva, E.M.; Larichev, K.T.; Salina, E.A.; Kochetov, A.V. Starch Metabolism in Potato Solanum tuberosum L. Vavilovskii Zhurnal Genet. Sel. 2022, 26, 250–263. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Ferreira-Lazarte, A.; Amirruddin, N.S.; Lin, A.H.M. Generating Slow Digestibility in Cooked Potatoes by Modulating Starch Accessibility to A-Amylase and Mucosal A-Glucosidase to Different Levels. Food Hydrocoll. 2023, 141, 108718. [Google Scholar] [CrossRef]
- Sołtys-Kalina, D.; Szajko, K.; Stefańczyk, E.; Smyda-Dajmund, P.; Śliwka, J.; Marczewski, W. eQTL Mapping of the 12S Globulin Cruciferin Gene PGCRURSE5 As a Novel Candidate Associated with Starch Content in Potato Tubers. Sci. Rep. 2020, 10, 17168. [Google Scholar] [CrossRef]
- Sołtys-Kalina, D.; Szajko, K.; Wasilewicz-Flis, I.; Mankowski, D.; Marczewski, W.; Sliwka, J. Quantitative Trait Loci for Starch-Corrected Chip Color After Harvest, Cold Storage and After Reconditioning Mapped in Diploid Potato. Mol. Genet. Genom. 2020, 295, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, A.G.J.; Jacobsen, E.; Visser, R.G.F. Formation and Deposition of Amylose in the Potato-Tuber Starch Granule Are Affected by the Reduction of Granule-Bound Starch Synthase Gene-Expression. Plant Cell 1994, 6, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, J.F.; Khan, A.; Fettke, J. Gradual Analytics of Starch-Interacting Proteins Revealed the Involvement of Starch-Phosphorylating Enzymes during Synthesis of Storage Starch in Potato (Solanum tuberosum L.) Tubers. Molecules 2023, 28, 6219. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, N.; Liu, L.; Ali, A.; Mughal, N.; Yu, G.; Huang, Y. Molecular Functions and Pathways of Plastidial Starch Phosphorylase (Pho1) in Starch Metabolism: Current and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 10450. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Friberg, M.; Vogel, P.; Turesson, H.; Olsson, N.; Andersson, M.; Hofvander, P. Pho1a (Plastid Starch Phosphorylase) is Duplicated and Essential for Normal Starch Granule Phenotype in Tubers of Solanum tuberosum L. Front. Plant Sci. 2023, 14, 1220973. [Google Scholar] [CrossRef]
- Tu, W.; Li, J.; Dong, J.; Wu, J.; Wang, H.; Zuo, Y.; Cai, X.; Song, B. Molecular Marker-Assisted Selection for Frost Tolerance in A Diallel Population of Potato. Cells 2023, 12, 1226. [Google Scholar] [CrossRef]
- Naeem, M.; Demirel, U.; Yousaf, M.F.; Caliskan, S.; Caliskan, M.E. Overview on Domestication, Breeding, Genetic Gain and Improvement of Tuber Quality Traits of Potato Using Fast Forwarding Technique (GWAS): A review. Plant Breed. 2021, 140, 519–542. [Google Scholar] [CrossRef]
- Xiao, X.O.; Zhang, N.; Jin, H.; Si, H. Genetic Analysis of Potato Breeding Collection Using Single-Nucleotide Polymorphism (SNP) Markers. Plants 2023, 12, 1895. [Google Scholar] [CrossRef]
- Zia, M.A.B.; Demirel, U.; Nadeem, M.A.; Caliscan, M.E. Genome-Wide Association Study Identifies Various Loci Underlying Agronomic and Morphological Traits in Diversified Potato Panel. Physiol. Mol. Biol. Plants 2020, 26, 1003–1020. [Google Scholar] [CrossRef]
- Ahmad, D.; Zhang, Z.; Rasheed, H.; Xu, X.; Bao, J. Recent Advances in Molecular Improvement for Potato Tuber Traits. Int. J. Mol. Sci. 2022, 23, 9982. [Google Scholar] [CrossRef]
- Pandey, J.; Scheuring, D.C.; Koym, J.W.; Vales, M.I. Genomic Regions Associated with Tuber Traits in Tetraploid Potatoes and Identification of Superior Clones for Breeding Purposes. Front. Plant Sci. 2022, 13, 952263. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Massa, A.N.; Douches, D.; Coombs, J.; Akdemir, D.; Yencho, G.C.; Whitworth, J.L.; Novy, R.G. Linkage and QTL Mapping for Tuber Shape and Specific Gravity in a Tetraploid Mapping Population of Potato Representing the Russet Market Class. BMC Plant Biol. 2021, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, Z.; Tang, D.; Zhu, Y.; Wang, P.; Li, D.; Zhu, G.; Xiong, X.; Shang, Y.; Li, C.; et al. Genome Design of Hybrid Potato. Cell 2021, 184, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- González, M.N.; Massa, G.A.; Andersson, M.; Turesson, H.; Olsson, N.; Fält, A.S.; Storani, L.; Décima Oneto, C.A.; Hofvander, P.; Feingold, S.E. Reduced Enzymatic Browning in Potato Tubers by Specific Editing of a Polyphenol Oxidase Gene Via Ribonucleoprotein Complexes Delivery of the CRISPR/Cas9 System. Front. Plant Sci. 2020, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.; Ahmad, D.; Bao, J. Genetic Diversity and Health Properties of Polyphenols in Potato. Antioxidants 2022, 11, 603. [Google Scholar] [CrossRef]
- Yousaf, M.F.; Demirel, U.; Naeem, M.; Naawe, E.K.; Caliskan, M.E. SNP Markers Associated with Some Root, Stolon, and Tuber Traits in Tetraploid Potatoes (Solanum tuberosum L.) Grown Under Diverse Growing Systems. Potato Res. 2024. [Google Scholar] [CrossRef]
- Ozkaynak, E. Development of PVX Resistant Potato Breeding Lines Using Marker-Assisted Selection. Turk. J. Field Crops 2020, 25, 66–73. [Google Scholar] [CrossRef]
- State Commission for Variety Testing of Agricultural Crops of the Republic of Kazakhstan. Available online: https://gcomsort.kz (accessed on 9 August 2024).
- Guerrero, H.R.; Meza, N.M.; Leon, B.M.D. Evaluation of Quality Characteristics of Promising Clones and Potato Varieties (Solanum tuberosum L.). Rev. Fac. Agron. 2018, 35, 477–792. [Google Scholar]
- PotatoPro. Available online: https://www.potatopro.com/potato-varieties/fontane (accessed on 10 September 2024).
- PotatoPro. Available online: https://www.potatopro.com/potato-varieties/santana (accessed on 9 August 2024).
- Germicopa Potato Varieties. Available online: https://www.germicopa.com/en/pommedeterre/punchy-en/ (accessed on 9 August 2024).
- Hara-Skrzypiec, A.; Sliwka, J.; Jakuczun, H.; Zimnoch-Guzowska, E. QTL for Tuber Morphology Traits in Diploid Potato. J. Appl. Genet. 2018, 59, 123–132. [Google Scholar] [CrossRef]
- Sulli, M.; Mandolino, G.; Sturaro, M.; Onofri, C.; Diretto, G.; Parisi, B.; Giuliano, G. Molecular and Biochemical Characterization of a Potato Collection with Contrasting Tuber Carotenoid Content. PLoS ONE 2017, 12, e0184143. [Google Scholar] [CrossRef]
- Destefano-Beltrán, L.; Knauber, D.; Huckle, L.; Suttle, J.C. Effects of Postharvest Storage and Dormancy Status on ABA Content, Metabolism, and Expression of Genes Involved in ABA Biosynthesis and Metabolism in Potato Tuber Tissues. Plant Mol. Biol. 2006, 61, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; Welsch, R.; Tavazza, R.; Mourgues, F.; Pizzichini, D.; Beyer, P.; Giuliano, G. Silencing of Beta-Carotene Hydroxylase Increases Total Carotenoid and Beta-Carotene Levels in Potato Tubers. BMC Plant Biol. 2007, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.M.; Hackett, C.A.; Voorrips, R.E.; Visser, R.G.F.; Maliepaard, C. Quantifying the Power and Precision of QTL Analysis in Autopolyploids Under Bivalent and Multivalent Genetic Models. G3 Genes|Genomes|Genetics 2019, 7, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, I.; Ngounou Wetie, A.G.; Roy, U.; Woods, A.G.; Darie, C.C. Mass Spectrometry Investigation of Glycosylation on the NXS/T Sites in Recombinant Glycoproteins. Biochim. Biophys. Acta 2013, 1834, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Wang, C.; Chen, L.; Liu, H.; Yang, G.; He, G. Expression of Phytoene Synthase1 and Carotene Desaturase CrtI Genes Result in an Increase in The Total Carotenoids Content in Transgenic Elite Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2009, 57, 8652–8660. [Google Scholar] [CrossRef] [PubMed]
- Werij, J.S.; Furrer, H.; van Eck, H.J.; Visser, R.G.F.; Bachem, C.W.B. A Limited Set of Starch Related Genes Explain Several Interrelated Traits in Potato. Euphytica 2012, 186, 501–516. [Google Scholar] [CrossRef]
- Hussen, E.S.; Mohammed, W.; Abebe, T. Genetic Diversity in Potato (Solanum tuberosum L.) Genotypes for Yield and Processing Attributes at Holetta, Central Highlands of Ethiopia. East Afr. J. Agric. Biotechnol. 2020, 2, 34–50. [Google Scholar] [CrossRef]
- Workayehua, M.; Mohammed, W.; Abebe, T.; Derebe, B. Estimation of Genetic Distance among Potato (Solanum tuberosum L.) Crosses in Ethiopia. Abyss. J. Sci. Technol. 2021, 6, 23–32. Available online: https://abjol.org.et/index.php/ajst/article/view/215 (accessed on 9 August 2024).
- Wassu, M. Genotype X Environment Interaction, Stability and Coheritability of Tuber Internal Quality Traits in Potato (Solanum Tuberosum L.) Cultivars in Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12930–12952. [Google Scholar] [CrossRef]
- Masson, M.F. Mapping, Combining Abilities, Heritabilities and Heterosis with 4x X 2x Crosses in Potato (Haploids, Gametes, Species, Solanum, Tubers). PhD Thesis, The University of Wisconsin-Madison, Madison, WI, USA, 1985. [Google Scholar]
- De Jong, H.; Burns, V.J. Inheritance of Tuber Shape in Cultivated Diploid Potatoes. Am. Potato J. 1993, 70, 267–284. [Google Scholar] [CrossRef]
- Van Eck, H.J.; Jacobs, J.M.; Stam, P.; Ton, J.; Stiekema, W.J.; Jacobsen, E. Multiple Alleles for Tuber Shape in Diploid Potato Detected by Qualitative and Quantitative Genetic Analysis Using RFLPs. Genetics 1994, 137, 303–309. [Google Scholar] [CrossRef] [PubMed]
- van Eck, H.J. Genetics of Morphological and Tuber Traits. In Potato Biology and Biotechnology; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Mackerron, D.K.L., Taylor, M.A., Ross, H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 91–115. [Google Scholar] [CrossRef]
- Wolters, A.M.A.; Uitdewilligen, J.G.A.M.L.; Kloosterman, B.A.; Hutten, R.C.B.; Visser, R.G.F.; van Eck, H.J. Identification of Alleles of Carotenoid Pathway Genes Important for Zeaxanthin Accumulation in Potato Tubers. Plant Mol. Biol. 2010, 73, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.; Zhang, S.; Yu, Z.; Li, J.; Jin, X.; Zhang, X.; Yang, D. Identification of Starch Candidate Genes Using SLAF-Seq and BSA Strategies and Development of Related SNP-CAPS Markers in Tetraploid Potato. PLoS ONE 2021, 16, e0261403. [Google Scholar] [CrossRef] [PubMed]

| Name | Origin | Maturity Type | Tuber Shape | Flesh Color | References |
|---|---|---|---|---|---|
| Yagodnyi 19 | North-West Scientific and Production Center of Agriculture, Kazakhstan | Medium early | Round oval | White | [50] |
| CIP 397079-6 | Clonal selection cross between “386768.10” and “392820.1”, International Potato Center (CIP), Peru | Early | Oblong | White creme | [51] |
| Fontane | Svalof Weibull BV, Netherlands | Early | Oval to long oval | Medium yellow | [52] |
| Santana | HZPC Holland BV, Netherlands | Medium early | Big long oval | Crème | [53] |
| Punchy | Germicopa, France | Medium early | Round | Yellow | [54] |
| Name | Primer sequence (5′-3′) | Product Length (bp) | Reference |
|---|---|---|---|
| Early maturity | |||
| SCARA2-2 | ACAGCTCGGCGAGAAAACAG TCAAGCAATTAGGGCGGTG | 500 | Hui et al., 2022 [8] |
| SCARA4-21 | TCACTTTGGCGACCACACTT CAGACCCGCTTACGCTAAGAT | 700 | Hui et al., 2022 [8] |
| SCARA5-16 | TTTTTGTGATCAGGGGCGG TGCATTGCATCCTCCCAAC | 100 | Hui et al., 2022 [8] |
| Tuber shape | |||
| SCAR14S6 | CTTTTTTATGCTCTTTCATACCTAAC CAAAAATTGTCACAACTTATATACTGAC | 570 | Chen et al., 2019 [18] |
| SCAR14S15 | TTCGATGGAGTATATTAGTCAGAGG GCAGAGACGAAACTAGAATTTCAAA | 750 | Chen et al., 2019 [18] |
| SCAR17S9 | CAGGCTACCGCCATTTTTAC TTTCACATCTCACAAAGTTTAGCAAT | 700 | Chen et al., 2019 [18] |
| SCAR20S2 | ATTAGACTTGTCTGTAAATGTGAGTAAA CTCTAGGAGATAGCCTAGAACCTAAAT | 850 | Chen et al., 2019 [18] |
| SCAR26S35 | CGTGGCATATTTAAGACGACG GAATCAATAGTTAGGACAAATGAATTG | 300 | Chen et al., 2019 [18] |
| Flesh color | |||
| AWZEP25/AWZEP20 | CTGGCTGCATCACTGGTCAAAG TCATTCATAATTGTATCCTCCC | 572 | Sulli et al., 2017 [56] |
| StZEP_RT_F/StZEP_RT_R | AAGTGCCGAGTCAGGAAGCC CAAGTCCGACGCCAAGATAAGC | 2046 | Destefano-Beltrán et al., 2006 [57] |
| AS-chy | TAGAGCTCGGGATTACTTC ATGGATCCTCCTTTTCCAA | 2900 | Diretto et al., 2007 [58] |
| Chy1 | CTTGGCCCAAAACCCACTT CCTCAAATTGAGGTTTCAGCTTCT | 152 | Goo et al., 2015 [19] |
| Chy2 | TTTTGCTGTCTCGAAGAAAGCC AGCCAACAGGCAGCTAAACTCT | 148 | Goo et al., 2015 [19] |
| StChy2 | CGAGATGGGCTCATAGAGCACT GAAAGTAAGGCACGTTGGCAAT | 4390 | Bourke et al., 2019 [59] |
| Nxs | CTTGGAGGAGACTTCTTTGGTGA CGGAAGTGGTCCTCCCATAG | 100 | Sokolowska et al., 2013 [60] |
| Psy1 | CGGTCTGCTATTGTTGCTACTCC CAGGAACAGGTATGTCTGGCTTC | 141 | Goo et al., 2015 [19] |
| Psy2 | AGCTTTAGATAGGTGGGAGGCA CAAGTCCATACGCATTCCTTCAA | 162 | Goo et al., 2015 [19] |
| PDS | AGAGACTTTGCATGCCGATTGT AAAGCATCGCCCTCAACTGT | 151 | Goo et al., 2015 [19] |
| ZDS | TTGCCATGTCAAAGGCCA ACAGGCACTCCGACCAATTT | 141 | Goo et al., 2015 [19] |
| CrtISO | TTGGCAGCAGTAGGACGTAAAC TCCCTTCCTTTTCATGTGGAA | 151 | Goo et al., 2015 [19] |
| Lcy-e | GCCAAAATGGATGTGGCAG CAATGTTGCACCAGTAGGATCAG | 151 | Goo et al., 2015 [19] |
| Lut1 | CGTTCTCCGCCCAAAAAAC TTGGCCTAAAGTAAGTGACCTGG | 140 | Goo et al., 2015 [19] |
| Lcy-b | AATGGGTGGTCCACTTCCAGTA GGATGGATGAACCATGCCAG | 76 | Goo et al., 2015 [19] |
| CrtI | GCGACCAGTAGCATCTAC GTTAGATGCCACGGCTTG | 623 | Cong et al., 2009 [61] |
| Starch-related genes | |||
| AGPaseS | AAGCCTAATATCTGCATGTCA GAGCACATCTTCTATGTCCTT | 500 | Werij et al., 2012 [62] |
| SSSIII | AACAAAAGTTCAGGTCCTCTCTC AAATCCCACCATCTTCTCTCTC | 1500 | Werij et al., 2012 [62] |
| StPho1b | ACACACTATGTTCTGCTTCTCTTC ACTATCCTCCACCTCAACCTTC | 6000 | Werij et al., 2012 [62] |
| StPho2 | GCATACTATGCTGCTACTGCTG GCACATCATATGCAAGAGCCTG | 850 | Werij et al., 2012 [62] |
| StUCP | GAACCCTTTTAGTTTCTCTTT TGCCAACAGTACCTAATAATC | 1300 | Werij et al., 2012 [62] |
| Traits | Molecular Markers | Fontane | Santana | Punchy | Yagodnyi 19 | CIP 397079-6 |
|---|---|---|---|---|---|---|
| Maturity type | SCARA2-2 | 0 * | 1 * | 1 | 1 | 0 |
| SCARA4-21 | 0 | 1 | 1 | 0 | 0 | |
| SCARA5-16 | 0 | 1 | 1 | 0 | 0 | |
| Tuber shape | SCAR14S6 | 0 | 0 | 1 | 1 | 0 |
| SCAR17S9 | 0 | 0 | 1 | 1 | 0 | |
| SCAR26S35 | 0 | 0 | 1 | 1 | 0 | |
| Flesh color | AWZEP25/AWZEP20 | 0 | 0 | 1 | 0 | 0 |
| AS-chy | 0 | 0 | 1 | 0 | 0 | |
| CHY2 | 0 | 1 | 1 | 0 | 1 | |
| CrtISO | 1 | 0 | 1 | 0 | 0 | |
| Lcy-e | 0 | 1 | 1 | 0 | 0 | |
| Lcy-b | 1 | 1 | 1 | 0 | 0 | |
| Starch-related genes | AGPaseS | 0 | 0 | 1 | 0 | 1 |
| SSSIII | 0 | 0 | 1 | 0 | 0 | |
| StPho1b | 0 | 1 | 1 | 0 | 0 | |
| StPho2 | 0 | 0 | 1 | 0 | 0 | |
| StUCP- | 0 | 0 | 1 | 0 | 0 |
| Name of the Breeding Lines | Maturity Type | Tuber Shape | Starch-Related Genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCARA202 | SCARA4021 | SCARA5016 | SCAR14S6 | SCAR14S15 | SCAR17S9 | SCAR20S2 | SCAR26S35 | AGPaseS | SSSIII | StPho1b | StPho2 | StUCP0 | |
| YF-1 | 1 * | 0 * | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| YF-2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| YF-3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| YF-4 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| YF-5 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| YF-6 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| YF-7 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| YF-8 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| YF-9 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| YF-10 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| YF-11 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| YF-12 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| YF-13 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| YF-14 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| YF-15 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| YF-16 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| YF-17 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| YF-18 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| YF-19 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| YF-20 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| YF-21 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| YF-22 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| YF-23 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| YF-24 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| YF-25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| YF-26 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| YF-27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| YF-28 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| YF-29 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| YF-30 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| YF-31 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| YF-32 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| YF-33 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| YF-34 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| YF-35 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| YF-36 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| YF-37 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| YF-38 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| YF-39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| YF-40 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| YF-41 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| YF-42 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Name of the Breeding Lines | AWZEP20/AWZEP25 | StZEP_RT | AS0chy | Chy1 | Chy2 | StChy2 | Nxs | Psy1 | Psy2 | PDS | ZDS | Crtlso | Lcy0e | Lut1 | Lcy0b | CrtI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YF-1 | 1 * | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| YF-2 | 0 * | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| YF-3 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| YF-4 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| YF-5 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| YF-6 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| YF-7 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| YF-8 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| YF-9 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| YF-10 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| YF-11 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| YF-12 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| YF-13 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| YF-14 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| YF-15 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| YF-16 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| YF-17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| YF-18 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| YF-19 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| YF-20 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| YF-21 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| YF-22 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| YF-23 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| YF-24 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| YF-25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| YF-26 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| YF-27 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| YF-28 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| YF-29 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| YF-30 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| YF-31 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| YF-32 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| YF-33 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| YF-34 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| YF-35 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| YF-36 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| YF-37 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| YF-38 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| YF-39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| YF-40 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| YF-41 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| YF-42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
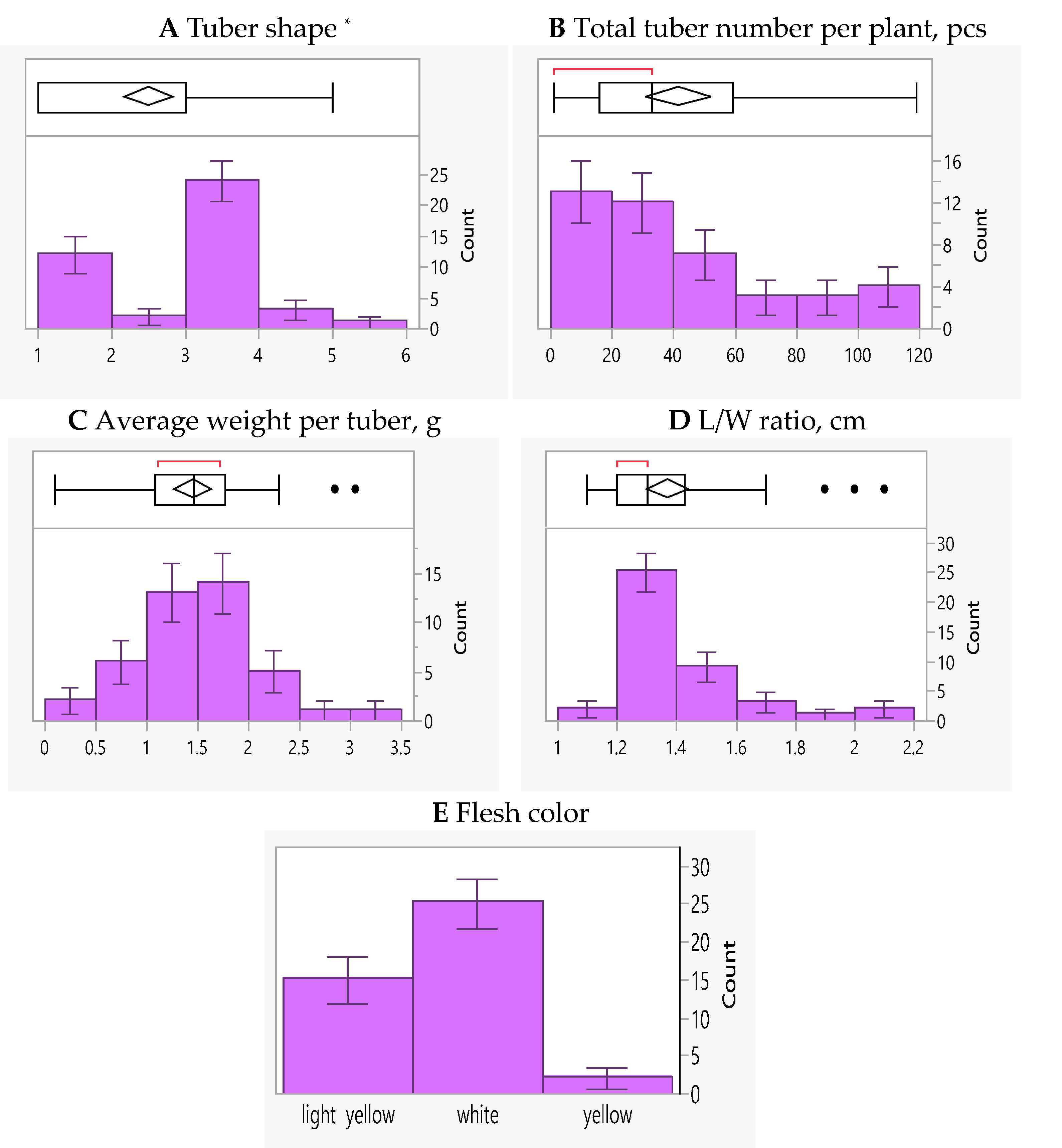
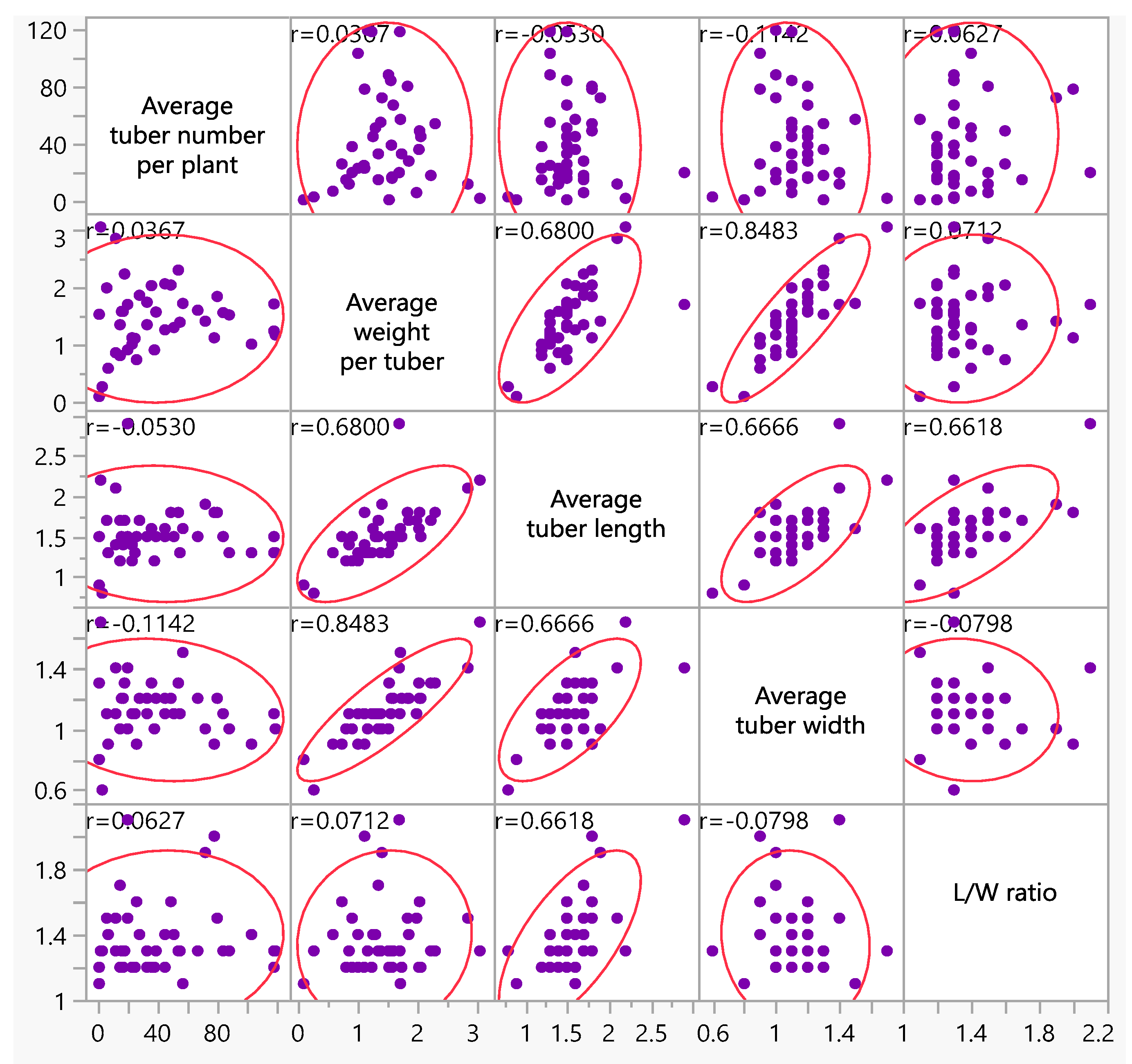
| Breeding Lines | Tuber Shape | Total Tuber Number per Plant | Average Tuber Weight per Plant | L/W Ratio | Flesh Color |
|---|---|---|---|---|---|
| BLUE | 2.50 | 41.71 | 1.45 | 1.37 | 1.93 |
| Range | 0.83–5.00 | 0.90–118.92 | 0.09–3.05 | 1.04–2.08 | 0.89–4.08 |
| SD | 1.07 | 33.79 | 0.61 | 0.23 | 1.12 |
| CV | 42.86 | 81.02 | 41.77 | 16.77 | 57.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapakhova, Z.; Abilda, Z.; Toishimanov, M.; Daurov, D.; Daurova, A.; Raissova, N.; Sidorik, A.; Kanat, R.; Zhambakin, K.; Shamekova, M. Early Generation Selection of Potato Breeding Lines. Horticulturae 2024, 10, 1121. https://doi.org/10.3390/horticulturae10101121
Sapakhova Z, Abilda Z, Toishimanov M, Daurov D, Daurova A, Raissova N, Sidorik A, Kanat R, Zhambakin K, Shamekova M. Early Generation Selection of Potato Breeding Lines. Horticulturae. 2024; 10(10):1121. https://doi.org/10.3390/horticulturae10101121
Chicago/Turabian StyleSapakhova, Zagipa, Zhanar Abilda, Maxat Toishimanov, Dias Daurov, Ainash Daurova, Nurgul Raissova, Alexander Sidorik, Rakhim Kanat, Kabyl Zhambakin, and Malika Shamekova. 2024. "Early Generation Selection of Potato Breeding Lines" Horticulturae 10, no. 10: 1121. https://doi.org/10.3390/horticulturae10101121
APA StyleSapakhova, Z., Abilda, Z., Toishimanov, M., Daurov, D., Daurova, A., Raissova, N., Sidorik, A., Kanat, R., Zhambakin, K., & Shamekova, M. (2024). Early Generation Selection of Potato Breeding Lines. Horticulturae, 10(10), 1121. https://doi.org/10.3390/horticulturae10101121






