Application of Ethephon Manually or via Drone Enforces Bud Dormancy and Enhances Flowering Response to Chilling in Litchi (Litchi chinensis Sonn.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Plant Materials for the Study
2.2. Treatments
2.3. Bud Status Observation
2.4. Leaf Drop Observation
2.5. Net Photosynthetic Rate Measurement
2.6. LcFT1 Expression Analysis
2.7. Statistics
3. Results and Analysis
3.1. Effects of Manually Spraying Ethephon at Different Concentrations
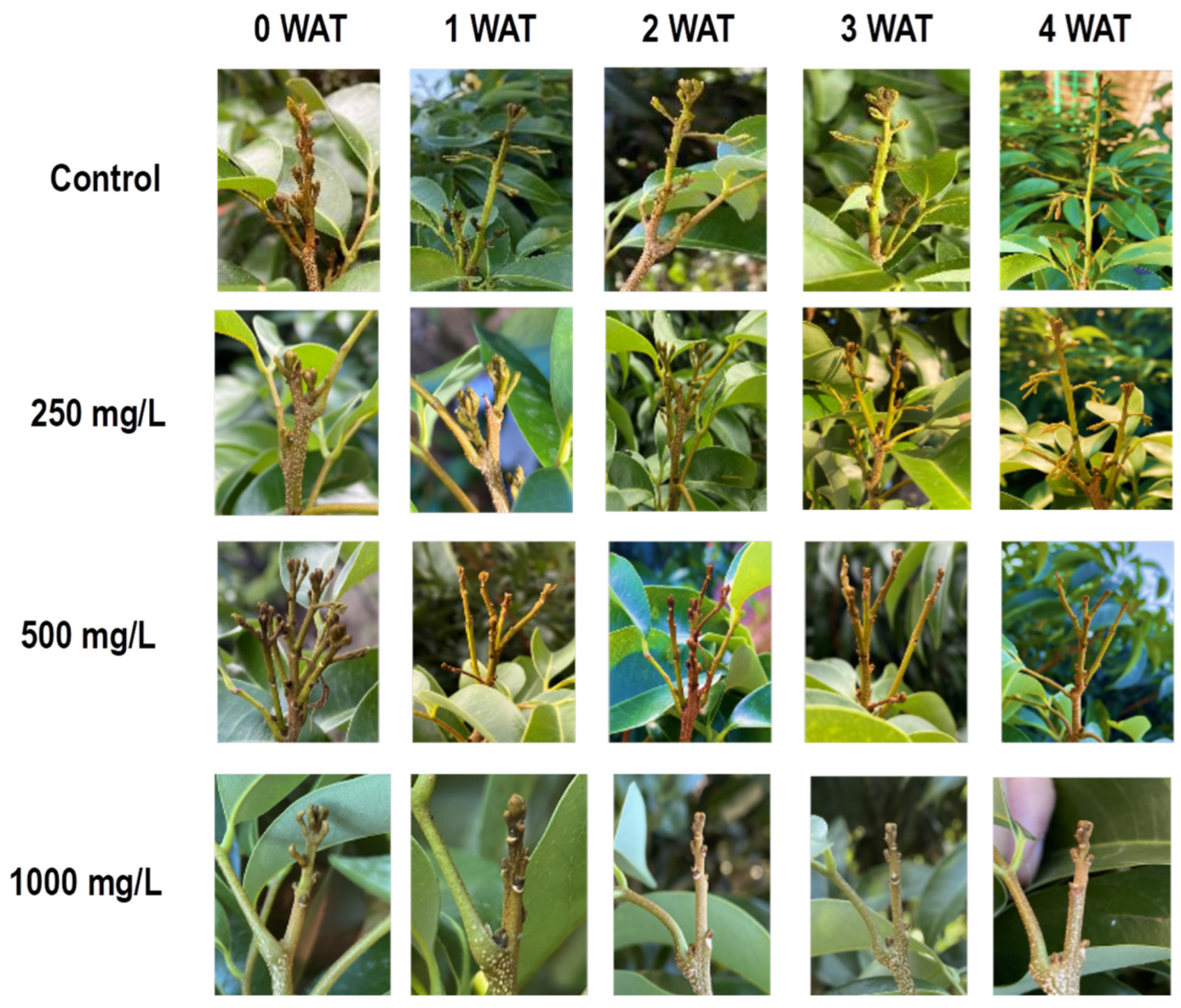
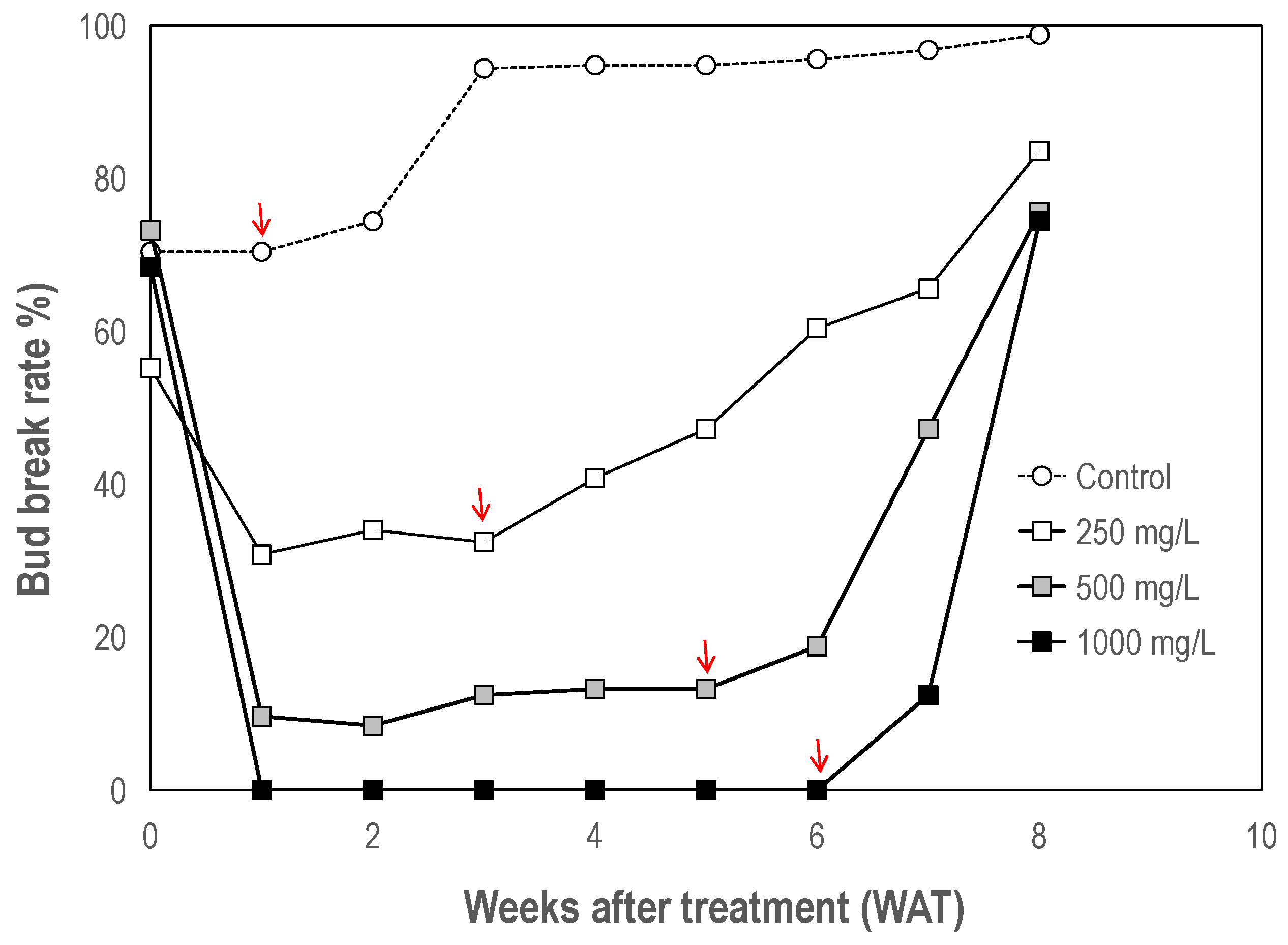
3.1.1. Effect on Bud Growth
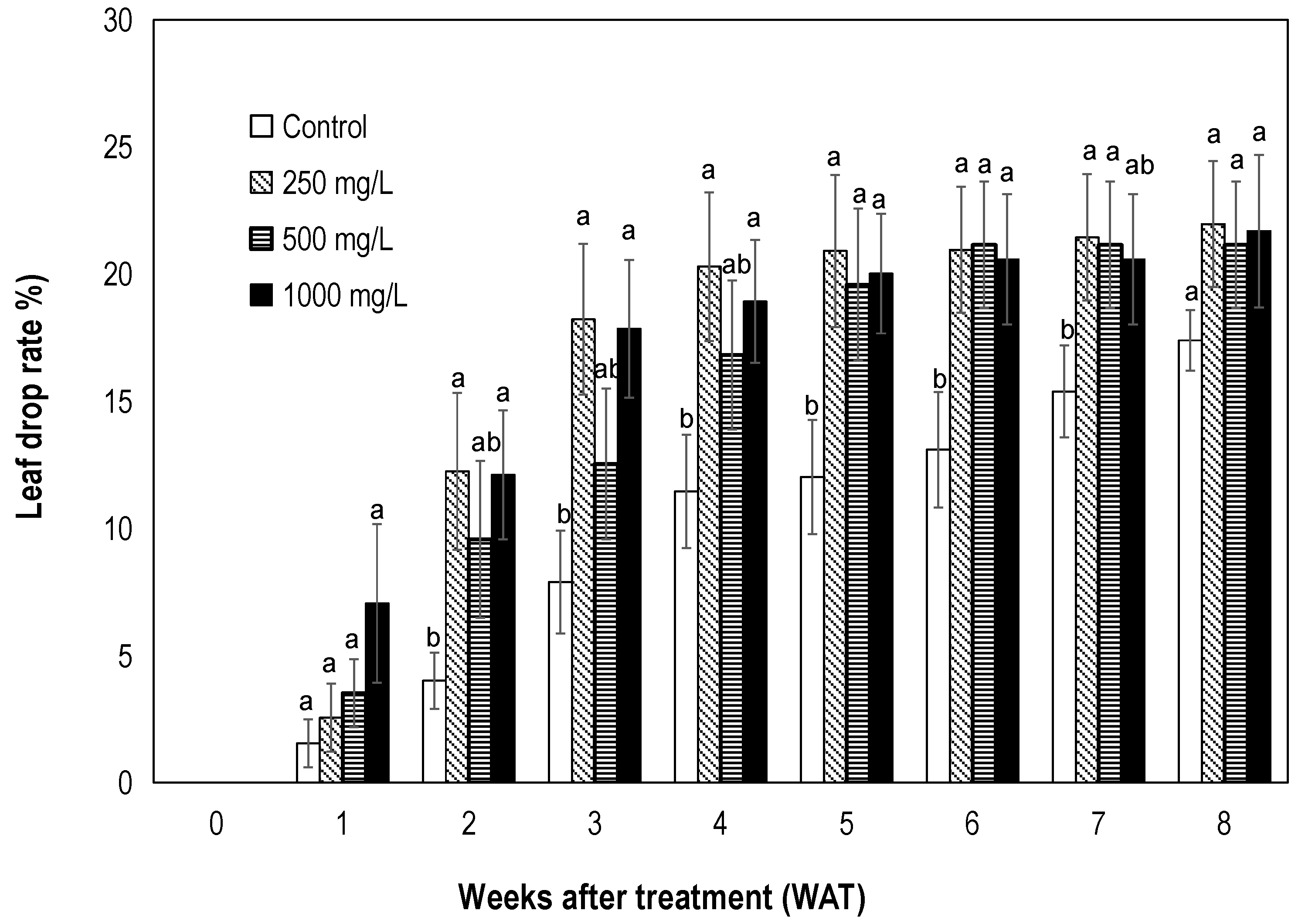
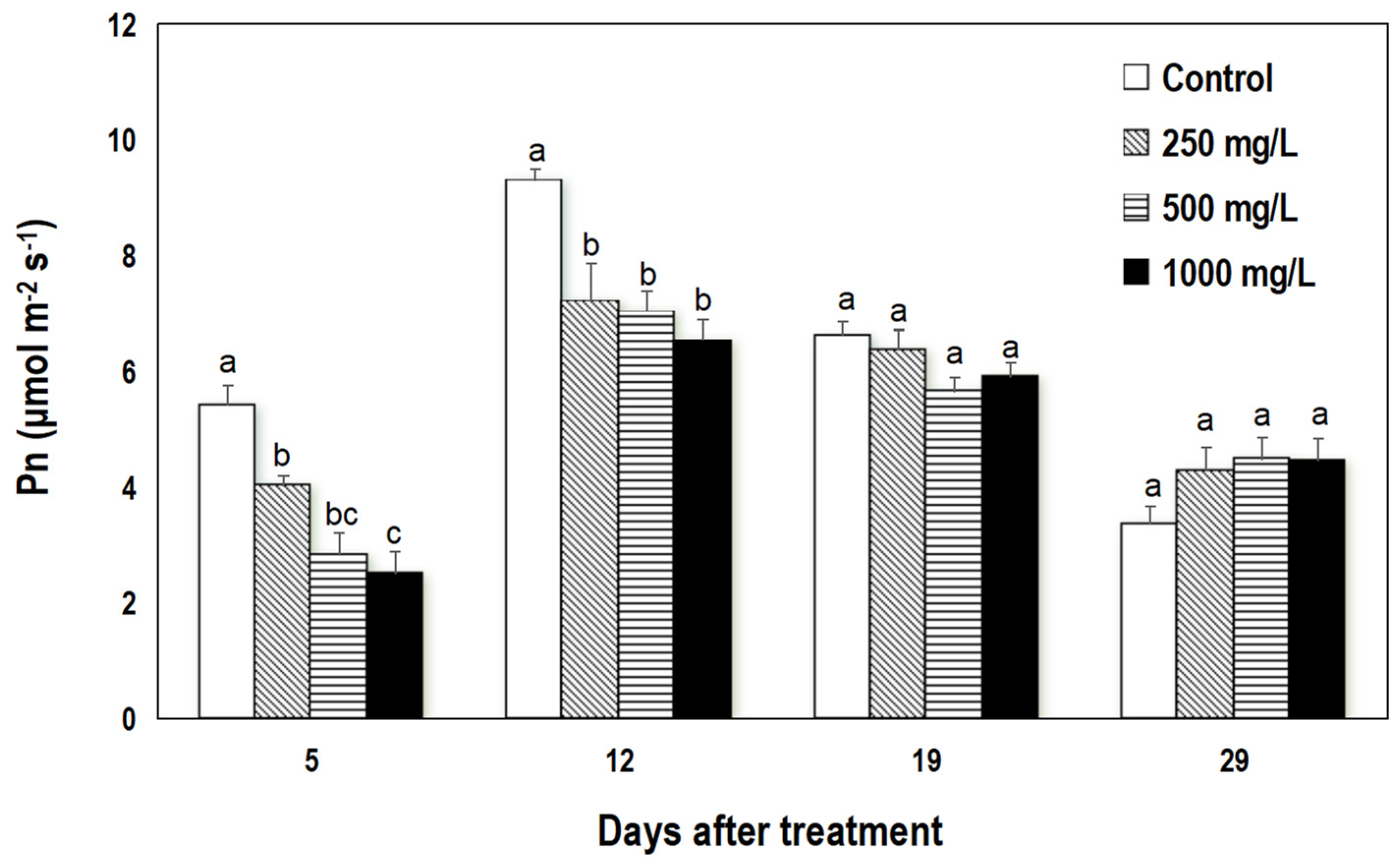
3.1.2. Effect on Leaf Drop and Net Photosynthetic Rate
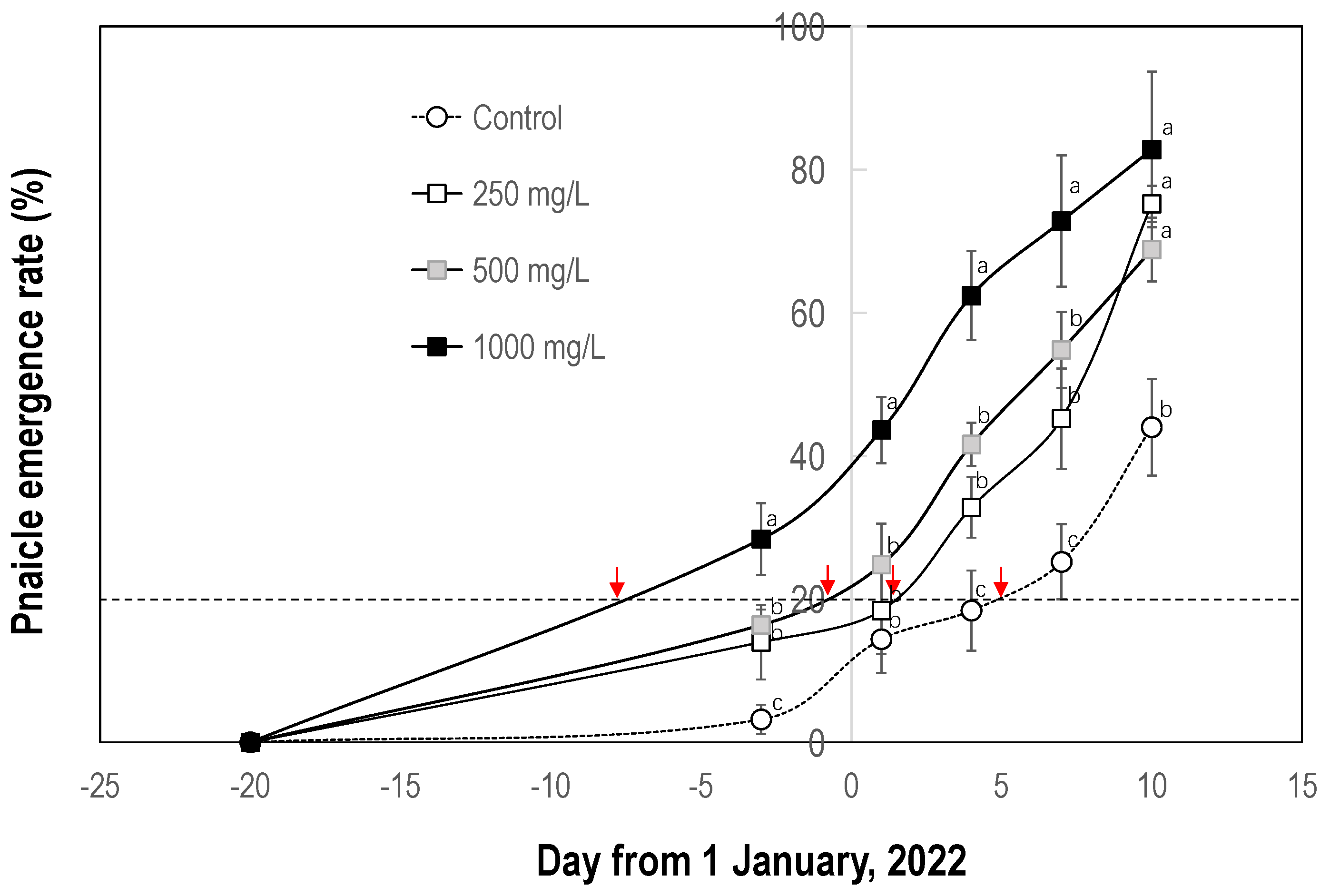
3.1.3. Effect on Floral Characters
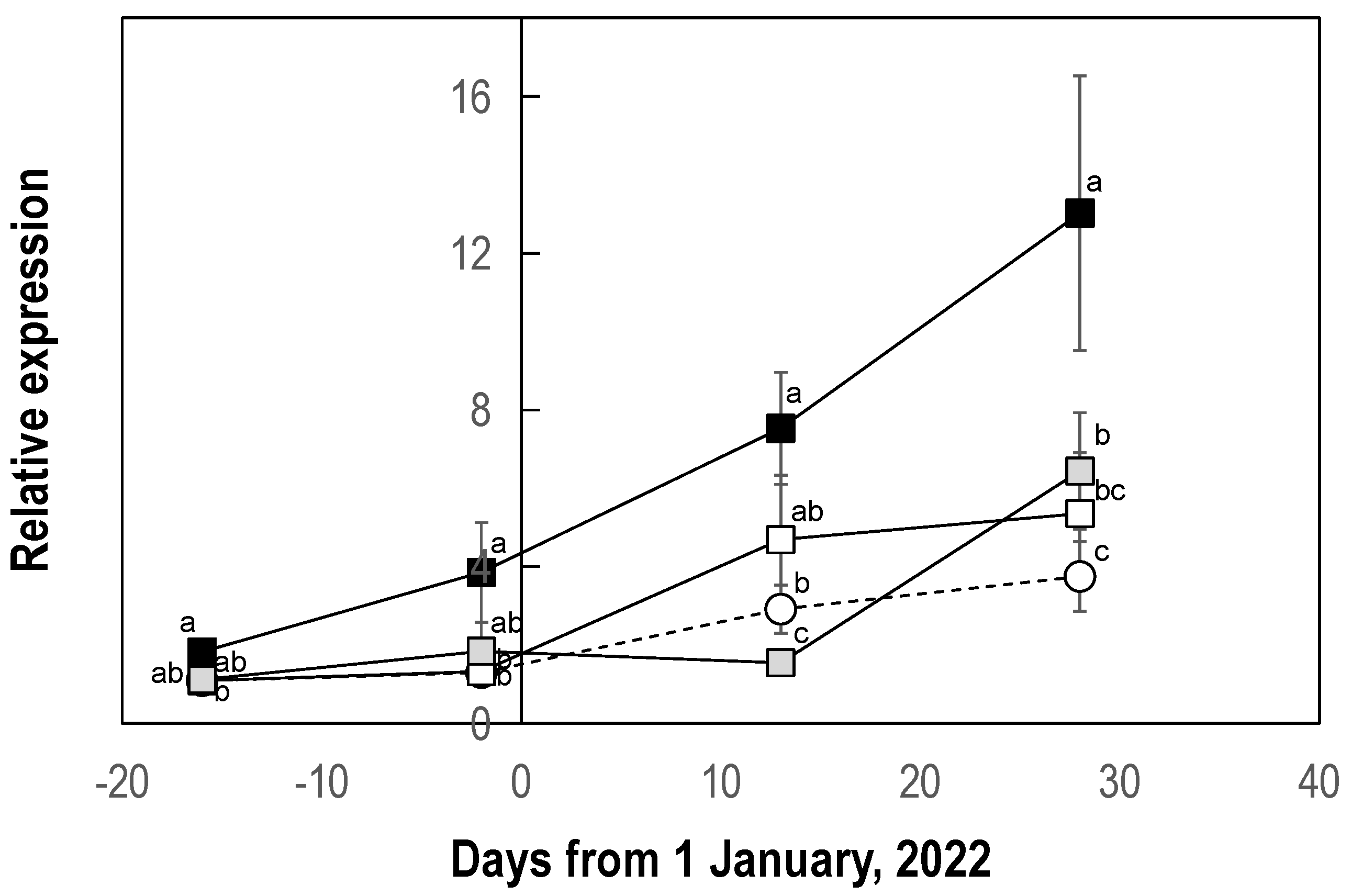
3.1.4. Effect on Expression of the Florigen Gene LcFT1
3.2. Trial of Spraying 1000 mg/L of Ethephon with a Drone
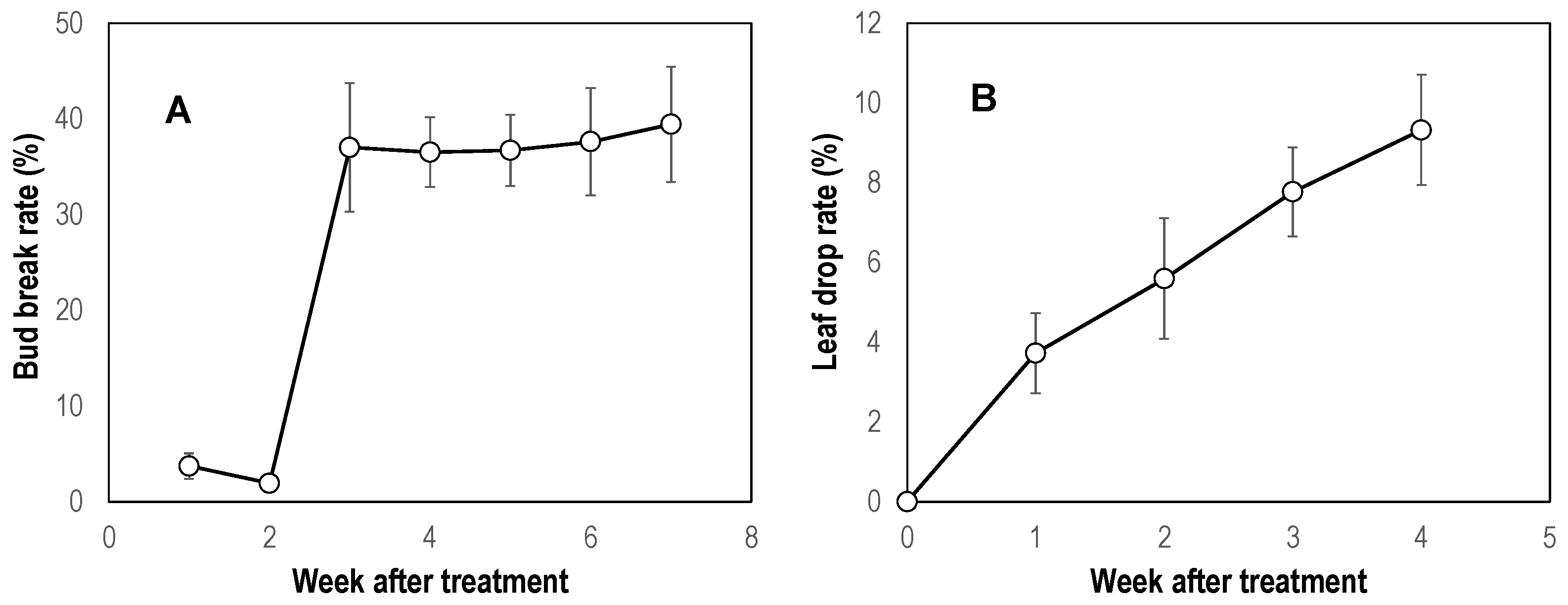
3.2.1. Effect on Bud Break and Leaf Drop
3.2.2. Effect on Flowering
4. Discussion
4.1. Ethephon Enforces Bud Dormancy in Litchi
4.2. Ethephon Causes Leaf Drop and a Short-Term Reduction in Photosynthesis
4.3. Ethephon Enhances the Flowering Response to Chilling in Litchi
4.4. Spraying Ethephon with a Drone for Flush Management Is Highly Feasible
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Menzel, C.M.; Simpson, D.R. Effect of temperature on growth and flowering of litchi (Litchi chinensis Sonn.) cultivars. J. Hortic. Sci. Biotechnol. 1988, 63, 349–360. [Google Scholar] [CrossRef]
- Chen, H.B.; Huang, H.B. Low temperature requirements for floral induction in lychee. Acta Hortic. 2005, 665, 195–202. [Google Scholar] [CrossRef]
- Huang, X.B.; Chen, H.B. Studies on shoot, flower and fruit development in litchi and strategies for improved litchi production. Acta Hortic. 2014, 1029, 127–136. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, J.; Wei, Y.; Chen, H. Transcriptome profiling of litchi leaves in response to low temperature reveals candidate regulatory genes and key metabolic events during floral induction. BMC Genom. 2017, 18, 363. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, S.; Chen, H.; Su, Z.; Zhang, R.; Xiao, Q.; Li, H. Promoter difference of LcFT1 is a leading cause of natural variation of flowering timing in different litchi cultivars (Litchi chinensis Sonn.). Plant Sci. 2015, 241, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Studies of Floral Induction and Initiation in Litchi (Litchi chinensis Sonn.). Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2014. [Google Scholar]
- Yang, M.C.; Wu, Z.C.; Chen, R.Y.; Abbas, F.; Hu, G.B.; Huang, X.M.; Guan, W.S.; Xu, Y.S.; Wang, H.C. Single-nucleus RNA sequencing and mRNA hybridization indicate key bud events and LcFT1 and LcTFL1-2 mRNA transportability during floral transition in litchi. J. Exp. Bot. 2023, 74, 3613–3629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Li, H.; Lai, B.; Xia, H.Q.; Wang, H.C.; Huang, X.M. Morphological characterization and gene expression profiling during bud development in a tropical perennial, Litchi chinensis Sonn. Front. Plant Sci. 2016, 7, 1517. [Google Scholar] [CrossRef]
- Fu, X.Y.; Mo, W.P.; Zhang, J.Y.; Zhou, L.Y.; Wang, H.C.; Huang, X.M. Shoot growth pattern and quantifying flush maturity with SPAD value in litchi (Litchi chinensis Sonn.). Sci. Hortic. 2014, 174, 29–35. [Google Scholar] [CrossRef]
- Batten, D.; Mcconchie, C. Floral induction in growing buds of lychee (Litchi chinensis) and mango (Mangifera indica). Aust. J. Plant Physiol. 1995, 22, 783–791. [Google Scholar] [CrossRef]
- Chen, H.; Huang, H.; Liu, Z. Flower formation and patterns of carbohydrate distribution in litchi trees. Acta Hortic. Sin. 2004, 31, 1–6. [Google Scholar]
- Cronje, R.B.; Ratlapane, I.M.; Rohwer, E.A.; Hoffman, E.W.; Huang, X.M. Carbohydrate reserve dynamics as influenced by shoot control strategies and climatic conditions prior to flowering in ‘Mauritius’ litchi. Acta Hortic. 2020, 1293, 155–165. [Google Scholar] [CrossRef]
- Menzel, C.M.; Rasmussen, T.S.; Simpson, D.R. Carbohydrate reserves in lychee trees (Litchi chinensis Sonn.). J. Hortic. Sci. 1995, 70, 245–255. [Google Scholar] [CrossRef]
- Yang, H.F.; Kin, H.J.; Chen, H.B.; Rahman, J.; Lu, X.Y.; Zhou, B.Y. Carbohydrate accumulation and flowering-related gene expression levels at different developmental stages of terminal shoots in Litchi chinensis. Hortic. Sci. 2014, 49, 1381–1391. [Google Scholar] [CrossRef]
- Cronje, R.B.; Hajari, E.; Jonker, A.; Ratlapane, I.M.; Huang, X.; Theron, K.I.; Hoffman, E.W. Foliar application of ethephon induces bud dormancy and affects gene expression of dormancy- and flowering-related genes in ‘Mauritius’ litchi (Litchi chinensis Sonn.). J. Plant Physiol. 2022, 276, 153768. [Google Scholar] [CrossRef]
- De Greef, J.A.; De Proft, M.P.; Mekers, O.; Van Dijck, R.; Jacobs, L.; Philippe, L. Floral Induction of Bromeliads by Ethylene. In Biochemical and Physiological Aspects of Ethylene Production in Lower and Higher Plants; Clijstersv, H., Proft, M., Marcelle, R., Poucke, M., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1989; pp. 313–322. [Google Scholar]
- Bukovac, M.J.; Sabbatini, P.; Schwallier, P.G. Modifying alternate bearing of spur-type ‘Delicious’ apple with ethephon. HortScience 2006, 41, 1606–1611. [Google Scholar] [CrossRef]
- Pasa, M.D.S.; Carra, B.; Brighenti, A.F.; Pinto, F.A.M.F.; de Mello-Farias, P.C.; Herter, F.G. Ethephon as a potential tool to manage alternate bearing of ‘Fuji’ apple trees. Rev. Ceres 2021, 68, 180–184. [Google Scholar] [CrossRef]
- Hayashi, T.; Heins, R.D.; Cameron, A.C.; Carlson, W.H. Ethephon influences flowering, height, and branching of several herbaceous perennials. Sci. Hortic. 2001, 91, 305–324. [Google Scholar] [CrossRef]
- Elfving, D.C.; Lang, G.A.; Visser, D.B. Prohexadione-Ca and ethephon reduce shoot growth and increase flowering in young, vigorous sweet cherry trees. HortScience 2003, 38, 293–298. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.Q.; Wang, G.; Liang, Z.Q. Techniques in flush control and flower promotion in litchi. China Trop. Agric. 2012, 46, 82–83. (In Chinese) [Google Scholar]
- Cronje, R.B.; Ratlapane, I.M. Effect of full-cover ethephon applications on flowering and yield of ‘Mauritius’ litchi in South Africa. Acta Hortic. 2018, 1211, 71–78. [Google Scholar] [CrossRef]
- Khan, N.A. An evaluation of the effects of exogenous ethephon, an ethylene releasing compound, on photosynthesis of mustard (Brassica juncea) cultivars that differ in photosynthetic capacity. BMC Plant Biol. 2004, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, L.X.; Wang, Y.B.; Han, L.B. Ethephon treatment reduced Mondo grass (Ophiopogon japonicus) gas exchange rate and gene expression of Rbcs. Euro. J. Hortic. Sci. 2019, 84, 106–112. [Google Scholar] [CrossRef]
- Li, K.T.; Burns, J.K.; Syvertsen, J.P. Recovery from phytotoxicity after foliar application of fruit-loosening abscission compounds to citrus. J. Am. Soc. Hortic. Sci. 2008, 133, 535–541. [Google Scholar] [CrossRef]
- Chen, H. Studies on Flower Induction and Differentiation in Litchi Chinensis Sonn. with Emphasis on Their Relations to Temperature. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2002. [Google Scholar]
- Liu, J.; Sherif, M. Hormonal orchestration of bud dormancy cycle in deciduous woody perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 2014, 5, 39. [Google Scholar] [CrossRef]
- Ophir, R.; Pang, X.; Halaly, T.; Venkateswari, J.; Lavee, S.; Galbraith, D.; Or, E. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Mol. Biol. 2009, 71, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Halaly-Basha, T.; Zheng, C.; Weissberg, M.; Ophir, R.; Galbraith, D.W.; Pang, X.; Or, E. Transient induction of a subset of ethylene biosynthesis genes is potentially involved in regulation of grapevine bud dormancy release. Plant Mol. Biol. 2018, 98, 507–523. [Google Scholar] [CrossRef]
- Ruttink, T.; Arend, M.; Morreel, K.; Storme, V.; Rombauts, S.; Fromm, J.; Bhalerao, R.P.; Boerjan, W.; Rohde, A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 2007, 19, 2370–2390. [Google Scholar] [CrossRef]
- Pan, W.; Liang, J.; Sui, J.; Li, J.; Liu, C.; Xin, Y.; Zhang, Y.; Wang, S.; Zhao, Y.; Zhang, J.; et al. ABA and bud dormancy in perennials: Current knowledge and future perspective. Genes 2021, 12, 1635. [Google Scholar] [CrossRef]
- Suttle, J.C. Physiological regulation of potato tuber dormancy. Am. J. Potato Res. 2004, 81, 253–262. [Google Scholar] [CrossRef]
- Ruonala, R.; Rinne, P.L.H.; Baghour, M.; Moritz, T.; Tuominen, H.; Kangaja¨rvi, J. Transitions in the functioning of the shoot apical meristem in birch (Betula pendula) involve ethylene. Plant J. 2006, 46, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, K.; Narumi, T.; Satoh, S.; Hisamatsu, T. Involvement of the ethylene response pathway in dormancy induction in chrysanthemum. J. Exp. Bot. 2008, 59, 4075–4082. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, T.; Zhuo, X.; Zhang, M.; Yong, X.; Li, L.; Wang, J.; Cheng, T.; Zhang, Q. Photoperiod- and temperature-mediated control of the ethylene response and winter dormancy induction in Prunus mume. Hortic. Plant J. 2021, 7, 232–242. [Google Scholar] [CrossRef]
- Durner, E.F.; Gianfagna, T.J. Ethephon prolongs dormancy and enhances supercooling in peach flower buds. J. Am. Soc. Hortic. Sci. 1991, 116, 500–506. [Google Scholar] [CrossRef]
- Liu, J.Y.; Islam, M.T.; Laliberte, S.; Haak, D.C.; Sherif, S.M. Fall applications of ethephon modulates gene networks controlling bud development during dormancy in peach (Prunus persica). Int. J. Mol. Sci. 2022, 23, 6801. [Google Scholar] [CrossRef]
- Ma, M.M.; Zhang, H.F.; Tian, Q.; Wang, H.C.; Zhang, F.Y.; Tian, X.; Zeng, R.F.; Huang, X.M. MIKC type MADS-box transcription factor LcSVP2 is involved in dormancy regulation of the terminal buds in evergreen perennial litchi (Litchi chinensis Sonn). Hortic. Res. 2024, 11, uhae150. [Google Scholar] [CrossRef]
- Burns, J.K.; Ferguson, L.; Glozer, K.; Krueger, W.H.; Rosecrance, R.C. Screening fruit loosening agents for black ripe processed table olives. HortScience 2008, 43, 1449–1453. [Google Scholar] [CrossRef]
- Dai, Y.W. A Study on Sensitive Difference of Flush and Panicle at Different Developmental Stages to Ethylene in Litchi and Related Physiological Mechanisms. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2016. [Google Scholar]
- Tian, Q. A Study on the Sensitivity of ‘Guiwei’ Shoots to Ethylene and Exploration of Using Ethephon for Flush Control and Flower Promotion. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2024. [Google Scholar]
- Woodrow, L.; Jiao, J.; Tsujita, M.J.; Grodzinski, B. Whole plant and leaf steady state gas exchange during ethylene exposure in Xanthium strumarium L. Plant Physiol. 1989, 90, 85–90. [Google Scholar] [CrossRef]
- Subhadrabandhu, S.; Koo-Duang, A. Effect of ethephon on flowering of two lychee (Litchi chinensis Sonn.) cultivars. Acta Hortic. 1987, 201, 181–186. [Google Scholar] [CrossRef]
- Hafeez, A.; Husain, M.A.; Singh, S.P.; Chauhan, A.; Khan, M.T.; Kumar, N.; Chauhan, A.; Soni, S.K. Implementation of drone technology for farm monitoring & pesticide spraying: A review. Inf. Process. Agric. 2023, 10, 192–203. [Google Scholar] [CrossRef]


| Gene | Sequence (F: Upper Stream Primer; R: Lower Stream Primer) |
|---|---|
| LcFT1 | F: CAAAGAATTTGCTGAGCTTTGCA R:ACTTTATCGTCTCCTTCCACC |
| LcActin | F: AGTTTGGTTGATGTGGGAGAC R: TGGCTGAACCCGAGATGAT |
| Treatment | Control | 250 mg/L | 500 mg/L | 1000 mg/L |
|---|---|---|---|---|
| Date of beginning of panicle emergence | 24 December 2021 | 31 December 2021 | 3 January 2022 | 6 January 2022 |
| Cumulative chilling hours below 15 °C (h) | 468.5 | 443.8 | 416.7 | 298.7 |
| Treatment | Number of Nodes with Panicles in a Terminal Shoot | Pure Panicle (%) | Leafy Panicle (%) | Vegetative Flush (%) |
|---|---|---|---|---|
| Control | 0.44 ± 0.14 d | 23.0 ± 11.20 c | 31.0 ± 6.50 b | 46.0 ± 13.00 a |
| 250 mg/L | 1.66 ±0.10 c | 38.0 ± 10.05 b | 45.0 ± 7.50 a | 22.0 ± 9.60 b |
| 500 mg/L | 4.35± 0.82 b | 50.2 ± 14.00 b | 30.0 ± 7.50 b | 17.0 ± 6.10 b |
| 1000 mg/L | 8.23 ±0.11 a | 67.0 ± 12.50 a | 20.0 ± 8.10 c | 13.0 ± 4.50 a |
| Treatment | Panicle Emergence Rate (%) | Pure Panicle Rate (%) | Number of Nodes with Panicle |
|---|---|---|---|
| Control | 37.8 ± 4.94 b | 14.5 ± 2.68 b | 0.59 ± 0.03 b |
| Traditional treatment | 65.7 ± 8.45 a | 43.8 ± 4.57 a | 3.78 ± 1.51 a |
| Drone spray | 60.5 ± 9.62 a | 40.5 ± 3.61 a | 5.65 ± 1.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, B.; Deng, C.; Tian, Q.; Ouyang, J.; Zeng, R.; Wang, H.; Huang, X. Application of Ethephon Manually or via Drone Enforces Bud Dormancy and Enhances Flowering Response to Chilling in Litchi (Litchi chinensis Sonn.). Horticulturae 2024, 10, 1109. https://doi.org/10.3390/horticulturae10101109
Wen B, Deng C, Tian Q, Ouyang J, Zeng R, Wang H, Huang X. Application of Ethephon Manually or via Drone Enforces Bud Dormancy and Enhances Flowering Response to Chilling in Litchi (Litchi chinensis Sonn.). Horticulturae. 2024; 10(10):1109. https://doi.org/10.3390/horticulturae10101109
Chicago/Turabian StyleWen, Bingyi, Cailian Deng, Qi Tian, Jianzhong Ouyang, Renfang Zeng, Huicong Wang, and Xuming Huang. 2024. "Application of Ethephon Manually or via Drone Enforces Bud Dormancy and Enhances Flowering Response to Chilling in Litchi (Litchi chinensis Sonn.)" Horticulturae 10, no. 10: 1109. https://doi.org/10.3390/horticulturae10101109
APA StyleWen, B., Deng, C., Tian, Q., Ouyang, J., Zeng, R., Wang, H., & Huang, X. (2024). Application of Ethephon Manually or via Drone Enforces Bud Dormancy and Enhances Flowering Response to Chilling in Litchi (Litchi chinensis Sonn.). Horticulturae, 10(10), 1109. https://doi.org/10.3390/horticulturae10101109






