Technical and Economic Assessment of Tomato Cultivation Through a Macro-Tunnel Production System with the Application of Gluconacetobacter diazotrophicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Bacteria
2.3. Preparation of Bacterial Suspension
2.4. Experimental Design
2.5. Economic Viability Assessment
3. Results
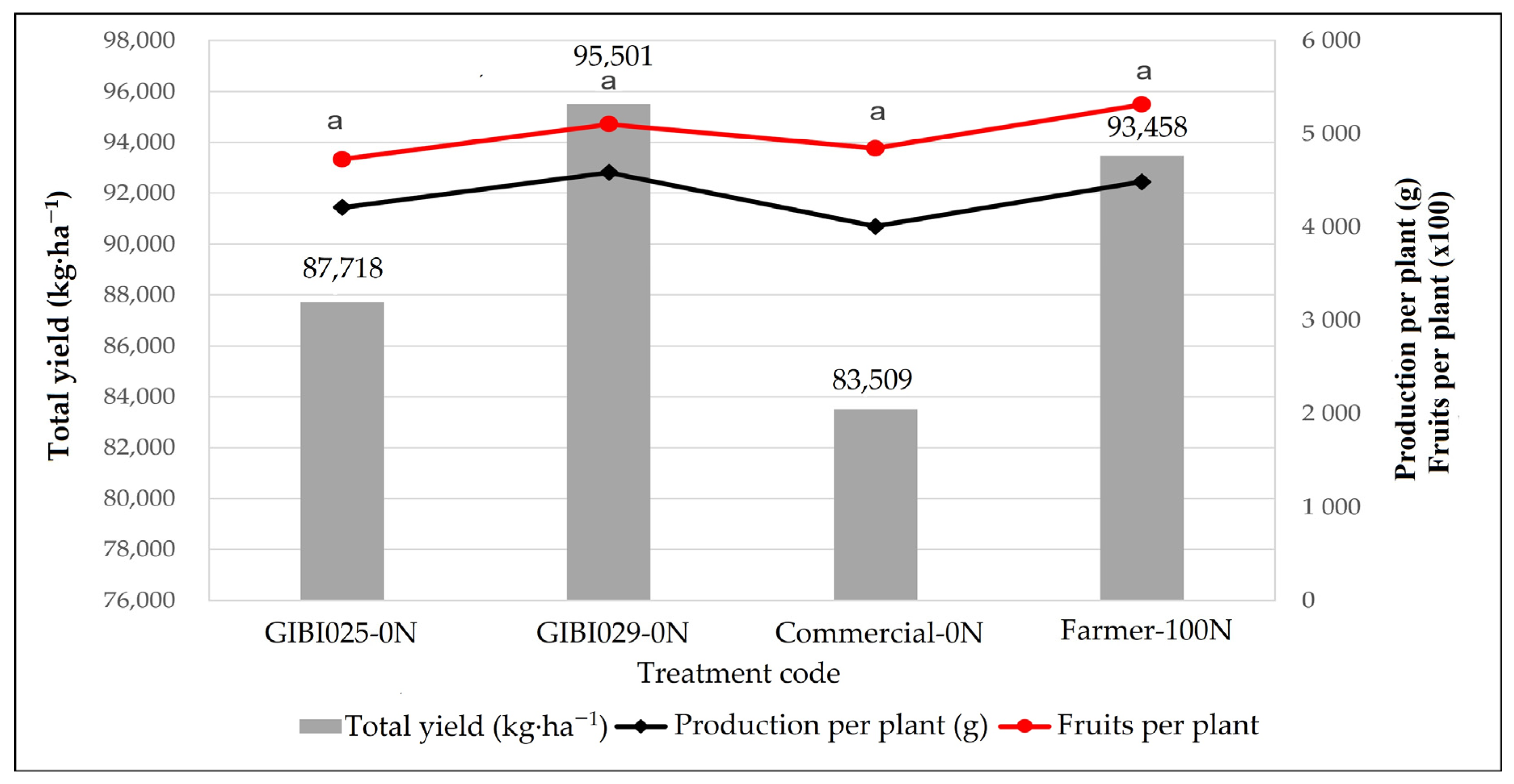
3.1. Effects of Biofertilization on Production per Plant, Total Yield, and Fruits per Plant
3.2. Impact of Biofertilization on Cost Structure and Economic Analysis of the Tomato Crop
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Construction and Installation Costs | Unit | Total Quantity | Unit Value (USD) | Total Value (USD) | Annual Value (USD) | Cycle Value (USD) | % |
|---|---|---|---|---|---|---|---|
| Structure—Inputs (A) | |||||||
| Galvanized pipe 1″ × 6 m C 2 mm | Unit | 667 | 17.63 | 11,759.21 | 587.96 | 293.98 | 6.82 |
| 1 1/2″ × 6 m galvanized pipe | Unit | 400 | 2.77 | 1108.00 | 55.40 | 27.70 | 0.64 |
| 3/4″ × 6 m black pipe | Unit | 67 | 9.47 | 634.49 | 31.72 | 15.86 | 0.37 |
| 1/8″ steel cable | m | 4444 | 0.17 | 755.48 | 37.77 | 18.89 | 0.44 |
| Welding reference 6013 × 1/8 | kg | 67 | 4.14 | 277.38 | 13.87 | 6.93 | 0.16 |
| Staples 50–19 | Box | 22 | 2.94 | 64.68 | 21.56 | 10.78 | 0.25 |
| Tamping rope roll × 800 | Roll | 11 | 6.94 | 76.34 | 25,45 | 12.72 | 0.30 |
| 3/8″ galvanized threaded rod | m | 67 | 1.82 | 121.94 | 6.10 | 3.05 | 0.07 |
| 3/8″ galvanized nut | Unit | 1111 | 0.04 | 44.44 | 2.22 | 1.11 | 0.03 |
| 3/8″ galvanized washer | Unit | 1111 | 0.19 | 211.09 | 10.55 | 5.28 | 0.12 |
| Agroclear 7 × 7 × 50 | kg | 2667 | 4.23 | 11,281.41 | 3760.47 | 1880.24 | 43.63 |
| Agroclear 1 × 8 × 50 | kg | 289 | 4.23 | 1222.47 | 407.49 | 203.75 | 4.73 |
| Anticorrosive paint | Gallon | 6 | 8.79 | 52.74 | 17.58 | 8.79 | 0.20 |
| Total (A) | 27,609.67 | 4978.15 | 2489.07 | 57.75% | |||
| Construction work (B) | |||||||
| Plastic construction and installation | Wage | 178 | 12.72 | 2264 | 754.52 | 377.26 | 8.75 |
| Total (B) | 2264 | 754.52 | 377.26 | 8.75 | |||
| Irrigation (C) | |||||||
| Hose 2″ 40 gauge | m | 1458 | 0.77 | 1122.66 | 224.53 | 112.27 | 2.60 |
| Suction hose 2″ water inlet | m | 2083 | 2.75 | 5728.25 | 1145.65 | 572.83 | 13.29 |
| Tank 2000 L | Unit | 21 | 132.07 | 2773.47 | 554.69 | 277.35 | 6.44 |
| Dripline 16 mm dripline 40 cm drippers | m | 8333 | 0.32 | 2666.56 | 533.31 | 266.66 | 6.19 |
| Motor pump 0.75 HP | Unit | 21 | 92.46 | 1941.66 | 194.17 | 97.08 | 2.25 |
| Accessories 2″ motor pump | Unit | 21 | 9.61 | 201.81 | 20.18 | 10.09 | 0.23 |
| Total (C) | 14,434.41 | 2886.88 | 1443.44 | 31.01% | |||
| Total (A + B + C) | 44,307.41 | 8619.55 | 4309.77 | 100.00% | |||
References
- Crops and Livestock Products. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 15 April 2024).
- Gil, R.; Bojacá, C.R.; Schrevens, E. Datasets of the environmental factors and management practices of the smallholder tomato production systems in the Colombian Andes. Data in Brief 2019, 24, 103844. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Duque, A.; Morales-Londoño, C.S.; Hurtado-Salazar, A.; Ceballos-Aguirre, N. Nitrogen-fixing bacteria and nitrogen fertilization on economic feasibility of tomato. Rev. Colomb. Cienc. Hortic. 2022, 16, e13623. [Google Scholar] [CrossRef]
- Cifras agropecuarias (Agricultural Figures). Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1 (accessed on 15 April 2024). (In Spanish)
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J.; Konvalina, P.; Kopecký, M. Impacts of environmental factors and nutrients management on tomato grown under controlled and open field conditions. Agronomy 2023, 13, 916. [Google Scholar] [CrossRef]
- Lamont, W.J. Overview of the use of high tunnels worldwide. HortTechnology 2009, 19, 25–29. [Google Scholar] [CrossRef]
- Arthur, J.D.; Li, T.; Lalk, G.T.; Bi, G. High tunnel production of containerized hybrid and heirloom tomatoes using grafted plants with two types of rootstocks. Horticulturae 2021, 7, 319. [Google Scholar] [CrossRef]
- Ángel-Hernández, M.D.; Zermeño-Gonzalez, A.; Melendres-Alvarez, A.I.; Campos-Magaña, S.G.; Cadena-Zapata, M.; Bosque-Villarreal, D.; Arturo, G. Characteristics of a tunnel cover effect on radiation, chlorophyll and zucchini yield. Rev. Mexicana Cienc. Agric. 2017, 8, 1127–1142. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, M.; Bi, G.; Evans, B.; Harkess, R. Planting date effect on yield of tomato, eggplant, pepper, zinnia, and snapdragon in high tunnel in Mississippi. J. Crop Improv. 2014, 28, 27–37. [Google Scholar] [CrossRef]
- Gude, K.M.; Pliakoni, E.D.; Cunningham, B.; Ayub, K.; Kang, Q.; Rajashekar, C.B.; Rivard, C.L. High tunnel coverings alter crop productivity and microclimate of tomato and lettuce. HortScience 2022, 57, 265–272. [Google Scholar] [CrossRef]
- Rubio, S.A.; Alfonso, A.M.; Grijalba, C.; Pérez, M.M. Determinación de los costos de producción de la fresa cultivada a campo abierto y bajo macrotúnel (Determination of production costs of strawberries grown in open fields and under macro tunnel). Rev. Colomb. Cienc. Hortic. 2014, 8, 67–69. (In Spanish) [Google Scholar] [CrossRef]
- Flórez-Hernández, E.A.; Montes-Ciro, E.; Hurtado-Salazar, A.; Aristizábal, J.C.; Ceballos-Aguirre, N. Technical-economic evaluation of bacterial consortia in strawberry cultivation across two production systems. Rev. Colomb. Cienc. Hortic. 2023, 17, e16506. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Cavalcante, V.A.; Döbereiner, J. A new acid-tolerant nitrogen-fixing bacterium associated with sugar cane. Plant Soil 1988, 108, 23–31. [Google Scholar] [CrossRef]
- Paula, M.A.; Reis, V.M.; Döbereiner, J. Interactions of Glomus clarum with Acetobacter diazotrophicus in infection of sweet potato (Ipomoea batatas), sugarcane (Saccharum spp.), and sweet sorghum (Sorghum vulgare). Biol. Fertil. Soils 1991, 11, 111–115. [Google Scholar] [CrossRef]
- Döbereiner, J.; Reis, V.; Paula, M.; Olivares, F.d. Endophytic diazotrophs in sugar cane, cereals and tuber plants. In New Horizons in Nitrogen Fixation; Proceedings of the 9th International Congress on Nitrogen Fixation, Cancún, Mexico, 6–12 December 1992; Springer: Berlin/Heidelberg, Germany, 1993; pp. 671–676. [Google Scholar]
- Jiménez, T.; Fuentes, L.E.; Tapia, A.; Macarua, M.; Martínez, E.; Caballero, J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl. Environ. Microbiol. 1997, 63, 3676–3683. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Nair, S. Crop-specific endophytic colonization by a novel, salt-tolerant, N2-fixing and phosphate-solubilizing Gluconacetobacter sp. from wild rice. Biotechnol. Lett. 2003, 25, 497–501. [Google Scholar] [CrossRef]
- Tapia, A.; Bustillos, M.R.; Jiménez, T.; Caballero, J.; Fuentes, L.E. Natural endophytic occurrence of Acetobacter diazotrophicus in pineapple plants. Microb. Ecol. 2000, 39, 49–55. [Google Scholar] [CrossRef]
- Muthukumarasamy, R.; Revathi, G.; Loganathan, P. Effect of inorganic N on the population, in vitro colonization and morphology of Acetobacter diazotrophicus (syn. Gluconacetobacter diazotrophicus). Plant Soil 2002, 243, 91–102. [Google Scholar] [CrossRef]
- Pedraza, R.O. Recent advances in nitrogen-fixing acetic acid bacteria. Int. J. Food Microbiol. 2008, 125, 25–35. [Google Scholar] [CrossRef]
- Ceballos-Aguirre, N.; Cuellar, J.A.; Restrepo, G.M.; Sánchez, Ó.J. Effect of the application of Gluconacetobacter diazotrophicus and its interaction with nitrogen and phosphorus fertilization on carrot yield in the field. Int. J. Agron. 2023, 2023, 6899532. [Google Scholar] [CrossRef]
- Caballero-Mellado, J.; Onofre-Lemus, J.; Estrada-De Los Santos, P.; Martínez-Aguilar, L. The tomato rhizosphere, an environment rich in nitrogen-fixing Burkholderia species with capabilities of interest for agriculture and bioremediation. Appl. Environ. Microbiol. 2007, 73, 5308–5319. [Google Scholar] [CrossRef]
- Luna, M.F.; Aprea, J.; Crespo, J.M.; Boiardi, J.L. Colonization and yield promotion of tomato by Gluconacetobacter diazotrophicus. Appl. Soil Ecol. 2012, 61, 225–229. [Google Scholar] [CrossRef]
- Adriano-Anaya, M.; Salvador-Figueroa, M.; Ocampo, J.; García-Romera, I. Hydrolytic enzyme activities in maize (Zea mays) and sorghum (Sorghum bicolor) roots inoculated with Gluconacetobacter diazotrophicus and Glomus intraradices. Soil Biol. Biochem. 2006, 38, 879–886. [Google Scholar] [CrossRef]
- Restrepo, G.M.; Sánchez, Ó.J.; Marulanda, S.M.; Galeano, N.F.; Taborda, G. Evaluation of plant-growth promoting properties of Gluconacetobacter diazotrophicus and Gluconacetobacter sacchari isolated from sugarcane and tomato in West Central region of Colombia. Afr. J. Biotechnol. 2017, 16, 1619–1629. [Google Scholar] [CrossRef]
- Saravanan, V.; Madhaiyan, M.; Osborne, J.; Thangaraju, M.; Sa, T. Ecological occurrence of Gluconacetobacter diazotrophicus and nitrogen-fixing Acetobacteraceae members: Their possible role in plant growth promotion. Microb. Ecol. 2008, 55, 130–140. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Soares, H.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019, 682, 779–799. [Google Scholar] [CrossRef]
- Dibut, B.; Martínez, R.; Ríos, Y.; Plana, L.; Rodríguez, J.; Ortega, M.; Tejada, G. Estudio de la asociación Gluconacetobacter diazotrophicus-viandas tropicales en suelo ferralítico rojo. I. selección de cepas efectivas para la biofertilización de boniato, yuca y malanga (Study of the association Gluconacetobacter diazotrophicus-tropical foods in red ferralite soil. I. Selection of effective strains for the biofertilization of sweet potato, cassava and taro). Cultiv. Tropic. 2010, 31, 51–57. Available online: http://scielo.sld.cu/pdf/ctr/v31n3/ctr17310.pdf (accessed on 25 April 2024). (In Spanish).
- Restrepo, G.M.; Ceballos, N.; Valencia, L.F.; Sánchez, Ó.J. Plant growth promotion by Gluconacetobacter diazotrophicus and its interaction with genotype and phosphorus availability in tomato seedlings. Org. Agr. 2021, 11, 601–614. [Google Scholar] [CrossRef]
- Granja Tesorito (Tesorito Farm). Available online: https://cienciasagropecuarias.ucaldas.edu.co/granja-tesorito/ (accessed on 25 April 2024). (In Spanish).
- Ríos Rocafull, Y.; Sánchez López, M.; Dibut Álvarez, B.; Ortega García, M.; Tejeda González, G.; Rodríguez Sánchez, J.; Rojas Badía, M. The culture medium effect in plant growth promotion activity of Gluconacetobacter diazotrophicus in carrot and sugar beet. Rev. Bio Cienc. 2019, 6, e470. [Google Scholar] [CrossRef]
- Reis, V.M.; Olivares, F.L.; Döbereiner, J. Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J. Microbiol. Biotechnol. 1994, 10, 401–405. [Google Scholar] [CrossRef]
- DeBolt, D.C. A high sample volume procedure for the colorimetric determination of soil organic matter. Commun. Soil Sci. Plant Anal. 1974, 5, 131–137. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Gillman, G.P.; Bruce, R.C.; Davey, B.G.; Kimble, J.M.; Searle, P.L.; Skjemstad, J.O. A comparison of methods used for determination of cation exchange capacity. Commun. Soil Sci. Plant Anal. 1983, 14, 1005–1014. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Lopez-Valdivia, L.M.; Novillo, J.; Obrador, A.; Rico, M.I. Comparison of EDTA and sequential extraction tests for phytoavailability prediction of manganese and zinc in agricultural alkaline soils. Geoderma 2006, 132, 450–463. [Google Scholar] [CrossRef]
- John, K.M.; Chuah, H.H.; Neufeld, J.H. Application of improved Azomethine-H method to the determination of boron in soils and plants. Anal. Lett. 1975, 8, 559–568. [Google Scholar] [CrossRef]
- Herrera, H.J.; Hurtado-Salazar, A.; Ceballos-Aguirre, N. Estudio técnico y económico del tomate tipo cereza élite (Solanum lycopersicum L. var. cerasiforme) bajo condiciones semicontroladas (Economic study of the elite cherry tomato type (Solanum lycopersicum L. var. cerasiforme) under semicontrolled conditions). Rev. Colomb. Cienc. Hortic. 2015, 9, 290–300. (In Spanish) [Google Scholar] [CrossRef]
- ATCC. Available online: https://www.atcc.org/search#q=Gluconacetobacter%20diazotrophicus&sort=relevancy (accessed on 16 April 2024). In Spanish.
- Boletín de Precios Diarios (Daily Price Bulletin). Available online: https://corabastos.com.co/inicio/precios/ (accessed on 16 April 2024). (In Spanish).
- Índice de Precios al Consumidor (IPC) (Consumer Price Index). Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/precios-y-costos/indice-de-precios-al-consumidor-ipc/ipc-informacion-tecnica#:~:text=Informaci%C3%B3n%20julio%202024,la%20anual%206%2C86%25 (accessed on 20 July 2024). (In Spanish)
- Colombia Central Bank key rates. Available online: https://countryeconomy.com/key-rates/colombia (accessed on 20 July 2024).
- Congreso de la República. Ley 2277 de 2022 (Act 2277 of 2022); Congreso de la República de Colombia: Bogotá, Colombia, 2022; 76p. (In Spanish) [Google Scholar]
- Rodríguez-Andrade, O.; Fuentes-Ramírez, L.E.; Morales-García, Y.E.; Molina-Romero, D.; Bustillos-Cristales, M.R.; Martínez-Contreras, R.D.; Muñoz-Rojas, J. The decrease in the population of Gluconacetobacter diazotrophicus in sugarcane after nitrogen fertilization is related to plant physiology in split root experiments. Rev. Argent. Microbiol. 2015, 47, 335–343. [Google Scholar] [CrossRef]
- Caballero-Mellado, J.; Fuentes-Ramirez, L.E.; Reis, V.M.; Martinez-Romero, E. Genetic structure of Acetobacter diazotrophicus populations and identification of a new genetically distant group. Appl. Environ. Microbiol. 1995, 61, 3008–3013. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Nguyen, C.T.; Armengaud, P.; Jossier, M.; Diatloff, E.; Filleur, S.; Daniel-Vedele, F. From the soil to the seeds: The long journey of nitrate in plants. J. Exp. Bot. 2011, 62, 1349–1359. [Google Scholar] [CrossRef]
- Marschner, P.; Gerendás, J.; Sattelmacher, B. Effect of N concentration and N source on root colonization by Pseudomonas fluorescens 2-79RLI. Plant Soil 1999, 215, 135–141. [Google Scholar] [CrossRef]
- Muñoz-Rojas, J.; Caballero-Mellado, J. Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microb. Ecol. 2003, 46, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.; Polidoro, J.; Reis, V. Nitrogen source effect on Gluconacetobacter diazotrophicus colonization of sugarcane (Saccharum spp.). Plant Soil 2006, 279, 141–152. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, S.; Fan, J.; Zhang, F.; Zheng, J.; Guo, J.; Xiang, Y. Combined application of soluble organic and chemical fertilizers in drip fertigation improves nitrogen use efficiency and enhances tomato yield and quality. J. Sci. Food Agric. 2020, 100, 5422–5433. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Delgado, J.; Abad-Rodríguez, E.M.; Salgado-Pulido, J.M. Efecto de Gluconacetobacter diazotrophicus en el cultivo del tomate (Solanum lycopercicum L.) (Efect of Gluconacetobacter diazotrophicus on tomato (Solanum lycopercicum L.) cultivation). Avances 2019, 21, 264–275. (In Spanish) [Google Scholar]
- Ceballos-Aguirre, N.; Restrepo, G.M.; Hurtado-Salazar, A.; Cuellar, J.A.; Sánchez, Ó.J. Economic feasibility of Gluconacetobacter diazotrophicus in carrot cultivation. Rev. Ceres 2022, 69, 40–47. [Google Scholar] [CrossRef]
- Chawla, N.; Phour, M.; Suneja, S.; Sangwaan, S.; Goyal, S. Gluconacetobacter diazotrophicus: An overview. Res. Environ. Life Sci. 2014, 7, 1–10. [Google Scholar]
- Srebot, M.S.; Tano, J.; Carrau, A.; Ferretti, M.D.; Martínez, M.L.; Orellano, E.G.; Rodriguez, M.V. Bacterial wilt biocontrol by the endophytic bacteria Gluconacetobacter diazotrophicus in Río Grande tomato cultivar. Biol. Control 2021, 162, 104728. [Google Scholar] [CrossRef]
- Suárez, O.; Salazar, A.H.; Aguirre, N.C. Número de racimos y la sostenibilidad económica del tomate bajo condiciones semicontroladas (Number of bunches and the economic sustainability of tomato under semi-controlled conditions). Temas Agrarios 2018, 23, 55–61. (In Spanish) [Google Scholar] [CrossRef]
- Rajula Shanthy, T.; Venkatesaperumal, M. Bio-fertilizers for sustainable sugarcane production: A socio-economic analysis. J. Sugarcane Res. 2018, 8, 127–137. [Google Scholar]
- Aechra, S.; Meena, R.; Jat, G.; Sharma, J.; Doodhwal, K.; Jat, H. Effect of biofertilizers and split application of vermicompost on productivity and profitability of wheat (Triticum aestivum L.) crop in clay loam soils. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1129–1139. [Google Scholar] [CrossRef]

| pH | Nitrogen (%) | Organic Matter (%) | Phosphorus (mg·kg−1) | Potassium (cmol·kg−1) | Calcium (cmol·kg−1) | Magnesium (cmol·kg−1) | Sodium (cmol·kg−1) | Iron (mg·kg−1) |
| 5.8 | 0.36 | 8.59 | 203 | 0.60 | 6.37 | 1.65 | 0.231 | 272 |
| Manganese (mg·kg−1) | Zinc (mg·kg−1) | Copper (mg·kg−1) | Sulfur (mg·kg−1) | Boron (mg·kg−1) | Sand (%) | Silt (%) | Clay (%) | Texture |
| 16.39 | 26.27 | 6.40 | 22.79 | 1.24 | 60 | 23 | 17 | Sandy loam |
| Microorganism | Concentration (CFU·mL−1) | Nitrogen Fertilization (%) | Other Nutrients | Code |
|---|---|---|---|---|
| GIBI025 | 1 × 108 | 0 | For all the treatments: 200 kg·ha−1 K2SO4 (18% S + 50% K2O), 200 kg·ha−1 MgSO4 (16% S + 50% MgO), and 250 kg·ha−1 KCl (60% K2O) | GIBI025-0N |
| GIBI029 | 1 × 108 | 0 | GIBI029-0N | |
| Azotobacter chrococcum + Azospirillium sp. | 1 × 108 | 0 | Commercial-0N | |
| No addition of bacteria | 0 | 100 | Farmer-100N |
| Parameter | Value | Reference |
|---|---|---|
| Year of analysis | 2024 | |
| Year construction starts | 2023 | |
| Construction period (months) | 18 | |
| Start-up period (months) | 7 | |
| Project life (years) | 15 | |
| Depreciation period (years) | 10 | |
| Depreciation method | Straight–line method | |
| Salvage cost | 0 | |
| Inflation (%) | 6.86 | [43] |
| Opportunity interest rate (year 2024) (%) | 9.09 | [44] |
| Income tax (year 2024) (%) | 35 | [45] |
| Treatment 1 | GIBI029-0N | GIBI025-0N | Commercial-0N | Farmer-100N | ||||
|---|---|---|---|---|---|---|---|---|
| Total Value (USD·ha−1) | % | Total Value (USD·ha−1) | % | Total Value (USD·ha−1) | % | Total Value (USD·ha−1) | % | |
| A. Labor (1 + 2 + 3) | 15,938 | 54.26 | 15,773 | 54.03 | 15,684 | 53.90 | 15,900 | 52.18 |
| (1) Adequacy of land | 1895 | 6.45 | 1895 | 6.49 | 1895 | 6.51 | 1895 | 6.22 |
| Preparation | 1615 | 5.50 | 1615 | 5.53 | 1615 | 5.55 | 1615 | 5.30 |
| Sowing | 280 | 0.95 | 280 | 0.96 | 280 | 0.96 | 280 | 0.92 |
| (2) Crop maintenance | 12,021 | 40.92 | 12,021 | 41.18 | 12,021 | 41.32 | 12,021 | 39.45 |
| Crop cultivation | 9758 | 33.22 | 9758 | 33.42 | 9758 | 33.54 | 9758 | 32.03 |
| Application of inputs | 1907 | 6.49 | 1907 | 6.53 | 1907 | 6.56 | 1907 | 6.26 |
| Application of treatments | 356 | 1.21 | 356 | 1.22 | 356 | 1.22 | 356 | 1.17 |
| (3) Harvest and post-harvest | 2022 | 6.88 | 1857 | 6.36 | 1768 | 6.07 | 1984 | 6.51 |
| B. Inputs (4 + 5 + 6 + 7 + 8 + 9 + 10) | 6798 | 23.14 | 6798 | 23.29 | 6798 | 23.37 | 7830 | 25.70 |
| (4) Seed | 2325 | 7.91 | 2325 | 7.96 | 2325 | 7.99 | 2325 | 7.63 |
| (5) Edaphic fertilizer | 2375 | 8.08 | 2375 | 8.13 | 2375 | 8.16 | 2375 | 7.79 |
| (6) Treatments | 755 | 2.57 | 755 | 2.58 | 755 | 2.59 | 1786 | 5.86 |
| (7) Fungicide | 92 | 0.31 | 92 | 0.32 | 92 | 0.32 | 92 | 0.30 |
| (8) Insecticide | 133 | 0.45 | 133 | 0.46 | 133 | 0.46 | 133 | 0.44 |
| (9) Pita cord, 13 gauge wire, cushioned | 1042 | 3.55 | 1042 | 3.57 | 1042 | 3.58 | 1042 | 3.42 |
| (10) Tutoring (amortization) | 76 | 0.26 | 76 | 0.26 | 76 | 0.26 | 76 | 0.25 |
| Direct cost (A + B) | 22,737 | 77.40 | 22,571 | 77.31 | 22,482 | 77.27 | 23,730 | 77.88 |
| C. Indirect Cost (11 + 12 + 13) | 2436 | 8.29 | 2420 | 8.29 | 2411 | 8.29 | 2536 | 8.32 |
| (11) Lease | 148 | 0.50 | 148 | 0.51 | 148 | 0.51 | 148 | 0.49 |
| (12) Administration | 1144 | 3.90 | 1136 | 3.89 | 1132 | 3.89 | 1194 | 3.92 |
| (13) Technical assistance | 1144 | 3.90 | 1136 | 3.89 | 1132 | 3.89 | 1194 | 3.92 |
| D. Macro-tunnel cost per cycle 2 | 4309.77 | 14.31 | 4309.77 | 14.40 | 4309.77 | 14.45 | 4309.77 | 13.80 |
| TOTAL (A + B + C + D) | 29,376 | 100.00 | 29,194 | 100.00 | 29,097 | 100.00 | 30,469 | 100.00 |
| Treatment 1 | Gross Income (USD·ha−1) | Total Cost 2 (USD·ha−1) | Net Income (USD·ha−1) | Unit Value of Production (USD·ha−1) | B/C | Rate of Return (%) |
|---|---|---|---|---|---|---|
| GIBI025-0N | 42,327 | 29,194 | 13,133 | 0.33 | 1.45 | 44.98 |
| GIBI029-0N | 46,083 | 29,376 | 16,707 | 0.31 | 1.57 | 56.87 |
| Commercial-0N | 40,296 | 29,097 | 11,200 | 0.35 | 1.38 | 38.49 |
| Farmer-100N | 45,097 | 30,469 | 14,628 | 0.33 | 1.48 | 48.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceballos-Aguirre, N.; Hurtado-Salazar, A.; Restrepo, G.M.; Sánchez, Ó.J.; Hernández, M.C.; Montoya, M. Technical and Economic Assessment of Tomato Cultivation Through a Macro-Tunnel Production System with the Application of Gluconacetobacter diazotrophicus. Horticulturae 2024, 10, 1110. https://doi.org/10.3390/horticulturae10101110
Ceballos-Aguirre N, Hurtado-Salazar A, Restrepo GM, Sánchez ÓJ, Hernández MC, Montoya M. Technical and Economic Assessment of Tomato Cultivation Through a Macro-Tunnel Production System with the Application of Gluconacetobacter diazotrophicus. Horticulturae. 2024; 10(10):1110. https://doi.org/10.3390/horticulturae10101110
Chicago/Turabian StyleCeballos-Aguirre, Nelson, Alejandro Hurtado-Salazar, Gloria M. Restrepo, Óscar J. Sánchez, María C. Hernández, and Mauricio Montoya. 2024. "Technical and Economic Assessment of Tomato Cultivation Through a Macro-Tunnel Production System with the Application of Gluconacetobacter diazotrophicus" Horticulturae 10, no. 10: 1110. https://doi.org/10.3390/horticulturae10101110
APA StyleCeballos-Aguirre, N., Hurtado-Salazar, A., Restrepo, G. M., Sánchez, Ó. J., Hernández, M. C., & Montoya, M. (2024). Technical and Economic Assessment of Tomato Cultivation Through a Macro-Tunnel Production System with the Application of Gluconacetobacter diazotrophicus. Horticulturae, 10(10), 1110. https://doi.org/10.3390/horticulturae10101110





