Physiological and Transcriptomic Analyses Reveal the Role of the Antioxidant System and Jasmonic Acid (JA) Signal Transduction in Mulberry (Morus alba L.) Response to Flooding Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatment
2.2. Methods Used in the Determination of Parameters
2.2.1. Determination of Reactive Oxygen Species (ROS) and Malondialdehyde (MDA) Content
2.2.2. Determination of Antioxidant Metabolites
2.2.3. Determination of Antioxidant Enzyme Activity
2.2.4. Determination of Physiological Indicators
2.2.5. Determination of Transcriptome
- (1)
- Sequencing Experiment
- (2)
- Quality Control
- (3)
- Differential Gene Screening
2.2.6. RT-PCR Analysis
2.3. Statistical Analysis
3. Results
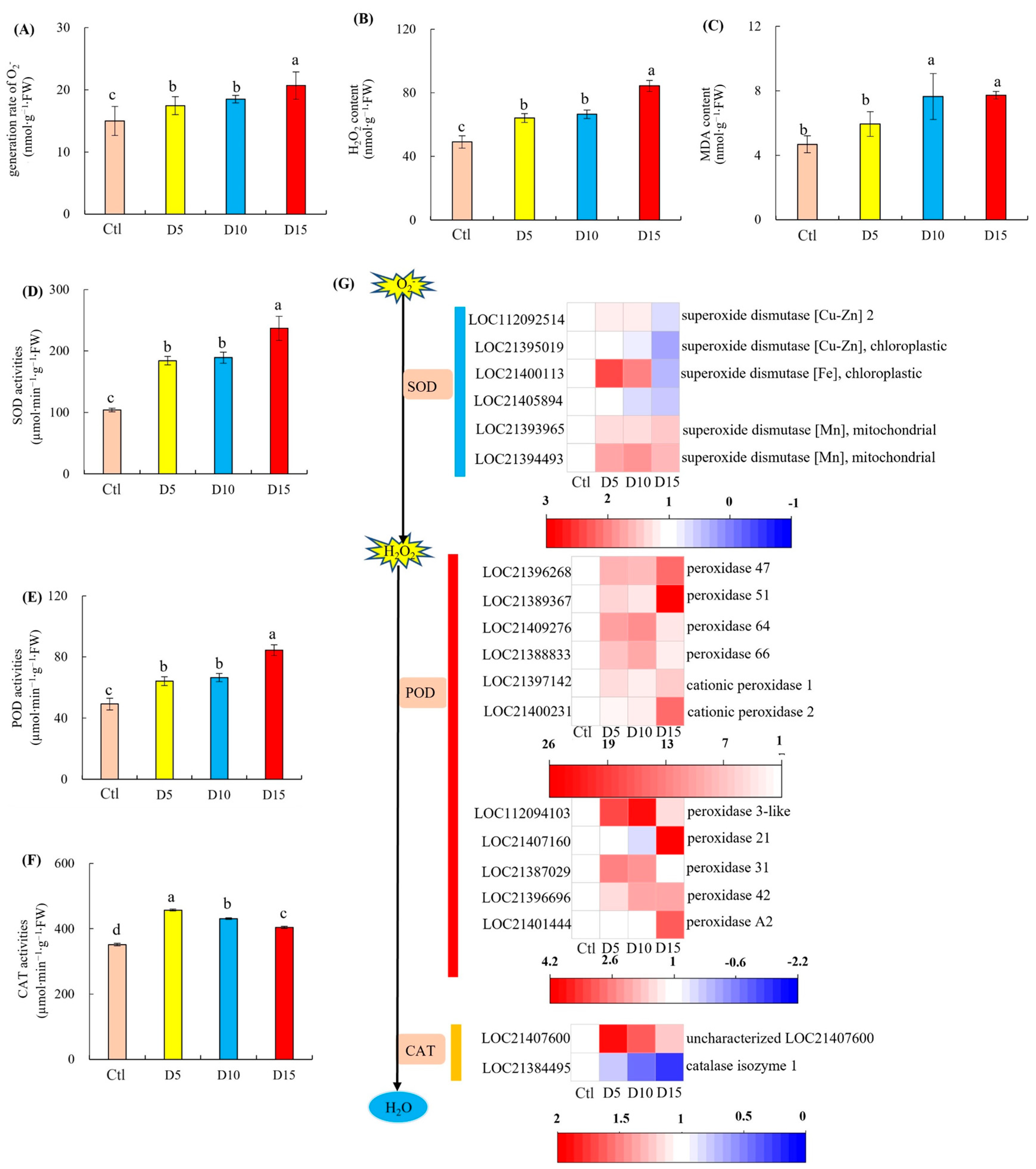
3.1. ROS Contents and SOD, POD, and CAT Activities
3.2. AsA-GSH Cycle and the Expression of Related Protein
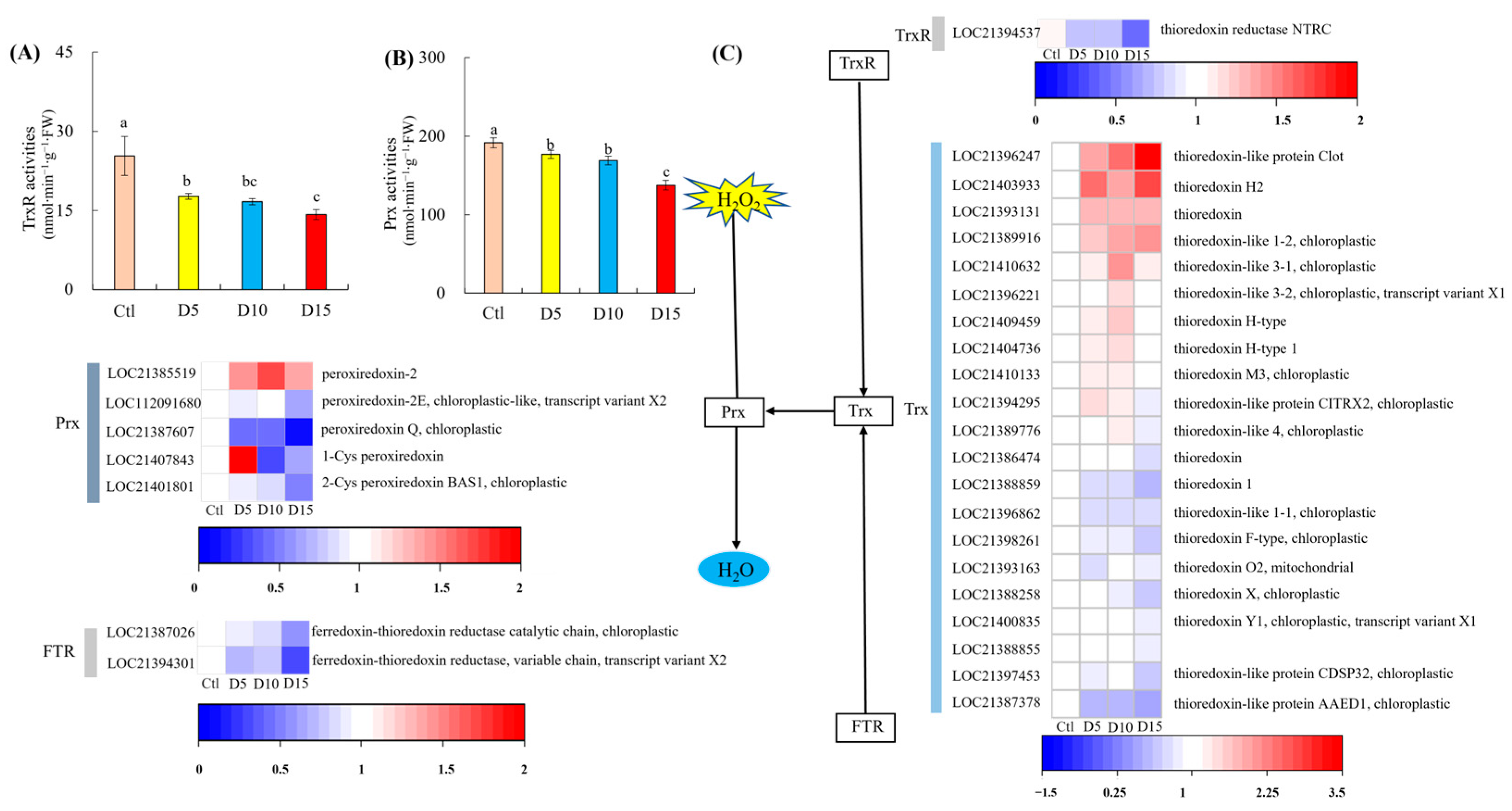
3.3. Expression of Trx-Prx Pathway-Related Proteins
3.4. JA Signal
3.5. Impact of Flooding Duration on Pro, SS, and SP Contents in Mulberry Leaves
3.6. RT-qPCR Validation of Transcriptome Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, X.B.; Yang, D.; Sun, H.; Liu, J.; Song, H.Y.; Xiong, Y.Q.; Wang, Y.M.; Ma, J.Y.; Zhang, M.H.; Li, J.; et al. Time-course analysis and transcriptomic identification of key response strategies to complete submergence in Nelumbo nucifera. Hortic. Res. 2022, 9, uhac001. [Google Scholar] [CrossRef]
- Ahmed, M.; Stockle, C.O. Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Tanoue, M.; Hirabayashi, Y.; Ikeuchi, H. Global-scale river flood vulnerability in the last 50 years. Sci. Rep. 2016, 6, 36021. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Kanae, S.; Seneviratne, S.I.; Handmer, J.; Nicholls, N.; Peduzzi, P.; Mechler, R.; Bouwer, L.M.; Arnell, N.; Mach, K.; et al. Flood risk and climate change: Global and regional perspectives. Hydrol. Sci. J. 2014, 59, 1–28. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, S.M.; Cao, L.G.; Cai, X.; Zhang, K.X.; Xu, Y.H.; Xu, W. Changes in extreme climate events in eastern China during 1960–2013: A case study of the Huaihe River Basin. Quat. Int. 2015, 380, 22–34. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Gomez, C.; Zubair, M.; Ilyas, N.; Khan, N.; Shahid, M.A. Silicon alleviate hypoxia stress by improving enzymatic and non-enzymatic antioxidants and regulating nutrient uptake in muscadine grape (Muscadinia rotundifolia Michx.). Front. Plant Sci. 2021, 11, 618873. [Google Scholar] [CrossRef]
- Geigenberger, P. Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef]
- Kaelke, C.M.; Dawson, J.O. Seasonal flooding regimes influence survival, nitrogen fixation, and the partitioning of nitrogen and biomass in Alnus incana ssp rugosa. Plant Soil 2003, 254, 167–177. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Ethylene—A key regulator of submergence responses in rice. Plant Sci. 2008, 175, 43–51. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; Lopez-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plantarum. 2008, 132, 452–466. [Google Scholar] [CrossRef]
- Olgun, M.; Kumlay, A.M.; Adiguzel, M.C.; Caglar, A. The effect of waterlogging in wheat (Triticum aestivum L.). Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 193–198. [Google Scholar]
- Greenway, H.; Armstrong, W.; Colmer, T.D. Conditions leading to high CO2 (>5kPa) in waterlogged–flooded soils and possible effects on root growth and metabolism. Ann. Bot. 2006, 98, 9–32. [Google Scholar] [CrossRef]
- Akhtar, I.; Nazir, N. Effect of waterlogging and drought stress in plants. Int. J. Water Resour. Environ. Sci. 2013, 2, 34–40. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Asif, M.; Kamran, A. Plant breeding for water-limited environments. Crop Sci. 2011, 51, 2911–2912. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia—Is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef]
- Islam, M.A.; Macdonald, S.E. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding and nutrition stress. Trees 2004, 18, 35–42. [Google Scholar] [CrossRef]
- Green, J.; Crack, J.C.; Thomson, A.J.; Lebrun, N.E. Bacterial sensors of oxygen. Curr. Opin. Microbiol. 2009, 12, 145–151. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Ushimaru, T.; Shibasaka, M.; Tsuji, H. Development of the O2•−-detoxification system during adaptation to air of submerged rice seedlings. Plant Cell Physiol. 1992, 33, 1065–1071. [Google Scholar]
- Hurng, W.P.; Kao, C.H. Effect of flooding on the activities of some enzymes of activated oxygen metabolism, the levels of antioxidants, and lipid peroxidation in senescing tobacco leaves. Plant Growth Regul. 1994, 14, 37–44. [Google Scholar] [CrossRef]
- Hurng, W.P.; Kao, C.H. Lipid peroxidation and antioxidative enzymes in senescing tobacco leaves during post-flooding. Plant Sci. 1994, 96, 41–44. [Google Scholar] [CrossRef]
- Grassini, P.; Indaco, G.V.; Pereira, M.L.; Hall, A.J.; Trapani, N. Responses to short-term waterlogging during grain filling in sunflower. Field Crops Res. 2007, 101, 352–363. [Google Scholar] [CrossRef]
- Zhang, G.P.; Tanakamaru, K.; Abe, J.; Morita, S. Influence of waterlogging on some anti-oxidative enzymatic activities of two barley genotypes differing in anoxia tolerance. Acta Physiol. Plant. 2007, 29, 171–176. [Google Scholar] [CrossRef]
- Shi, F.; Yamamoto, R.; Shimamura, S.; Hiraga, S.; Nakayama, N.; Nakamura, T.; Yukawa, K.; Hachinohe, M.; Matsumoto, H.; Komatsu, S. Cytosolic ascorbate peroxidase 2 (cAPX 2) is involved in the soybean response to flooding. Phytochemistry 2008, 69, 1295–1303. [Google Scholar] [CrossRef]
- Zhao, N.; Li, C.W.; Yan, Y.J.; Cao, W.; Song, A.P.; Wang, H.B.; Chen, S.M.; Jiang, J.F.; Chen, F.D. Comparative transcriptome analysis of waterlogging-sensitive and waterlogging-tolerant chrysanthemum morifolium cultivars under waterlogging stress and reoxygenation conditions. Int. J. Mol. Sci. 2018, 19, 1455. [Google Scholar] [CrossRef]
- Ayano, M.; Kani, T.; Kojima, M.; Sakakibara, H.; Kitaoka, T.; Kuroha, T.; Angeles-Shim, R.B.; Kitano, H.; Nagai, K.; Ashikari, M. Gibberellin biosynthesis and signal transduction is essential for internode elongation in deepwater rice. Plant Cell Environ. 2014, 37, 2313–2324. [Google Scholar] [CrossRef]
- Schmitz, A.J.; Folsom, J.J.; Jikamaru, Y.; Ronald, P.; Walia, H. SUB1A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytol. 2013, 198, 1060–1070. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, S.J.; Wagas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef]
- Alisofi, S.; Einali, A.; Sangtarash, M.H. Jasmonic acid-induced metabolic responses in bitter melon (Momordica charantia) seedlings under salt stress. J. Pomol. Hortic. Sci. 2019, 95, 247–259. [Google Scholar] [CrossRef]
- Wan, S.W.; Xin, X.F. Regulation and integration of plant jasmonate signaling: A comparative view of monocot and dicot. J. Genet. Genom. 2022, 49, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.J.; Zou, C.H.; Liu, Z.B.; Wei, G.; Yang, B.Z.; Zou, X.X. Mitigation of waterlogging-induced damages to pepper by exogenous MeJA. Pak. J. Bot. 2017, 49, 1127–1135. [Google Scholar]
- Balfagon, D.; Sengupta, S.; Gomez-Cadenas, A.; Fritschi, F.B.; Azad, R.; Mittler, R.; Zandalinas, S.I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Razmjoo, J.; Cheema, M. Foliage applications of jasmonic acid modulate the antioxidant defense under water deficit growth in sugar beet. Span. J. Agric. Res. 2019, 17, e0805. [Google Scholar] [CrossRef]
- Sun, Y.P.; Wang, F.W.; Wang, N.; Dong, Y.Y.; Liu, Q.; Zhao, L.; Chen, H.; Liu, W.C.; Yin, H.L.; Zhang, X.M. Transcriptome exploration in Leymus chinensis under saline-alkaline treatment using 454 pyrosequencing. PLoS ONE 2013, 8, e53632. [Google Scholar] [CrossRef]
- Geng, G.; Lv, C.H.; Stevanato, P.; Li, R.R.; Liu, H.; Yu, L.H.; Wang, Y.G. Transcriptome analysis of salt-sensitive and tolerant genotypes reveals salt-tolerance metabolic pathways in sugar beet. Int. J. Mol. Sci. 2020, 20, 5910. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Carter, A.; Tucker, J.R.; Yao, Z.; Xu, W.; Badea, A. Genome-wide analysis of gene expression provides new insights into waterlogging responses in Barley (Hordeum vulgare L.). Plants 2020, 9, 240. [Google Scholar] [CrossRef]
- Fu, S.H.; Chang, P.L.; Friesen, M.L.; Teakle, N.L.; Tarone, A.M.; Sze, S.H. Identifying similar transcripts in a related organism from de Bruijn graphs of RNA-Seq data, with applications to the study of salt and waterlogging tolerance in Melilotus. BMC Genom. 2019, 20, 425. [Google Scholar] [CrossRef]
- Alam, I.; Lee, D.G.; Kim, K.H.; Park, C.H.; Sharmin, S.A.; Lee, H.; Oh, K.W.; Yun, B.W.; Lee, B.H. Proteome analysis of soybean roots under waterlogging stress at an early vegetative stage. J. Biosci. 2010, 35, 49–62. [Google Scholar] [CrossRef]
- Zeng, B.; Zhang, Y.J.; Zhang, A.L.; Qiao, D.D.; Ren, J.C.; Li, M.Y.; Cai, K.; Zhang, J.H.; Huang, L.K. Transcriptome profiling of two Dactylis glomerata L. cultivars with different tolerance in response to submergence stress. Phytochemistry 2020, 175, 112378. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Zhou, Z.X.; Zhang, Z.; Yu, X.L.; Zhang, X.Y.; Du, K.B. Molecular and physiological responses in roots of two full-sib poplars uncover mechanisms that contribute to differences in partial submergence tolerance. Sci. Rep. 2018, 8, 12829. [Google Scholar] [CrossRef] [PubMed]
- Li, R.X.; Su, X.Q.; Zhou, R.; Zhang, Y.P.; Wang, T.C. Molecular mechanism of mulberry response to drought stress revealed by complementary transcriptomic and iTRAQ analyses. Bmc Plant Biol. 2022, 22, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, D.F.; Turgeon, R.; Chen, J.; Lin, T.B.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.K.; Lv, Z.Q. Physiological and Proteomic Responses of Mulberry Trees (Morus alba L.) to Combined Salt and Drought Stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Yu, C.; Mo, R.; Zhu, Z.; Dong, Z.; Hu, X.; Zhuang, C.; Deng, W. Physiological and Transcriptome Analyses of Photosynthesis in Three Mulberry Cultivars within Two Propagation Methods (Cutting and Grafting) under Waterlogging Stress. Plants 2023, 12, 2066. [Google Scholar] [CrossRef]
- Zhang, C.G.; Leung, K.K.; Wong, Y.S.; Tam, N.F.Y. Germination, growth and physiological responses of mangrove plant (Bruguiera gymnorrhiza) to lubricating oil pollution. Environ. Exp. Bot. 2007, 60, 127–136. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 2004, 52, 302–310. [Google Scholar]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.W.; Liang, D.; Ma, F.W. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2012, 54, 292–302. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide dismutases. Annu. Rev. Biochem. 2013, 44, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Bernroitner, M.; Zamocky, M.; Furtmuller, P.G.; Peschek, G.A.; Obinger, C. Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J. Exp. Bot. 2009, 60, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyuki, N.; Kozi, A. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Arrigoni, O.; Dipierro, S.; Borraccino, G. Ascorbate free radical reductase, a key enzyme of the ascorbic acid system. FEBS Lett. 1981, 125, 242–244. [Google Scholar] [CrossRef]
- Bocova, B.; Huttova, J.; Liptakova, L.; Mistrik, I.; Olle, M.; Tamas, L. Impact of short-term cadmium treatment on catalase and ascorbate peroxidase activities in barley root tips. Biol. Plant. 2012, 56, 724–728. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 2010, 16, 259–272. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Hossain, M.D.; Fujita, M. Induction of pumpkin glutathione S-transferases by different stresses and its possible mechanisms. Biol. Plant. 2006, 50, 210–218. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.T.; Zhang, H.B.; Huo, Y.Z.; Liu, X.Q.; Che, Y.H.; Wang, J.C.; Sun, G.Y.; Zhang, H.H. The phytotoxicity of exposure to two polybrominated diphenyl ethers (BDE47 and BDE209) on photosynthesis and the response of the hormone signaling and ROS scavenging system in tobacco leaves. J. Hazard. Mater. 2022, 424, 127265. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Inze, D.; Montagu, V.M. Oxidative Stress in Plants. In Taylor and Francis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Ruma, D.; Gitika, B.; Sharma, S.K.; Pauline, T.; Ram, M.S.; Ilavazhagan, G.; Sawhney, R.C.; Kumar, D.; Banerjee, P.K. Antioxidant activities of seabuckthorn (Hippophae rhamnoides) during hypoxia induced oxidative stress in glial cells. Mol. Cell. Biochem. 2005, 278, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Anee, T.I.; Nahar, K.; Rahman, A.; Al-Mahmud, J.; Bhuiyan, T.F.; Ul-Alam, M.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Chen, Y.Z.; Lu, H.Q.; Kong, X.Q.; Dai, J.L.; Li, Z.H.; Dong, H.Z. Growth, lint yield and changes in physiological attributes of cotton under temporal waterlogging. Field Crops Res. 2016, 194, 83–93. [Google Scholar] [CrossRef]
- Labudda, M. Lipid Peroxidation as a Biochemical Marker for Oxidative Stress during Drought. An Effective Tool for Plant Breeding. E-Wydawnictwo, Poland. 2013. Available online: http://www.e-wydawnictwo.eu/Document/DocumentPreview/334 (accessed on 6 March 2013).
- Pallavi, S.; Bhushan, J.A.; Shanker, D.R.; Mohammad, P. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar]
- Damanik, R.I.; Maziah, M.; Ismail, M.R.; Ahmad, S.; Zain, A. Responses of the antioxidative enzymes in Malaysian rice (Oryza sativa L.) cultivars under submergence condition. Acta Physiol. Plant. 2010, 32, 739–747. [Google Scholar] [CrossRef]
- Ushimaru, T.; Ogawa, K.; Ishida, N.; Shibasaka, M.; Kanematsu, S.; Asada, K.; Tsuji, H. Changes in organelle superoxide dismutase isoenzymes during air adaptation of submerged rice seedlings: Differential behaviour of isoenzymes in plastids and mitochondria. Planta 1995, 196, 606–613. [Google Scholar] [CrossRef]
- Cheng, X.X.; Yu, M.; Zhang, N.; Zhou, Z.Q.; Xu, Q.T.; Mei, F.Z.; Qu, L.H. Reactive oxygen species regulate programmed cell death progress of endosperm in winter wheat (Triticum aestivum L.) under waterlogging. Protoplasma 2016, 253, 311–327. [Google Scholar] [CrossRef]
- Hernandez, M.; Fernandez-Garcia, N.; Diaz-Vivancos, P.; Olmos, E. A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J. Exp. Bot. 2010, 61, 521–535. [Google Scholar] [CrossRef]
- Hossain, Z.; Lopez-Climent, M.F.; Arbona, V.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J. Plant Physiol. 2009, 166, 1391–1404. [Google Scholar] [CrossRef]
- Tian, L.X.; Li, J.; Bi, W.S.; Zuo, S.Y.; Li, L.J.; Li, W.L.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Yadav, D.K.; Srivastava, J.P. Temporal changes in biochemical and antioxidant enzymes activities in Maize (Zea mays L.) under waterlogging stress during early growth stage. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 351–362. [Google Scholar] [CrossRef]
- Kumutha, D.; Ezhilmathi, K.; Sairam, R.K.; Srivastava, G.C.; Deshmukh, P.S.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol. Plant. 2009, 53, 75–84. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Pena-Castro, J.M. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Hayes, D.; Nichols, D.S.; Zhou, M.X. Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 2015, 394, 355–372. [Google Scholar] [CrossRef]
- Chen, L.Z.; Wang, W.Q.; Lin, P. Photosynthetic and physiological responses of Kandelia candel L. Druce seedlings to duration of tidal immersion in artificial seawater. Environ. Exp. Bot. 2005, 54, 256–266. [Google Scholar]
- Frank, V.B.; Eva, V.; James, F.D.; Dirk, I. The role of active oxygen species in plant signal transduction. Plant Sci. 2001, 161, 405–414. [Google Scholar]
- Tang, B.; Xu, S.Z.; Zou, X.L.; Zheng, Y.L.; Qiu, G.Z. Changes of antioxidative enzymes and lipid peroxidation in leaves and roots of waterlogging-tolerant and waterlogging-sensitive maize genotypes at seedling stage. Agric. Sci. China 2010, 9, 651–661. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Rahantaniaina, M.S.; Li, S.; Chatel-Innocenti, G.; Tuzet, A.; Issakidis-Bourguet, E.; Mhamdi, A.; Noctor, G. Cytosolic and chloroplastic DHARs cooperate in oxidative stress-driven activation of the salicylic acid pathway. Plant Physiol. 2017, 174, 956–971. [Google Scholar] [CrossRef]
- Liu, N.; Li, J.W.; Lv, J.; Yu, J.H.; Xie, J.M.; Wu, Y.; Tang, Z.Q. Melatonin alleviates imidacloprid phytotoxicity to cucumber (Cucumis sativus L.) through modulating redox homeostasis in plants and promoting its metabolism by enhancing glutathione dependent detoxification. Ecotoxicol. Environ. Saf. 2021, 217, 112248. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, M.; Zhou, Q.; Cai, J.; Dai, T.B.; Cao, W.X.; Jiang, D. Physiological and proteomic mechanisms of waterlogging priming improves tolerance to waterlogging stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 132, 175–182. [Google Scholar] [CrossRef]
- Luo, Q.X.; Peng, M.; Zhang, X.L.; Lei, P.; Ji, X.M.; Chow, W.; Meng, F.J.; Sun, G.Y. Comparative mitochondrial proteomic, physiological, biochemical and ultrastructural profiling reveal factors underpinning salt tolerance in tetraploid black locust (Robinia pseudoacacia L.). BMC Genom. 2017, 18, 648. [Google Scholar] [CrossRef]
- Wu, X.; Lu, X.M. Effects of brassinolide on the growth and ascorbate-glutathione cycle of cucumber seedling roots under suboptimal temperature, light and salt environment. Chin. J. Ecol. 2015, 34, 2149–2154. [Google Scholar]
- Zhang, H.H.; Li, X.; Guan, Y.P.; Li, M.B.; Wang, Y.; An, M.J.; Zhang, Y.H.; Liu, G.J.; Xu, N.; Sun, G.Y. Physiological and proteomic responses of reactive oxygen species metabolism and antioxidant machinery in mulberry (Morus alba L.) seedling leaves to NaCl and NaHCO3 stress. Ecotoxicol. Environ. Saf. 2020, 193, 110259. [Google Scholar]
- Jin, Y.H.; Tao, D.L.; Hao, Z.Q.; Ye, J.; Du, Y.J.; Liu, H.L.; Zhou, Y.B. Environmental stresses and redox status of ascorbate. Acta Bot. Sin. 2003, 45, 795–801. [Google Scholar]
- Yoshimura, K.; Miyao, K.; Gaber, A.; Takeda, T.; Kanaboshi, H.; Miyasaka, H.; Shigeoka, S. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. Plant J. 2004, 37, 21–33. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Cummins, I.; Cole, D.J.; Edwards, R. A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. Cell Mol. Biol. 1999, 18, 285–292. [Google Scholar] [CrossRef]
- Wu, J.W.; Zhao, H.B.; Yu, D.; Xu, X.W. Transcriptome profiling of the floating-leaved aquatic plant Nymphoides peltata in response to flooding stress. BMC Genom. 2017, 18, 119. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ying, Y.; Chen, J.; Wang, X.C. Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci. 2004, 167, 671–677. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, S.S.; Chung, J.M.; Jung, H.S.; Singh, S.; Mondal, S.; Jang, H.H.; Cho, J.Y.; Bae, H.J.; Chung, B.Y. Site-specific mutagenesis of yeast 2-Cys peroxiredoxin improves heat or oxidative stress tolerance by enhancing its chaperone or peroxidase function. Protoplasma Int. J. Cell Biol. 2018, 254, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.N.; Bhatt, I.; Dietz, K.J. Peroxiredoxins: A less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 2009, 235, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Komatsu, S. Characteristics of soybean 1-Cys peroxiredoxin and its behavior in seedlings under flooding stress. Plant Biotechnol. 2011, 28, 83–88. [Google Scholar] [CrossRef]
- Balmer, Y.; Buchanan, B.B. Yet another plant thioredoxin. Trends Plant Sci. 2002, 7, 191–193. [Google Scholar] [CrossRef]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef]
- Zou, X.L.; Tan, X.Y.; Hu, C.W.; Zeng, L.; Lu, G.Y.; Fu, G.P.; Cheng, Y.; Zhang, X.K. The transcriptome of Brassica napus L. roots under waterlogging at the seedling stage. Int. J. Mol. Sci. 2013, 14, 2637–2651. [Google Scholar] [CrossRef]
- Benkova, E. Plant hormones in interactions with the environment. Plant Mol. Biol. 2016, 91, 597. [Google Scholar] [CrossRef]
- Liu, H.W.; Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Effects of jasmonic acid signalling on the wheat microbiome differ between body sites. Sci. Rep. 2017, 7, 41766. [Google Scholar] [CrossRef]
- Arbona, V.; Gomez-Cadenas, A. Hormonal modulation of citrus responses to flooding. J. Plant Growth Regul. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- Xu, X.W.; Wang, H.H.; Qi, X.H.; Xu, Q.; Chen, X.H. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar]
- O’Donnell, P.J.; Schmelz, E.; Block, A.; Miersch, O.; Wasternack, C.; Jones, J.B.; Klee, H.J. Multiple hormones act sequentially to mediate a susceptible tomato pathogen defense response. Plant Physiol. 2003, 133, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Pedranzani, H.; Racagni, G.; Alemano, S.; Miersch, O.; Ramirez, I.; Pena-Cortes, H.; Taleisnik, E.; Machado-Domenech, E.; Abdala, G. Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul. 2003, 41, 149–158. [Google Scholar] [CrossRef]
- Mahouachi, J.; Arbona, V.; Gómez-Cadenas, A. Hormonal changes in papaya seedlings subjected to progressive water stress and re-watering. Plant Growth Regul. 2007, 53, 43–51. [Google Scholar] [CrossRef]
- Suza, W.P.; Staswick, P.E. The role of JAR1 in Jasmonoyl-l-isoleucine production during Arabidopsis wound response. Planta 2008, 227, 1221–1232. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Kumari, P.H.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef]
- Zheng, F.L.; Liang, S.M.; Chu, X.N.; Yang, Y.L.; Wu, Q.S. Mycorrhizal fungi enhance flooding tolerance of peach through inducing proline accumulation and improving root architecture. Plant Soil Environ. 2020, 66, 624–631. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Popova, L.P. Photosynthetic response of barley plants to soil flooding. Photosynthetica 2001, 39, 515–520. [Google Scholar] [CrossRef]
- Sairam, R.K.; Dharmar, K.; Chinnusamy, V.; Meena, R.C. Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J. Plant Physiol. 2009, 166, 602–616. [Google Scholar] [CrossRef]
- Sarkar, R.K.; Das, A. Changes in anti-oxidative enzymes and antioxidants in relation to flooding tolerance in rice. J. Plant Biol. 2000, 27, 307–311. [Google Scholar]
- Lbrecht, G.; Mustroph, A.; Fox, T.C. Sugar and fructan accumulation during metabolic adjustment between respiration and fermentation under low oxygen conditions in wheat roots. Physiol. Plant. 2010, 120, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, A.; Albrecht, G. Tolerance of crop plants to oxygen deficiency stress: Fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol. Plant. 2010, 117, 508. [Google Scholar] [CrossRef]
- Ou, L.J.; Dai, X.Z.; Zhang, Z.Q.; Zou, X.X. Responses of pepper to waterlogging stress. Photosynthetica 2011, 49, 339–345. [Google Scholar] [CrossRef]
- Nataa, L.; Tanja, T.; Danijela, K.; Biljana, K. Modulations of the antioxidants defence system in two maize hybrids during flooding stress. J. Plant Res. 2021, 134, 237–248. [Google Scholar]
- Tian, L.X.; Bi, W.S.; Liu, X.; Sun, L.; Li, J. Effects of waterlogging stress on the physiological response and grain-filling characteristics of spring maize (Zea mays L.) under field conditions. Acta Physiol. Plant. 2019, 41, 63. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, C.X.; Zhang, Y. Effects of submergence-drought stresses on growth and physiological characteristics of salix rosthornii seedlings. Sci. Silvae Sin. 2013, 49, 164–170. [Google Scholar]
- Zhan, J.H.; Lan, Z.H. Effect of flooding on some physiological indexes of Panicum repens. Guihaia 2011, 31, 823–826. [Google Scholar]




| Gene Name | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| actin (Reference genes) | GGAACGGGTTGAGGAGAAAGAAG | GCAAGAACAAGATGAAGCACAGAGC |
| SOD (LOC21393965) | TTTTGGGAAACTGTTTTGGGG | ATGTTATTCGCCTGTCGCCT |
| SOD (LOC21405894) | GCCCACCTCCTACATGCTTA | GGTAATATGCGTGCTGCGTG |
| POD (LOC21396268) | CTTCCAACAAGCTCTACTACTCAT | GAGCACCGAGCTACTCCAAG |
| POD (LOC21389367) | ATCCACATTGTACCCAGCGT | ATAATGTAGGGCCGGGGGAT |
| CAT (LOC21407600) | TTTCTTACGATGGCCGCACT | AACAGTCACAGCCTACTTCGC |
| APX (LOC21409302) | TATGCTGAAGCCCATGCCAA | GGACTAAGATACCAGGCAGGC |
| MDHAR (LOC21393482) | TCCATCTGCAAGCTTGTTTTTCA | GGCACTGGTTGATCGTCTCA |
| GPX (LOC21407427) | TTGGAGGCGGAGTCTTCCTA | TTCACGGCGTAAACGCAAAG |
| GST (LOC21394898) | TCCAAAGGCGACCACAAGAA | TGTGGGTGTTTGAATTTACCGAA |
| GST (LOC21386514) | CGGCATGAGGGTCAGAGTAG | AGCTAGAACGGAGGCTTGG |
| OPR (LOC21397444) | TGCTAAAGTTCTCTGCTTGTTACT | AGCCGCATGGATCTCAACTC |
| OPR (LOC21409989) | GATCACCTTGATGCCGTGGA | CCATAGGCCGTGTAACGAGG |
| JAZ (LOC21402520) | GGTTCTTGGAAAAGCGACGG | AACTTGGTGAACCGCCTCC |
| JAZ (LOC21384207) | ACCACCTCAGATGCCTACCA | ACTTGAGCCAAATGCTCGCC |
| AOS (LOC21409613) | TCTGTTCAAGTGGCTCGGAC | CGATAAGCCAAGGGTCTCAGG |
| MYC2 (LOC21406020): | TGGTCGAATTGGGATCCACG | GAGCTGATCTGCTGGTTCGT |
| MYC2 (LOC21406208) | TCCCGACTTCTACCGGAGTT | ACTGTATTGGCCCTGTGTCG |
| MYC2 (LOC21398450) | AGGCTTAGCACATCCGATCA | CAATCACCGGCTTTCTCCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Huang, H.; Li, D.; Yang, F.; Cong, X.; Wu, S.; Zhu, W.; Qin, S.; Wen, Y. Physiological and Transcriptomic Analyses Reveal the Role of the Antioxidant System and Jasmonic Acid (JA) Signal Transduction in Mulberry (Morus alba L.) Response to Flooding Stress. Horticulturae 2024, 10, 1100. https://doi.org/10.3390/horticulturae10101100
Bai X, Huang H, Li D, Yang F, Cong X, Wu S, Zhu W, Qin S, Wen Y. Physiological and Transcriptomic Analyses Reveal the Role of the Antioxidant System and Jasmonic Acid (JA) Signal Transduction in Mulberry (Morus alba L.) Response to Flooding Stress. Horticulturae. 2024; 10(10):1100. https://doi.org/10.3390/horticulturae10101100
Chicago/Turabian StyleBai, Xuejiao, He Huang, Dan Li, Fei Yang, Xinyao Cong, Siqi Wu, Wenxu Zhu, Shengjin Qin, and Yibo Wen. 2024. "Physiological and Transcriptomic Analyses Reveal the Role of the Antioxidant System and Jasmonic Acid (JA) Signal Transduction in Mulberry (Morus alba L.) Response to Flooding Stress" Horticulturae 10, no. 10: 1100. https://doi.org/10.3390/horticulturae10101100
APA StyleBai, X., Huang, H., Li, D., Yang, F., Cong, X., Wu, S., Zhu, W., Qin, S., & Wen, Y. (2024). Physiological and Transcriptomic Analyses Reveal the Role of the Antioxidant System and Jasmonic Acid (JA) Signal Transduction in Mulberry (Morus alba L.) Response to Flooding Stress. Horticulturae, 10(10), 1100. https://doi.org/10.3390/horticulturae10101100






