Research Progress on Physical Preservation Technology of Fresh-Cut Fruits and Vegetables
Abstract
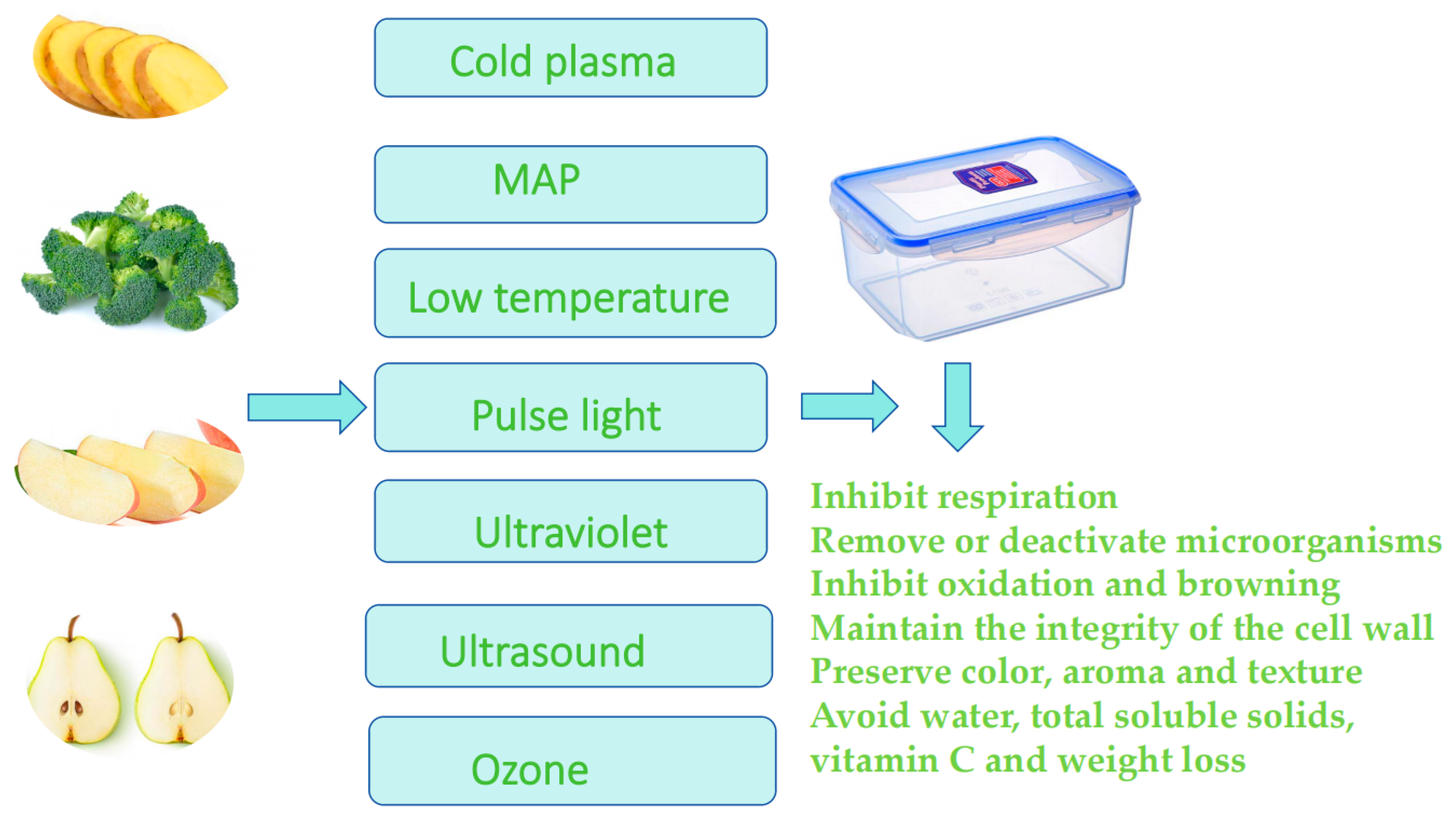
1. Introduction
2. Different Methods and Their Effects
2.1. The Preservative Effect of Low-Temperature Treatment on Fresh-Cut Fruits and Vegetables
2.2. Preservation of Fresh-Cut Fruits and Vegetables by Modified Atmosphere Packaging
| Species | Shape | Processing Conditions | Fresh-Keeping Effects | Reference Literature |
|---|---|---|---|---|
| Cucumber | Slices | Inoculated with Escherichia coli O157:H7, packaged in atmospheres with different gas compositions. | E. coli O157:H7 in fresh-cut cucumbers was efficiently inhibited under MAP (atmosphere = 2% O2, 7% CO2, 91% N2); visual appeal also maintained. | [42] |
| Grape | Clusters | Modified passive atmosphere (air), used as control. Modified active atmosphere (10 kPa CO2 and 20 kPa O2) plus chitosan. Stored at 5 °C for 14 d. | High-CO2-MAP plus chitosan was best for preservation of quality, nutrients, and sensorial parameters, and delayed spoilage of minimally processed table grapes. | [43] |
| Pear | Slice | Modified atmospheric packaging (MAP) in combination with 2% NatureSeal®. | Inhibited microbial growth and nutrient loss. | [44] |
| Lily | Bulbs | MAP1: 5% O2 + 5% CO2 + 90% N2; MAP2: 10% O2 + 5% CO2 + 85% N2; MAP3: 5% O2 + 10%CO2 + 85% N2; MAP4: 10% O2 + 10% CO2 + 80% N2. | Lipid peroxidation was inhibited, and the membrane integrity of Lanzhou lily bulbs was maintained. | [45] |
| Broccoli | Single-florets | HOMAP storage boxes were filled with 100% O2 and sealed, and control boxes contained normal air. | HOMAP lowered the content of substances with undesirable odors in fresh-cut broccoli by inhibiting the expression of specific enzymes. | [46] |
| Cucumber | Cubes | 0.5 MPa Ar, 1.0 MPa Ar, 1.5 MPa Ar and 1.5 MPa air for 1 h at 20 °C. (control without pressure), and then stored at 4 °C and 90% RH for 12 d. | Pressurized Ar treatments inhibited respiration, water loss, softening, chlorophyll degradation, and color change, and also prevented a decrease in ascorbic acid and soluble solids. Treated cucumbers had lower bacterial load. | [51] |
| Red chard leaves | Leaves | O2, He, N2 or N2O MAP. | MAP with He and O2 inhibited bacterial growth and decreased chlorophyll and vitamin C (VC) contents. | [52] |
| Potato | Slices | After 60 min of pressure at 4 MPa, 4% O2 + 2% CO2 + 94% N2 was applied. | Argon treatment successfully delayed the loss of moisture, VC, color and hardness, and microbial growth. | [53] |
2.3. Effects of Cold Plasma Treatment on Preservation of Fresh-Cut Fruits and Vegetables
2.4. Effects of Pulsed-Light Treatment on Preservation of Fresh-Cut Fruits and Vegetables
2.5. Effects of Ultrasound Treatment on Preservation of Fresh-Cut Fruits and Vegetables
2.6. Effect of UV Treatment on Preservation of Fresh-Cut Fruits and Vegetables
2.7. Effect of Ozone Treatment on Preservation of Fresh-Cut Fruits and Vegetables
3. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sucheta; Singla, G.; Chaturvedi, K.; Sandhu, P.P. Status and recent trends in fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA; London, UK, 2020; pp. 17–49. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Xie, B.; Sun, Z. Effect of ultrasound combined with ultraviolet treatment on microbial inactivation and quality properties of mango juice. Ultrason. Sonochem. 2020, 64, 105000. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Song, K.B. Antibacterial activity of the noni fruit extract against Listeria monocytogenes and its applicability as a natural sanitizer for the washing of fresh-cut produce. Food Microbiol. 2019, 84, 103260. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Paramasivam, N.; Vadivel, V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Res. Int. 2018, 111, 509–523. [Google Scholar] [CrossRef]
- Thakur, R.J.; Shaikh, H.; Gat, Y.; Waghmare, R.B. Effect of calcium chloride extracted from eggshell in maintaining quality of selected fresh-cut fruits. Int. J. Recycl. Org. Waste Agric. 2019, 8, 27–36. [Google Scholar] [CrossRef]
- Sandarani, M.; Dasanayaka, D.; Jayasinghe, C. Strategies used to prolong the shelf life of fresh commodities. J. Agric. Sci. Food Res. 2018, 9, 1–6. [Google Scholar]
- Mendoza, I.C.; Luna, E.O.; Pozo, M.D.; Vásquez, M.V.; Montoya, D.C.; Moran, G.C.; Romero, L.G.; Yépez, X.; Salazar, R.; León, J.C.; et al. Conventional and non-conventional disinfection methods to prevent microbial contamination in minimally processed fruits and vegetables. LWT 2022, 165, 113714. [Google Scholar] [CrossRef]
- Koh, P.; Noranizan, M.; Karim, R.; Hanani, Z.; Rosli, S.; Hambali, N. Enzymatic activity of alginate coated and pulsed light treated fresh-cut cantaloupes (Cucumis melo L. var. reticulatus cv. Glamour) during chilled storage. Int. Food Res. J. 2019, 26, 547–556. [Google Scholar]
- Muhammad, R.K.; Lukas, V.; Muhammad, B.S.; Elena, T.; Ales, R. Comparative influence of active PLA and PP films on the quality of minimally processed cherry tomatoes. Food Packag. Shelf 2024, 44, 101313. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Novel technologies to improve food safety and quality. Curr. Opin. Food Sci. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Khouryieh, H.A. Novel and emerging technologies used by the US food processing industry. Innov. Food Sci. Emerg. Technol. 2020, 67, 102559. [Google Scholar] [CrossRef]
- Byun, S.; Chen, C.H.; Yin, H.B.; Patel, J. Antimicrobial effect of natural fruit extracts against salmonella on whole and fresh-cut cucumbers. J. Food Process. Preserv. 2022, 46, e16437. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality control of fresh-cut apples after coating application. Foods 2019, 8, 189. [Google Scholar] [CrossRef]
- Irazoqui, M.; Romero, M.; Paulsen, E.; Barrios, S.; Pérez, N.; Faccio, R.; Lema, P. Effect of power ultrasound on quality of fresh-cut lettuce (cv. Vera) packaged in passive modified atmosphere. Food Bioprod. Process 2019, 117, 138–148. [Google Scholar] [CrossRef]
- Sarkar, S.; Akhter, S.; Roy, J.; Wazed, M.A.; Abedin, R.; Neogie, S.; Mishat, K.B.; Sarker, M.S.H. Preventing enzymatic browning of freshly cut green bananas through immersion in normal water, lemon juice, and coconut water. Food Sci. Nutr. 2024, 12, 6612–6626. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Fan, L. Quality changes in fresh-cut asparagus with ultrasonic-assisted washing combined with cinnamon essential oil fumigation. Postharvest Biol. Technol. 2022, 187, 111873. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, M.A. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The effects of cold plasma-activated water treatment on the microbial growth and antioxidant properties of fresh-cut pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT 2019, 115, 108447. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.; Aheto, J.H.; Agyekum, A.A.; Zhou, C. Effect of sequential multi-frequency ultrasound washing processes on quality attributes and volatile compounds profiling of fresh-cut Chinese cabbage. LWT 2020, 117, 108666. [Google Scholar] [CrossRef]
- Valdivia-Nájar, C.G.; Martín-Belloso, O.; Soliva-Fortuny, R. Impact of pulsed light treatments and storage time on the texture quality of fresh-cut tomatoes. Innov. Food Sci. Emerg. Technol. 2018, 45, 29–35. [Google Scholar] [CrossRef]
- Pace, B.; Capotorto, I.; Cefola, M.; Minasi, P.; Montemurro, N.; Carbone, V. Evaluation of quality, phenolic and carotenoid composition of fresh-cut purple Polignano carrots stored in modified atmosphere. J. Food Compos. Anal. 2020, 86, 103363. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Ghahfarrokhi, M.G.; Eş, I. Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest Biol. Technol. 2017, 125, 26–34. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Zheng, H.; Zheng, L. Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 125562. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, G.B.; Fawole, O.A.; Verboven, P.; Zhang, X.R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K. Postharvest precooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. 2019, 60, 940–975. [Google Scholar] [CrossRef]
- Han, C.; Ji, Y.; Li, M.; Li, X.; Jin, P.; Zheng, Y. Influence of wounding intensity and storage temperature on quality and antioxidant activity of fresh-cut Welsh onions. Sci. Hortic. 2016, 212, 203–209. [Google Scholar] [CrossRef]
- Berno, N.D.; Tezotto-Uliana, J.V.; Tadeu, D.S.D.C.; Kluge, R.A. Storage temperature and type of cut affect the biochemical and physiological characteristics of fresh-cut purple onions. Postharvest Biol. Technol. 2014, 93, 91–96. [Google Scholar] [CrossRef]
- Feng, K.; Hu, W.; Jiang, A.; Saren, G.; Shao, W. Growth of Salmonella spp. and Escherichia coli O157:H7 on fresh-cut fruits stored at different temperatures. Foodborne Pathog. Dis. 2017, 14, 510–517. [Google Scholar] [CrossRef]
- Feng, K.; Sarengaowa; Ma, J.; Hu, W. Modelling the growth of Listeria monocytogenes on fresh-cut cucumbers at various storage temperatures. Horticulturae 2024, 10, 667–672. [Google Scholar] [CrossRef]
- Aguila, J.S.D.; Sasaki, F.F.; Heiffig, L.S.; Edwin, M.M.O.; Jacomino, A.P.; Kluge, R.A. Fresh-cut radish using different cut types and storage temperatures. Postharvest Biol. Technol. 2006, 40, 149–154. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, T.; Jiang, Z.; Zhu, Y. Low temperature preservation for perishable ready to eat foods: Not entirely effective for control of L. monocytogenes. Trends Food Sci. Technol. 2023, 142, 104228. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT 2021, 144, 111223. [Google Scholar] [CrossRef]
- Marrero, A.; Kader, A.A. Optimal temperature and modified atmosphere for keeping quality of fresh-cut pineapples. Postharvest Biol. Technol. 2006, 39, 163–168. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Liu, Y.; Li, L.; Liang, F.; Wang, X.; Li, D.; Pan, N.; Li, X.; Yang, X. Effects of short-term high oxygen pre-stimulation on browning resistance and low-temperature tolerance of fresh-cut potatoes in supercooled storage. Food Bioprocess Technol. 2024, 17, 709–721. [Google Scholar] [CrossRef]
- Zhao, S.; Han, X.; Liu, B.; Wang, S.; Guan, W.; Wu, Z.; Theodorakis, P.E. Shelf-life prediction model of fresh-cut potato at different storage temperatures. J. Food Eng. 2022, 317, 110867. [Google Scholar] [CrossRef]
- Opara, U.L.; Caleb, O.J.; Belay, Z.A. Modified Atmosphere Packaging for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–259. [Google Scholar]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Impacts of low and super-atmospheric oxygen concentrations on quality attributes, phytonutrient content and volatile compounds of minimally processed pomegranate arils (cv. Wonderful). Postharvest Biol. Technol. 2017, 124, 119–127. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, X.; Ma, Y.; Guan, H.; Wang, D. Inhibitory effect of modified atmosphere packaging on Escherichia coli O157:H7 in fresh-cut cucumbers (Cucumis sativus L.) and effectively maintain quality during storage. Food Chem. 2021, 369, 130969. [Google Scholar] [CrossRef]
- Liguori, G.; Sortino, G.; Gullo, G.; Inglese, P. Effects of modified atmosphere packaging and chitosan treatment on quality and sensorial parameters of minimally processed cv. ‘italia’ table grapes. Agronomy 2021, 11, 328. [Google Scholar] [CrossRef]
- Siddiq, R.; Auras, R.; Siddiq, M.; Dolan, K.D.; Harte, B. Effect of modified atmosphere packaging (MAP) and NatureSeal® treatment on the physico-chemical, microbiological, and sensory quality of fresh-cut d’Anjou pears. Food Packag. Shelf 2020, 23, 100454. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wang, X.; Liu, X.; Zhu, X.; Zhang, J. Integration of metabolome and transcriptome profiling reveals the effect of modified atmosphere packaging (MAP) on the browning of fresh-cut Lanzhou lily (Lilium davidii var. unicolor) bulbs during storage. Foods 2023, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, L.; Tao, J.; Han, L.; Wang, H.; Zhao, X.; Zuo, J.; Zheng, Y. High-oxygen-modified atmospheric packaging delays flavor and quality deterioration in fresh-cut broccoli. Food Chem. 2024, 450, 139517. [Google Scholar] [CrossRef]
- Pinela, J.; Barreira, J.C.M.; Barros, L.; Antonio, A.L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Postharvest quality changes in fresh-cut watercress stored under conventional and inert gasenriched modified atmosphere packaging. Postharvest Biol. Technol. 2016, 112, 55–63. [Google Scholar] [CrossRef]
- Farina, V. Effects of argon-based and nitrogen-based modified atmosphere packaging technology on the quality of pomegranate (Punica granatum L. cv. Wonderful) arils. Foods 2021, 10, 370. [Google Scholar] [CrossRef]
- Silveira, A.C.; Araneda, C.; Hinojosa, A.; Escalona, V.H. Effect of non-conventional modified atmosphere packaging on fresh cut watercress (Nasturtium officinale R. Br.) quality. Postharvest Biol. Technol. 2014, 92, 114–120. [Google Scholar] [CrossRef]
- Mao, L.; Mhaske, P.; Zing, X.; Kasapis, S.; Majzoobi, M.; Farahnaky, A. Cold plasma: Microbial inactivation and effects on quality attributes of fresh and minimally processed fruits and ready-to-eat vegetables. Trends Food Sci. Technol. 2021, 116, 146–175. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, M.; Zhan, Z.; Adhikari, B. Changes in quality characteristics of fresh-cut cucumbers as affected by pressurized argon treatment. Food Bioprocess Technol. 2014, 7, 693–701. [Google Scholar] [CrossRef]
- Tomás-Callejas, A.; María, B.; Pedro, A.R.; Artés, F.; Artés-Hernández, F. Innovative active modified atmosphere packaging improves overall quality of fresh-cut red chard baby leaves. LWT-Food Sci. Technol. 2011, 44, 1422–1428. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, M.; Sakamon, D.; Guo, Z. Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biol. Technol. 2019, 149, 159–165. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Bagheri, H.; Abbaszadeh, S. Effect of cold plas1ma on quality retention of fresh-cut Produce. J. Food Qual. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Segura-Ponce, L.A.; Reyes, J.E.; Troncoso-Contreras, G.; Valenzuela-Tapia, G. Effect of low-pressure cold plasma (LPCP) on the wettability and the inactivation of escherichia coli and listeria innocua on fresh-cut apple (Granny Smith) skin. Food Bioprocess Technol. 2018, 11, 1075–1086. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Wang, S. Study on the bactericidal mechanism of atmospheric-pressure low-temperature plasma against escherichia coli and its application in fresh-cut cucumbers. Molecules 2018, 23, 975. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, R.L.; Pietro, R. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food Sci. Emerg. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Zheng, H.; Miao, T.; Shi, J.; Tian, M.; Wang, L.; Geng, X.; Zhang, Q. Effect of cold plasma treatment on the quality of fresh-cut hami melons during chilling storage. Horticulturae 2024, 10, 735. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, W.; Liu, Z.; Jia, M.; Zhang, Q.; Gao, G.; Zhang, Z. Optimization of cold plasma processing conditions for fresh-cut kiwifruit slices by using response surface methodology. Food Sci. Technol. Int. 2024, 10820132231225778. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Liu, D.; Wang, W.; Niu, J.; Xia, Y.; Qi, Z.; Zhao, Z.; Song, Y. Effect of nonthermal plasma-activated water on quality and antioxidant activity of fresh-cut kiwifruit. IEEE Trans. Plasma Sci. 2019, 47, 4811–4817. [Google Scholar] [CrossRef]
- Schnabel, U.; Handorf, O.; Stachowiak, J.; Boehm, D.; Weit, C.; Weihe, T.; Schäfer, J.; Below, H.; Bourke, P.; Ehlbeck, J. Plasma-functionalized water: From bench to prototype for fresh-cut lettuce. Food Eng. Rev. 2021, 13, 115–135. [Google Scholar] [CrossRef]
- Aihaiti, A.; Maimaitiyiming, R.; Wang, L.; Wang, J. Processing of fresh-cut potato using plasma-activated water prepared by decreasing discharge frequency. Foods 2023, 12, 2285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, T.; Cong, K.; Suo, A.; Wu, C. Influence of cold plasma on quality attributes and aroma compounds in fresh-cut cantaloupe during low temperature storage. LWT 2022, 154, 112893. [Google Scholar] [CrossRef]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of cold plasma treatment on the functional properties of fresh-cut apples. J. Agric. Food Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef]
- Misra, N.N.; Martynenko, A.; Chemat, F.; Paniwnyk, L.; Barba, F.J.; Jambrak, A.R. Thermodynamics, transport phenomena, and electrochemistry of external field-assisted nonthermal food technologies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1832–1863. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Ramos-Villarroel, A.; Martín-Belloso, O.; Soliva-Fortuny, R. Intense light pulses: Microbial inactivation in fruits and vegetables. CyTA-J. Food 2013, 11, 234–242. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Bolton, J.R. An approach to standardize methods for fluence determination in bench-scale pulsed light experiments. Food Bioprocess Technol. 2016, 9, 1040–1048. [Google Scholar] [CrossRef]
- Llano, K.R.A.; Marsellés-Fontanet, A.R.; Martín-Belloso, O.; Soliva-Fortuny, R. Impact of pulsed light treatments on antioxidant characteristics and quality attributes of fresh-cut apples. Innov. Food Sci. Emerg. 2016, 33, 206–215. [Google Scholar] [CrossRef]
- Gómez, P.L.; García-Loredo, A.; Nieto, A.; Salvatori, D.M.; Guerrero, S.; Alzamora, S.M. Effect of pulsed light combined with an antibrowning pretreatment on quality of fresh-cut apple. Innov. Food Sci. Emerg. 2012, 16, 102–112. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Pobiega, K.; Witrowa-Rajchert, D.; Nowacka, M. Impact of pulsed light treatment on the quality properties and microbiological aspects of red bell pepper fresh-cuts. LWT 2021, 149, 111906. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A. Microbiological stability and quality of pulsed light treated cantaloupe (Cucumis melo L. reticulatus cv. Glamour) based on cut type and light fluence. J. Food Sci. Technol. 2016, 53, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Lopes, M.M.; Silva, E.O.; Laurent, S.; Charles, F.; Urban, L.; de Miranda, M.R.A. The influence of pulsed light exposure mode on quality and bioactive compounds of fresh-cut mangoes. J. Food Sci. Technol. 2017, 54, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A. Sensory quality and flavour of alginate coated and repetitive pulsed light treated fresh-cut cantaloupes (Cucumis melo L. Var. Reticulatus Cv. Glamour) during storage. J. Food Sci. Technol. 2019, 56, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, A.E.D.; Ribeiro, L.B.; da Silveira, M.R.S.; de Oliveira Silva, E.; Germano, T.A.; Aziz, S.; de Miranda, M.R.A.; Gallão, M.I.; Fonseca, K.S.; Puschmann, R. Effect of pulsed light fluences on quality, biochemistry and physiology of fresh-cut mangoes during refrigerated storage. Sci. Hortic. 2023, 321, 112328. [Google Scholar] [CrossRef]
- Demirci, A.; Krishnamurthy, K. Pulsed ultraviolet light. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Cánovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; Blackwell Publishing Ltd.: New Delhi, India, 2011; pp. 249–261. [Google Scholar]
- Food and Drug Administration (FDA). CFR-Code of Federal Regulations. Title 21 Food and Drugs; Food and Drug Administration: Silver Spring, MD, USA, 2020; pp. 500–599.
- Salehi, F. Application of pulsed light technology for fruits and vegetables disinfection: A review. J. Appl. Microbiol. 2022, 132, 2521–2530. [Google Scholar] [CrossRef]
- Bhilwadikar, T.; Pounraj, S.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Decontamination of microorganisms and pesticides from fresh fruits and vegetables: A comprehensive review from common household processes to modern techniques. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1003–1038. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2020, 70, 105293. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Mason, T.J.; Riera, E.; Vercet, A.; Lopez-Buesa, P. Application of ultrasound. In Emerging Technologies for Food Processing; Sun, D., Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA; London, UK, 2005; pp. 323–351. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, X.; Zheng, J.; Wang, T.; You, X.; Liu, H.; Liu, X. The effect of ultrasonic on reducing anti-browning minimum effective concentration of purslane extract on fresh-cut potato slices during storage. Food Chem. 2021, 343, 128401. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, L.; Pang, L.; Chen, X.; Jia, X.; Li, X. Ultrasound treatment inhibits browning and improves antioxidant capacity of fresh-cut sweet potato during cold storage. RSC Adv. 2020, 10, 9193–9202. [Google Scholar] [CrossRef]
- Shi, J.; Wang, S.; Yao, J.; Cui, M.; Hu, B.; Wang, J.; Li, F.; Wang, S.; Tong, R.; Li, M.; et al. Ultrasound treatment alleviates external pericarp browning and improves fruit quality of pomegranate during storage. J. Sci. Food Agric. 2024, 104, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, M.; Chen, H. Effect of ultrasound treatment combined with carbon dots coating on the microbial and physicochemical quality of fresh-cut cucumber. Food Bioprocess Technol. 2020, 13, 648–660. [Google Scholar] [CrossRef]
- Yildiz, G.; Izli, G.; Aadil, R.M. Comparison of chemical, physical, and ultrasound treatments on the shelf life of fresh-cut quince fruit (Cydonia oblonga Mill.). J. Food Process. Preserv. 2020, 44, e14366. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Jiang, F. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochem. 2019, 54, 162–170. [Google Scholar] [CrossRef]
- Yildiz, G.; Aadil, R.M. Comparative analysis of antibrowning agents, hot water and high-intensity ultrasound treatments to maintain the quality of fresh-cut mangoes. J. Food Sci. Technol. 2022, 59, 202–211. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, W.; Zhao, W.; Zhao, S.; Wang, P.; Zhao, X.; Wang, D. Effect of ultrasound combinated with sodium hypochlorite treatment on microbial inhibition and quality of fresh-cut cucumber. Foods 2023, 12, 754. [Google Scholar] [CrossRef]
- Song, L.; Yang, H.; Cheng, S.; Zhang, Z.; Zhang, L.; Su, R.; Li, Y.; Zhan, X.; Yang, B.; Lin, L.; et al. Combination effects of ultrasound and citral nanoemulsion against Shigella flexneri and the preservation effect on fresh-cut carrots. Food Control 2024, 155, 110069. [Google Scholar] [CrossRef]
- Santos, J.G.; Fernandes, F.A.N.; de Siqueira Oliveira, L.; de Miranda, M.R.A. Influence of ultrasound on fresh-cut mango quality through evaluation of enzymatic and oxidative metabolism. Food Bioprocess Technol. 2015, 8, 1532–1542. [Google Scholar] [CrossRef]
- Hägele, F.; Nübling, S.; Schweiggert, R.M.; Baur, S.; Weiss, A.; Schmidt, H.; Alexander Menegat, A.; Gerhards, R.; Carle, R. Quality improvement of fresh-cut endive (Cichorium endivia L.) and recycling of washing water by low-dose UV-C irradiation. Food Bioprocess Technol. 2016, 9, 1979–1990. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Balbinot Filho, C.A.; Borges, C.D. Effects of UV-C radiation on minimally processed lettuce and apple: A review. Braz. J. Food Technol. 2020, 23, 1–13. [Google Scholar] [CrossRef]
- Gutiérrez, D.R.; Char, C.; Escalona, V.H.; Chaves, A.R.; Rodríguez, S.d.C. Application of UV-C radiation in the conservation of minimally processed rocket (Eruca sativa Mill.). J. Food Process. Preserv. 2015, 39, 3117–3127. [Google Scholar] [CrossRef]
- Ortiz-Araque, C.; Darré, M.; Civello, P.; Vicente, A.R. Short UV-C treatments extend the shelf-life of fresh-cut strawberries (Fragaria x ananassa Duch cv. Camarosa). Ing. Investig. 2022, 42. [Google Scholar] [CrossRef]
- Avalos-Llano, K.R.; Molina, R.S.; Sgroppo, S.C. UV-C Treatment Applied alone or combined with orange juice to improve the bioactive properties, microbiological, and sensory quality of fresh-cut strawberries. Food Bioprocess Technol. 2020, 13, 1528–1543. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Robles, P.A.; Gómez, P.A.; Tomás-Callejas, A.; Artés, F.; Martínez-Hernández, G.B. Quality changes of fresh-cut watermelon during storage as affected by cut intensity and UV-C pre-treatment. Food Bioprocess Technol. 2021, 14, 505–517. [Google Scholar] [CrossRef]
- Han, C.; Zhen, W.; Chen, Q.; Fu, M. UV-C irradiation inhibits surface discoloration and delays quality degradation of fresh-cut stem lettuce. LWT 2021, 147, 111533. [Google Scholar] [CrossRef]
- Gutiérrez, D.R.; Rodríguez, S.D.C. Combined effect of UV-C and ozone on bioactive compounds and microbiological quality of fresh-cut rocket leaves. Am. J. Food Sci. Technol. 2019, 7, 71–78. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. UV-C treatment maintains quality and enhances antioxidant capacity of fresh-cut strawberries. Postharvest Biol. Technol. 2019, 156, 110945. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z. Minimal processing of produce using a combination of UV-C irradiation and ultrasound-assisted washing. LWT 2023, 182, 114901. [Google Scholar] [CrossRef]
- Garzón-García, A.M.; Ruiz-Cruz, S.; Dussán-Sarria, S.; Hleap-Zapata, J.I.; Márquez-Ríos, E.; Del-Toro-Sánchez, C.L.; Tapia-Hernández, J.A.; Canizales-Rodríguez, D.F.; Ocaño-Higuera, V.M. Effect of UV-C Postharvest Disinfection on the Quality of Fresh-Cut ‘Tommy Atkins’ Mango. Pol. J. Food Nutr. Sci. 2023, 73, 39–49. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Plessas, S.; Ceciu, S.; Lazar, V.; Mantzourani, I.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Evaluation of ozone efficacy on the reduction of microbial population of fresh-cut lettuce (Lactuca sativa) and green bell pepper (Capsicum annuum). Food Control 2013, 30, 491–496. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A.; Forney, C.F. Effects of ozone on major antioxidants and microbial populations of fresh-cut papaya. Postharvest Biol. Technol. 2014, 89, 56–58. [Google Scholar] [CrossRef]
- Sripong, K.; Uthairatanakij, A.; Jitareerat, P. Impact of gaseous ozone on microbial contamination and quality of fresh-cut durian. Sci. Hortic. 2022, 294, 110799. [Google Scholar] [CrossRef]
- Calder, B.L.; Skonberg, D.I.; Davis-Dentici, K.; Hughes, B.H.; Bolton, J.C. The effectiveness of ozone and acidulant treatments in extending the refrigerated shelf life of fresh-cut potatoes. J. Food Sci. 2011, 76, S492–S498. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Zhang, Y.; Zhao, Q.; Hu, W. Effects of aqueous ozone treatment on microbial growth, quality, and pesticide residue of fresh-cut cabbage. Food Sci. Nutr. 2021, 9, 52–61. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Ölmez, H. Effectiveness of organic acid, ozonated water and chlorine dippings on microbial reduction and storage quality of fresh-cut iceberg lettuce. J. Sci. Food Agric. 2007, 87, 2609–2616. [Google Scholar] [CrossRef]
- Lin, F.; Lv, K.; Ma, S.; Wang, F.; Li, J.; Wang, L. Effects of ozone treatment on storage quality and antioxidant capacity of fresh-cut water fennel [Oenanthe javanica]. Food Sci. Technol. 2023, 43, e108422. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yang, S.; Li, C.; Li, L.; Gao, S.; Wu, Z. Mechanism of ozone treatment in delayed softening of fresh-cut kiwifruit during storage. Postharvest Biol. Technol. 2023, 204, 112469. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, L.; Wang, T.; Gao, S.; Zhang, S.; Wu, Z. Transcriptome analysis reveals ozone treatment maintains ascorbic acid content in fresh-cut kiwifruit by regulating phytohormone signalling pathways. Food Res. Int. 2024, 191, 114699. [Google Scholar] [CrossRef]
- Li, C.; Tao, J.; Wu, Z. Gaseous ozone regulates reactive oxygen species metabolism and ascorbate–glutathione cycle to delay the senescence of fresh-cut red pitaya (Selenicereus undatus) fruit. Sci. Hortic. 2023, 312, 111839. [Google Scholar] [CrossRef]
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A.A. Ozone-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov. Food Sci. Emerg. 2010, 11, 666–671. [Google Scholar] [CrossRef]
- Liu, C.; Ma, T.; Hu, W.; Tian, M.; Sun, L. Effects of aqueous ozone treatments on microbial load reduction and shelf life extension of fresh-cut apple. Int. J. Food Sci. Technol. 2016, 51, 1099–1109. [Google Scholar] [CrossRef]
- Botondi, R.; de Sanctis, F.; Moscatelli, N.; Vettraino, A.M.; Catelli, C.; Mencarelli, F. Ozone fumigation for safety and quality of wine grapes in postharvest dehydration. Food Chem. 2015, 188, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Chrysargyris, A. Postharvest ozone application for the preservation of fruits and vegetables. Food Rev. Int. 2017, 33, 270–315. [Google Scholar] [CrossRef]
- Shezi, S.; Magwaza, L.; Mditshwa, A.; Zeray Tesfay, S.Z. Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Sci. Hortic. 2020, 269, 109397. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changs in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
| Species | Shape | Processing Condition | Fresh-Keeping Effect | Reference |
|---|---|---|---|---|
| Welsh onion | Slices, pieces, and shreds | Stored at 4 and 20 °C. | High storage temperature reduces quality and shelf life. | [30] |
| Onion | Slices and cubes | Stored at different temperatures (0, 5, 10 and 15 °C) with 85–90% relative humidity (RH) for 15 d. | 0 °C storage resulted in lower pungency and respiration and fewer changes in total phenolics, anthocyanin and quercetin levels. Physicochemical properties and appearance were maintained at 0 °C. | [31] |
| Honeydew melon, cantaloupe, watermelon, pitaya, mango, papaya, and pineapple | Slices or wedges | Stored at 5 °C, 13 °C, and 25 °C after inoculation with pathogens. | Pathogens grew on fresh-cut fruits (except pineapple) at 13 °C and 25 °C. | [32] |
| Cucumber | 0.5 cm cubes | Stored at 5, 10, 15, 20, 25, 30, and 35 °C. | The conservation of fresh-cut cucumbers at temperatures below 5 °C to guarantee product safety. | [33] |
| Radish | Slices and shreds | Three storage temperatures (1, 5 and 10 °C). | Fresh-cut radish cubes stored at 5 °C and 1 °C had lower respiration rates and nutrient content. | [34] |
| Apple | Slices | Packaged in tannin-loaded chitosan+gelatin films and stored at 4 °C. | Decreased weight loss, browning, lipid oxidase activity, and malondialdehyde (MDA) during 10 d storage < 4 °C. | [36] |
| Pineapple | Wedges 1 cm thick | Stored at 0 °C and 10 °C. | The shelf life at 10 °C ranges from 4 to 10 d, and the shelf life at 0 °C exceeds 14 d. | [37] |
| Potato | 2 cm cubes | SHOP (80% O2, 4 °C), SC (21% O2, −2 °C), and SHOP + SC (80% O2, −2 °C). | The browning and softening of fresh-cut potato were delayed by inhibiting the activity of related enzymes. | [38] |
| Potato | 3 mm slices | Dipped in chlorine dioxide solution (100 mg L−1), citric acid solution (1.5%) and potassium sorbate solution (0.1%), stored at 0 °C, 4 °C, 7 °C and 10 °C. | The weight loss rate, polyphenol oxidase (PPO) activity and total number of colonies decreased significantly, and 0 °C was the best. | [39] |
| Species | Shape | Processing Condition | Fresh-Keeping Effect | Reference Literature |
|---|---|---|---|---|
| Cucumbers | Slices | E. coli was inoculated and then treated with atmospheric low-temperature plasma. | Inhibited bacterial growth, retained moisture, sugar, acidity, VC and color, and improved the aroma of fresh-cut cucumbers. | [57] |
| Melon | Trapezoidal | Gas plasma for 30 or 60 min. | The 30-min treatment group showed better antibacterial and antioxidant capacity. | [58] |
| Hami melon | Long strips | Voltage 120 kV, frequency 130 Hz, distance 60 mm, time 150 s. | Inhibited oxidation, reduced microbial contamination, and extended the shelf life of fresh-cut cantaloupes. | [59] |
| Kiwifruit | Slices | Voltages of 15, 25 and 35 kV, discharge times of 90 s (45 s each side), 110 s (55 s each side) and 130 s (65 s each side). | Significantly decreased bacterial level in fresh-cut kiwi fruit. | [60] |
| Kiwifruit | cubes | 1 mL of plasma-activated water (PAW) and 1 mL of sterile water as control sample were separately sprayed on fresh-cut kiwifruit (FCK) and stored at 4 °C for 8 d. | PAW reduced the microbial population of FCK by 1.8 log CFU/g. The activities of superoxide dismutase, peroxidase, and catalase in PAW-treated FCK samples were higher. | [61] |
| Lettuce | Slices | Rinse 30 s with plasma-functionalized water. | The effective removal of microorganisms did not affect the organelles of lettuce tissue. | [62] |
| Potato | Cubes | PAW was prepared using a frequency of 200 Hz (200 Hz-PAW). Its efficacy was compared with that of PAW prepared using 10 kHz. | PAW inactivated the browning-related enzymes PPO and POD, lowering the browning index and inhibiting browning. The fewest aerobic mesophilic, mold, and yeast counts during storage were measured with 200 Hz-PAW treatment. | [63] |
| Cantaloupe | Circular sections | Treatment with cold plasma at 40 kV for 90 s. | Microbial growth was significantly inhibited and the surface color, soluble solid content (SSC), VC content and pulp firmness were maintained. | [64] |
| Species | Shape | Processing Condition | Fresh-Keeping Effect | Reference Literature |
|---|---|---|---|---|
| Apple | Wedges | Rinsed with 1% w/v N-acetylcysteine and 0.5% w/v CaCl2 and pulsed with broad-spectrum light at an overall radiant exposure of 4, 8, 12 and 16 J cm−2. | Inhibited microbial growth and maintained antioxidant capacity. | [70] |
| Apple | Slices | 1% (w/v) VC plus 0.1% (w/v) CaCl2 and 2.4, 11.9, 23.9, 71.6 and 119.4 J cm−2 PL. | Inhibited microbial growth and prevented decline of soluble solids and hardness. | [71] |
| Red bell pepper | Pieces | 4 and 32 J cm−2 PL treatments with exposure times varying between 3.5 and 26.5 min. | Inhibited microbial growth and retained content of VC, phenols and carotenoids. | [72] |
| Cantaloupe | Cuboid, triangular prism and spherical | Number of pulses were 9, 26, 39 and 52 with corresponding fluences of 2.7, 7.8, 11.7 and 15.6 J cm−2. | The spherical samples treated with 7.8 J cm−1 PL had lower microbial count and higher VC content. | [73] |
| Mangos | Cubes | Control (0 P), 1 pulse (1 P; 0.7 J cm−2), 4 successive pulses (4 P; 2.80 J cm−2) and 1 pulse per day for 4 d (1 P 4 D; 2.80 J cm−2) before storage for 7 d at 6 °C. | Treatment with four continuous pulses (4 P; 2.80 J cm−2) resulted in higher VC and carotenoid content as well as antioxidant activity. | [74] |
| Cantaloupe | Spherical | A fluence of 0.9 J cm−2 was applied every 48 h during storage until day 26. | Sensory quality was maintained effectively with no change in the contents of sugar and organic acids, and minimal loss of total aroma compound concentration. | [75] |
| Mango | Cubes | Xenon gas, 190 mm width, lateral position, with a capacity of one pulse every 15 s and 0.3 × 104 J m−2 per pulse power (100% lamp potency). | PL irradiation maintained the integrity of the cell wall and inhibited the decrease in VC content and the color change during storage. | [76] |
| Species | Shape | Processing Condition | Fresh-Keeping Effect | Reference Literature |
|---|---|---|---|---|
| Cucumber | Slices | The power intensity was 226 W cm−2, the frequency was 20 kHz, and the treatment lasted for 10 min. | Bacterial growth was inhibited significantly, the minimum respiration rate, the weight, and the SSC content were maintained, and the MDA concentration of fresh-cut cucumber was low. | [87] |
| Quince | Slices | Slices were treated in ultrasonic bath with frequency of 28 kHz, intensity of 100 kWm−3, power of 50 W and time of 15 min. | Inactivated browning enzymes to prevent enzymatic browning. | [88] |
| Cucumber | Slices | Cucumber was treated with ultrasound (20 kHz) at different times (5, 10 and 15 min) and then stored in the modified atmospheric packaged at 4 °C for 15 d. | US treatment inhibited the growth of mold and yeast in MAP fresh-cut cucumber. | [89] |
| Mango | Slices | Immerse in 0.5% (w/w) anti-browning preservative for 3 min and ultrasonic water bath for 4 min. | The browning enzyme activity was effectively inhibited, and the color and bioactive substance content were maintained. | [90] |
| Cucumber | Slices | US for 5 min, 10 min, and 15 min plus rinsing with 50, 75, 100 ppm NaOCl. | Inhibited microbial contamination, maintained the integrity of cell membranes and tissue hardness, and reduced water loss. | [91] |
| Carrot | Cubes | US plus citral nanoemulsion (CLON): combined treatment with CLON (0.10, 0.15 mg mL−1) and US (115, 230 and 345 W cm−2). | 0.15 mg mL−1 of CLON combined with US (20 kHz, 345 W cm−2) for 9 min significantly improved the bactericidal effect against Sh. flexneri. | [92] |
| Species | Shape | Processing Condition | Fresh-Keeping Effect | Reference Literature |
|---|---|---|---|---|
| Strawberries | Wedges | Brief UV-C treatments with different combinations of radiation intensity (0, 9, or 36 W m−2) and dose (0, 2, or 4 kJ m−2). | UV-C irradiation with 4 kJ m−2 at an intensity of 36 W m−2 reduced decay, juice leakage, dehydration softening, and yeast/mold spores. Freshness, color, and shelf life were maintained. | [98] |
| Strawberries | Slices | UV-C treatment (5.8 kJ m−2) alone and combined with the addition of orange juice for 12 d at 0 °C. | Both UV-C treatments alone and combined with juice immersion reduced microbial load; UV-C applied alone allowed obtaining the highest microbial reductions. | [99] |
| Watermelon | Cylinders | Fruit cylinders irradiated with UV-C at a dose of 2.4 (exposure time of 60 s), 4.8 (120 s) and 7.2 kJ m−2 (190 s). | The growth of microorganisms was significantly inhibited, and phenolic content was maintained. | [100] |
| Stem lettuce | Slices | Treated with different doses (0, 1, 4, 8 or 12 kJ m−2) of UV-C, then stored at 4 °C for 6 d. | UV-C treatment decreased the degradation of chlorophyll, the loss of VC and the accumulation of phenolic compounds. UV-C treatment did not affect PPO or POD activity, but it did inhibit PAL. | [101] |
| Rocket leaves | Slices | Separate application of UV-C (25 kJ m−2, 380 s) and O3 gaseous (2.5 mg L−1 for 10 min) treatments and of their combination were studied to evaluate the effect of combined treatments on microbial counts. | The UV-C treatment was better at reducing microorganisms present, and non-significant differences were found regarding the combined treatment. | [102] |
| Strawberries | Wedges | The dose of UV-C used in this experiment was 4.0 kJ m−2. | Inhibited microbial growth, promoted the production of ROS, and increased the content of total phenols, total anthocyanins and individual phenolic compounds. | [103] |
| Lettuce and cherry tomatoes | Pieces | US-free chlorine (FC)/peracetic acid (PAA) (5 min), US-FC/PAA-UV (3 min; 1.71 kJ m−2). | US-FC/PAA-UV was more effective in reducing microbial colonization on products with smooth surfaces, such as cherry tomatoes. | [104] |
| Mango | Spear | A UV-C dose of 6 kJ m−2 established. | The microbiological safety and surface color of fresh-cut mango were maintained, and the total carotenoid content was increased. | [105] |
| Species | Shape | Processing Condition | Fresh-Keeping Effect | Reference Literature |
|---|---|---|---|---|
| Lettuce and bell peppers | Slices | Dipped in continuously ozonated (0.5 mg L−1) water. | Approx. 2-log reduction in microbial load after 15 min and 3.5-log after 30 min of exposure. | [106] |
| Papaya | Cubes | Treated with 9.2 pl L−1 ozone for 10, 20, and 30 min. | Microbial population of fresh-cut papaya was significantly reduced, and the total phenolics content was somewhat increased. | [107] |
| Durian | Peeled flesh | Ozone at 500 and 900 mg L−1 was selected to reduce microbial contamination in vivo. | Significantly reduced microbial counts with 900 mg L−1 being the most effective. | [108] |
| Potato | Slices | Soak in acidic dip with aqueous ozone and stir for 5 min. | Reduced enzymatic browning of fresh-cut potatoes. | [109] |
| Cabbage | Slices | Wash for 1, 5, and 10 min with ozone concentration of 1.4 mg L−1. | Inhibited aerobic bacteria, coliform bacteria and yeast and removed some pesticide residues. | [110] |
| Iceberg lettuce | Pieces | Soak in ozonated water at 20 °C for 2 min. | Controlled the growth of Enterobacteriaceae, thermophilic and psychrophilic bacteria. | [111] |
| Water fennel | Slices | Treated with 18.52, 37.04, 55.56 and 74.07 mg m−3 ozone for 15 min. | Maintained ascorbic acid content, inhibited polyphenol oxidase activity, lowered content of reduced glutathione, and increased peroxidase, catalase, ascorbate peroxidase and superoxide dismutase. | [112] |
| Kiwi fruit | Pieces | One group was fumigated with 1 mg L−1 of gaseous ozone for 10 min, while the other group was unfumigated. | Inhibited activity of polysaccharide-degrading enzymes and electrolyte leakage, reduced MDA and hydrogen peroxide content, and maintained the original pectin and cellulose levels, thus preventing the softening of fresh-cut kiwi fruit. | [113] |
| Kiwi fruit | Pieces | Subjected to fumigation with gaseous ozone (1 mg L−1) for 10 min. | Ozone treatment maintained AsA/dehydroascorbic acid content and reduced the total soluble solids/titratable acidity. | [114] |
| Red pitaya | Strips | Fumigated with 10 μL L−1 ozone in enclosed chamber; control treated with air. | The activity of antioxidant enzymes was increased, the ascorbate–glutathione cycle was activated, and the loss of hardness and total soluble solids/titratable acids was delayed. | [115] |
| Honey pineapple, banana and guava | Cubes | Exposure to ozone for 0, 10, 20 and 30 min. | Levels of total phenols and flavonoids in pineapples and bananas were increased; vitamin C levels in pineapples, bananas and guava were significantly reduced. | [116] |
| Apple | Slices | Ozone (1.4 mg L−1) treatment for 1, 5, and 10 min. | Activities of polyphenol oxidase and peroxidase were reduced as well as total phenols and MDA concentration; antioxidant capacity was enhanced. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Zhang, Y.; Zhao, J.; Liu, L.; Zhao, L. Research Progress on Physical Preservation Technology of Fresh-Cut Fruits and Vegetables. Horticulturae 2024, 10, 1098. https://doi.org/10.3390/horticulturae10101098
Chen D, Zhang Y, Zhao J, Liu L, Zhao L. Research Progress on Physical Preservation Technology of Fresh-Cut Fruits and Vegetables. Horticulturae. 2024; 10(10):1098. https://doi.org/10.3390/horticulturae10101098
Chicago/Turabian StyleChen, Dixin, Yang Zhang, Jianshe Zhao, Li Liu, and Long Zhao. 2024. "Research Progress on Physical Preservation Technology of Fresh-Cut Fruits and Vegetables" Horticulturae 10, no. 10: 1098. https://doi.org/10.3390/horticulturae10101098
APA StyleChen, D., Zhang, Y., Zhao, J., Liu, L., & Zhao, L. (2024). Research Progress on Physical Preservation Technology of Fresh-Cut Fruits and Vegetables. Horticulturae, 10(10), 1098. https://doi.org/10.3390/horticulturae10101098






