1. Introduction
The raspberry (
Rubus idaeus L.) is a high-value fruit, but its shelf life is impacted by high perishability. In 2020, the United States produced 111,000 tons of raspberries at a value of USD 469 million, and California alone contributed fresh raspberries worth USD 395 million [
1]. Raspberry’s delicate morphology coupled with high respiration and transpiration rates make the fruit vulnerable to rapid deterioration after harvest. The typical shelf life of a raspberry ranges from 3 to 5 d [
2]. Decay, leakiness, loss of firmness, darkening of the red color, and off-flavors are common limiting factors contributing to the short storage life of raspberries [
3]. It is well established that cooling is by far the best technology for increasing the shelf life of horticultural produce. Low temperatures slow pathogen growth and reduce the rate of deterioration of freshly harvested commodities, thus extending their shelf life and the marketing period [
4]. The recommended temperature for raspberry storage is 0 to 1 °C [
3], but it is challenging to maintain this recommended temperature during transportation and marketing. Although low storage temperatures can slow the development of
Botrytis cinerea infections, they do not provide adequate control when the inoculum loads are high [
5]. Atmospheres enriched in CO
2 can create fungistatic conditions and, therefore, inhibit the growth of fungi. Raspberries exposed to CO
2 levels of 20 kPa or higher showed delayed gray mold decay and an extended shelf life [
6]. Nine red raspberry genotypes were stored in controlled atmospheres (CA) with 12.5 kPa CO
2 and 7.5 kPa O
2 for 50 d at 1 °C, and decay development was strongly suppressed across all genotypes [
7]. Our objective was to determine the optimum atmosphere to extend the raspberry’s shelf life and maximize its quality during transit or storage by assessing the fruit’s response to a range of CO
2 atmospheres. We also developed a novel method to assess raspberry leakiness.
2. Materials and Methods
Freshly harvested, non-organic, ready-to-eat red raspberries (Rubus idaeus L., cv. Maravilla) were obtained immediately after harvest from a commercial grower in Watsonville, CA, in the fall of 2020 and 2021. The raspberries were field packed into clamshells (170 g) and precooled at 1 °C at a commercial facility in Watsonville, California. The cooled fruits were transported on the same day in an air-conditioned vehicle to the UC Davis postharvest laboratory within three hours. The raspberries were held at 5 °C overnight, and the next day, the baseline quality of a sample of fruit was analyzed before randomly assigning the remaining clamshells to different atmosphere treatments at 5 °C. The fruit was removed from the atmosphere treatments after 6, 10, and 14 d in 2020 and 5, 10, and 13 d in 2021 and immediately evaluated to assess the changes in the fruit’s physical quality over time in storage. The performance of the fruit in each treatment atmosphere was evaluated from the perspective of the raspberry’s shelf life and quality.
2.1. Treatment Atmospheres and Experimental Setup
In both years, the raspberries packed in clamshells were stored at 5 °C for up to 14 or 13 d in 2020 or 2021, respectively, in one of four atmosphere treatments: 15 kPa CO
2 and 6 kPa O
2 (15 kPa); 8 kPa CO
2 and 13 kPa O
2 (8 kPa); 5 kPa CO
2 and 16 kPa O
2 (5 kPa); or 0.03 kPa CO
2 and 21 kPa O
2 (0.03 kPa). These atmospheric treatments simulate the mixture of O
2 and CO
2 concentrations that would be present in a modified-atmosphere package aiming for 15, 8, and 5 kPa CO
2 as well as an unmodified-atmosphere package (air). The gas concentrations were measured during set up and periodically with a CO
2/O
2 gas analyzer (Systec Gas Advance Micro-GS3, Boston, MA, USA). The four atmospheres were humidified by bubbling through water prior to fruit exposure in a continuous flow-through system at a rate of 100 mL/min (
Supplementary Figure S5), similar to the system described by Carmelo et al. [
8]. The raspberry fruit remained in the clamshells during treatment. For each atmospheric condition, there were nine plastic bags (0.127 mm thick transparent bag; 50.8 × 61.0 cm) with six clamshells inside each. There were three plastic bags for each assessment day (3 assessment time points). The bags were modified with an inlet and outlet port and connected to a separate gas flow board for each atmosphere.
2.2. Quality Evaluations
The respiration rate and ethylene production were measured at 5 °C on each evaluation date. Fruit for the 0 d evaluation was cooled overnight before measurement. After removing the stored raspberries from the different atmospheres, the fruit was held at 5 °C in air for 18 to 20 h to off-gas before being sealed inside a 10 L container for 1 h at 5 °C prior to gas sampling. Headspace gas samples were collected and analyzed for CO2 (Horiba infrared gas analyzer, Irvine, CA, USA) and ethylene (Carle gas chromatograph, Tulsa, OK, USA) concentrations. The respiration and ethylene production rates were calculated and expressed as mL CO2·kg−1·h−1 and µL·ethylene·kg−1·h−1, respectively.
One clamshell per treatment and replication was weighed before being sealed in the plastic bags. The percent weight loss was calculated by deducting the measured final weight from the initial weight, dividing the resulting value by the initial weight, and multiplying it by 100. Leakiness was assessed subjectively on one clamshell per treatment and replication. In 2020, a single layer of paper towel was laid on a tray. The whole clamshell of raspberries was gently poured onto the tray, and then the tray was shaken five times back and forth, gently, but enough to move the berries. The tissue paper was evaluated for the juice marks resulting from the berries’ leaking and ranked based on their intensity, where 1 = none, 2 = very slight, 3 = slight, 4 = moderate, and 5 = severe. In 2021, an improved method was developed. The raspberries from one clamshell were arranged on a white paper divided into 40 square blocks; an individual raspberry was placed horizontally on each block for leakiness evaluation. A similar paper (with printed square blocks) was used to cover the raspberries and pressed very gently onto the fruit for 1 s. The top paper and the fruit were removed, and the papers’ printed square blocks (bottom and top) were evaluated and scored for liquid stains resulting from berry leakage. The scores for each fruit (block) were assigned based on the intensity, where 1 = none, 2 = very slight, 3 = slight, 4 = moderate, and 5 = severe (
Supplementary Figure S1). The number of berries with a score of 2 or higher were divided by the total number of berries to determine the percentage of affected fruit. The leakiness severity was calculated by summing up the severity scores of the fruit with a score of 2 or higher (leaky fruit) and dividing by the total number of leaky berries.
Decay was evaluated visually on the fruit from the same clamshell as leakiness. The severity of infection on each fruit was scored using a scale of 1 to 5, where 1 = none; 2 = very slight, 1–3 decayed drupelets; 3 = slight, 4–6 decayed drupelets; 4 = moderate, 7–9 decayed drupelets; and 5 = severe, >9 decayed drupelets (
Supplementary Figure S2). The number of berries with a score of 2 or higher was divided by the total number of berries and multiplied by 100 to determine the percentage of decayed fruit. The decay severity was calculated by summing up the severity scores of the fruit with a score of 2 or higher (decayed fruit) and dividing by the total number of decayed berries.
Ten raspberries were randomly selected from one clamshell per treatment and replication to evaluate their color using a chroma meter (Konica Minolta Sensing Americas, Inc., Ramsey, NJ, USA) with the CIELAB color space. The color of the external surface of the raspberries was measured and expressed as L* C* h color coordinates, indicating the lightness, chroma, and hue angle, respectively. Only one side, close to the apex of the fruit, was measured.
The same fruit was evaluated for glossiness, which refers to the light reflection intensity of the fruit. The fruit was visually inspected and subjectively scored from 1 to 3, where 1 = dull, 2 = moderately glossy, and 3 = glossy (
Supplementary Figure S3). The same ten berries were used to evaluate the degree of discoloration based on the number of discolored (whitish/pale) drupelets and scored on a 1 to 5 scale, where 1 = none; 2 = very slight, 1–3 discolored drupelets; 3 = slight, 4–7 discolored drupelets; 4 = moderate, 8–11 discolored drupelets; and 5 = severe, >11 discolored drupelets (
Supplementary Figure S4).
The fruit’s firmness was also assessed subjectively using the same raspberries that were used to evaluate glossiness and discoloration. Each raspberry was pressed slightly with the thumb and middle fingers. Based on the palpability, the berries were scored from 1 to 5, where 1 = very firm, rebounds from compression, high resistance; 2 = firm, partial rebound; 3 = soft, partial rebound; 4 = very soft, partial rebound; and 5 = no resistance.
A second clamshell of raspberries from each treatment and replication was frozen with liquid N
2 and immediately broken into drupelets with a mortar and pestle. The drupelets were mixed among the fruit from each clamshell and stored in a −80 °C freezer until analysis. These frozen raspberries were used to measure the total anthocyanin content (TAC). The TAC was measured using a microvolume UV-Vis spectrophotometer (Nanodrop, Thermo Fisher Scientific, Waltham, MA, USA) by adapting a method from Abdel-Aal and Hucl [
9]. Liquid N
2 was added to the frozen raspberry drupelets and then immediately ground with a blender (Osterizer 12 speed blender, Mexico) for 1 min and turned into a fine powder. An aliquot (400 mg) of raspberry powder was added to 10 mL of acidified ethanol solution (96% ethanol and 1 N HCL 85:15
v/
v) and vortexed for 1 min. The solution was incubated for 30 min at 50 °C and then filtered through a 0.45 micron polytetrafluorethylene filter (Agilent Technologies, Santa Clara, CA, USA). The supernatant was collected and held in a −20 °C freezer until it was evaluated using spectrophotometry. The absorbance (A) was measured at 530 and 700 nm on cyanidin 3-glucoside equivalents. The acidified ethanol solution was used as a blank. The total anthocyanin content per sample (mg·kg
−1) was calculated as the cyanidin 3-glucoside equivalent, the most dominant anthocyanin in raspberry:
where C is the concentration of total anthocyanin (mg·kg
−1), A is the difference (530–700 nm) between the absorbance readings, ε is the molar absorptivity (cyanidin 3-glucoside = 25,965 cm
−1 M
−1), vol is the total volume of anthocyanin extract, and MW is the molecular weight of cyanidin 3-glucoside = 449 [
9].
The data were statistically analyzed using R statistical program [
10]. In addition to base statistical analysis, ggplot2 and dplyr packages were used. A total of 4 atmospheres (treatments) and 3 replications across the 4 evaluation dates were analyzed for the quality characteristics of raspberry fruit. The data were assessed through ANOVA followed by the Honestly Significant Difference (HSD) Tukey test to reveal significant differences (
p < 0.05) among treatments and evaluation days.
4. Discussion
Firmness is an important indicator of quality in raspberry fruit, as well as many other fruits. The decrease in raspberry firmness after harvest was inhibited or slowed by storage under increasing CO
2 concentrations, and the fruit had significantly higher firmness than air-stored raspberries. In accordance with our findings, Alamar et al. [
11] reported that strawberries held in high CO
2 atmosphere (15 kPa CO
2 and 5 KPa O
2) at 5 °C for 14 days were significantly firmer than air-stored fruit. The effect of CO
2 in delaying further ripening, as evidenced by other quality parameters such as color, may be one reason why firmness was maintained.
In addition to delayed ripening, CO
2 has other effects on fruit physiology: reduced respiration and altered metabolism of ethylene and other volatile compounds [
12]. CO
2 influences ethylene biosynthesis by regulating 1-aminocyclopropane-1-carboxylic acid (ACC) synthesis and oxidization. ACC synthase is inhibited by high (5–20 kPa) CO
2. ACC oxidase activity is stimulated by low levels (<5 kPa) of CO
2 and inhibited by higher CO
2 [
13]. The association of high CO
2 atmospheres with and maintenance of raspberry fruit firmness was further supported by Gonzalez et al. [
14], who found that raspberries stored in a continuous flow of CO
2 (15 or 10 kPa CO
2) for 14 days had higher firmness than berries exposed to CO
2 for 3 days or an intermittent CO
2 treatment. In strawberries, elevated CO
2 has also been shown to enhance firmness [
15]. Strawberry fruit exposed to high CO
2 atmospheres exhibited changes in apoplastic pH levels and, in turn, may have increased cell-to-cell adhesion via the precipitation of soluble pectin [
16]. The solubilization of CO
2 produces H
+ and HCO
3−, which could influence the pH [
17]. The increase in firmness following exposure to high CO
2 atmospheres, as related to pectin polymerization, is mediated by calcium. In strawberries, the modification of pectic polymers decreased the amount of water-soluble pectin (WSP) and increased the chelator-soluble pectin (CSP), which is a major factor in the firmness increase [
18]. However, in our study, we did not find any increases in raspberry firmness as a result of exposure to up to 15% CO
2 for 14 days, although the rate of softening was reduced. However, Forney et al. [
7] found that CA (12.5 KPa CO
2 with 7.5 KPa O
2) did not maintain raspberry firmness during 2–3 days of storage at 1 °C and resulted in the fruit softening compared with air-stored raspberry.
In our study, we observed an increase in leakiness and a decrease in glossiness during storage. Leakiness is initiated in a raspberry via the physiological breakdown (PB) of the cells, a typical symptom of plant tissue senescence [
19]. Physiological breakdown is evidenced as juice leakage and softening and contributes to the fast deterioration of the raspberry fruit’s quality [
20]. We observed a significant increase in leakiness over time after harvest; however, the rate of increase was slower, with less leaky raspberries, when the fruit was stored in 15 KPa CO
2. The effect of high CO
2 in slowing further ripening and over-ripening likely contributed to the slower rate of leakiness development. When evaluating different raspberry cultivars, Harshman et al. [
21] did not detect a clear association between fruit firmness and PB resistance, indicating that initial fruit firmness is not related to PB incidence. Forney et al. [
7] reported that storage in 12.5 KPa CO
2 and 7.5 KPa O
2 was less effective in delaying PB than delaying decay. Perhaps their fruit had already begun senescence prior to CA exposure.
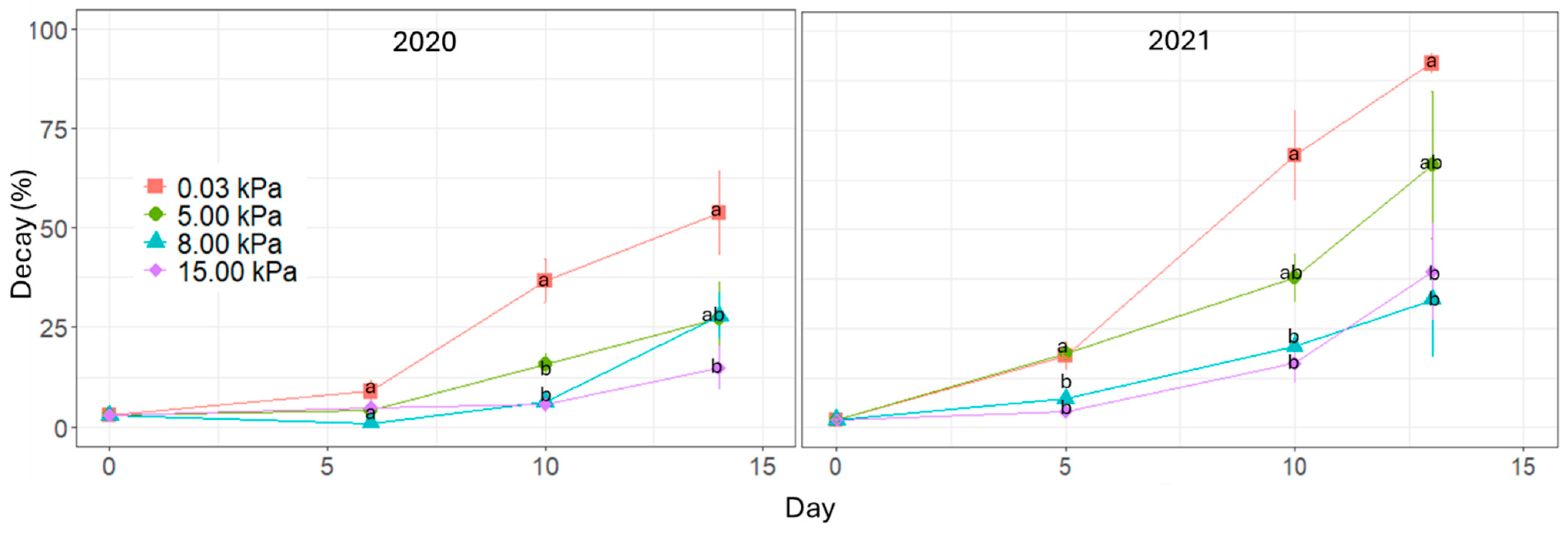
Visible decay on the fruit surface significantly reduces raspberry fruit’s quality and marketability. In our study, decay incidence was reduced by storage under high CO
2 concentrations, with the maximum effect achieved at 8 kPa CO
2. In agreement with our study, Haffner et al. [
3] found significant inhibition of raspberry decay by using high CO
2 atmospheres (10–30 kPa CO
2 in combination with 10 kPa O
2) as compared with air-stored fruit. High CO
2 concentrations create a fungistatic effect that slows the activity of fungi as well as the metabolic activity of the fruit. The fungistatic effect of high CO
2 is due to its solubility in the aqueous phase of produce and fungi. CO
2 in the intercellular environment lowers the pH, which inhibits enzyme-catalyzed processes and enzyme production, interacts with cell membranes, and affects the physicochemical characteristics of proteins [
22]. The altered expression of proteins in both fungi and fruit tissues can therefore alter decay development [
23]. In addition, maintaining cellular integrity as a result of the firming effect of CO
2 may have also inhibited fungal activity. Petrasch et al. [
24] also reported that mycelium developed faster on softer strawberry fruit than firmer fruit. Modified atmospheres reduce the respiration rates and delay the ripening of fruit [
12], which is also in agreement with our findings. In addition, higher firmness can reduce fruit damage and stronger cell walls resist cell wall-degrading enzymes produced by pathogens, hindering a microbe’s capacity to infect the fruit.
Anthocyanins play a vital role in raspberry’s color expression. Maintaining a bright red color is an important postharvest quality attribute for raspberries, as a dark red color is associated with over-ripe fruit [
25]. The visual appeal of raspberry fruit decreases with time after harvest, along with increased levels of certain anthocyanins [
26]. High hue angle values indicate more orange-red color, and low values indicate more blue-red color. Our results showed that raspberry fruit stored in 15 kPa CO
2 maintained a stable hue angle after 5 d, but the hue angle declined over time in the raspberries stored in air or lower CO
2 concentrations. Strawberries held in 15 kPa CO
2 and 5 kPa O
2 had decreased ethylene biosynthesis and a lighter, brighter hue [
11]. This finding is also aligned with our finding of a significantly lower ethylene production rate in the raspberries stored in high CO
2 compared with air storage.
CO
2 in the intercellular environment lowers the cellular pH [
22]. The hydration of CO
2 and production of HCO
3− and H
+ may reduce the intracellular pH [
17], and the pH plays a crucial role in raspberry’s fruit color. In the strawberry, reducing the pH from 3.81 to 3.21 resulted in a 37 to 13 percent shift in flavylium formation and also increased the stability of the fruit’s color more than any other factors [
27]. The red flavylium cation (AH
+) remains stable only in acidic conditions [
28].
In our study, the total anthocyanins increased over time, except in the raspberries stored in 15 kPa CO
2; storing raspberries in 15 kPa CO
2 maintained the anthocyanin content (as well as hue angle) close to the levels at harvest. In agreement with our findings, Gil et al. [
29] found that high CO
2 concentrations inhibited the increase in anthocyanin content after harvest by affecting its biosynthesis, degradation, or both. These results indicate the ability of high CO
2 atmospheres to maintain raspberry fruit’s color tone, even after 2 weeks of storage. Palonen et al. [
30] found a significant correlation between anthocyanin concentration and color values, as the darkest raspberries had a higher anthocyanin content. In our study, we also found a higher anthocyanin content and lower hue angle in the raspberries stored in air or low CO
2 atmospheres. Moore [
31] showed that raspberry’s anthocyanin content could be predicted by the hue angle or a*/b*.
We observed an increase in raspberry discoloration after harvest, which has not been reported previously to our knowledge. Discoloration occurred when the raspberry drupelets changed color from red to light pink. In blackberries, a similar phenomenon, red drupelet reversion (RDR), occurs, which is a type of physiological disorder [
32]. Edgley et al. [
33] reported that RDR was associated with a decrease in the anthocyanin content and was primarily caused by mechanical damage during harvest, which causes membrane integrity loss and a decrease in cellular structural integrity. There may also be some change in the pH from membrane leakiness, leading to color changes in the anthocyanins. Slight changes in the pH have a significant impact on anthocyanins, as the acidity of the solution impacts the ratio between different forms (colors) of the pigments [
34]. In our study, discoloration increased with time in storage but was inhibited by high CO
2; the anthocyanin content was also maintained close to harvest levels with high CO
2. Also, high CO
2 atmospheres maintained fruit firmness and the integrity of the cell wall and reduced senescence. These results suggest that discoloration in raspberries is related to the loss of membrane integrity and/or a decrease in cellular structural integrity, and the incidence is reduced by storage in high CO
2 atmospheres.