Abstract
The main mango cultivars produced in the southern Pacific region of Mexico are Ataulfo, Manila, Haden, and Criollo. However, mineral, nutritional, and nutraceutical composition studies are limited. This study aimed to evaluate the effect of cultivars on the nutritional, mineral, and nutraceutical qualities of mango produced in Mexico. The cultivar significantly affected (p ≤ 0.05) the fruit composition across these indices. Criollo had the highest potassium, magnesium, sodium, and zinc concentrations, while Haden showed the highest calcium content. Manila had the highest iron content, contributing 0.76% of the recommended daily intake. Ataulfo and Haden were statistically similar in manganese content, both higher than Criollo, with Ataulfo reaching the maximum copper concentration. Ataulfo also had the highest sugar content, while Criollo had the most dietary fiber (3.1%), double that of Ataulfo and Haden. Haden had the lowest dry matter (14.8%) and lowest protein content (0.46%), with Manila showing the lowest ash content. The cultivars also differed in nutraceutical composition: Ataulfo was highest in total phenols, DPPH, and vitamin A; Haden in carotenoids and flavonoids; and Criollo in vitamin C. Cultivar selection induces changes in mango nutritional composition.
1. Introduction
Mango (Mangifera indica L.) is cultivated in 23 states of Mexico, but Sinaloa, Guerrero, Nayarit, and Chiapas concentrate most of the national production, generating approximately 76% of the total. Guerrero stands out for its contribution, representing about 18.7% of the national volume, with a cultivated area exceeding 27,000 hectares. The main cultivars are Ataulfo (30%); Manila, Kent, and Tommy Atkins (≈15%); Haden (9%); and Criollo (7%), each with distinctive characteristics that influence their market acceptance [].
Mango quality is assessed through various parameters, such as sugar content (13.8%), acidity (0.42%), firmness (8N), and color (Hue 72.4°), which are crucial for its marketability [,]. Mango is a rich source of vitamins in mg or μg 100−1 g of fresh pulp weight (A with 300 to 1800 μg retinol equivalents, B1 0.01–0.04 mg, C 13.2–92.8 mg, E 0.79–1.02 mg, and K 4.2 μg). On the other hand, it stands out for its levels (g or mg 100–1 g of dry pulp weight) of water (78–83 g), protein (0.36–0.40 g), carbohydrates (16.20–17.18 g), fat (0.30–0.53 g), ash (0.34–0.52 g), dietary fiber (0.85–1.06 g), energy (62.1–190 kcal), and minerals such as potassium (120–211 mg), calcium (7–16 mg), magnesium (8–19 mg), sodium (0–3 mg), iron (0.09–0.41 mg), manganese (0.03–0.12 mg), copper (0.04–0.32 mg), zinc (0.06–0.15 mg), and selenium (0–0.6 mg). It also contains essential amino acids like lysine (0–0.06 g), leucine (0–0.05 g), valine (0–0.04 g), and nutraceutical compounds like total polyphenols (0.418 g) and gallic acid (98.7 mg) among other substances that make it a highly beneficial food for human health [,,]. Although there are reports on its nutritional composition, they do not always specify the cultivar, and studies addressing similar topics [,,] do not include the cultivars most produced in Guerrero, one of the leading mango-producing regions in Mexico.
Studies on the nutritional and mineral qualities of mangoes in Mexico are limited. On the one hand, research has focused on the agronomic and environmental factors that affect yield [,,,]; on the other hand, other studies have concentrated on the physical attributes and postharvest quality of the fruit, which determine its potential for international markets [,,]. In the state of Guerrero, studies conducted between 2016 and 2017 identified that although producers are interested in exporting, only a few meet the necessary standards. This underscores the need for more comprehensive studies on the nutritional and mineral qualities of mangoes, particularly those most produced in Guerrero, one of the leading mango-producing regions in Mexico [].
Studies have documented the postharvest quality and some nutraceutical aspects of the mangoes grown in Guerrero. However, there is still a need to explore the nutritional, mineral, and functional qualities to understand the potential benefits for consumers receiving mangoes produced in Mexico. The lack of this information limits producers’ ability to compete in broader markets, where consumers increasingly value quality and nutritional value. Guerrero, located in the southern Pacific region of Mexico, primarily grows ‘Ataulfo’, ‘Manila’, ‘Haden’, and ‘Criollo’ mangoes. Therefore, studying the effect of cultivars under standard management practices on the nutritional aspects is essential. This research aimed to evaluate the effect of cultivars on the nutritional, mineral, and nutraceutical qualities of mangoes cultivated in the southern Pacific region of Mexico.
2. Materials and Methods
2.1. Study Area and Sample Collection
In March 2020, fruit and soil samples were collected from mango orchards located in San Marcos (16°47′38.09″ N, 99°23′14.51″ W), Las Vigas (16°45′47.50″ N, 99°13′49.56″ W), Alto de Ventura (16°45′47.50″ N, 99°13′49.56″ W), and Cuajinicuilapa (16°28′28.92″ N, 98°24′51.69″ W) in Guerrero, Mexico (Figure 1).
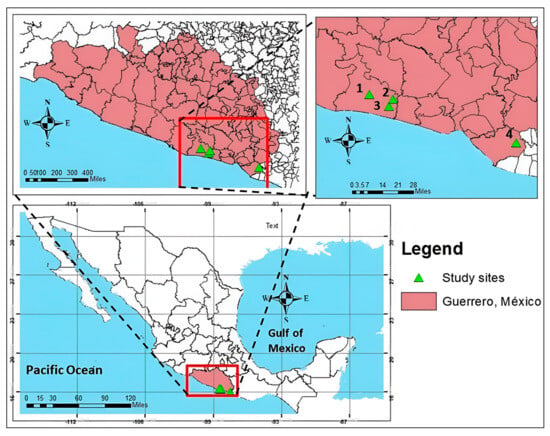
Figure 1.
Geographical location of sampling points; sites 1 and 2 San Marcos (Manila and Ataulfo), 3 Las Vigas (Haden), and 4 Cuajinicuilapa (Criollo).
The orchards, with less than 2% slopes and a predominant loamy-sand texture, were located between 30 and 60 m above sea level. In the study area, the meteorological conditions for the current production cycle were recorded, with an average temperature of 23 °C (Figure 2), an annual precipitation of 1200 mm, and a predominantly subhumid climate [].
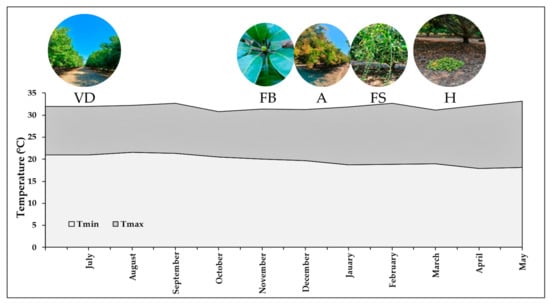
Figure 2.
Average monthly maximum (Tmax) and minimum (Tmin) temperatures. Data from meteorological station Las Vigas, San Marcos, Guerrero. The mango production cycle begins in July with vegetative development (VD), in November the flower buds appear (FB) and in December–January the anthesis (A), from January–February the fruit set (FS), and in March–April the harvest (H) takes place.
2.2. Fruit and Soil Sample Collection
In 2020, a survey was conducted in the mango-producing region of Guerrero, Mexico, to identify the orchards of the cultivars ‘Ataulfo’, ‘Manila’, ‘Haden’, and ‘Criollo’. Usually, ‘Criollo’ is self-rooted and is used as rootstock for the ‘Ataulfo’, ‘Manila’, and ‘Haden’ cultivars. The selected orchards were 12 years old, with uniform tree health, canopy diameter, height, and standard pest and disease control practices. Mango fertilization included the application of 4.5 kg per tree by mixing fertilizers based on urea (46% N), diammonium phosphate (18% N and 46% P2O5), and muriate of potash (60% K2O), used in a combination (100 kg urea + 100 kg diammonium phosphate + 50 kg as muriate of potash) that resulted in a dose of 129–93−60 kg ha−1 in terms of N-P2O5-K2O, respectively []. Midway through the harvest period in March, the trees were marked for fruit and soil sampling.
The soil sampling process was meticulous and comprehensive. Using the zig-zag method [,], twelve trees were carefully selected, and samples were obtained with a ‘T-type’ auger from the 0 to 30 cm soil profile. The samples were taken at the four cardinal points and halfway between the trunk and the drip perimeter of the tree canopy. The samples were combined in a plastic container, followed by homogenization and quartering, resulting in a composite sample of 1 kg per orchard []. The collected soil samples were then transported to the laboratory for processing, which involved drying them on kraft paper in the shade. Following this, the soil was ground and sieved for physicochemical profile analysis according to standard procedures NOM-021-RECNAT-2000 [] (Table 1).

Table 1.
Physicochemical components of soil salinity in orchards of four mango cultivars produced in Guerrero, México.
As previously described, the fruits were collected from the same trees where the soil samples were obtained. For each cultivar, the experimental unit or replication consisted of four trees, from which 10 fruits per tree were selected based on the absence of physical damage, uniform size, and health. The mangoes were manually harvested by experienced mango pickers, considering the size and shoulder filling of the fruits. After that, physiological maturity was confirmed by the fact that the total soluble solids values were greater than or equal to 9 ± 0.1% in a subsample of 10 fruits []. The mango fruits were transported in cardboard boxes to the ‘Centro de Investigación en Alimentación y Desarrollo’ (Food and Development Research Center) Nutrition Laboratory in Culiacan, Sinaloa, Mexico. The fruits were washed with a chlorine solution at 150 ppm and allowed to dry. Any fruits with physical damage or heterogeneity were discarded until 10 fruits per replication were obtained for proximal and mineral determinations and 10 fruits for assessing functional quality. The fruits were stored at 20 °C until they reached maturity (Figure 3) after 8 days, at which point they achieved stage 5 of internal color, indicating suitability for consumption according to the scale reported for mangoes NMX-FF-058-SCFI-2006 [].

Figure 3.
Mango cultivars at consumption maturity (fruit visual condition). ‘Ataulfo’ and ‘Manila’ grown in San Marcos, ‘Haden’ from Las Vigas, and ‘Criollo’ from Cuajinicuilapa, all of Guerrero, Mexico.
The ripe fruits were peeled, sliced, and diced into 1 cm cubes using a knife. For functional determinations, 10 samples of 1 g of fresh pulp from each replication and cultivar were placed into individual 50 mL Eppendorf tubes and stored at -80 °C until analysis. A similar sampling procedure was used for proximal and mineral analyses. However, four 50 g samples of fresh pulp per replication for each cultivar were collected and stored at minus 20 °C for the subsequent analyses.
2.3. Evaluated Variables
2.3.1. Mineral Analysis
A 2 g sample of fresh pulp was weighed in a pre-weighed porcelain crucible and then placed in a muffle furnace for ashing at 650 °C for 12 h. The ash was then resuspended with 5 mL of HCl, and the resulting mineral solution was diluted to 100 mL with Type 2 purified water. Potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), and boron (B) were quantified via atomic absorption spectrometry (AA FS flame AA 280FS + SIPS 20, Agilent Technologies, Santa Clara, CA, USA) according to the AOAC official methods (AOAC, 1998) [].
2.3.2. Nutritional Analysis
The determination of moisture, ash, protein (Nitrogen × 6.25), fat, and carbohydrates was performed using the methods 925.45, 942.05, 988.05, 920.39, and 993.21, respectively []. The analysis was conducted on fresh mango pulp at consumption maturity.
For moisture determination, 2 g of sample was placed in a pre-weighed and tared crucible and then dried in an oven (70–80 °C) for 24 h until a constant weight was achieved. The percentage of protein was obtained by multiplying the nitrogen content analyzed by the semi-micro Kjeldahl method by the conversion factor of 6.25. For lipid extraction, 2 g of fresh pulp was used with a Goldfish apparatus (Buchi® E-816 SOX, BÜCHI Labortechnik AG, Flawil, Switzerland) utilizing anhydrous petroleum ether. The lipid content was calculated by the weight difference in the receiving flask before and after the extraction. Energy in calories was obtained from protein, fat, and sugars according to NOM-051-SCFI/SSA 1-2010 [].
Dietary fiber was obtained by analyzing 2 g of mango pulp dried at 60 °C in a forced-air oven (Isotemp, Fisher Scientific, Waltham, MA, USA) for 24 h. The sample was then ground to particle size and sieved (Mesh 40). Total dietary fiber, insoluble dietary fiber, and soluble dietary fiber were determined following the method 991.43 [].
The total sugars in g 100−1 g of fresh pulp were the sum of glucose, fructose, and sucrose determined in Section 2.3.3.
2.3.3. Available Sugars
Sugars were analyzed in 2 g of fresh mango pulp through three successive 80% (v/v) ethanol extractions according to Nikolidaki (2017) and subsequently following the instructions of the Megazyme enzymatic kit [,]. The sum of glucose, fructose, and sucrose constituted the total sugars.
2.3.4. Nutraceutical and Vitamin Analysis
The functional quality of fresh mango pulp was evaluated based on the content of soluble and insoluble dietary fiber, vitamin C, total carotenoids, β-carotene, phenolic compounds, total flavonoids, and antioxidant activity.
The determination of vitamin C in 1 g of frozen sample Whatman No. 1 paper using a vacuum pump and Kitasato flask, and then collected in a separation funnel to obtain the organic phase. This phase was analyzed at a wavelength (λ) of 446 nm using a spectrophotometer (Cary 60, Agilent Technologies, Santa Clara, CA, USA). Quantification was performed using a molar extinction coefficient of 2500, with the results reported as mg of carotenoids per 100 g of fresh pulp (FP), ensuring was a comprehensive process. The sample was homogenized with 45 mL of cold HPLC-grade water, filtered through Whatman No. 1 filter paper, and then through a SepPak C18 cartridge (Waters Corporation, Milford, MA, USA) []. A 2 mL aliquot of the extract was mixed with 2 mg of dithiothreitol to reduce dehydroascorbic acid, allowed to stand for 1 h, and then filtered through a 0.45 µm nylon membrane. A 20 μL sample was analyzed at a wavelength (λ) of 268 nm using a high-performance liquid chromatography (HPLC) system (Varian, 9050, Spectralab Scientific Inc., Mississauga, ON, Canada) equipped with a UV-Vis detector and a C18 column (25 cm length × 4.6 mm diameter). The mobile phase, consisting of a KH2PO4 solution (pH 2.4 adjusted with H3PO4), was eluted at a flow rate of 1 mL min⁻¹ over a 10 min analysis time.
Vitamin C was quantified based on a calibration curve ranging from 0 to 0.2 mg mL⁻¹, using L-ascorbic acid as the reference standard (Sigma Aldrich Chemical Co., St. Louis, MO, USA). The analysis of total carotenoids was a meticulous process. In total, 5 g of sample was homogenized and mixed with 15 mL of hexane and 17.5 mL of acetone in three successive extractions []. The extracts from each sample were combined, filtered through the thoroughness and accuracy of our research.
β-Carotene was analyzed by liquid chromatography []. A 1 g sample of frozen mango pulp was homogenized with 10 mL of 50 mM sodium phosphate for 1 min. After homogenization, the sample was centrifuged at 10,000 rpm for 10 min at 4 °C, and the supernatant was transferred to a flask. The pellet was then resuspended in 10 mL of ethyl acetate, homogenized, and centrifuged under the same conditions. The supernatant was collected, and this process was repeated three more times until the sample became colorless. The five extracts from each sample were combined in a separation funnel, and the organic phase was collected and made up to 50 mL for storage at −20 °C until determination by HPLC []. A β-carotene analysis was performed (λ 460 nm) using 5 mL of the extract injected into an HPLC system (Varian 9050) equipped with a UV-Vis detector and a 55 cm × 4.6 mm C18 Reselut column. The sample was eluted with a mixture of acetonitrile–methanol (55:35:10 v/v) at a 1 mL min⁻1 flow rate over a 10 min analysis period. The identification and quantification of carotenoids were performed by comparison with a β-carotene standard (Sigma Aldrich Chemical Co.) used to construct a calibration curve with the concentrations of 0, 20, 50, 60, 80, and 100 mg mL⁻1 [].
The analysis of total phenols was performed using 2.5 g of fresh mango pulp []. The sample was homogenized (Ultra-Turrax T 25, IKA IKA Works, Inc., Wilmington, NC, USA), mixed with 10 mL of methanol, and incubated at 200 rpm for 2 h at room temperature. After incubation, the sample was centrifuged at 10,000 rpm for 15 min at 4 °C, and the supernatant was collected. Following methanolic extraction, the quantification was carried out in a 96-well plate. In each well, 20 μL of the supernatant was added, followed by 180 μL of Folin–Ciocalteu reagent and 120 μL of Na2CO3. The mixture was gently mixed and allowed to rest in the dark for 1 h. Absorbance was then measured at 720 nm using a microplate reader (Synergy TM HT Multidetection, BioTek, Inc., Winooski, VT, USA). Phenols were quantified using a calibration curve from 0 to 0.4 mg mL⁻1 with gallic acid as the standard, expressing the concentration as milligrams of gallic acid equivalents per 100 g of mango pulp (mg GAE 100 g⁻1).
The antioxidant capacity using the DPPH method, a widely accepted method for measuring antioxidant activity, was quantified by homogenizing 2.5 g of fresh mango pulp (Ultra-Turrax T 25, IKA Works, Inc.) with 10 mL of 80% methanol. The mixture was then stirred at 200 rpm for 2 h at room temperature in the dark. Afterward, the sample was centrifuged at 10,000 rpm for 15 min at 4 °C, and the supernatant was collected for measurement.
The antioxidant capacity in the methanolic extracts to deactivate the stable DPPH radical was determined using the method described by Palafox-Carlos et al. []. A DPPH (2,2-diphenyl-1-picrylhydrazyl) radical solution in methanol was prepared, adjusting the initial absorbance to 0.7 ± 0.05. The reaction was performed by mixing 140 µL of the DPPH radical with 10 µL of the supernatant. The mixture was allowed to stand in the dark for 30 min, and the absorbance was measured at 515 nm using a spectrophotometer equipped with a microplate reader (Synergy TM HT Multi detection, BioTek, Inc., Winooski, VT, USA). The activity was expressed as the percentage of DPPH radical inhibition.
The total flavonoids were analyzed following the methodology described by Ebrahimzadeh et al. with slight modifications []. To a sample of 2.5 g fresh pulp (FP) of mango, 10 mL of cold methanol was added, and the mixture was homogenized (Ultraturrax T 25, IKA Works, Inc.). The mixture was stirred at 200 rpm for 2 h at room temperature and in the dark. Afterward, the mixture was centrifuged at 10,000 rpm for 15 min at 4 °C, and the supernatant was collected for analysis.
In a 96-well plate, 30 µL of the extract was transferred to each well, and then 250 µL of distilled water, 10 µL of 10% aluminum chloride, and 10 µL of 1 M potassium acetate were added. The mixture was gently mixed and incubated in the dark for 30 min. The plate was then read using a microplate reader Synergy HT (BioTek, Agilent Technologies, Inc., Santa Clara, CA, USA) at a wavelength of 415 nm. The flavonoid concentration was determined using a quercetin standard with a calibration curve ranging from 0 to 0.4 mg mL−1, and the results were expressed as milligrams of quercetin equivalents per gram of fresh pulp (mg EQ·g−1 FP) [].
2.4. Experimental Design and Statistical Analysis
The experimental design was completely randomized. The factor under study was the cultivar with four replications. The experimental unit consisted of four mango trees in total production, with 10 fruits collected from each tree at physiological maturity. An analysis of variance was performed with a completely randomized effects model, followed by mean comparisons using Tukey’s test (p < 0.05). All the analyses were conducted using the SAS statistical software version 9.0.
3. Results
3.1. Mineral Analysis
The mineral composition of the fresh mango pulp grown in Guerrero, Mexico, showed significant variations due to cultivar effects (Table 2). Based on the mineral concentration in fresh mango pulp and assuming a consumption of 100 g, each element contributes to human health according to the average recommended daily intake (RDI). Potassium (K) was the most abundant mineral, with ‘Criollo’ having the highest value (157.91 mg 100−1 g), representing potentially 3.3% of the RDI. In contrast, Haden and Ataulfo only reached 2.2% and 2.0% of the RDI, respectively. For magnesium (Mg) and sodium (Na), Criollo was statistically superior to the other cultivars, achieving 7.4% and 0.1% of the RDI, respectively.

Table 2.
Effect of the cultivar (p-value) on the mineral concentration of fresh mango pulp (fp) at consumption ripeness (according to NMX-FF-058-SCFI-2006).
For calcium, Haden achieved the highest statistically significant level (3.61 mg 100−1 g), representing 0.40% of the recommended daily intake (RDI), which was 185% higher compared to the lowest RDI of 0.14% in the ‘Criollo’ mango. Iron was the most concentrated in the fresh pulp among the important micronutrients for human health. Manila statistically surpassed the other cultivars in iron concentration, contributing up to 1.3% of the RDI. For manganese, Ataulfo and Haden reached the highest concentrations, covering 9% of the RDI. For copper, Ataulfo achieved the highest level compared to the other cultivars, equivalent to 6.4% of the RDI. For zinc, Criollo exceeded the other cultivars, and its consumption could reach an RDI of 0.6%, which is 200% higher than Manila, with an RDI of 0.2%. Meanwhile, for boron, Ataulfo achieved the highest concentration and surpassed the other evaluated cultivars, although its RDI for human health is unknown.
3.2. Nutritional Analysis
The cultivar significantly affected mango pulp nutritional content (Table 3). The mango ‘Haden’ exhibited the highest moisture content (85.8%) and the lowest protein content (0.46%) compared to the other cultivars. On the other hand, Manila showed statistically the lowest ash content, which is related to its lower mineral content (Table 2). A total of 100 g of fresh mango pulp can provide between 49 and 73 calories (205 to 305 kJ). After water, the main component in mangoes is carbohydrates, represented by the sugars present in ripe fruit, whereas Ataulfo and Manila exhibited significantly higher amounts of sugars and, therefore, also provided the highest energy contribution (Table 3).

Table 3.
Effect (p-value) of the cultivar on the proximate properties, fiber, and sugars of fresh mango (fp) at consumer maturity (according to NMX-FF-058-SCFI-2006) [].
The dietary fiber content of the fresh pulp showed significant differences among the evaluated cultivars (Table 3). The ‘Criollo’ cultivar stood out for having the highest amount of dietary fiber at 3.1%, double that of the ‘Ataulfo’ and ‘Haden’ cultivars (Table 3). The recommended daily intake of fiber is 28 g/day [].
When analyzing the individual components of dietary fiber, both soluble and insoluble, and considering the fibrous characteristics of some mango fruit pulps, it was observed that insoluble fiber was greater than soluble fiber (2:1, except for ‘Haden’, which had a 1:2 ratio).
3.3. Available Sugars
The concentration of sugars among the four mango cultivars showed statistical differences (Figure 4). Sucrose represented more than 77% of the total sugars, followed by fructose and glucose. ‘Ataulfo’ achieved the highest sucrose value at 14%, statistically similar to the sucrose content in the ‘Manila’ cultivar but different from the other cultivars. For fructose, Ataulfo, Manila, and Haden showed the highest values and were statistically similar (1.7–2.8%), whereas for glucose, with levels less than one percent, ‘Haden’ and ‘Ataulfo’ excelled with 0.4% and were superior to the other cultivars (Figure 4).
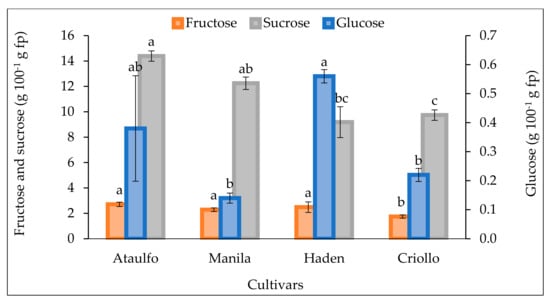
Figure 4.
Concentration of glucose, fructose, and sucrose in fresh mango pulp (fp) based on cultivar at consumption maturity. Bars ± standard deviation with different letters for each type of sugar indicate significant differences (Tukey, p ≤ 0.05).
3.4. Nutraceutical and Vitamin Analysis
Table 4 shows the results for crucial nutraceutical compounds in the fresh pulp of the four mango cultivars. Ataulfo, Haden, and Criollo had statistically similar values for the total phenols, flavonoids, and antioxidant capacity (DPPH). Mango pulp is recommended for its provitamin A content, with Ataulfo showing the highest statistical content compared to the other cultivars. Regarding vitamin C, Criollo had the highest concentration, with 66 mg of ascorbic acid, surpassing the other cultivars.

Table 4.
Effect (p-value) of the cultivar on the functional indices of fresh mango pulp (fp) at consumption ripeness.
4. Discussion
In this study, it was confirmed that mango cultivars exhibit differences in nutritional, mineral, sugar, carbohydrate, and nutraceutical compositions. These variations in nutritional content, which align with previous reports [,], contribute to our understanding of the diversity in mango cultivars.
The average mineral content reported by the USDA [] for Tommy Atkins, Keitt, Kent, or Haden mango cultivars is similar to those studied, indicating good fruit development under the growing conditions with an important contribution of minerals. Among the mineral content of the pulp from the four mango cultivars analyzed, potassium was found to have the highest concentration, particularly in ‘Criollo’, contributing 3.3% of the Daily Recommended Intake (DRI) according to reports by []. This finding contrasts with the potassium levels reported in the mango pericarp and seed [,], suggesting that mango pulp is a rich source of this mineral, which aligns with previous reports []. On the other hand, the iron content in the Manila pulp is comparable to that of the pericarp, while the zinc content in the pericarp exceeds that in the pulp. Calcium is identified as the third most concentrated mineral in the pulp, with Haden showing the highest concentration of this mineral []. These results highlight the variability in the mineral content among the different cultivars, a phenomenon also observed in the mineral content of the pericarp, which varies according to the cultivation region []. This aspect underscores the need to consider cultivation conditions, as they significantly influence the mineral composition of mangoes, as noted by the Institute of Tropical Fruit Research [].
The average values of proximate composition for mango fruit recorded by the Agricultural Research Service of the United States Department of Agriculture (USDA) [] are consistent with the average data obtained for each component of the proximate composition of the four cultivars analyzed in this study. Similarly, the proximate composition tables published by the National Institute of Medical Sciences and Nutrition Salvador Zubirán (INCMNSZ) report the nutritional content of mango in general and for specific cultivars such as Ataulfo, Criollo, and Manila []. The results agree with the specifications in these tables. Of the four cultivars evaluated, ‘Ataulfo’ has the highest content of available sugars and caloric contribution, while the protein, fat, and ash levels are below 1%. Likewise, it is confirmed that the ‘Criollo’ mango has the highest dietary fiber content, indicating that consuming 100 g of ‘Criollo’ mango can provide 10% of the recommended daily intake for fiber.
Regarding the dietary fiber content, the INCMNSZ does not specify the soluble and insoluble fiber content, which is information obtained in this study. The different proportions of these two types of dietary fiber are relevant due to their distinct biological functionalities, which depend on their different physicochemical properties. A balanced content of soluble and insoluble fiber is desirable, as both contribute differently and their effects on the body complement each other. While insoluble dietary fiber (IDF) has a high capacity for bile acid absorption and cation exchange, soluble dietary fiber (SDF) can reduce cholesterol and glucose absorption and is efficiently fermented by intestinal bacteria, which induces more significant development and diversity in the gut microbiota [,,]. The latter is associated with a lower incidence of metabolic diseases and obesity []. The proportion of both types of dietary fiber in the evaluated mango showed differences, with the proportion of IDF and SDF being similar in the Ataulfo and Criollo cultivars. With this characteristic, these two cultivars might contribute to better physiological effects on human health [].
Regarding nutraceutical components, Ataulfo, a popular variety known for its sweet and creamy texture, stood out with the highest contents of total phenolic compounds and flavonoids, while Manila, another widely grown variety, had the lowest levels of these phytochemical compounds. These differences between the cultivars have been previously reported []. Other studies have demonstrated that ‘Ataulfo’ contains significantly higher amounts of bioactive compounds and exhibits greater in vitro antioxidant capacity [,].
A strong correlation has also been demonstrated between higher in vitro antioxidant capacity and more content of phenolic compounds [], including the individual contribution of specific phenolic acids to antioxidant activity []. Similarly, it has been documented that Ataulfo exhibits a significant anticancer effect associated with a high concentration of polyphenols [].
The highest carotenoid content was found in the Haden cultivar, which can be related to its characteristic intense color, whereas Criollo had the lowest content. Ataulfo stood out for its high carotenoid pigment content, primarily vitamin A (beta-carotene). It has been reported to contain up to three times more vitamin A than the other commercial varieties in North America. It has a pigment and polyphenol content similar to that of the Ubá cultivar, which is popular in Brazil and distinguished by its abundance of phytochemical compounds compared to the other commercial cultivars []. ‘Criollo’ excelled in vitamin C content compared to the other cultivars, with a vitamin C contribution of 90 mg []; so, 100 g of fresh mango pulp from the cultivars in this study can provide between 12% and 73% of the RDI for vitamin C.
5. Conclusions
The nutritional, mineral, and nutraceutical qualities are dependent on the mango cultivar produced on the Southern Pacific coast of Mexico. The mango, and especially the Criollo cultivar, are considered nutritious and functional foods in diets because they have high concentrations of potassium, magnesium, sodium, zinc, ash, protein, fat, dietary fiber, and vitamin C. Manila fruits bring the iron necessary for diet. Haden mangoes provide calcium, manganese, carotenoids, and flavonoids, essential parameters in human recommended daily intake. Ataulfo fruits are appreciated for their international market sensory attributes, and in this study, it showed to be competitive in copper, sugars, phenolic compounds, DPPH, and vitamin A. The variability in the quality and composition of mango pulp highlights the importance of selecting appropriate cultivars that offer consumers the most significant nutritional benefits.
6. Limitations and Future Perspectives
This work is one of the first investigations carried out regarding the nutritional, chemical, and postharvest qualities of different mango cultivars produced in the southeastern region of Mexico (state of Guerrero); however, data were generated from only one harvest period. It is necessary to continue researching the quality of mangoes produced in this important production area, and to expand the research to fertilization protocols and/or technologies that help increase productivity and quality.
Author Contributions
Conceptualization, N.B.P.-M., M.D.M.-R. and C.S.-M.-H.; methodology, N.B.P.-M., J.Á.M.-G., F.A.-T. and R.V.-d.l.R.; software, C.A.L.-O.; validation, F.A.-T. and C.S.-M.-H.; formal analysis, J.Á.M.-G., M.D.M.-R., C.A.L.-O. and C.S.-M.-H.; investigation, N.B.P.-M., R.V.-d.l.R. and C.S.-M.-H.; resources, M.D.M.-R. and C.S.-M.-H.; data curation, N.B.P.-M., C.A.L.-O., and J.Á.M.-G.; writing—original draft preparation, N.B.P.-M.; writing—review and editing, M.D.M.-R., J.Á.M.-G., R.V.-d.l.R. and F.A.-T.; visualization, R.V.-d.l.R., F.A.-T. and J.Á.M.-G.; supervision, C.S.-M.-H. and C.A.L.-O.; project administration, C.S.-M.-H.; funding acquisition, C.S.-M.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
Fernando Mascada Bibiano, Rosario Morales, Enrique Soto Vaquera, and Fernando Mascada Jr. for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Servicio de Información Agroalimentaria y Pesquera. SIAP. Available online: http://nube.siap.gob.mx/cierreagricola/ (accessed on 22 March 2024).
- Echevarria Victorio, J.P.; Malpartida Yapias, R.J.; Castro Galarza, C.R.; Pérez Sullcaray, W. Características fisicoquímicas del mango (Mangifera indica L.) en dos variedades para su comercialización en la provincia de Chanchamayo–Junín. Kanyú 2023, 1, 55–65. [Google Scholar] [CrossRef]
- Maldonado-Astudillo, Y.I.; Navarrete-García, H.A.; Ortiz-Morales, Ó.D.; Jiménez-Hernández, J.; Salazar-López, R.; Alia-Tejacal, I.; Álvarez-Fitz, P. Propiedades físicas, químicas y antioxidantes de variedades de mango crecidas en la costa de Guerrero. Rev. Fitotec. Mex. 2016, 39, 207–214. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Yahia, E.M.; de Jesús Ornelas-Paz, J.; Brecht, J.K.; García-Solís, P.; Celis, M.E.M. The contribution of mango fruit (Mangifera indica L.) to human nutrition and health. Arab. J. Chem. 2023, 16, 104860. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Nutrient Data Laboratory. 2018. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169910/nutrients (accessed on 24 July 2024).
- Lazarte, C.V.; Nader-Macías, M.E.F. Aceptabilidad, Conocimiento, Consumo y Composición Química-Nutricional del Mango (Mangifera indica L.) y Productos Elaborados. Publitec SA 2016. Available online: http://wwww.publitec.com.ar/contenido/objetos/MANGO.pdf (accessed on 18 June 2024).
- Baeza-Herrera, L.; Jaen-Contreras, D.; San-Martín-Hernández, C.; Chávez-Franco, S.H.; López-Jiménez, A.; Muratalla-Lua, A.; García-Osorio, C. Changes in mango postharvest quality due to nitrogen-phosphorus-potassium dose and production season. Not. Bot. Horti Agrobot. 2024, 52, 13522. [Google Scholar] [CrossRef]
- Baeza-Herrera, L.; Jaén-Contreras, D.; San-Martín-Hernández, C.; Chávez-Franco, S.H.; López-Jiménez, A.; Muratalla-Lua, A. Efecto de la reducción de la fertilización en la calidad de mango ‘Ataulfo’ producido en la Costa Chica de Guerrero. Agro-Divulg. 2023, 3, 4. [Google Scholar] [CrossRef]
- Velasquez, A.M.; Contreras, R.; Baez, M.A.; Crisosto, C.H. La decoloración y el ablandamiento de la piel son los principales factores que afectan la calidad poscosecha del mango tratado con agua caliente (Mangifera indica L.). Sci. Hortic. 2023, 316, 112005. [Google Scholar] [CrossRef]
- Solis-Navarrete, J.A.; Bucio-Mendoza, S.; Stezano-Pérez, F. Innovaciones en biotecnología agroalimentaria: Mega tendencias en frutas tropicales mexicanas. World Pat. Inf. 2023, 74, 102207. [Google Scholar] [CrossRef]
- Astudillo-Miller, M.X.; Maldonado-Astudillo, R.I.; Segura-Pacheco, H.R.; Pallac Maldonado, Y. Cadenas de comercialización de mango y potencial exportador en la Costa Grande, Guerrero. Rev. Mex. Cienc. Agrícolas 2020, 11, 111–124. [Google Scholar] [CrossRef]
- CONAGUA. Comisión Nacional del Agua, Base de Datos del Gobierno de México. Estación Meteorológica Las Vigas, Guerrero, México. 2020. Available online: https://smn.conagua.gob.mx/es/ (accessed on 30 March 2024).
- Peverill, K.I.; Sparrow, L.A.; Reuter, D.J. Soil Analysis: An Interpretation Manual; CSIRO Publishing: Clayton, Australia, 1999; pp. 1–42. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 1224. [Google Scholar] [CrossRef]
- Aguilar-Santelises, A. Métodos de Análisis de Suelos; Sociedad Mexicana de la Ciencia del Suelo/Secretaría de Agricultura y Recursos Hidráulicos-Universidad Autónoma Chapingo: Chapingo, Estado de México, 1988. [Google Scholar]
- NOM-021-RECNAT-2000; Que Establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudio, Muestreo y Análisis. Declaratoria de Vigencia Publicada en el Diario Oficial de la Federación el 17 de octubre de 2000. SAGARPA: Mexico City, Mexico, 2000.
- NMX-FF-058-SCFI-2006; Especificaciones de Productos Alimenticios No Industrializados Para Consumo Humano–Fruta Fresca–Mango (Mangifera indica L.). Publicado por Instituto Mexicano de Normalización y Certificación: Mexico City, Mexico, 2006.
- AOAC. Official Methods of Analysis, 16th ed.; William, S., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1998. [Google Scholar]
- NOM-051-SCFI/SSA 1-2010; Especificaciones Generales de Etiquetado Para Alimentos y Bebidas No Alcohólicas Preenvasados-Información Comercial y Sanitaria. Publicada en el Diario Oficial de la Federación: Mexico City, Mexico, 2010.
- Megazyme. Sucrose, D-Fructose and D-Glucose Assay Procedure; K-SUFRG 04/18; USDA National Nutrient Database for Standard Reference; United States Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2018. [Google Scholar]
- Nikolidaki, E.K.; Chiou, A.; Christea, M.; Gkegka, A.P.; Karvelas, M.; Karathanos, V.T. Sun dried Corinthian currant (Vitis vinifera L., var. Apyrena) simple sugar profile and macronutrient characterization. Food Chem. 2016, 221, 365–372. [Google Scholar] [CrossRef]
- Gökmen, V.; Kahraman, N.; Demir, N.; Acar, J. Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. J. Chromat. 2000, 881, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.J.; Russell, R.M. Biodisponibilidad y bioconversión de carotenoides. Revisión Anu. Nutr. 2002, 22, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Gross, J. Pigments in Vegetables: Chlorophylls and Carotenoids; Van Nostrand Reinhold: New York, NY, USA, 1991; p. 351. [Google Scholar]
- Bushway, R.J.; Wilson, A.M. Determination of α– and β–Carotene in fruit and vegetables by high performance liquid chromatography. Can. Inst. Food Sci. Technol. J. 1982, 15, 165–169. [Google Scholar] [CrossRef]
- Pérez, T.; Aldana, M.I.; Rodríguez, L.I. IV. Validación de un Método para la Determinación de Beta-Caroteno en Aceite de Palma por HPLC con Detector UV. 2007. Available online: http://hdl.handle.net/20.500.12010/12650 (accessed on 10 December 2023).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC-DAD-MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Ghasemi, K.; Ghasemi, Y. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pakistan J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar] [PubMed]
- FDA. Nutrition Facts Labels. Daily Value and Percent. Daily Value on the Nutrition and Supplement Facts Labels; FDA: White Oak, MD, USA, 2023. [Google Scholar]
- Lebaka, V.R.; Wee, Y.-J.; Sí, W.; Korivi, M. Composición nutricional y compuestos bioactivos en tres partes diferentes de la fruta del mango. Int. J. Medio Ambiente Res. Salud Pública 2021, 18, 741. [Google Scholar] [CrossRef]
- Quintana-Obregón, E.; San Martín-Hernández, C.; Muy-Rangel, M.; Vargas-Ortiz, M. Valorization of mango (Mangifera indica L.) pericarp powders as an alternative for the generation of functional foods. TIP Rev Esp. Cienc. Quim. Biol. 2019, 22, 1–5. [Google Scholar] [CrossRef]
- Patel, G.N.; Kheni, J. Mango seed kernel, a highly nutritious food, should we continue to trash or use? J. Pharmacogn. Phytochem. 2018, 7, 4–7, 2349-8234. [Google Scholar]
- Shi, S.; Ma, X.; Xu, W.; Zhou, Y.; Wu, H.; Wang, S. Evaluation of 28 mango genotypes for physicochemical characters, antioxidant capacity, and mineral content. J. Appl. Bot. Food Qual. 2015, 88, 264–273. [Google Scholar] [CrossRef]
- Instituto de Investigaciones de Fruticultura Tropical. Apoyo al Fortalecimiento de Cadenas de Frutales a Nivel Local; Programa de las Naciones Unidas para el Desarrollo. Unión Europea; Ministerio de la Agricultura: La Habana, Cuba, 2023; ISBN 978-959-296-068-8.
- INCMNSZ. Tablas de Composición de Alimentos y Productos Alimenticios (Versión Condensada 2015); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Dirección de Nutrición, Departamento de Ciencia y Tecnología de los Alimentos: Ciudad de México, México, 2016; pp. 230–233. ISBN 978-607-7797-19-7. [Google Scholar]
- Daou, C.; Zhang, H. Functional and physiological properties of total, soluble, and insoluble dietary fibers derived from defatted rice bran. J. Food Sci. Technol. 2014, 51, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Isken, F.; Klaus, S.; Osterhoff, M.; Pfeiffer, A.F.H.; Weickert, M.O. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J. Nutr. Biochem. 2010, 21, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Fibra dietética soluble, uno de los nutrientes más importantes para la microbiota intestinal. Moléculas 2021, 26, 6802. [Google Scholar] [CrossRef]
- Rocha Ribeiro, S.M.; de Queiroz, J.H.; López Ribeiro De Queiroz, M.E.; Campos, F.M.; Pinheiro Sant’Ana, H.M. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum. Nutr. 2007, 62, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Perkins-Veazie, P. Influences of harvest date and location on the levels of β-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Bertoldi, M.C.; Krenek, K.; Talcott, S.T.; Stringheta, P.C.; Mertens-Talcott, S.U. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem. 2010, 58, 4104–4112. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Evaluación comparativa del contenido fenólico y la capacidad antioxidante in vitro en la pulpa y cáscara de cultivares de mango. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).